Abstract
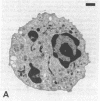
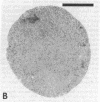
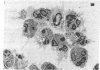
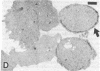
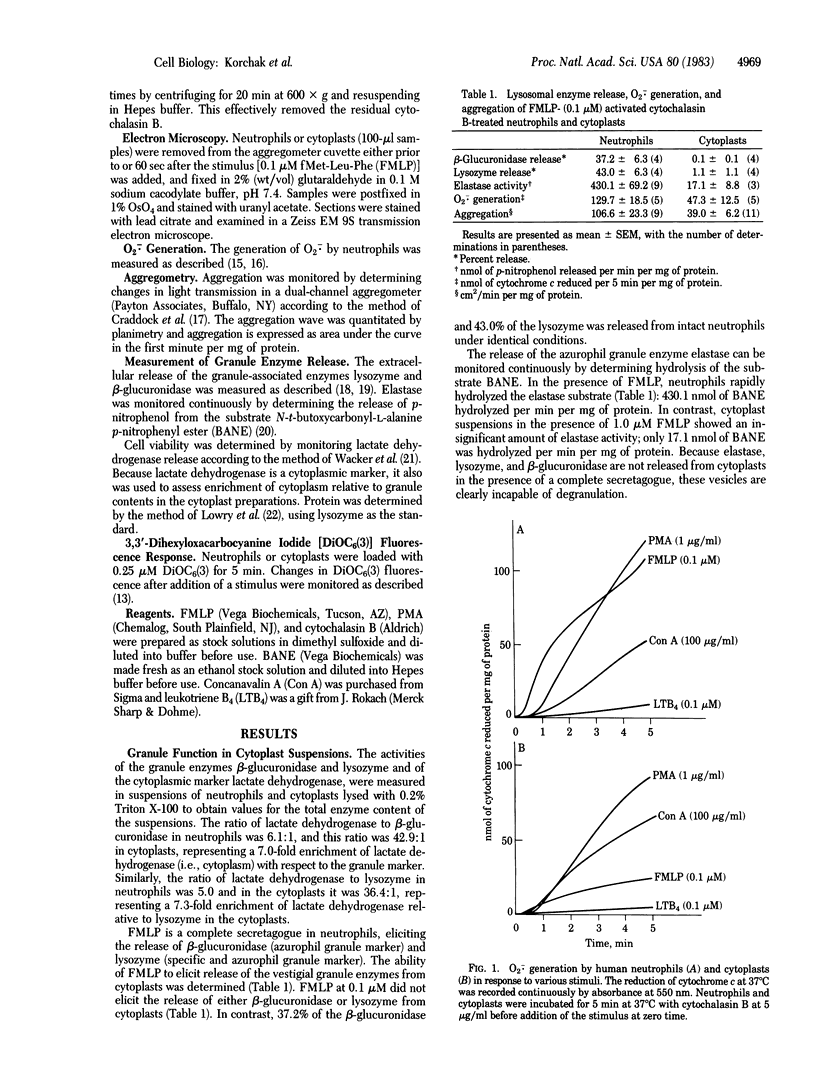
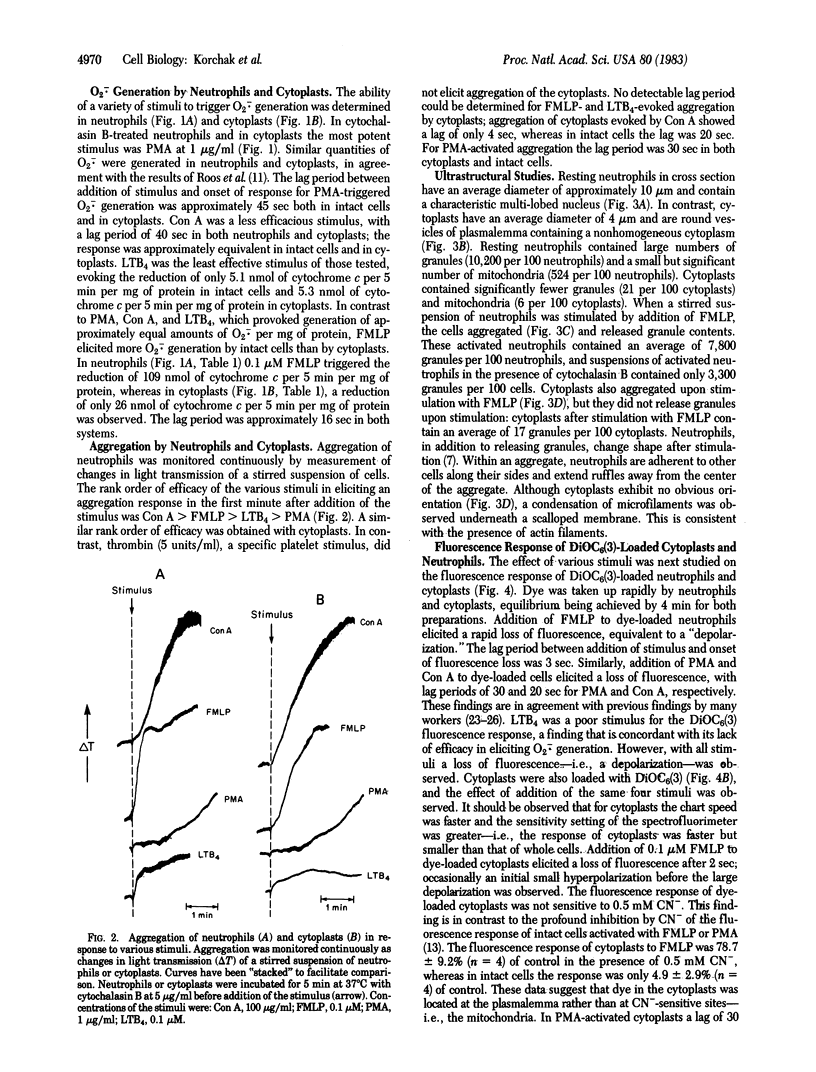
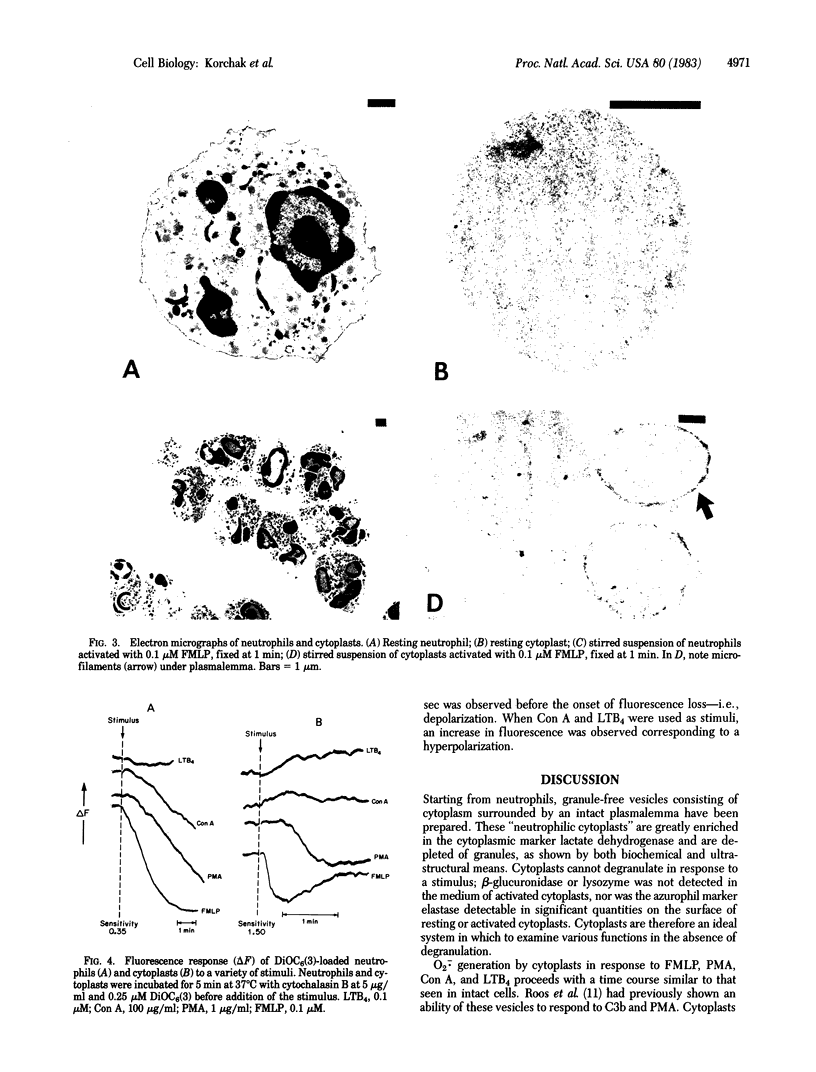
Neutrophils respond to a variety of stimuli by generating superoxide anion, degranulating, and aggregating. Because it has been suggested that fusion of granules with the plasmalemma (degranulation) is necessary for aggregation and superoxide anion generation, we have tested whether these responses can be demonstrated in "neutrophilic cytoplasts" (granule-free vesicles of cytoplasm enclosed by plasmalemma). When examined by electron microscopy, cytoplasts were found to be approximately 4 microns in diameter and essentially granule free. Cytoplasts exposed to fMet-Leu-Phe (0.1 microM) generated superoxide anion after a lag of 16 sec but released no detectable beta-glucuronidase, lysozyme, or elastase. Aggregation of cytoplasts, as measured by changes in light transmission, was also activated by fMet-Leu-Phe; no lag period was observed. Electron microscopy of the aggregates demonstrated clusters of cytoplasts with a scalloped appearance. Superoxide anion generation and aggregation of cytoplasts were also activated by phorbol 12-myristate 13-acetate, concanavalin A, and leukotriene B4. Exposure of cytoplasts to the dye 3,3'-dihexyloxacarbocyanine iodide (DiOC6(3)] led to dye uptake and enhancement of fluorescence, implying that the vesicles were sealed and maintained a membrane potential across the plasmalemma. Exposure of DiOC6(3)-loaded cytoplasts to fMet-Leu-Phe and PMA caused a rapid loss of dye fluorescence that was not inhibited by CN-, compatible with their lack of mitochondria. Exposure of dye-loaded cytoplasts to concanavalin A or leukotriene B4 caused an increase in fluorescence--i.e., a