Abstract
Background:
Proinflammatory role of serum cholesterol in asthma has been recently explored with contradicting results. Clarity on the link between serum cholesterol and asthma may lead to new evolutions in planning management strategies. The objective of our study was to examine the relationship between the serum cholesterol, asthma and its characteristics.
Materials and Methods:
A total of 40 asthmatics and 40 normal subjects were examined cross-sectionally and their serum fasting cholesterol and serum high sensitivity C reactive protein (hsCRP) levels were measured along with other baseline investigations. All subjects were non-smokers.
Results:
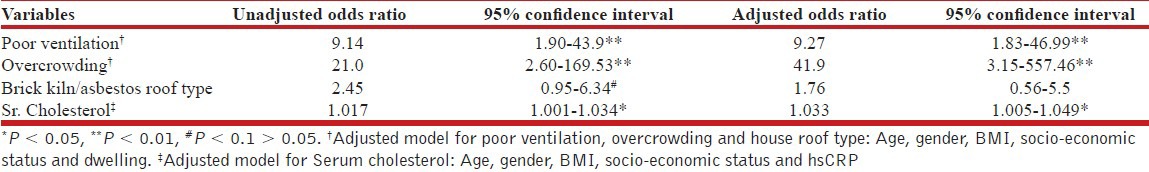
Serum total cholesterol (mean ± SD) among asthmatics was 176.45 ± 30.77 mgs/dL as compared to 163.33 ± 26.38 mgs/dL among normal subjects (P < 0.05). This higher serum cholesterol level was found to be associated with asthma independent of age, gender, body mass index (BMI), socio-economic status and serum hsCRP levels. However, the association was only modest (adjusted odds ratio 1.033; 95% confidence interval [CI] 1.008-1.059). There was no association between the serum cholesterol and asthma characteristics such as duration of illness, intake of inhaled steroids and frequency of emergency department visits. Other risk factors identified were poor ventilation (adjusted odds ratio 9.27; 95%CI 1.83-46.99) and overcrowding (adjusted odds ratio 41.9; 95% CI 3.15-557.46) at home.
Conclusion:
Our study found a modest but significant association between higher levels of serum cholesterol and asthma, which is independent of age, gender, BMI, socio-economic status and serum hsCRP. Future research is required in a larger population to substantiate above association and its clinical implications. Poor ventilation and overcrowding at home are risk factors for asthma possibly facilitating increased exposure to indoor allergens.
KEY WORDS: Asthma, cholesterol, high sensitivity C reactive protein, indoor hygiene, inflammation
INTRODUCTION
Asthma currently affects about 300 million people world-wide,[1] and with this, number of patients seeking specialized asthma care has also increased.[2] Global Initiative for Asthma (GINA) 2011, has defined asthma as a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role.[3] The chronic airway inflammation is associated with airway hyperresponsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning. Such a chronic inflammatory state in asthma is triggered and maintained at various levels by environmental factors. There are many host related factors that add upto inflammation and consequently increasing the occurrence of asthma. One such proinflammatory host factor that has gained interest among researchers in recent years is serum cholesterol level. It is well established that hypercholesterolemia is associated with enhanced expression of proinflammatory mechanisms leading to increased levels of proinflammatory cytokines,[4] cellular adhesion molecules[5] and inflammation sensitive plasma proteins.[6]
Going by these proinflammatory properties, it has been hypothesized that serum cholesterol may also potentiate eosinophilic inflammation in those with genetic susceptibility for asthma thereby leading to its increased phenotypic expression [Figure 1]. Studies at cellular level[7,8,9] have indicated an undeniable role of cholesterol metabolism in the pathogenesis of asthma and few studies have suggested that cholesterol trafficking and inflammation are coupled in the lung.[10,11,12] Evidence from animal models have substantiated above mentioned cellular mechanisms.[13,14,15] Most representative of these studies was by Yeh and Huang who showed in the murine model that increase in dietary cholesterol resulted in enhanced pulmonary allergic inflammation.[14] There was a significant correlation between serum cholesterol and elevated inflammatory markers in bronchoalveolar lavage fluid such as eosinophil counts, interleukin-5, prostaglandin E (2) and monocyte chemoattractant protein-1. Authors also found that the administration of pravastatin decreased pulmonary allergic inflammation. Similar anti-inflammatory effect of statins at varying doses has been demonstrated in other animal studies indicating its therapeutic potential in asthma.[16,17,18,19,20]
Figure 1.
