Abstract
Background:
HIV–TB (tuberculosis) coinfection has emerged as a major public health threat. Given the multifactorial enabling environment in a resource-constrained setting like India, the consequences are of epidemic proportions.
Aims:
This study was aimed at identifying the clinical and epidemiological determinants underlying HIV–TB coinfection.
Settings and Design:
A retrospective review of patient records was done from the antiretroviral therapy center (ART) center at a district hospital in southern India between May and August 2012.
Materials and Methods:
Secondary data of 684 patients on ART as well as pre-ART were collected between July 2008 and June 2012 and were analyzed.
Statistical Analysis:
Descriptive analysis, χ2, and Wilcoxon signed rank tests were used with SPSS version 15.0 to draw significant statistical inferences.
Results:
HIV–TB coinfection was diagnosed in 18.9% with higher prevalence among males (75.3%), in the sexually active age group 31-45 years (61.3%), with less than primary education (44.15%), who were married (56.1%), laborers (42.4%), from rural backgrounds (88.2%), and having low income-earning capacity (94.4%). Transmission was predominantly through the heterosexual route. The key entry point was the integrated counseling and testing center (ICTC) (47.4%). Pulmonary tuberculosis (58.8%) was predominantly found followed by extrapulmonary tuberculosis (38.2%) and both in 3.1%. A favorable outcome was observed in 69.3% of coinfected patients with 89.2% on ART and 97.2% currently on DOTS therapy. The Wilcoxon signed-rank test found significant association between rises in CD4 counts after the 6th-month follow up (P < 0.05). Coinfected patients had a case fatality rate of 25%.
Conclusions:
The prevalence of HIV–TB coinfection recorded in this sample was 18.86%. ICTC implemented by NACO emerged as an effective entry point, while Revised National Tuberculosis Control Program referred 1.6% (n = 11) of the patients to the ART center. Coinfection is associated with lower CD4 counts than those with HIV alone, which could translate into increased morbidity and progression of HIV to AIDS.
KEY WORDS: ART, CD4, DOTS, HIV–TB coinfection, ICTC
INTRODUCTION
While HIV/AIDS and tuberculosis (TB) can individually be the major causes for concern as stand-alone public health threats, the combination of the two has proven to have a far greater impact on the epidemiologic progression and consequently on the impact it has on the global health scene. The dual infection has been termed “accursed duet”.[1] Research shows that of the opportunistic infections affecting HIV-infected patients, TB is found to be the most common with high risk for mortality[2,3] and the risk of coinfection with TB is about 20-37 times higher among those infected with HIV according to WHO. The 2009 report of UNAIDS estimated that 33.4 million people are living with HIV/AIDS with a third of them showing coinfection with TB. Globally, about 14.8% of patients with TB are coinfected with HIV.[4] About one in four deaths among people living with HIV are reportedly because of TB.[5,6] A 2010 report by the WHO reported that 360,000 people had died with active TB and HIV infection, indicating an increase from 2010 to 2011.[7]
As evidenced by several research reports globally, susceptibility to TB increases manifold with concurrent HIV infection. HIV increases the probability of recently acquired TB infection to progress to the status of active disease[8,9,10] and the co-occurrence of TB is not limited to the stage of HIV. It is fast becoming evident that the TB population should be seen as an important cohort to screen for HIV.[9,10] It has been documented that coinfection with HIV and Mycobacterium tuberculosis has a synergistic effect on each other, and in later stages of HIV infection, TB may present as extrapulmonary disease.[11]
India has a very high burden of TB according to the WHO, and infection with M. tuberculosis ranks foremost among opportunistic infections causing comorbidity with HIV infection.[1] The potentiating effect between HIV and TB is well established in studies from Africa, and evidence is gradually mounting in the Asian and Pacific regions as well. Rapid spread of HIV could lead to increasing burden of TB. India bears the burden of 2.5 million people infected with HIV. Of these, 40% suffer coinfection with TB.[12,13] There is wide variation in HIV seropositivity among TB patients in India, ranging from 9.4% in New Delhi and 30% in Mumbai.[14] In a resource-limited setting like India, this could have far reaching consequences.[15] Research has demonstrated that in resource-constrained settings up to 50% of patients with HIV without treatment but with concurrent TB would die prior to completion of the 6 to 8 months of treatment for TB, some as early as within the first 2 to 3 months. However, with the addition of prophylactic therapy for opportunistic infections, this proportion can be brought down to <10%. Thus, the importance of concurrent treatment for HIV and TB cannot be emphasized enough. Studies show TB is attributed to be the one of commonest causes of death among people living with HIV/AIDS (PLWHA) and development of multidrug resistant and extremely drug-resistant TB have increased morbidity and mortality.[16]
With the emergence of TB as a lethal counterpart in the epidemiology of HIV, there is an urgent need to understand possible multifactorial associations to this partnership. This study attempts to do just that in describing the underlying correlates to HIV-TB coinfection.
