Abstract
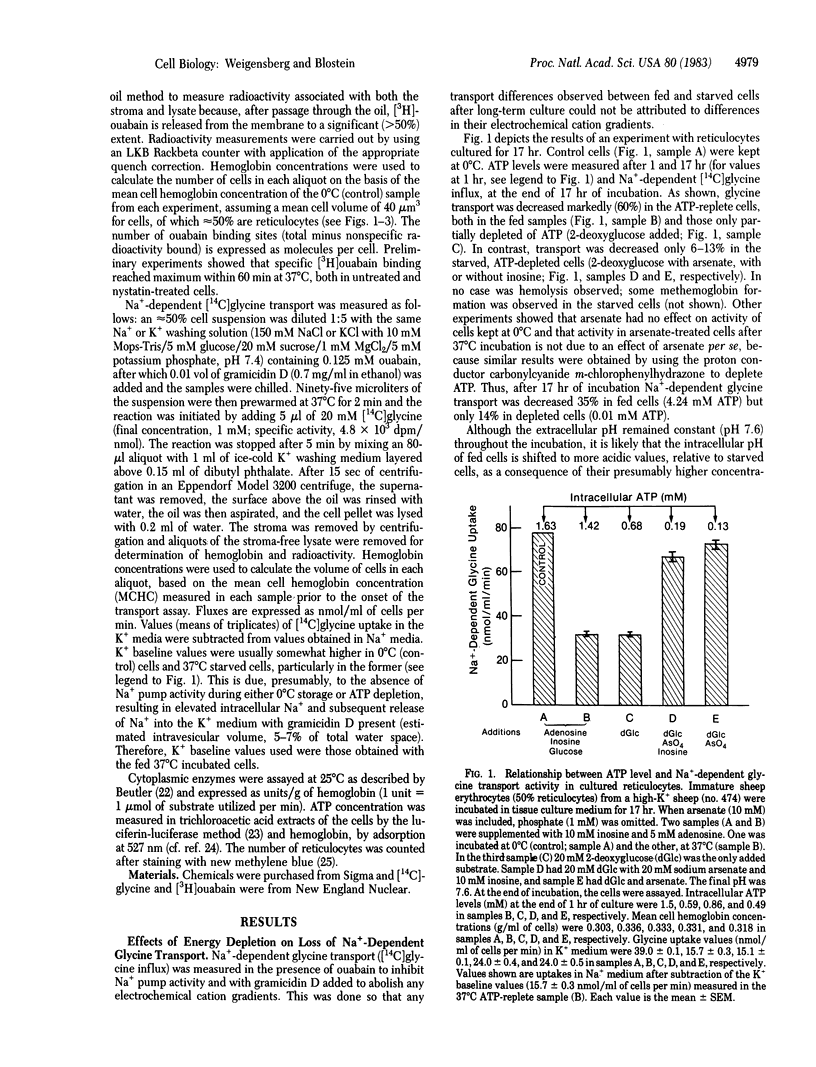
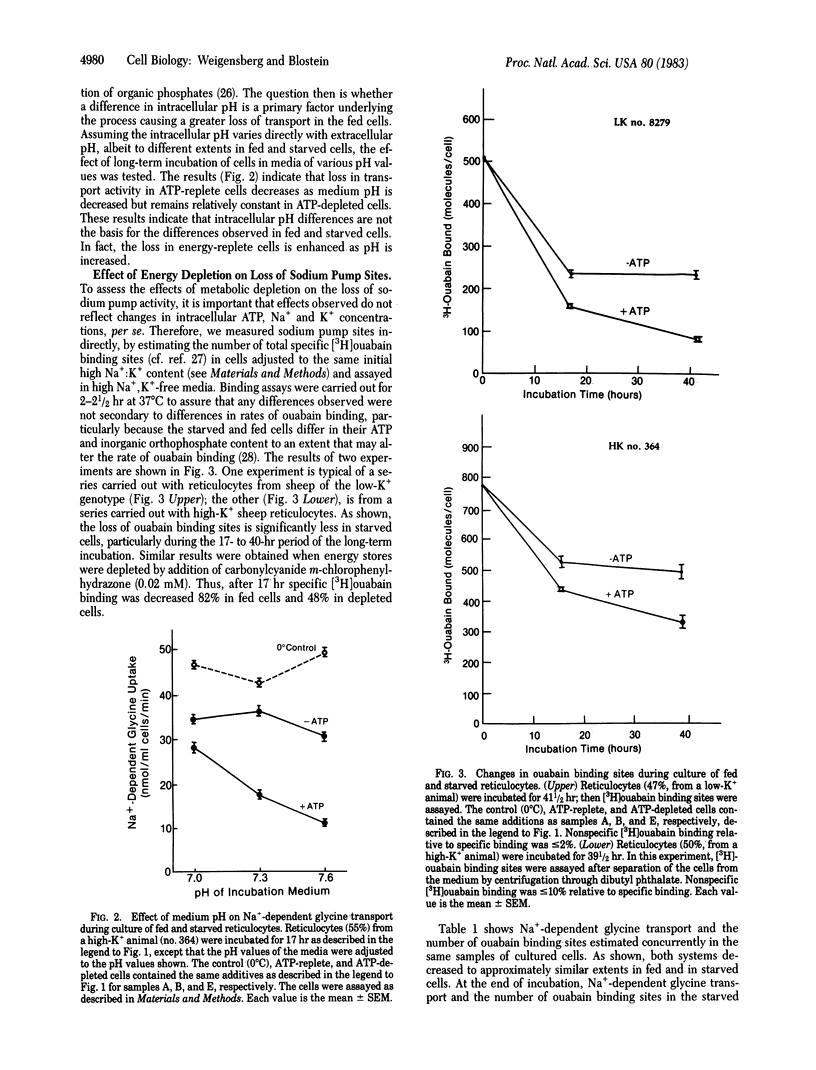
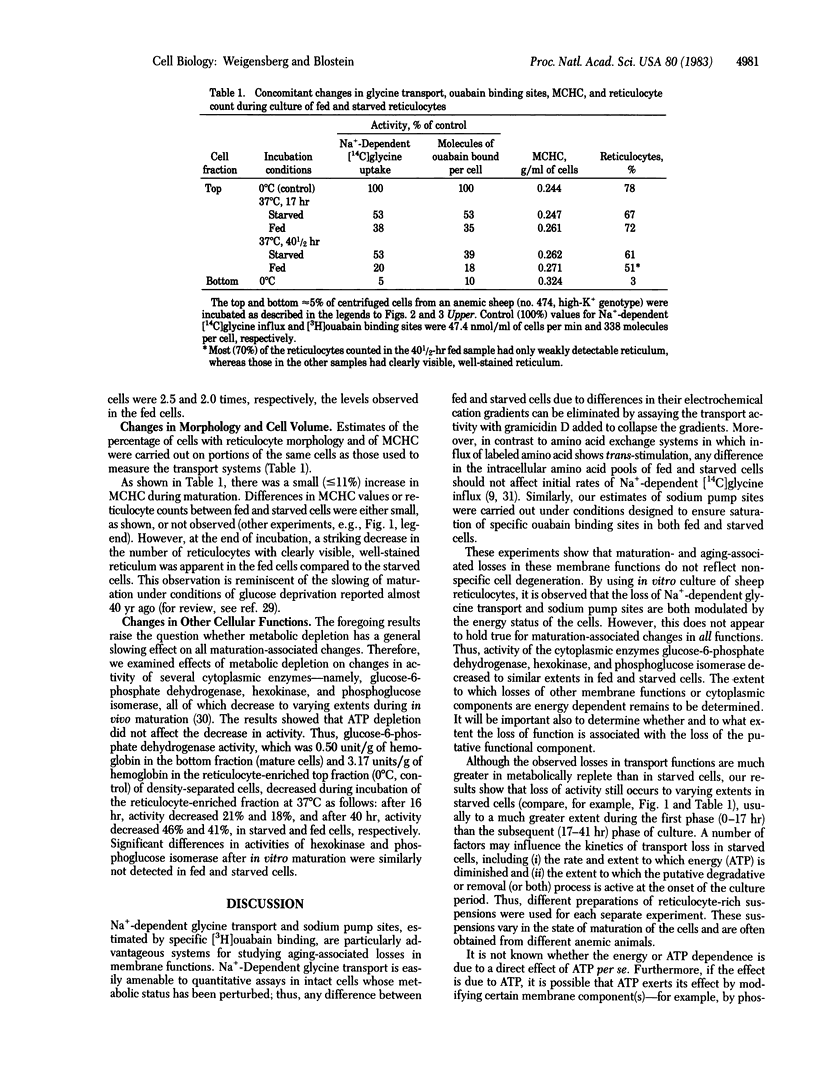
The effect of metabolic depletion on the maturation-associated loss of membrane functions has been studied by using sheep reticulocytes incubated in vitro at 37 degrees C for periods up to 41 hr. ATP was either maintained with glucose, adenosine plus inosine, or depleted with 2-deoxyglucose plus arsenate. Two membrane transport systems were studied: Na+-dependent glycine transport activity and the sodium pump, estimated from measurements of the number of [3H]ouabain binding sites per cell. Both transport systems were decreased during maturation. However, the decrease was much less in ATP-depleted cells compared to ATP-replete cells. It is concluded that the loss of certain functions during reticulocyte maturation is retarded by metabolic depletion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., BURN G. P. Enzyme activity as a function of age in the human erythrocyte. Br J Haematol. 1955 Jul;1(3):291–303. doi: 10.1111/j.1365-2141.1955.tb05511.x. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN R. E. Alterations in metabolic energetics and cation transport during aging of red cells. J Clin Invest. 1959 Sep;38:1572–1586. doi: 10.1172/JCI103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benderoff S., Blostein R., Johnstone R. M. Changes in amino acid transport during red cell maturation. Membr Biochem. 1978;1(1-2):89–106. doi: 10.3109/09687687809064161. [DOI] [PubMed] [Google Scholar]
- Benderoff S., Johnstone R. M., Blostein R. Electrogenic sodium-dependent glycine transport in sheep reticulocytes. Can J Biochem. 1978 Jun;56(6):545–551. doi: 10.1139/o78-083. [DOI] [PubMed] [Google Scholar]
