Abstract
Chronic obstructive pulmonary disease (COPD) exacerbations admitted in intensive care units (ICUs) occur rarely due to fungal respiratory tract infections, but may occur when associated co-morbidities like diabetes mellitus coexist. Candida ciferrii is a new agent, recently was isolated from lung infections but usually resistant to fluconazole. Here, we report a rare case of pneumonia due to fluconazole sensitive Candida ciferrii in a COPD patient with known diabetes, admitted in our ICU.
KEY WORDS: Candida ciferrii, chronic obstructive pulmonary disease, fluconazole sensitive, pneumonia
INTRODUCTION
Intensive care unit (ICU) admission in chronic obstructive pulmonary disease (COPD) patients is generally due to infections by bacterial or viral agents, but fungal agent may occur in immunocompromised states. Among fungal agents Candida is the most common species, of which a new fluconazole resistant strain of Candida named Candida ciferrii has been found to be frequently associated with systemic mycosis in immunocompromised hosts.[1] Here, we report a rare case of pneumonia due to fluconazole sensitive Candida ciferrii in a patient of COPD exacerbation with underlying diabetes.
CASE REPORT
A 55-year-old, female, bidi smoker, suffering from moderate COPD (GOLD guidelines) for 2 years, presented to the emergency room with progressive increase in dyspnea and cough with copious mucopurulent expectoration for 15 days and 10 days respectively. Before her exacerbation, her symptoms were well controlled with tiotropium and a fixed dose combination of formoterol and budesonide. On general examination, she was tachypneic (respiratory rate 30/min); had cyanosis and used her accessory muscles of respiration. Respiratory system examination revealed diminished vesicular breath sound with prolonged expiration on left lower zone; bilateral polyphonic rhonchi all over the chest and coarse biphasic crackles over bilateral lung bases more on the right side. Her peripheral oxygen saturation was 74% in room air during the admission and improved to 88-92% with oxygen through face mask at a flow of 8 L/min. Her arterial blood gas showed pH 7.43, PaO2 70 mmHg, PaCO2 36 mmHg, PaO2/FiO2 234, suggestive of acute lung injury. She was a known diabetic for 15 years and had needed insulin for the control of diabetes for the past 6 months. On admission blood culture; sputum for gram stain, Ziehl-Neelsen (Z-N) stain and pyogenic culture with sensitivity were asked for. She received piperacillin-tazobactum (4.5 g) 6 hourly and azithromycin (500 mg) once daily, along with nebulization by salbutamol + ipratropium, injectable deriphylline and intravenous fluids. Her chest X-ray showed bilateral lower zone heterogeneous opacity, more on left side with clear costophrenic angles on lateral chest X-ray suggestive of bilateral pneumonia [Figure 1a]. Total leukocyte count was 11,700/mm3 with neutrophil 67%, lymphocytes 28%, eosinophils 2%, basophil 1% and monocytes 2%. Serum urea was 34 mg%, creatinine 0.7 mg%, sodium 138 mEq/L, potassium 3.5 mEq/L. Her glycosylated hemoglobin level was 11.8 indicating uncontrolled diabetes. Sputum gram staining and Z-N staining were negative. Since, her condition did not improve after 2 days a computed tomographic scan of thorax was requested for further evaluation, which revealed left lower lobe collapse with consolidation along with patchy pulmonary infiltrations in the right middle and lower lobe [Figure 1b]. Fiber-optic bronchoscopy was carried out to know the cause of collapse, which showed patchy whitish lesion in the lateral wall of trachea just above the carina with a mucous plug occluding the lumen of the left main bronchus and purulent discharge from right main bronchus [Figure 2]. Bronchoalveolar lavage fluid (BAL) was taken from those segments with clearing of that mucous plug and BAL fluid was sent for cytological examination, gram staining, fungal staining, Z-N staining, malignant cells, mycobacterial culture, and fungal culture. The antimicrobial susceptibility test was performed by microbroth dilution method by VITEK-2. Fungal staining of BAL and mucosal biopsy specimen showed yeast forms suggestive of Candida species [Figure 3]. The fungal culture showed moderate growth of Candida ciferrii. Intravenous liposomal amphotericin B (150 mg daily) was initiated and changed to oral fluconazole (150 mg daily) after 4 days, when drug sensitivity revealed that the strain was fluconazole sensitive [Table 1]. After 4 days, she showed remarkable recovery and maintained oxygen saturation on room air. Patient was discharged after 2 weeks on oral fluconazole 150 mg daily. Chest X-ray on follow-up after 6 weeks showed marked improvement [Figure 4]. Antifungal therapy stopped after 3 months.