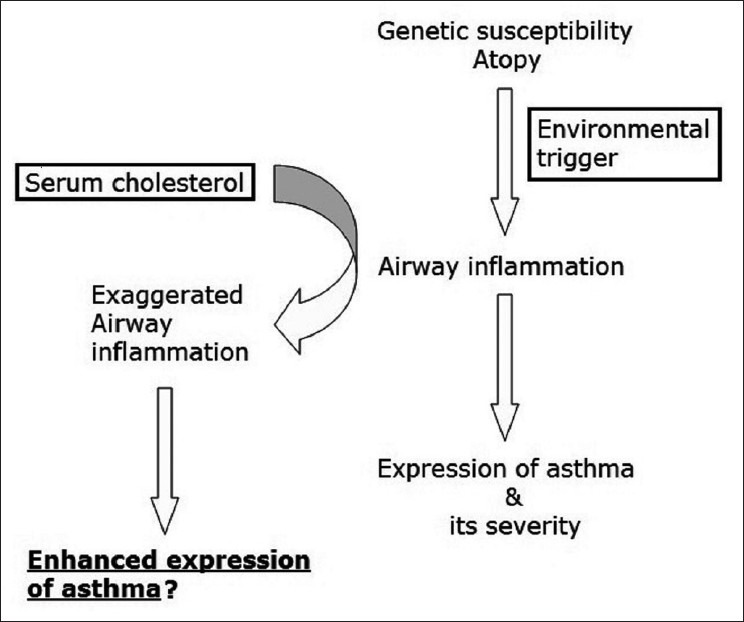
Hypothetical model of pro-inflammatory role of serum cholesterol in enhanced expression of asthma
Despite of encouraging results from fundamental research in support of the hypothetical link between serum cholesterol and asthma, clinical studies have yielded divergent results. While some studies have denied[21,22] any relationship between asthma and serum cholesterol, few others have shown positive[23] as well as negative[24,25] correlations. This has led to unclear knowledge about the subject of interest. Furthermore, the inflammatory link between asthma and cholesterol needs to be explored. Serum highly sensitive C reactive protein (hsCRP) as a surrogate marker of systemic inflammation may be useful in decoding this link. Addressing these gaps in knowledge is essentially important and may lead to newer strategies to tackle simultaneously two major diseases: Asthma and dyslipidemia. We attempted to evaluate the risk of asthma in relation to serum cholesterol level and to establish inflammatory link for the above relationship as proposed in the hypothetical model.
MATERIALS AND METHODS
This was a cross-sectional and case-control study involving asthmatic patients attending the chest clinic of a tertiary care center and an equal number of healthy volunteers. A sample size of 13 in each group was calculated to be sufficient to detect a mean difference of 14, with a power of 80% and significance of 5%, assuming a standard deviation of 12.8 based on previously published data.[23] Forty asthmatics were recruited whose diagnosis was established based on criteria suggested by the Expert Panel Report III of the National Institute of Health, USA.[26] Forty normal subjects were recruited for the control group. All study subjects were of age between 18 years and 40 years and were never smokers. Asthmatics did not have acute exacerbation or any clinically evident focus of infection, ongoing or within 2 weeks prior to the recruitment. Subjects with current or past history of diabetes mellitus, cardiovascular diseases, malignancy, systemic inflammatory disorders, nephrotic syndrome, familial hypercholesterolemia, liver diseases, thyroid disorders, and other conditions known to be associated with deranged lipid profile or C reactive protein (CRP) levels were excluded. Their treatment records were carefully reviewed and those with a history of current or recent intake of statins, systemic steroids and other drugs that are known or suspected to influence lipid metabolism were excluded. Besides above mentioned selection criteria, subjects recruited for the control group did not have current or past history of atopy or allergic diseases and also denied any family history of allergic diseases. This study was approved by institutional ethics committee and all subjects gave written informed consent before enrollment.