hyperpolarization. These results demonstrate that degranulation is not a prerequisite for aggregation or superoxide anion generation. The retention of ionic gradients and changes in membrane potential, as measured by DiOC6(3) fluorescence changes, suggest a fundamental role for ionic movements in activating superoxide anion generation and aggregation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baici A., Salgam P., Fehr K., Böni A. Inhibition of human elastase from polymorphonuclear leucocytes by gold sodium thiomalate and pentosan polysulfate (SP-54). Biochem Pharmacol. 1981 Apr 1;30(7):703–708. doi: 10.1016/0006-2952(81)90154-4. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Coates T. D., Haak R. A., Wolach J. B., Hoffstein S., Baehner R. L. Lactoferrin deficiency associated with altered granulocyte function. N Engl J Med. 1982 Aug 12;307(7):404–410. doi: 10.1056/NEJM198208123070704. [DOI] [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M. P., Seligmann B. E., Gallin J. I. Correlation of human neutrophil secretion, chemoattractant receptor mobilization, and enhanced functional capacity. J Immunol. 1982 Feb;128(2):941–948. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S. T., Friedman R. S., Weissmann G. Degranulation, membrane addition, and shape change during chemotactic factor-induced aggregation of human neutrophils. J Cell Biol. 1982 Oct;95(1):234–241. doi: 10.1083/jcb.95.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. S., VanDyke K., Castranova V. Transmembrane potential changes associated with superoxide release from human granulocytes. J Cell Physiol. 1981 Jan;106(1):75–83. doi: 10.1002/jcp.1041060109. [DOI] [PubMed] [Google Scholar]
