Testis development in the mammalian embryo requires the formation and assembly of several cell types that allow these organs to achieve their roles in male reproduction and endocrine regulation. In this review, Svingen and Koopman focus on the issues of cell differentiation and development of the testis architecture and highlight the questions that remain to be explored.
Keywords: sex determination, organogenesis, Sertoli cells, Leydig cells, disorder of sex development, fertility
Abstract
Development of testes in the mammalian embryo requires the formation and assembly of several cell types that allow these organs to achieve their roles in male reproduction and endocrine regulation. Testis development is unusual in that several cell types such as Sertoli, Leydig, and spermatogonial cells arise from bipotential precursors present in the precursor tissue, the genital ridge. These cell types do not differentiate independently but depend on signals from Sertoli cells that differentiate under the influence of transcription factors SRY and SOX9. While these steps are becoming better understood, the origins and roles of many testicular cell types and structures—including peritubular myoid cells, the tunica albuginea, the arterial and venous blood vasculature, lymphatic vessels, macrophages, and nerve cells—have remained unclear. This review synthesizes current knowledge of how the architecture of the testis unfolds and highlights the questions that remain to be explored, thus providing a roadmap for future studies that may help illuminate the causes of XY disorders of sex development, infertility, and testicular cancers.
The existence of two sexes complicates the genetics and biology of embryonic development: It requires a strategy of embryogenesis that can generate two physiologically different types of mature organism in each species. In eutherian mammals, early development of the embryo is similar in both sexes until the fetal stage, when males and females embark on different developmental trajectories. This divergence is marked by the formation of testes or ovaries, respectively.
Although the testis and ovary are functionally analogous and arise from a common primordial structure, the genital ridge, they are remarkably different organs, and their development is driven by distinct programs of gene regulation and cellular organization. The identification of the mammalian testis-determining gene Sry a quarter of a century ago (Gubbay et al. 1990; Sinclair et al. 1990; Koopman et al. 1991) provided an entry point to molecular studies of testis development. Since that time, much has been learned regarding the genetic networks responsible for orchestrating testis development, far more so than ovary development. This progress has been reviewed extensively elsewhere (Brennan and Capel 2004; Wilhelm et al. 2007b; Eggers and Sinclair 2012; Quinn and Koopman 2012; Warr and Greenfield 2012) and is not reiterated here.
In parallel with molecular studies, imaging techniques developed in the last two decades have led to a growing appreciation of the well-organized tissue architecture of the developing testis. The cellular makeup of the testis is now well understood (Fig. 1) due to the availability of antibodies and probes recognizing specific markers for most of the component cell types. How these cell types assemble into a functional organ remains an active topic of research. It has become clear that the development of the testis is in many ways a paradigm for the development of other organs, incorporating mechanisms for determining organ shape, size, internal architecture, vascularization, and interaction with other tissues physically, hormonally, and neurally. On the other hand, the development of the testis is unusual in several respects. First, several of the cell lineages involved are bipotential, since the genital ridges must be able to differentiate into testes or ovaries depending on signals received. Second, the differentiation of these cell lineages does not proceed independently but instead follows from differentiation of Sertoli cells, which then orchestrate the behavior of all other cell types (Fig. 2; Burgoyne et al. 1995). Finally, the testis is built from a combination of innate precursors and immigrant cells such as germ cells. Together, these idiosyncrasies present logistical challenges in terms of regulatory circuitry, canalization of outcomes, and coordination of developmental events between cell lineages.