A detailed history about the symptoms was taken from all patients and general and systemic examination was carried out. The details regarding patient's socio-demographics including education, marital status, family size, dwelling and employment were obtained. Socio-economic status of the subjects was assessed by modified Prasad scale adjusted for All India Whole Price Index during the study period.[27,28] This scale include five socio-economic classes based on per capita income monthly: Class I: Rs. 4110 and above; Class II: Rs. 2050-Rs. 4109; Class III: Rs. 1230-Rs. 2049; Class IV: Rs. 620-Rs. 1229 and Class V: Rs. 620 and below. Adequate ventilation was defined as each room at home has at least one window into open space, which along with a door facilitates adequate cross ventilation. Overcrowding at home was defined when two persons over 9 years of age, not husband and wife, of opposite sexes are obliged to sleep in the same room.[29] In females, menstrual, and obstetric history was taken. A detailed history of disease characteristics such as duration of illness, treatment in the past, comorbidities both related and unrelated to asthma, family history and burden of environmental triggers at home as well as workplace, was recorded. Frequency of unscheduled visits to a health facility, for acute wheezing during 6 months prior to the recruitment was noted. All relevant clinical and laboratory/radiological details as available from medical records were noted. Following a detailed clinical evaluation, all subjects were sampled five milliliters of blood after fasting for about 8-10 h. The samples were allowed to clot and then centrifuged at 3,000 rpm for 5 min. Serum collected from the centrifuged sample was used to measure high hsCRP level by a hsCRP assay (Latex immunoturbidimetry method) with measurement range of 0.01-2 mg/dL (Cobas Integra 400 plus with Roche reagents, USA). Serum total cholesterol level was estimated by enzymatic colorimetric method (Cobas Integra 400 by Roche Diagnostics, USA).
Statistical analysis
Statistical analysis was carried out using SPSS 17 software. The data were examined initially for normality of distribution and homogeneity of variance. The comparison of quantitative variables between the groups was carried out by using Student t-test. Categorical variables were compared by using Chi-square test and odds ratio was evaluated for significant variables using logistic regression after adjusting for important confounders. Serum cholesterol level among asthmatics was correlated with clinicolaboratory variables and Pearson's correlation coefficient was derived. A P value of <0.05 was considered significant.
RESULTS
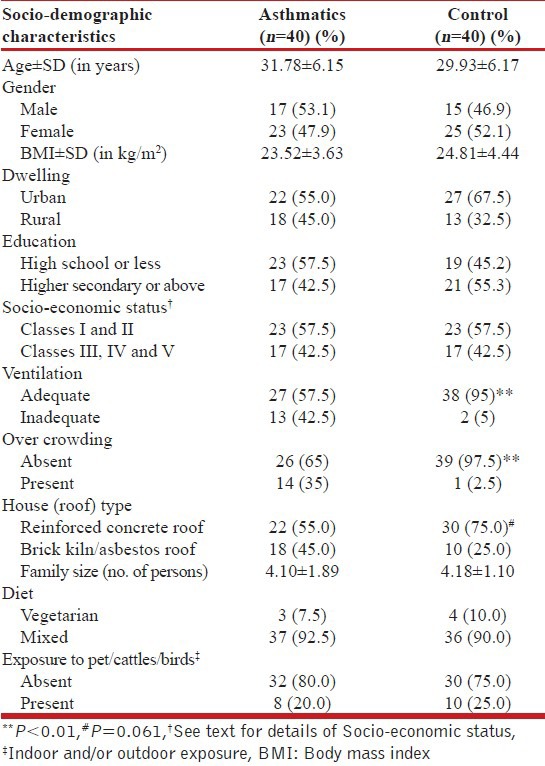
In all, 80 subjects were included in the study with 40 asthmatics and 40 normal subjects as control. Socio-demographic characteristics are summarized in Table 1. Both asthma and control groups were matched for age, gender, body mass index (BMI), socio-economic status and dwelling. There was no difference among the study groups in education level and exposure to pets, cattle or birds. Diet habits were not different between the groups with a vast majority of the study subjects consuming mixed diet, which included meat and sea food. Thirteen (32.5%) asthmatics reported inadequate ventilation at home compared to two (5%) control subjects reporting the same (P = 0.006). Similarly, 14 (35%) asthmatics reported overcrowding at home compared to only one (2.5%) in the control group (P = 0.004). There was a trend towards statistical significance for asthmatics (45%) living more commonly in houses with brick kiln or asbestos roofs than control subjects (25%) (P = 0.061).
Table 1.