Figure 1.
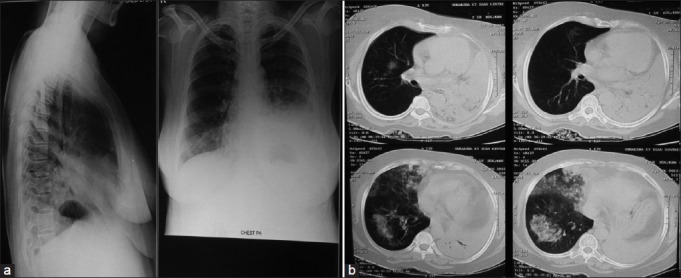
Chest X-ray (a) showing bilateral lower zone heterogeneous opacity, more on left side with clear costophrenic angles on lateral X-ray suggestive of bilateral pneumonia and computed tomographic scan of thorax (b) Showing left lower lobe collapse with consolidation along with patchy pulmonary infiltrations in right middle and lower lobes
Figure 2.
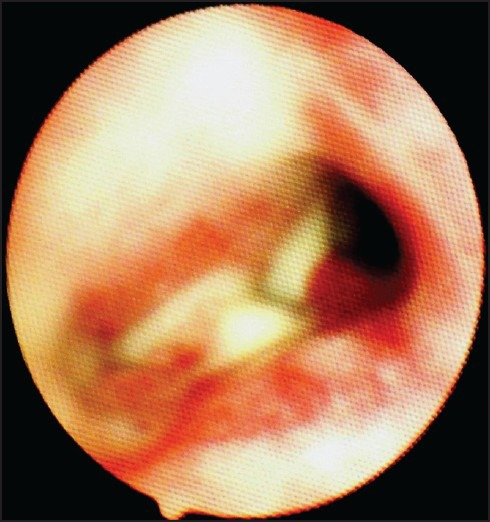
Fibre-optic bronchoscopy showing mucous plug occluding the lumen of the left main bronchus and pus coming out from right main bronchus
Figure 3.
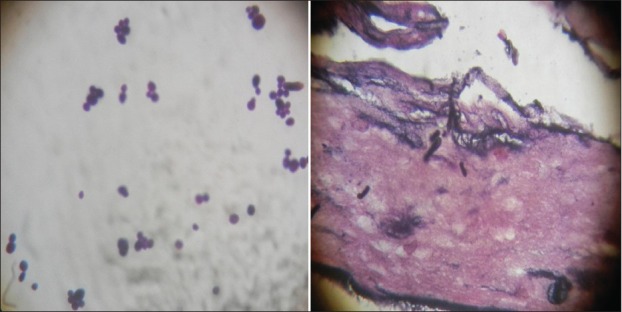
Fungal staining of bronchoalveolar lavage fluid and mucosal biopsy specimen showing yeast forms suggestive of Candida species
Table 1.
Sensitivity pattern of Candida ciferrii to antimicrobial agents
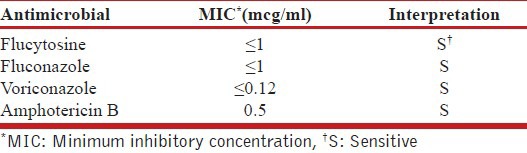
Figure 4.