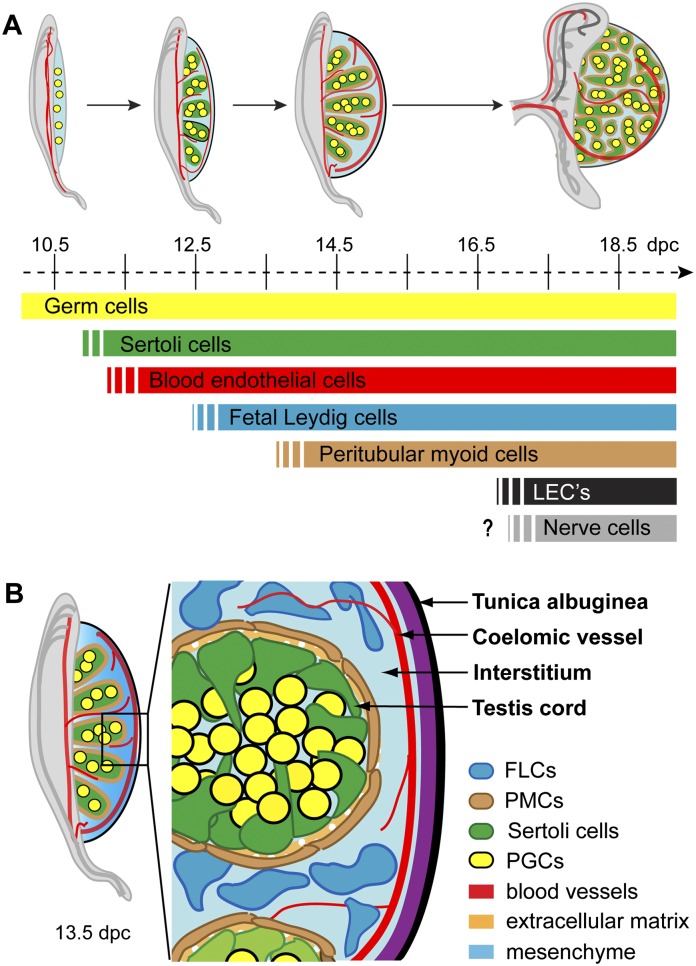
Figure 1.
Anatomy of the developing mouse testis. (A) Schedule of cell lineage differentiation during testis organogenesis. The genital ridges are colonized by germ cells (yellow) prior to testis specification. The first somatic cells to differentiate are the Sertoli cells (green), with blood endothelial cells also migrating into the gonad at this early stage to lay down a primitive arterial vasculature (red). Following a period of Sertoli cell proliferation, fetal Leydig cells (FLCs; blue) and peritubular myoid cells (PMCs; brown) differentiate. The vasculature becomes more complete with additional development of venous vessels and lymphatic endothelial cells (LECs; black). Although the postnatal testis also contains neurons, the developmental stage at which they first appear remains unclear. (B) Fetal testis architecture. By 13.5 dpc, the mouse testis is compartmentalized into testis cords and interstitial space, with the majority of the cell types of the mature testis already in place. The testis cords comprise mitotically arrested germ cells surrounded by Sertoli cells, with an outer layer of PMCs and an extracellular matrix (ECM) giving structural support. The interstitium consists of mesenchymal tissue, steroidogenic FLCs, and a prominent blood vascular network. At this stage, the protective testis cap, the tunica albuginea, has also begun to develop.
Figure 2.
Gonadal sex determination: a choice between two mutually opposing fates. The genital ridges contain at least three types of unspecified, bipotential precursor cells. In XY gonads, cells of the supporting cell lineage start to express Sry and then Sox9, causing them to differentiate into Sertoli cells. In the absence of Sry, as in XX genital ridges, the same precursor cells differentiate into granulosa cells under the influence of genes encoding transcription factors, including Ctnnb1 and Foxl2. In addition to promoting the Sertoli cell differentiation pathway, Sry and Sox9 also (directly or indirectly) suppress female-specific cell differentiation pathways. Sertoli cells induce (dotted arrows) other cell populations to differentiate into the steroidogenic FLCs that otherwise would differentiate into ovarian theca cells and enter the spermatogenic pathway as opposed to the oocyte differentiation pathway.
Here, we review current knowledge, mostly gained from studies in mice, regarding the origin, early differentiation, interaction, and function of the cell lineages of the mammalian fetal testis. Discussion of these issues provides a framework for further research into the cell biology and tissue morphogenesis of the testis, promotes a deeper appreciation of potential causes of testicular dysmorphology syndromes, and provides a point of reference for comparative studies of testis development in nonmammalian species and of organogenesis more broadly, including the relatively mysterious process of ovarian morphogenesis.