Socio-demographic characteristics of study subjects
Mean serum cholesterol level (±SD) was 176.45 ± 30.77 mg/dL for asthma group, which was significantly higher than that of control subjects who had mean level of 163.33 ± 26.38 mg/dL [P = 0.044; Figure 2]. Results of logistic regression analysis of variables significantly associated with asthma are given in Table 2. Serum cholesterol level was significantly associated with asthma with an odds ratio of 1.033 (95% confidence interval [CI] 1.008-1.059) after adjusting for important covariates such as age, gender, BMI, socio-economic status and serum hsCRP level. Poor ventilation and overcrowding had a direct relationship with asthma with odds ratio of 9.27 (95% CI 1.83-46.99) and 41.9 (95% CI 3.15-557.46) respectively, after adjusting for age, gender, BMI, socio-economic status and dwelling. Relationship between house roof type and asthma did not withstand in the adjusted model.
Figure 2.
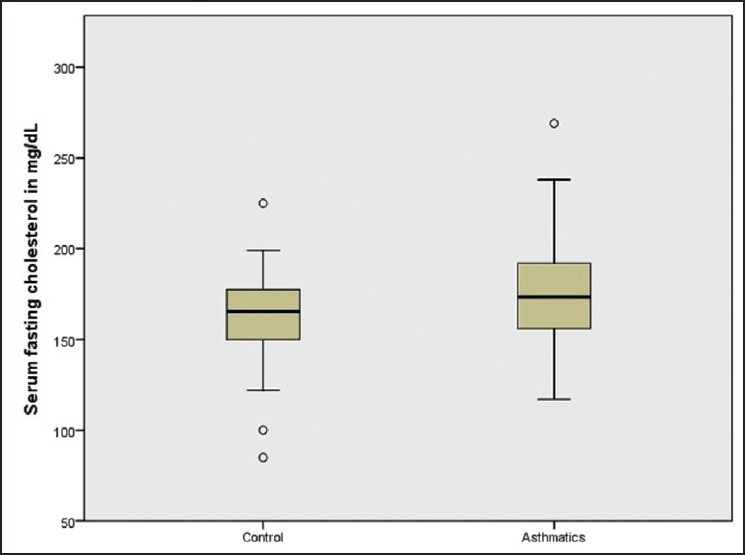
Box plot graphical representation of association between serum cholesterol and asthma. Mean value of serum cholesterol among asthmatics was 176.45±30.77 mg/dL as compared to 163.33±26.38 mg/dL among normal (control) subjects (P<0.05)
Table 2.
Logistic regression analysis of association between serum cholesterol and clinico-laboratory variables
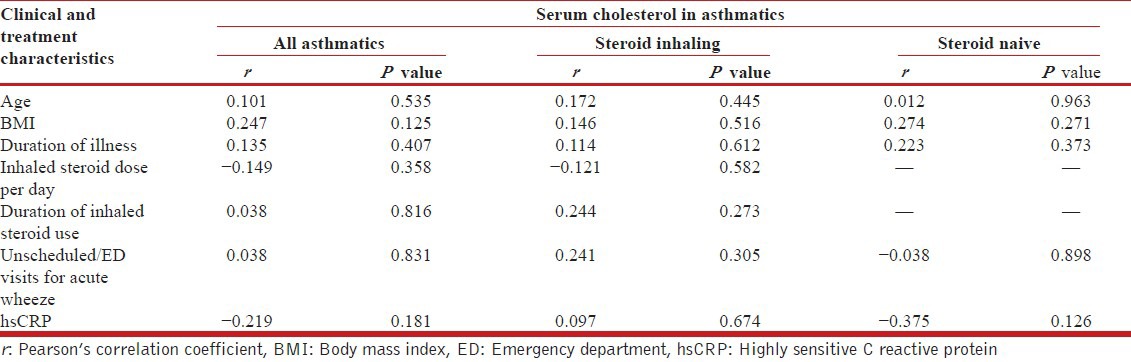
Further, we attempted to correlate serum cholesterol level with important clinical and laboratory variables among asthmatics [Table 3]. There was no significant correlation found between serum cholesterol and any of the tested variables among all asthmatics as well as steroid naïve and steroid inhaling subgroups.
Table 3.
Correlation between serum cholesterol level and clinical and treatment characteristics among asthmatics
DISCUSSION
In our study, we found that serum total cholesterol was directly associated with the risk of asthma. This association was independent of important confounders such as age, gender, BMI, socio-economic status and systemic inflammation. The effect size of this relationship was however, only modest. There was no correlation between serum cholesterol and duration of asthma, duration of inhaled steroid use, frequency of emergency department (ED) use or unscheduled visits for acute wheezing and level of systemic inflammation.