- Blostein R., Drapeau P., Benderoff S., Weigensberg A. M. Changes in Na+-ATPase and Na,K-pump during maturation of sheep reticulocytes. Can J Biochem Cell Biol. 1983 Jan;61(1):23–28. doi: 10.1139/o83-004. [DOI] [PubMed] [Google Scholar]
- Blostein R., Whittington E. S., Kuebler E. S. Na+-ATPase of mammalian erythrocyte membranes: kinetic changes associated with postnatal development and following active erythropoiesis. Ann N Y Acad Sci. 1974;242(0):305–316. doi: 10.1111/j.1749-6632.1974.tb19099.x. [DOI] [PubMed] [Google Scholar]
- Bodemann H. H., Hoffman J. F. Comparison of the side-dependent effects of Na and K on orthophosphate-, UTP-, and ATP-promoted ouabain binding to reconstituted human red blood cell ghosts. J Gen Physiol. 1976 May;67(5):527–545. doi: 10.1085/jgp.67.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brok F., Ramot B., Zwang E., Danon D. Enzyme activities in human red blood cells of different age groups. Isr J Med Sci. 1966 May-Jun;2(3):291–296. [PubMed] [Google Scholar]
- Cass A., Dalmark M. Equilibrium dialysis of ions in nystatin-treated red cells. Nat New Biol. 1973 Jul 11;244(132):47–49. doi: 10.1038/newbio244047a0. [DOI] [PubMed] [Google Scholar]
- Choy Y. M., Wong S. L., Lee C. Y. Changes in surface carbohydrates of erythrocytes during in vivo aging. Biochem Biophys Res Commun. 1979 Nov 28;91(2):410–415. doi: 10.1016/0006-291x(79)91537-7. [DOI] [PubMed] [Google Scholar]
- Duhm J. Effects of 2,3-diphosphoglycerate and other organic phosphate compounds on oxygen affinity and intracellular pH of human erythrocytes. Pflugers Arch. 1971;326(4):341–356. doi: 10.1007/BF00586998. [DOI] [PubMed] [Google Scholar]