MATERIALS AND METHODS
A retrospective review of standardized patient records was conducted at the antiretroviral therapy center (ART) center of the Udupi district hospital in southern India between May and August 2012. Secondary data on 684 HIV–TB co-infected patients accessing services at the ART center including those on ART and pre-ART enrolled between July 2008 and June 2012 were included in the study.
The study aims at describing the sociodemographic and clinical profiles of HIV–TB coinfected patients. Following data collection, analysis was done using SPSS software version 15.0. The descriptive methods of analysis were used to profile the patients and to determine the relative burden of TB in this ART center. The χ2 Wilcoxon signed rank tests were used to draw significant statistical inferences.
RESULTS
Between July 2008 and June 2012, 3626 patients infected with HIV were registered at this ART center located at a district hospital in Southern India. The Voluntary Counseling and Testing Center (VCTC) now designated as the Integrated Counseling and Testing Center emerged as the key entry point for patients to this ART center (47.4%, n = 324). All patients were screened for PTB and EPTB with physical and sputum examinations, chest X-ray, and ultrasound of the abdomen. Records of patients diagnosed with active TB and presenting to the ART centre were reviewed. The diagnosis of PTB (58.8%, n = 402) dominated in the population sampled followed by EPTB in 38.2% (n = 261) and both (PTB and EPTB) in 3.1% (n = 21) with the trend of predominantly PTB being observed throughout the study duration. All coinfected patients were treated with DOTS under RNTCP. Infection with HIV was diagnosed at the center using three antibody tests as per the guidelines of the National AIDS Control Organization (NACO). The prevalence of HIV-TB coinfection in this sample was found to be 18.86% (n = 684).
Sociodemographic profile
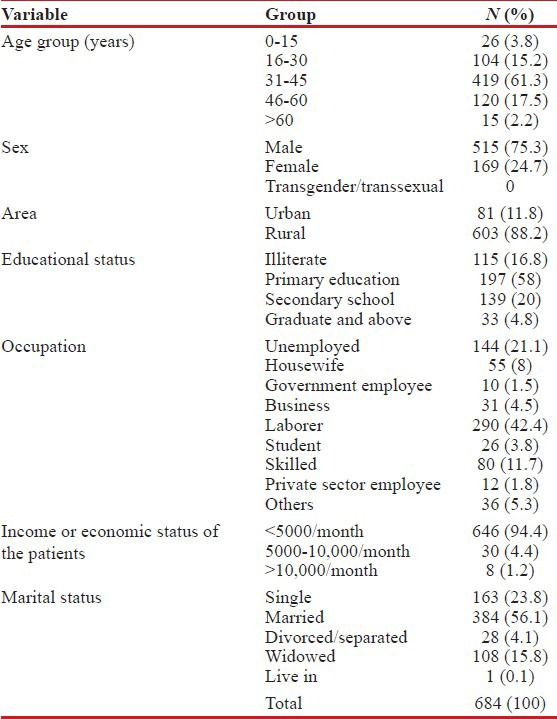
The rate of coinfection with HIV–TB in this study was found to be higher among males (75.3%, n = 515) in the sexually active age group of 31-45 years (61.3%, n = 419), hailing from rural areas (88.2%, n = 603), having less than primary level of education (74.9%, n = 512), working as laborers (42.4%, n = 290) and having low income-earning capacity (94.4%, n = 646). While married (56.1%, n = 384) individuals were seen to have higher rates of infection, the status of being divorced or separated was significantly associated (P = 0.037) with PTB. In addition comparable to studies across India,[12] the heterosexual route of transmission predominated in this population among both genders (males = 488 and females = 155) at 94% [Table 1].