The beginnings of gonad formation
The bipotential gonadal primordia
In mice, the genital ridges first appear between 10 and 10.5 d post-coitum (dpc) as regional thickenings of the epithelium overlying the ventromedial surfaces of the mesonephroi, two parallel structures lying dorsally in the coelomic cavity, each running along the head-to-tail axis of the embryo (Fig. 3). It is understood that proliferation of the coelomic epithelium overlying each mesonephros leads to genital ridge outgrowth (Merchant 1975; Pelliniemi 1975; Karl and Capel 1998; Schmahl et al. 2000) and that the genital ridge mesenchyme expands through a combination of ingression of cells from the coelomic epithelium, recruitment of cells from the adjacent mesonephros, and proliferation. Mainly through mouse knockout studies, several genes—including Lhx9, Emx2, Wt1, Cbx2, Nr5a1, Six1/4, and genes encoding the insulin receptors Igf1r/Irr/Ir—have been implicated in establishing the bipotential genital ridges in both sexes (Kreidberg et al. 1993; Luo et al. 1994; Miyamoto et al. 1997; Katoh-Fukui et al. 1998; Birk et al. 2000; Nef et al. 2003; Fujimoto et al. 2013).
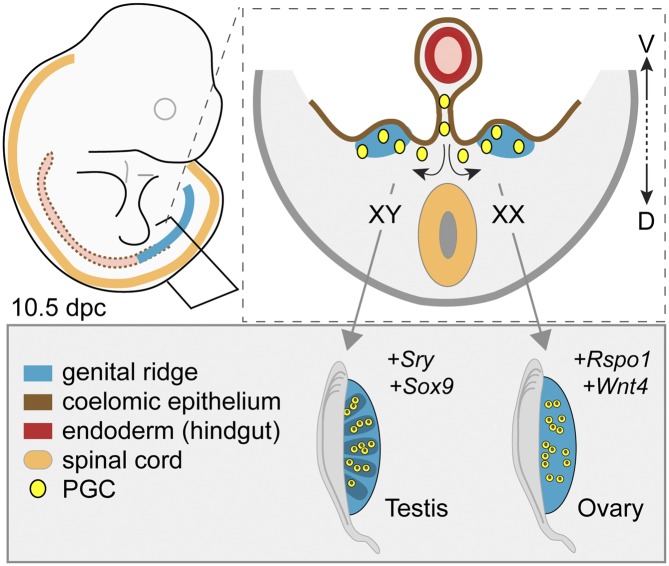
Figure 3.
The establishment of the bipotential genital ridges and gonadal sex determination. In mammals, the genital ridges (blue) typically appear as longitudinal outgrowths along the surfaces of the mesonephroi within the coelomic cavity. In mice, they emerge at ∼10 dpc through recruitment of cells from the overlying coelomic epithelium (brown). Primordial germ cells (yellow) colonize the genital ridges (arrows) after leaving the hindgut (red) via the dorsal mesentery. At this stage in development, the genital ridges are bipotential and can differentiate into testes or ovaries, depending on genetic cues. From ∼10.5 dpc, the Y-linked sex-determining gene Sry is expressed in XY genital ridges and initiates Sox9 expression and testis differentiation. In the absence of Sry, as in XX genital ridges, ovary differentiation is initiated by the action of genes such as Rspo1 and Wnt4. (D) Dorsal; (V) ventral.
There is no morphological difference between the genital ridges of XX and XY embryos. Moreover, XY genital ridges can be induced to differentiate as ovaries by deletion or malfunction of the protestis genes Sry and Sox9, and XX genital ridges can be induced to develop as testes by forced expression of these genes (Koopman et al. 1991; Foster et al. 1994; Wagner et al. 1994; Huang et al. 1999; Vidal et al. 2001; Chaboissier et al. 2004; Barrionuevo et al. 2006). These observations support the concept that the cell types of the genital ridges must be equally capable of testis and ovary development. Under normal circumstances, the molecular chain of events leading to ovarian development will proceed unless diverted by the action of Sry. In this sense, the ovarian fate can be viewed as the default fate. It is important to understand that “default” does not mean to imply that no genes or cellular activity are required to make an ovary: It is clear that both ovarian and testicular development involve active pathways of molecular regulation and that each pathway antagonizes the other (Nef et al. 2005; Kim et al. 2006; Ottolenghi et al. 2007; Jameson et al. 2012a,b).