- Gattegno L., Bladier D., Garnier M., Cornillot P. Changes in carbohydrate content of surface membranes of human erythrocytes during ageing. Carbohydr Res. 1976 Dec;52:197–208. doi: 10.1016/s0008-6215(00)85960-1. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside translocation in sheep reticulocytes and fetal erythrocytes: a proposed model for the nucleoside transporter. J Physiol. 1982 Mar;324:47–66. doi: 10.1113/jphysiol.1982.sp014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner C. H., Lauf P. K. The correlation between ouabain binding and potassium pump inhibition in human and sheep erythrocytes. J Physiol. 1978 Oct;283:155–175. doi: 10.1113/jphysiol.1978.sp012494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepner G. R., Tosteson D. C. Incubation of HK and LK sheep red cells in vitro for long periods. Biochim Biophys Acta. 1972 May 9;266(2):471–483. doi: 10.1016/0005-2736(72)90103-4. [DOI] [PubMed] [Google Scholar]
- Kim H. D., Theg B. E., Lauf P. K. LK sheep reticulocytosis: effect of anti-L on K influx and in vitro maturation. J Gen Physiol. 1980 Jul;76(1):109–121. doi: 10.1085/jgp.76.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P. A., Gartrell J. E., Jr, Gardner F. H., Carter J. R., Jr Biogenesis of erythrocyte membrane proteins. In vivo studies in anemic rabbits. Biochim Biophys Acta. 1975 Apr 21;389(1):162–176. doi: 10.1016/0005-2736(75)90394-6. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN L. M. The mammalian reticulocyte. Int Rev Cytol. 1959;8:135–174. doi: 10.1016/s0074-7696(08)62730-8. [DOI] [PubMed] [Google Scholar]