Table 1.
Sociodemographic profile of coinfected patients
Clinico-epidemiological profile
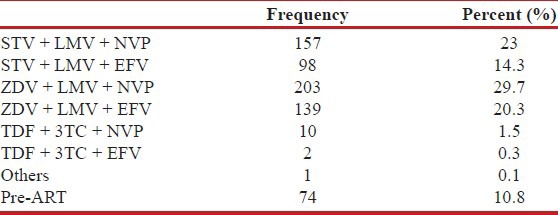
ART was started for eligible patients on the basis of CD4 counts in accordance with the National ART guidelines. Among this study population, 89.2% (n = 610) were on ART and 10.8% (n = 74) were pre-ART. Adherence was good with minimal loss to follow up of more than 3 months of 2.3% (n = 14) among those on treatment with the main cause of loss to follow up being death (18.9%, n = 129) [Table 2].
Table 2.
ART regimen among HIV–TB coinfected patients
Under the Revised National Tuberculosis Control Program (RNTCP) employing the Directly Observed Treatment, Short course (DOTS) initiative,[15] 97.2% (n = 665) of the sampled individuals with HIV-TB coinfection were under DOTS therapy at this facility.
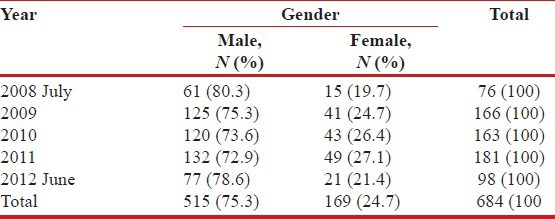
The proportion of HIV-TB coinfection among those registering at this particular ART center increased year wise from 10.6% (76/752) in 2008, 13.9% (166/1192) in 2009, 20.9% (163/779) in 2010, 30.6% (181/590) in 2011 to a high of 31.3% (98/313) in June 2012 until when data were included in this study.
The initial CD4 count was procured for 640 patients with HIV–TB. At initial presentation, the mean CD4 count was 174.47 cells/μL (median 156, range 18-755). Following 6 months of treatment, CD4 counts were assessed for 456 patients with a mean of 300.49 cells/μL (median 283, range 36-1181), and a mean rise of 118.13 cells/μL. A significant correlation was observed between CD4 rise at 6 months and initial CD4 count (P < 0.05). At the 12th month follow up, CD4 counts were available for 368 patients (mean 356.88, median 322, range 39-1389) and 288 patients were followed up at 24 months with a mean CD4 count of 409.26 cells/μL; all of whom exhibiting marked rise. The Wilcoxon signed rank test found significant association between rises in CD4 counts after the follow up at the sixth month (P < 0.05) [Graph 1].
Graph 1.
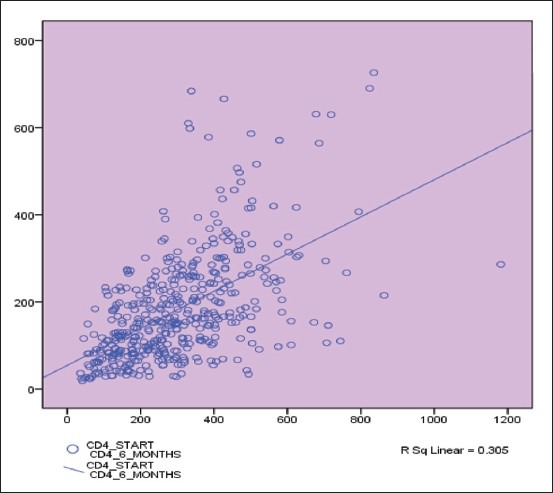
CD4 rise at 6 months correlated significantly to initial CD4 count (P < 0.05)
A diagnosis of PTB predominated at 60.2% (n = 103) among those who died, 33.9% (n = 58) with EPTB, and 2.1% (n = 11) with both PTB and EPTB. Patients with HIV–TB coinfection had an overall case fatality rate of 25% (n = 171) in this study. The case fatality rate due to PTB alone was 15.05%. Significant association was demonstrated between the vital status and type of TB (P = 0.032).