Contrary to the available evidence from fundamental research on the effect of hypercholesterolemia on airway inflammation, we find from clinical studies, variety of conclusions leading to unclear understanding about the role of serum cholesterol as a potentially modifiable risk factor of asthma. We found at least two studies that denied any association between asthma and serum cholesterol. Schäfer et al. in their nested case control study involving 1537 adult asthmatics in Germany found no significant relationship between serum cholesterol and asthma.[21] Similar findings were reported by Picado et al.,who found no difference in serum cholesterol between 121 controls and 118 asthmatics.[22] There are few studies, which have suggested an inverse relationship between serum cholesterol and asthma. Shenoi et al., found a lower level of cholesterol among 45 stable asthmatic children, which further lowered during exacerbations.[24] A report from the data base of National Health and Nutrition Examination Survey 2005-2006 showed an inverse relationship among Mexican Americans and no relationship among non-hispanic whites and blacks.[25] This report was the first to indicate a possible racial/ethnic disparity in the asthma–cholesterol association. To our knowledge, only one study that found serum cholesterol as a risk factor for asthma. Al-Shawwa et al. in a retrospective analysis of data from 188 subjects including 50 asthmatics aged between 4 years and 20 years, found significantly higher mean serum cholesterol level in asthmatics as compared to control subjects.[23] This study had several limitations including the fact that the study subjects had high risk for cardiovascular diseases, which might have contributed to very high effect size (odds ratio 7.54; 95% CI, 1.13-50.7) as reported by the authors. Thus, we find contradicting conclusions in literature, which may be explained by various factors like different age group and ethnicity studied as well as inherent methodological limitations in the study design.
We report a positive association between serum cholesterol level and asthma, which is consistent with findings of Al-Shawwa et al. However, we found only modest association. With every one SD increase in serum cholesterol level above the mean value, the risk of asthma increased by 3.3%. Given that the mean cholesterol value [Figure 2] in our study was far less than the cut-off level for cardiovascular risk,[30,31] the above reported modest risk for asthma cannot be neglected as clinically insignificant and requires further evaluation. Most of the cited studies were retrospective data analyses and hence had many confounders that could have biased their results. We examined the research question in a cross-sectional case control study design and attempted to address as many confounders as possible, known or suspected to influence the results. None of our study subjects had life style risk factors for dyslipidemia nor did they have any comorbid conditions that can influence the lipid metabolism independently. A meticulous review of their treatment history did not reveal any drug intake currently or in the recent past that could have contributed to a change in lipid levels. All asthmatic subjects had stable disease during and prior to enrollment in the study and hence did not use systemic steroids. Any incidental/undocumented use of systemic steroids for management of acute wheezing during last 6 months is unlikely to be a confounder since the frequency of ED use or unscheduled visits for acute wheezing did not correlate with serum cholesterol levels [Table 3]. Systemic effects of inhaled corticosteroids, in particular, its role in lipid metabolism, are shown to be none at doses of 400 μg or less of budesonide or equivalent daily.[3,22,32,33] Asthmatic subjects in our study were either steroidnaïve or on inhaled steroid doses not exceeding 400 μg of budesonide or equivalent per day, with variable treatment compliance. As cited, these doses of inhaled steroids are unlikely to have influenced the outcome of the study. Nevertheless, we found that inhaled steroid use by asthmatic subjects did not correlate with serum cholesterol level and thus, its influence on the study results was ruled out [Table 3].
Although we accept the hypothesis that serum cholesterol is a risk factor for asthma, our findings do not support the proposed inflammatory link between asthma and serum cholesterol. We found that the relationship between asthma and serum cholesterol was independent of systemic inflammation as measured by serum hsCRP. There may be other pathogenetic mechanisms such as cholesterol trafficking and mevalonate pathway, to explain the risk relating cholesterol with asthma, which require further evaluation. Recent research has found that homeostatic trafficking of cholesterol across the cell membrane is coupled with regulation of innate and adaptive immune response to environmental stimuli.[34,35] In brief, cholesterol synthesized in endoplasmic reticulum is partly internalized into lipid rafts of plasma membrane. Cholesterol content of these rafts is regulated by transporter mediated (ATP binding cassettes [ABC] type A1 and G1, scavenger receptor B1) efflux of free cholesterol in the plasma membrane to extracellular acceptors such as Apolipoprotein A1 and high density lipoprotein. Dysregulation of these transporter proteins stimulate cholesterol efflux thereby altering the content lipid rafts and sledding to hyperresponsiveness of cells (in particular macrophages) to external stimuli leading to inflammation.[8,36] In an animal model, abnormal cholesterol efflux has been shown to activate toll-like receptor signaling pathway leading to enhanced inflammation and lung damage.[12] Gupta et al. studied erythrocyte membrane of asthmatics and found significantly low cholesterol content in the lipid rafts that could have played a critical role in the pathogenesis of asthma.[7] Thus cholesterol trafficking rather than its absolute concentration in serum may be important in immunopathogenesis of asthma.