- Lee P., Woo A., Tosteson D. C. Cytodifferentiation and membrane transport properties in LK sheep red cells. J Gen Physiol. 1966 Nov;50(2):379–390. doi: 10.1085/jgp.50.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morville M., Reid M., Eddy A. A. Amino acid absorption by mouse ascites-tumour cells depleted of both endogenous amino acids and adenosine triphosphate. Biochem J. 1973 May;134(1):11–26. doi: 10.1042/bj1340011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Dubiel W., Rathmann J., Rapoport S. Determination and characteristics of energy-dependent proteolysis in rabbit reticulocytes. Eur J Biochem. 1980 Aug;109(2):405–410. doi: 10.1111/j.1432-1033.1980.tb04808.x. [DOI] [PubMed] [Google Scholar]
- Noble N. A., Tanaka K. R., Nathanielsz P. W. Erythrocyte enzymes in Ovis aries. Effect of cell age. Comp Biochem Physiol B. 1982;71(1):1–6. doi: 10.1016/0305-0491(82)90166-3. [DOI] [PubMed] [Google Scholar]
- Pan B. T., Blostein R., Johnstone R. M. Loss of the transferrin receptor during the maturation of sheep reticulocytes in vitro. An immunological approach. Biochem J. 1983 Jan 15;210(1):37–47. doi: 10.1042/bj2100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINSTEIN D., OTTOLENGHI P., DENSTEDT O. F. The metabolism of the erythrocyte. XIII. Enzyme activity in the reticulocyte. Can J Biochem Physiol. 1956 Mar;34(2):222–235. [PubMed] [Google Scholar]
- Shattil S. J., Cooper R. A. Maturation of macroreticulocyte membranes in vivo. J Lab Clin Med. 1972 Feb;79(2):215–227. [PubMed] [Google Scholar]
- Spooner R. J., Percy R. A., Rumley A. G. The effect of erythrocyte ageing on some vitamin and mineral dependent enzymes. Clin Biochem. 1979 Dec;12(6):289–290. doi: 10.1016/s0009-9120(79)80132-0. [DOI] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Winter C. G., Christensen H. N. Contrasts in neutral amino acid transport by rabbit erythrocytes and reticulocytes. J Biol Chem. 1965 Sep;240(9):3594–3600. [PubMed] [Google Scholar]