Sputum smear positivity for acid-fast bacilli was noted in 43.7% (n = 299) and negativity among 18.1% (n = 124) of the coinfected patients. Chest X-ray suggestive of PTB was seen in 21.6% (n = 148). The CD4 count among sputum positive cases was 176.68 cells/μL (median 153.50, range 19-666) and 189.59 cells/μL (median 156.50, range 24-684) among sputum negative cases. A favorable outcome was seen in 69.3% of the coinfected patients of whom 89.2% were on ART treatment and 97.2% (665) were under DOTS therapy as depicted in Table 3. During the course of TB treatment, all patients on ART were put on the Efavirenz regimen and after completion of the treatment, patients were substituted with Nevirapine [Table 4].
Table 3.
DOTS treatment and outcome among all HIV–TB coinfected patients
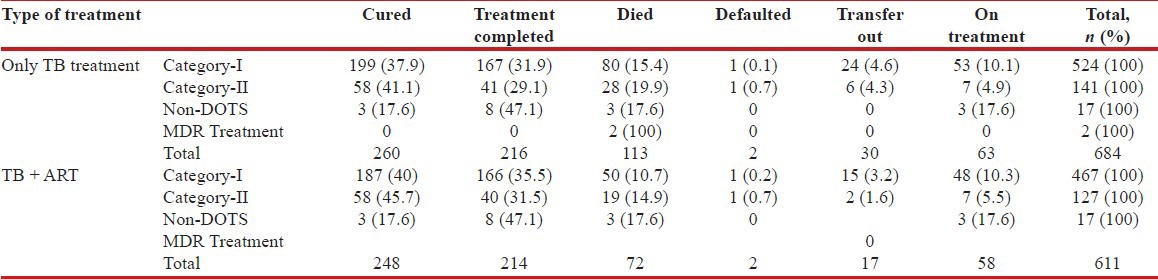
Table 4.
Year wise distribution of the HIV-TB coinfected patients (N=684)
DISCUSSION
This study aimed at drawing out the profile of individuals with dual infection of HIV–TB. A total 3626 HIV/AIDS patients reported in the ART center, Udupi district, between July 2008 and June 2012. Of them, 684 were reported HIV–TB coinfection, which indicates 18.86% prevalence in this sample. From this study, the profile emerged of higher prevalence of coinfection among males in the sexually active age group with little or no education, being married, working as laborers, living in the rural setting and belonging to the lower socioeconomic rung. These socio-demographic findings are comparable to other studies conducted in India.[17,18,19,20] Interestingly, married (56.1%) individuals were seen to have a higher rate of infection in comparison with single, divorced, or widowed individuals. This could be seen in light of the cultural drift toward the universality of marriage in the Indian context. However, the status of being divorced or separated was significantly associated (P = 0.037) with PTB in this population. The results of this study also showed that the heterosexual route of transmission was the most common indicating the need for intervention targeted at behavior modification.[21] Data accrued from this study pointed to the fact that VCTC, now designated as ICTC implemented by NACO emerged as an effective entry point for almost half (n = 324, 47.4%) of those sampled to access ART, while RNTCP referred 1.6% (n = 11) of the patients to the ART center. The mean CD4 count in this population at presentation was 174.47 cells/μL (median 156, range 18-755) with significant association (P < 0.05) between CD4 rise at 6 months, and the initial CD4 count was observed in this study comparable to a study conducted in Northern India.-[22] Coinfection is associated with lower CD4 counts than those with HIV alone, which could translate into increased morbidity and progression of HIV to AIDS. Several other research studies have pointed to the fact that CD4 counts are lower among coinfected patients as compared to HIV infected alone and severe immune suppression is seen in those with CD4 count below 200 cells/μL.[22,23] TB therapy is seen to have a positive influence on CD4 counts,[24] and the DOTS initiative has been demonstrated to prevent and even reverse the emergence of MDR-TB.[25]
It is worth noting that an increasing trend in the proportion of HIV–TB cases in this population from 10.6% in 2008 to 31.3% in June 2012 until when data were included in this study. In light of a WHO report in 2008 that only about 4% of individuals in India with TB get tested for concurrent HIV infection, this could be deciphered to mean that the case finding has improved since this last report. The Centers for Disease Control (CDC) has stated that TB is one of the few HIV related opportunistic infections that is both preventable as well as curable.[26] As observed in this study, treatment of HIV and TB comorbid conditions together had a favorable outcome with reduced risk of death comparable to a study by Cain et al.[27] Nevertheless, this rising trend needs to be further investigated to identify other underlying factors.
