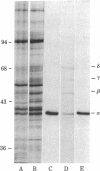
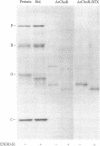
Abstract
The direct binding of alpha-bungarotoxin to the alpha subunit of the acetylcholine receptor from Torpedo electric organ immobilized onto protein blots was demonstrated. Protein blots were prepared by electrophoretically transferring resolved acetylcholine receptor subunits from 10% polyacrylamide gels onto Zetabind, positively charged nylon membrane filters. Such blots, when incubated with 125I-labeled alpha-bungarotoxin, washed, and autoradiographed, gave rise to a single labeled band corresponding to the alpha subunit of the receptor. The labeling with alpha-bungarotoxin could be inhibited by pretreating the receptor-containing membranes with the affinity ligand 4-(N-maleimido)-alpha-benzyltrimethylammonium iodide. In addition, the association of toxin with the alpha subunit could be inhibited by d-tubocurarine (IC50 = 0.9 mM). Furthermore, removal of high-mannose oligosaccharide chains from the alpha subunit by treatment with endoglycosidase H did not interfere with the observed toxin binding. Thus it is demonstrated that isolated, immobilized alpha subunit of the Torpedo acetylcholine receptor can bind alpha-bungarotoxin. However, the observed binding of alpha-bungarotoxin to immobilized alpha subunit is reduced in affinity to 1/1,000 to 1/10,000 of that obtained with native receptor. The endoglycosidase H-susceptible oligosaccharide side chain(s) is not required for this interaction. Binding of alpha-bungarotoxin is to the physiologically relevant acetylcholine binding site as defined by affinity ligand alkylation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. In vitro synthesis, glycosylation, and membrane insertion of the four subunits of Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5598–5602. doi: 10.1073/pnas.78.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Walter P., Blobel G. Signal recognition protein is required for the integration of acetylcholine receptor delta subunit, a transmembrane glycoprotein, into the endoplasmic reticulum membrane. J Cell Biol. 1982 May;93(2):501–506. doi: 10.1083/jcb.93.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Damle V. N., McLaughlin M., Karlin A. Bromoacetylcholine as an affinity label of the acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1978 Oct 30;84(4):845–851. doi: 10.1016/0006-291x(78)91661-3. [DOI] [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner S. C., Rafto S. Comparison of the subunits of Torpedo californica acetylcholine receptor by peptide mapping. Biochemistry. 1979 Jan 23;18(2):301–307. doi: 10.1021/bi00569a011. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to a positively charged membrane filter. Anal Biochem. 1982 Aug;124(2):396–405. doi: 10.1016/0003-2697(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Shochat S., Malkin S., Ohad I. Functional Organization of the Chlorophyll-Containing Complexes of Chlamydomonas reinhardi: A Study of Their Formation and Interconnection with Reaction Centers in the Greening Process of the y-1 Mutant. Plant Physiol. 1982 Sep;70(3):637–644. doi: 10.1104/pp.70.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty J. G., Froehner S. C. Restoration of 125I-alpha-bungarotoxin binding activity to the alpha subunit of Torpedo acetylcholine receptor isolated by gel electrophoresis in sodium dodecyl sulfate. J Biol Chem. 1981 Aug 25;256(16):8294–8297. [PubMed] [Google Scholar]
- Karlin A. Nicotinic acetylcholine receptors. Methods Enzymol. 1977;46:582–590. doi: 10.1016/s0076-6879(77)46072-5. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Anholt R., Einarson B., Engel A., Osame M., Montal M. Purification of acetylcholine receptors, reconstitution into lipid vesicles, and study of agonist-induced cation channel regulation. J Biol Chem. 1980 Sep 10;255(17):8340–8350. [PubMed] [Google Scholar]
- Lindstrom J., Einarson B., Tzartos S. Production and assay of antibodies to acetylcholine receptors. Methods Enzymol. 1981;74(Pt 100):432–460. doi: 10.1016/0076-6879(81)74031-x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Merlie J., Yogeeswaran G. Biochemical properties of acteylcholine receptor subunits from Torpedo californica. Biochemistry. 1979 Oct 16;18(21):4465–4470. doi: 10.1021/bi00588a003. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Sebbane R. Acetylcholine receptor subunits transit a precursor pool before acquiring alpha-bungarotoxin binding activity. J Biol Chem. 1981 Apr 25;256(8):3605–3608. [PubMed] [Google Scholar]
- Merlie J. P., Sebbane R., Tzartos S., Lindstrom J. Inhibition of glycosylation with tunicamycin blocks assembly of newly synthesized acetylcholine receptor subunits in muscle cells. J Biol Chem. 1982 Mar 10;257(5):2694–2701. [PubMed] [Google Scholar]
- Nathanson N. M., Hall Z. W. In situ labeling of Torpedo and rat muscle acetylcholine receptor by a photoaffinity derivative of alpha-bungarotoxin. J Biol Chem. 1980 Feb 25;255(4):1698–1703. [PubMed] [Google Scholar]
- Oblas B., Boyd N. D., Singer R. H. Analysis of receptor-ligand interactions using nitrocellulose gel transfer: application to Torpedo acetylcholine receptor and alpha-bungarotoxin. Anal Biochem. 1983 Apr 1;130(1):1–8. doi: 10.1016/0003-2697(83)90641-3. [DOI] [PubMed] [Google Scholar]
- Prives J., Bar-Sagi D. Effect of tunicamycin, an inhibitor of protein glycosylation, on the biological properties of acetylcholine receptor in cultured muscle cells. J Biol Chem. 1983 Feb 10;258(3):1775–1780. [PubMed] [Google Scholar]
- Weill C. L., McNamee M. G., Karlin A. Affinity-labeling of purified acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1974 Dec 11;61(3):997–1003. doi: 10.1016/0006-291x(74)90254-x. [DOI] [PubMed] [Google Scholar]
- Wu W. C., Moore H. P., Raftery M. A. Quantitation of cation transport by reconstituted membrane vesicles containing purified acetylcholine receptor. Proc Natl Acad Sci U S A. 1981 Feb;78(2):775–779. doi: 10.1073/pnas.78.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]