- Kaplan H. B., Edelson H. S., Friedman R., Weissmann G. The roles of degranulation and superoxide anion generation in neutrophil aggregation. Biochim Biophys Acta. 1982 Sep 13;721(1):55–63. doi: 10.1016/0167-4889(82)90023-4. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Rich A. M., Wilkenfeld C., Rutherford L. E., Weissmann G. A carbocyanine dye, DiOC6(3), acts as a mitochondrial probe in human neutrophils. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1495–1501. doi: 10.1016/s0006-291x(82)80076-4. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mehta J., Mandell B., Kleyman T., Spilberg I. Demonstration of calcium-dependent chemotactic factor activatable esterase activity in human neutrophils: relationship with chemotaxis and chemotactic deactivation. Inflammation. 1981 Jun;5(2):89–101. doi: 10.1007/BF00914199. [DOI] [PubMed] [Google Scholar]
- Oseas R. S., Allen J., Yang H. H., Baehner R. L., Boxer L. A. Rabbit cationic protein enhances leukocyte adhesiveness. Infect Immun. 1981 Aug;33(2):523–526. doi: 10.1128/iai.33.2.523-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Dri P., Fant L., Basford R. E., Rossi F. NADPH oxidizing activity in rabbit polymorphonuclear leukocytes: localization in azurophilic granules. Biochem Biophys Res Commun. 1973 Aug 6;53(3):830–837. doi: 10.1016/0006-291x(73)90168-x. [DOI] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin J. I. Use of lipophilic probes of membrane potential to assess human neutrophil activation. Abnormality in chronic granulomatous disease. J Clin Invest. 1980 Sep;66(3):493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan E. P., Crawford D. R., Schneider D. L. Isolation of plasma membrane from human neutrophils and determination of cytochrome b and quinone content. J Exp Med. 1981 May 1;153(5):1316–1328. doi: 10.1084/jem.153.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. Biochemical demonstration of the activatable esterase of the rabbit netrophil involved in the chemotactic response. J Immunol. 1970 Nov;105(5):1057–1067. [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]