CONCLUSIONS
The prevalence of HIV–TB coinfection in this sample was 18.86%. About half (n = 324, 47.4%) of those sampled accessed ICTC as an entry point to the ART center. Coinfection was seen to be associated with reduced CD4 counts, which could hasten the progression to AIDS. It is imperative that physicians treating HIV-infected patients should aggressively identify those with M. tuberculosis in order to reduce the associated comorbidity resulting from the pairing of the infections, notwithstanding the imminent threat of multidrug-resistant and extremely drug-resistant TB on the rise. The increasing trend of HIV–TB cases observed in this population from 10.6% in 2008 to 31.3% in June 2012 is also a cause for concern. Greater focus of health interventions should be on the rural populace as 88% of those coinfected were from rural areas in this study. Creating grass root level awareness coupled with aggressive case finding in suspected high-risk population may be key in preventing and early detection of the dual infections.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jaiswal RK, Srivastav S, Mahajan H. Socio demographic profile of TB-HIV co-infected patients in Bundelkhand Region, Uttar-Pradesh. Natl J Med Res. 2012;2:149–51. [Google Scholar]
- 2.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 3.Lawn S, Churchyard G. Epidemiology of HIV associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–33. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geneva (Switzerland): Global tuberculosis control: Epidemiology, strategy, financing; 2009. World Health Organization World Health Organization; p. 411. WHO/HTM/TB/2009. [Google Scholar]
- 5.UNAIDS 2012. UNAIDS Annual Report. 2009. [Last accessed on August 14, 2013]. Available from: http://www.unaids.org/en/ Available from: http://data.unaids.org/pub/Report/2010/2009_annual_report_en.pdf .
- 6.WHO 2012. World Health Organization: TB-HIV 2011 Factsheet Source. [Last accessed on August 14, 2013]. Available from: http://www.who.int/en/ Available from: http://www.who.int/tb/publications/TBHIV_Facts_for_2011.pdf .
- 7.Joint United Nations Programme on HIV/AIDS (2011) World AIDS Day report. 2011. [Last accessed on 2013 April 17]. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2216_WorldAIDSday_report_2011_en.pdf .