Primordial germ cell specification and gonadal colonization
Unlike the somatic cells of the fetal testis, the primordial germ cells originate far from their final location. They arise within the proximal epiblast and initially cluster at the base of the incipient allantois from 6.25 dpc in mice (Lawson and Hage 1994; Ying et al. 2000; Ying and Zhao 2001). From ∼7.5 dpc, the germ cells move from the posterior primitive streak to the subjacent embryonic endoderm, after which they become motile and migrate anteriorly through the hindgut toward the future genital ridges, from 8.0 to 10.5 dpc (Anderson et al. 2000; Molyneaux et al. 2001; Hara et al. 2009). This active movement involves several guidance cues (Richardson and Lehmann 2010), including Kitl/Kit (Mauduit et al. 1999), Cxcl12/Cxcr4 (Molyneaux et al. 2003), Foxc1 (Mattiske et al. 2006), Lhx1 (Tanaka et al. 2010), Wnt5a (Chawengsaksophak et al. 2012), and Ror2 (Laird et al. 2011), and potentially also further cues from nerve cells developing along the migratory route (Møllgård et al. 2010). Around 10 dpc, the germ cells finally come to occupy the genital ridges by migrating anteriorly through the hindgut mesentery. It has been speculated that the long and narrow structure of the genital ridges is important for capturing the migrating germ cells that are widely scattered along the hindgut (Harikae et al. 2013). At this point, the germ cells lose their motility and polarized morphology (Baillie 1964; Donovan et al. 1986) and are referred to as gonocytes.
Testis differentiation needs to be carefully orchestrated so that it does not begin until the germ cells have arrived in the gonads, since the germ cells must come to be encased in the somatic testis cords. With this in mind, it is curious that cord formation does not depend on the presence of germ cells as a trigger. Numerous germ cell-deficient mouse models exist, including the white spotting mutants of the c-kit/Kit ligand pathway, in which normal testicular histology and endocrine function are achieved (Merchant 1975; Hashimoto et al. 1990; Pellas et al. 1991; Buehr et al. 1993b). The mechanisms that coordinate testis differentiation and arrival of germ cells remain to be determined.
Sertoli cell specification and testis cord formation
Experiments conducted in the early 1990s revealed that in XX ↔ XY mouse chimeras, both XX and XY cells could contribute to several testicular cell types (Palmer and Burgoyne 1991; Burgoyne et al. 1995). However, Sertoli cells were almost exclusively XY, suggesting that Sertoli cell differentiation is normally driven by cell-autonomous action of the testis-determining gene in the XY cells of the lineage now known as “supporting cells” and that all other testicular cell types can differentiate as a result of signaling from the Sertoli cells to the other cells of the nascent gonad, whether XX or XY. This central role of the Sertoli cell in the signaling machinery of testis development is illustrated in Figure 4.
Figure 4.
Sertoli cells as the organizing center of testis differentiation. Sertoli cells are the first somatic cells to differentiate in the XY gonad. They then act as a regulatory hub during testis organogenesis, influencing testis cord formation, Müllerian duct regression, and the differentiation and function of several other cell types, including the germ cells, PMCs, FLCs, and endothelial cells (ECs). In turn, androgens produced by the FLCs are largely responsible for the masculinization of the embryo.
Specification of pre-Sertoli cells
Cell-tracing studies revealed that some cells of the supporting cell lineage originate from the coelomic epithelium overlying the genital ridges (Fig. 5; Karl and Capel 1998). Whether these cells enter the gonad by active migration or passive ingression caused by the high rate of division of surface epithelial cells (Rodemer-Lenz 1989; Karl and Capel 1998; Schmahl et al. 2000) is not clear. However, coelomic epithelial cells ingress into both XY and XX genital ridges, and it is only after they have colonized the gonad proper that the testis-determining factor Sry is activated in XY genital ridges (Bullejos and Koopman 2001). Therefore, this ingression cannot be a consequence of Sry expression.
Figure 5.
Known and proposed origins of the testicular cell lineages. The cells of the nascent genital ridges originate primarily from the overlying coelomic epithelium but also from the subjacent mesonephros. A subset of ingressing coelomic epithelial cells differentiates into Sertoli cells following Sry expression. Some of these supporting cells are also believed to differentiate into FLCs. It is unclear whether cells originating from the mesonephros contribute toward somatic cells other than blood endothelium, but they very likely contribute to the mesenchyme. The origin of PMCs remains unknown, but it is likely that they differentiate from a subset of mesenchymal cells or yet unidentified precursor cells of the testis interstitium. A second origin for the FLCs has also been proposed to include perivascular cells located at the gonad–mesonephric junction.