Another important mechanism that links cholesterol with inflammation is mevalonate pathway, which is on the limelight since the time statins were popularized for management of cardiovascular diseases. Statins non-selectively inhibit mevalonate pathway and thereby decreases cholesterol synthesis as well as nonsterol derivatives, which are called isoprenoids. Statins also exhibit anti-inflammatory effect, which is mediated by these nonsterol mevalonate products.[37] In asthma, anti-inflammatory effect of statins has been demonstrated in animal models.[16,17,18,19] However, in human studies statins’ effect on cellular and humoral mechanisms of inflammation did not translate well in clinical outcome parameters.[38,39,40,41] Lack of therapeutic effect of statins in asthma may be due to different dose concentrations used in animal and human studies as well as short term intervention protocol in most clinical trials.[42] Lipid independent effect of statins on inflammation suggest that higher cholesterol level in serum may merely indicate a hyperactive mevalonate pathway whose nonsterol products are responsible for exaggerated inflammation in asthma. Thus, a feeble relationship between serum cholesterol and asthma found in our study needs to be re-explored with specific reference to cholesterol trafficking and mevalonate pathway.
This study also found poor ventilation and overcrowding at home as independent risk factors for asthma. Subjects living in homes with poor ventilation or overcrowding had 9.27 times and 41.9 times greater risk of having asthma respectively. These variables common for Indian population may be seen as markers for poor indoor hygiene and consequently increased exposure to various domestic allergens such as house dustmite, animal dander, cockroach, fungi, molds etc. that are known to play an important role in the pathogenesis of asthma.
Our study has several limitations besides having unaccounted/unknown confounders that could have partly influenced our results. A smaller sample size could not accommodate interpretation of some variables at broader perspective. Age group of the sample was limited to 18-40 years because inclusion of wide range of age groups could have been possible only with larger sample size. Asthma control was not assessed with validated questionnaires or tools to evaluate for its effect of serum cholesterol. However, frequency of emergency use or unscheduled visits to general practitioners for acute wheezing during last 6 months as a marker of asthma control did not correlate with serum cholesterol levels. Role of other subtypes of lipids was not evaluated in our study, which might have facilitated further insight to the research question. In our attempt to explain the relationship between asthma and serum cholesterol, we used hs CRP, which is a non-specific inflammatory marker and may not reflect specific immunological interactions as discussed earlier. Our study design did not include such specific markers and hence could not conclude on inflammatory mechanism as core pathogenetic link.
In summary, our study has clearly established serum cholesterol as an independent risk factor for asthma. This risk is only modest whose clinical significance needs further evaluation. Serum cholesterol level does not correlate with duration of illness, duration of inhaled steroid use and asthma control. The association between serum cholesterol and asthma is found to be independent of systemic inflammation; however,we do not rule out other immunological and non-immunological pathways in the pathogenesis linking asthma and lipid metabolism. The findings of our study may represent specific age group and ethnicity of the sample and cannot be generalized for a multi-ethnic population or across all ages. Poor ventilation and overcrowding at home is associated with a significant risk for developing asthma possibly due to increased exposure to indoor allergens. This study emphasizes the need for re-exploration of pathogenetic link between lipid metabolism and asthma, which has paramount importance in this era when asthma and dyslipidemia are evolving as two epidemics possibly converging with each other at some point. Decoding this link may revolutionize the management of both these diseases. We recommend a large scale population based study to evaluate the role of serum cholesterol and its subtypes as modifiable risk factors of asthma. Future research with specific focus on cholesterol trafficking and nonsterol products of mevalonate pathway may be helpful in defining exact role of lipid metabolism in the pathogenesis of asthma. This direction may also provide targets for pharmacological interventions and paveway for interventional studies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.NHLBI/WHO workshop report: Global Strategy for asthma management and prevention. Global Initiative for Asthma (revised 2006) [Accessed 2012 Jan 27]. Available from: http://www.ginasthma.org .