- 8.Meya DB, McAdam KP. The TB pandemic: An old problem seeking new solutions. J Intern Med. 2007;261:309–29. doi: 10.1111/j.1365-2796.2007.01795.x. [DOI] [PubMed] [Google Scholar]
- 9.Girardi E, Raviglione MC, Antonucci G, Godfrey-Faussett P, Ippolito G. Impact of the HIV epidemic on the spread of other diseases: The case of tuberculosis. AIDS. 2000;14(Suppl 3):S47–56. [PubMed] [Google Scholar]
- 10.Gao L, Zhou F, Li X, Jin Q. HIV/TB co-infection in Mainland China: A meta-analysis. PLoS One. 2010;5:e10736. doi: 10.1371/journal.pone.0010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: Epidemiology, diagnosis and management. Indian J Med Res. 2005;121:550–67. [PubMed] [Google Scholar]
- 12.Marfatia YS, Sharma A, Modi M. Overview of HIV/AIDS in India. Indian J Sex Transm Dis. 2007;28:1–5. doi: 10.4103/2589-0557.55473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma SK. Co-infection of human immunodeficiency virus (HIV) and tuberculosis: Indian perspective. Indian J Tuberc. 2004;51:5–16. [Google Scholar]
- 14.Narain JP, Lo YR. Epidemiology of HIV-TB in Asia. Indian J Med Res. 2004;120:277–89. [PubMed] [Google Scholar]
- 15.Londhey VA. HIV and tuberculosis – A “cursed duo” in the HAART Era. [Last accessed on August 14, 2013]. Available from: http://www.japi.org/october_2009/article_01.pdf . [PubMed]
- 16.WHO 2012. [Last accessed onAugust 14, 2013]. Available from: http://www.who.int/en/ Available from: http://www.who.int/tb/publications/2010/factsheet_tb_2010.pdf .
- 17.Patel AK, Thakrar SJ, Ghanchi FD. Clinical and laboratory profile of patients with TB/HIV coinfection: A case series of 50 patients. Lung India. 2011;28:93–6. doi: 10.4103/0970-2113.80316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal U, Kumar A, Behera D. Profile of HIV associated tuberculosis at a tertiary institute in setting of free anti-retroviral therapy. J Assoc Physicians India. 2009;57:685–90. [PubMed] [Google Scholar]
- 19.Gupta P, Rawat J, Sindhwani G, Prasad R, Talekar M. HIV sero-prevalence and tuberculosis in Uttarakhand. Indian J Tuberc. 2006;53:96–100. [Google Scholar]
- 20.Ghiya R, Naik E, Beata C, Izurieta R, Marfatia Y. Clinico-epidemiological profile of HIV/TB coinfected patients in Vadodara, Gujarat. Indian J Sex Transm Dis. 2009;30:10–5. doi: 10.4103/2589-0557.55472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seifu L. Socio-demographic and clinical profile of AIDS patients in Jimma referral Hospital, Southwest Ethiopia. Ethiop J Health Dev. 2004;18:203–7. [Google Scholar]
- 22.Iredia CH, Oguntibeju OO, Lewis HA, Mokwena K. Trends and characteristics of patients admitted with musculoskeletal tuberculosis to a referral hospital from 2003 to 2008. Afr J Microbiol Res. 2011;5:532–40. [Google Scholar]
- 23.Vajpayee M, Kanswal S, Seth P, Wig N, Pandey RM. Tuberculosis infections in HIV-infected Indian Patients. AIDS Patient Care STDS. 2004;18:209–13. doi: 10.1089/108729104323038883. [DOI] [PubMed] [Google Scholar]
- 24.Martin DJ, Sim JG, Sole GJ, Rymer L, Shalekoff S, van Niekerk AB, et al. CD4+ lymphocyte count in African patients co-infected with HIV and tuberculosis. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:386–91. [PubMed] [Google Scholar]
- 25.Karnataka State AIDS prevention Society. 2012. [Last accessed on August 14, 2013]. Available from: http://www.ksaps.gov.in/ Available from: http://www.ksaps.gov.in/FAQ's.htm#12 .
- 26.Atlanta (GA): 2012. [Last updated on 2012 April 12; cited on 2012 Nov 1]. Tuberculosis: The connection between TB and HIV (the AIDS virus) Available from: http://www.cdc.gov/tb . Available from: http://www.cdc.gov/tb/publications/pamphlets/TB-HIVEng.PDF . [Google Scholar]
- 27.Cain KP, Anekthananon T, Burapat C, Akksilp S, Mankhatitham W, Srinak C, et al. Causes of death in HIV-infected persons who have tuberculosis, Thailand. Emerg Infect Dis. 2009;15:258–64. doi: 10.3201/eid1502.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]