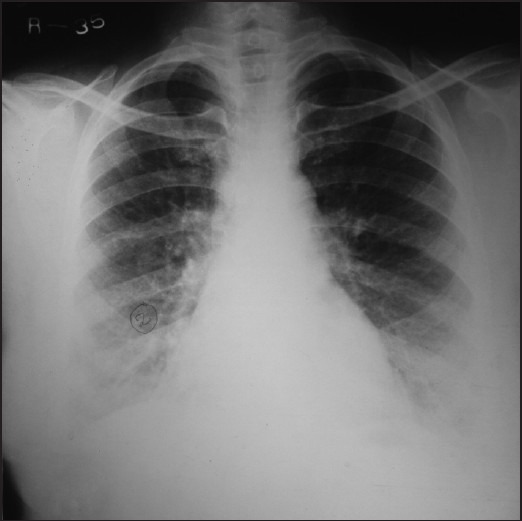
Chest X-ray showing marked improvement and clearance of alveolar opacities after 6 weeks of antifungal therapy
DISCUSSION
COPD exacerbation is an acute event characterized by worsening of patient symptoms that is beyond normal day to day variations and leads to change of medications.[2,3] The most common cause of COPD exacerbations appears to be respiratory tract infections mainly bacterial or viral. Fungal pneumonia may be a cause of exacerbation especially in immunocompromised patients. Candida spp. constituted to normal flora of the human skin, oropharynx, lower gastrointestinal tract, and genitourinary system.[4] The incidence of deep Candida infections resistant to common antifungal agents has increased in recent years especially among immunocompromised patients such as diabetes mellitus, prolonged high dose glucocorticoid (GC) use, transplant recipients, and human immunodeficiency virus infected patients.[5,6]
Candida is a part of the normal flora in the oropharynx and gastrointestinal tract, so growth of Candida from upper respiratory samples is frequently disregarded as a contaminant.[6,7] Although, Candida spp. can be isolated from bronchial washings, tracheal aspirates, and BAL samples of patients, but accompanying lung parenchymal invasion is infrequent; Hence, demonstration of Candida spp. from lower respiratory tract along with parenchymal invasion conclusively proves Candidal pneumonia.
Candida ciferrii is a newer strain of Candida, which has been rarely reported as a cause of human infection. However, in immunocompromised host it can cause human infection.[1,8] Most of the reported cases by Candida ciferrii include malignant otitis externa and onychomycosis.[9,10] In the literature there was only one reported case of lung involvement by Candida ciferrii in the form of spotted pulmonary infiltrations, which was resistance to fluconazole.[1] The Candida ciferrii strain in our case was sensitive to fluconazole with minimum inhibitory concentration ≤1 μg/ml and it implies the need for anti-microbial susceptibility testing before starting therapy.
To the best of our knowledge this is the first case report of recovery of fluconazole sensitive Candida ciferrii in a COPD patient admitted with pneumonia in an ICU.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gunsilius E, Lass-Flörl C, Kähler CM, Gastl G, Petzer AL. Candida ciferrii, a new fluconazole-resistant yeast causing systemic mycosis in immunocompromised patients. Ann Hematol. 2001;80:178–9. doi: 10.1007/s002770000252. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117:398S–401S. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- 3.Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29:1224–38. doi: 10.1183/09031936.00109906. [DOI] [PubMed] [Google Scholar]
- 4.Pappas PG. Invasive candidiasis. Infect Dis Clin North Am. 2006;20:485–506. doi: 10.1016/j.idc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Bouza E, Muñoz P. Epidemiology of candidemia in intensive care units. Int J Antimicrob Agents. 2008;32:S87–91. doi: 10.1016/S0924-8579(08)70006-2. [DOI] [PubMed] [Google Scholar]
- 6.Knox KS, Meinke L. Role of bronchoalveolar lavage diagnostics in fungal infections. Clin Chest Med. 2009;30:355–65. doi: 10.1016/j.ccm.2009.02.010. viii. [DOI] [PubMed] [Google Scholar]
- 7.el-Ebiary M, Torres A, Fàbregas N, de la Bellacasa JP, González J, Ramirez J, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156:583–90. doi: 10.1164/ajrccm.156.2.9612023. [DOI] [PubMed] [Google Scholar]
- 8.García-Martos P, Ruiz-Aragón J, García-Agudo L, Saldarreaga A, Lozano MC, Marín P. Candida ciferrii in an immunocompromised patient. Rev Iberoam Micol. 2004;21:85–6. [PubMed] [Google Scholar]
- 9.Hazen KC. New and emerging yeast pathogens. Clin Microbiol Rev. 1995;8:462–78. doi: 10.1128/cmr.8.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin Grandis J, Branstetter BF, 4th, Yu VL. The changing face of malignant (necrotising) external otitis: Clinical, radiological, and anatomic correlations. Lancet Infect Dis. 2004;4:34–9. doi: 10.1016/s1473-3099(03)00858-2. [DOI] [PubMed] [Google Scholar]