- 2.Stirling RG, Chung KF. Severe asthma: Definition and mechanisms. Allergy. 2001;56:825–40. doi: 10.1034/j.1398-9995.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2011. [Accessed 2012 Jan 15]. Available from: http://www.ginasthma.org/guidelines-gina-report-global-strategy-for-asthma.html .
- 4.Stokes KY, Cooper D, Tailor A, Granger DN. Hypercholesterolemia promotes inflammation and microvascular dysfunction: Role of nitric oxide and superoxide. Free Radic Biol Med. 2002;33:1026–36. doi: 10.1016/s0891-5849(02)01015-8. [DOI] [PubMed] [Google Scholar]
- 5.Scalia R, Appel JZ, 3rd, Lefer AM. Leukocyte-endothelium interaction during the early stages of hypercholesterolemia in the rabbit: Role of P-selectin, ICAM-1, and VCAM-1. Arterioscler Thromb Vasc Biol. 1998;18:1093–100. doi: 10.1161/01.atv.18.7.1093. [DOI] [PubMed] [Google Scholar]
- 6.Engström G, Lind P, Hedblad B, Stavenow L, Janzon L, Lindgärde F. Effects of cholesterol and inflammation-sensitive plasma proteins on incidence of myocardial infarction and stroke in men. Circulation. 2002;105:2632–7. doi: 10.1161/01.cir.0000017327.69909.ff. [DOI] [PubMed] [Google Scholar]
- 7.Gupta P, Vijayan VK, Bansal SK. Sphingomyelin metabolism in erythrocyte membrane in asthma. J Asthma. 2010;47:966–71. doi: 10.1080/02770903.2010.517590. [DOI] [PubMed] [Google Scholar]
- 8.Fessler MB, Arndt PG, Frasch SC, Lieber JG, Johnson CA, Murphy RC, et al. Lipid rafts regulate lipopolysaccharide-induced activation of Cdc 42 and inflammatory functions of the human neutrophil. J Biol Chem. 2004;279:39989–98. doi: 10.1074/jbc.M401080200. [DOI] [PubMed] [Google Scholar]
- 9.Baumruker T, Csonga R, Pursch E, Pfeffer A, Urtz N, Sutton S, et al. Activation of mast cells by incorporation of cholesterol into rafts. Int Immunol. 2003;15:1207–18. doi: 10.1093/intimm/dxg120. [DOI] [PubMed] [Google Scholar]
- 10.Baldán A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol. 2008;180:3560–8. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 11.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–82. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 12.Goldklang M, Golovatch P, Zelonina T, Trischler J, Rabinowitz D, Lemaître V, et al. Activation of the TLR4 signaling pathway and abnormal cholesterol efflux lead to emphysema in ApoE-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1200–8. doi: 10.1152/ajplung.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson AK, Zhou X, Strandvik B, Hansson GK. Severe hypercholesterolaemia leads to strong Th2 responses to an exogenous antigen. Scand J Immunol. 2004;59:285–93. doi: 10.1111/j.0300-9475.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 14.Yeh YF, Huang SL. Enhancing effect of dietary cholesterol and inhibitory effect of pravastatin on allergic pulmonary inflammation. J Biomed Sci. 2004;11:599–606. doi: 10.1007/BF02256124. [DOI] [PubMed] [Google Scholar]
- 15.Yeh YF, Huang SL. Dietary cholesterol enhances pulmonary eosinophilic inflammation in a murine model of asthma. Int Arch Allergy Immunol. 2001;125:329–34. doi: 10.1159/000053834. [DOI] [PubMed] [Google Scholar]
- 16.Imamura M, Okunishi K, Ohtsu H, Nakagome K, Harada H, Tanaka R, et al. Pravastatin attenuates allergic airway inflammation by suppressing antigen sensitisation, interleukin 17 production and antigen presentation in the lung. Thorax. 2009;64:44–9. doi: 10.1136/thx.2007.094540. [DOI] [PubMed] [Google Scholar]
- 17.Kim DY, Ryu SY, Lim JE, Lee YS, Ro JY. Anti-inflammatory mechanism of simvastatin in mouse allergic asthma model. Eur J Pharmacol. 2007;557:76–86. doi: 10.1016/j.ejphar.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: Implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med. 2009;180:731–40. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeki AA, Bratt JM, Rabowsky M, Last JA, Kenyon NJ. Simvastatin inhibits goblet cell hyperplasia and lung arginase in a mouse model of allergic asthma: A novel treatment for airway remodeling? Transl Res. 2010;156:335–49. doi: 10.1016/j.trsl.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol. 2004;172:2903–8. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]
- 21.Schäfer T, Ruhdorfer S, Weigl L, Wessner D, Heinrich J, Döring A, et al. Intake of unsaturated fattyacids and HDL cholesterol levels are associated with manifestations of atopy in adults. Clin Exp Allergy. 2003;33:1360–7. doi: 10.1046/j.1365-2222.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- 22.Picado C, Deulofeu R, Lleonart R, Agustí M, Casals E, Quintó L, et al. Lipid and protein metabolism in asthma. Effects of diet and corticosteroid therapy. Allergy. 1999;54:569–75. doi: 10.1034/j.1398-9995.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 23.Al-Shawwa B, Al-Huniti N, Titus G, Abu-Hasan M. Hypercholesterolemia is a potential risk factor for asthma. J Asthma. 2006;43:231–3. doi: 10.1080/02770900600567056. [DOI] [PubMed] [Google Scholar]
- 24.Shenoi A, Kumar L, Sarihyan S, Gangully NK. High density lipoprotein cholesterol and total cholesterol in children with asthma and allergic rhinitis. Acta Paediatr. 1992;81:150–2. doi: 10.1111/j.1651-2227.1992.tb12192.x. [DOI] [PubMed] [Google Scholar]
- 25.Fessler MB, Massing MW, Spruell B, Jaramillo R, Draper DW, Madenspacher JH, et al. Novel relationship of serum cholesterol with asthma and wheeze in the United States. (e1-15).J Allergy Clin Immunol. 2009;124:967–74. doi: 10.1016/j.jaci.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NHLBI. National asthma education and prevention program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report. 2007. [Accessed 2012 Feb 01]. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf/
- 27.Kumar P. Social classification – need for constant updating. Indian J Community Med. 1993;18:60–1. [Google Scholar]
- 28.Agarwal A. Social classification: The need to update in the present scenario. Indian J Community Med. 2008;33:50–1. doi: 10.4103/0970-0218.39245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park K. Environment and health. In: Park K, editor. Text book of Preventive and Social Medicine. 17th ed. Jabalpur: M/S Banarsidas Bhanot Publishers; 2002. p. 528. [Google Scholar]
- 30.Study group, European Atherosclerosis Society. Strategies for the prevention of coronary heart disease: A policy statement of the European atherosclerosis society. Eur Heart J. 1987;8:77–88. [PubMed] [Google Scholar]
- 31.Cleeman JL. USA: NIH Publication No 01-3670; 2001. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) [Google Scholar]
- 32.Turpeinen M, Sorva R, Juntunen-Backman K. Changes in carbohydrate and lipid metabolism in children with asthma inhaling budesonide. J Allergy Clin Immunol. 1991;88:384–9. doi: 10.1016/0091-6749(91)90101-s. [DOI] [PubMed] [Google Scholar]
- 33.Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. Am J Respir Crit Care Med. 1998;157:S1–53. doi: 10.1164/ajrccm.157.3.157315. [DOI] [PubMed] [Google Scholar]
- 34.Gowdy KM, Fessler MB. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm Pharmacol Ther. 2012 doi: 10.1016/j.pupt.2012.06.002. PMID: 22706330. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187:1529–35. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diomede L, Albani D, Sottocorno M, Donati MB, Bianchi M, Fruscella P, et al. In vivo anti-inflammatory effect of statins is mediated by nonsterol mevalonate products. Arterioscler Thromb Vasc Biol. 2001;21:1327–32. doi: 10.1161/hq0801.094222. [DOI] [PubMed] [Google Scholar]
- 38.Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol. 2007;119:328–35. doi: 10.1016/j.jaci.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Simvastatin in the treatment of asthma: Lack of steroid-sparing effect. Thorax. 2010;65:891–6. doi: 10.1136/thx.2010.138990. [DOI] [PubMed] [Google Scholar]
- 40.Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax. 2008;63:1070–5. doi: 10.1136/thx.2008.100198. [DOI] [PubMed] [Google Scholar]
- 41.Moini A, Azimi G, Farivar A. Evaluation of atorvastatin for the treatment of patients with asthma: A double-blind randomized clinical trial. Allergy Asthma Immunol Res. 2012;4:290–4. doi: 10.4168/aair.2012.4.5.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering – Are they clinically relevant? Eur Heart J. 2003;24:225–48. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]