In mice, Sry-positive supporting cells are first observed in the middle third of the XY genital ridges, with subsequent expansion of the expression domain outward toward the poles (Fig. 6A; Albrecht and Eicher 2001; Bullejos and Koopman 2001; Wilhelm et al. 2005). This expression pattern is also observed for some other genes expressed even earlier in both male and female embryos (Lee et al. 2009), so it seems likely that the “wave” of Sry up-regulation depends on the appropriate combination of transcription factors being present in different parts of the gonad at different times. While it remains unclear how the center-to-pole expansion of Sry expression occurs, the result is that Sertoli cells do not differentiate synchronously in all parts of the gonad, a situation that has consequences for the formation of ovotestes in some situations, as discussed below.
Figure 6.
Correct spatiotemporal expression of Sry within the genital ridges is required for testis differentiation. (A) Sry transcription (dark blue) is initiated in a subset of supporting lineage cells in the central region of the genital ridges. From here, the domain of Sry expression extends toward the gonadal poles. In mice, Sry is expressed along the entire length of the genital ridge for only a brief period before becoming down-regulated first within the center region, then at the anterior pole, and finally at the posterior pole. (In larger mammals, including humans, Sry expression is maintained after testis differentiation.) (B,C) Experimentally, it has been shown that Sry expression must reach a threshold level within a temporal window of 14–19 ts (∼11.2–11.75 dpc; yellow shading) in order to activate Sox9 expression (green) and hence Sertoli cell differentiation and testis cord formation appropriately along the entire length of the testis. (B) Crossing Ypos mice (harboring a Sry locus that causes retarded Sry expression) with C57BL/6 mice and delaying Sry initiation by a few hours does not allow it to be expressed along the entire length of the genital ridge at the critical time, with the result that Sox9 expression (green) is limited to the central region, with the ultimate result of ovotesticular development, characterized by testicular tissue in the center and ovarian tissue at the poles. (C) By experimentally delaying Sry expression until after 11.75 dpc in transgenic mice, Sox9 is not expressed during the critical time window in the majority of supporting cells, resulting in a complete failure of Sox9 up-regulation and Sertoli cell differentiation and hence ovarian differentiation.
Pre-Sertoli cell differentiation
Sry transcription in the supporting cells up-regulates Sox9 expression in those cells, which are then referred to as pre-Sertoli cells. Up-regulation of Sox9 follows a center-to-pole pattern similar to that of Sry, albeit ∼10 h later (Bullejos and Koopman 2005). Both waves still occur when the genital ridge is cut into three segments and cultured, indicating that they do not depend on intercellular signals radiating outward from the center of the genital ridges (Hiramatsu et al. 2010).
The presence, integrity, and activity of Sry are not always sufficient to activate Sox9 in a cell-autonomous manner. Several experimental observations indicate that a threshold level of Sry expression is required within any given supporting lineage cell during a defined temporal window to activate Sox9 expression. In B6-YDOM ovotestes (Eicher et al. 1982), where testis cords appear in the center and ovarian structures at the poles within the same gonad, Wilhelm et al. (2009) observed Sry expression along the entire gonadal axis, even where ovarian tissue would later develop. Sox9 expression, on the other hand, was confined to the center of the primitive gonad, in the region that later develops into testicular tissue (Fig. 6B). Furthermore, using a transgenic mouse system in which Sry was ectopically induced in XX genital ridges (Kidokoro et al. 2005), delayed Sry expression at 12 dpc failed to induce SOX9 expression in the majority of XX supporting cells (Fig. 6C; Hiramatsu et al. 2009). Both sets of experiments point to the existence of a limited time window within which cells remain competent to respond to SRY by activating Sox9 expression. Thus, although Sry is necessary for the initial up-regulation of Sox9 in pre-Sertoli cells, it is not always sufficient (Wilhelm et al. 2009).
Even if Sox9 is successfully activated, the number of Sox9-positive cells is a critical determinant of testis differentiation, and three mechanisms act to maximize the number of Sox9-positive pre-Sertoli cells in the developing testis. XX ↔ XY chimeric gonads typically develop as testes (Palmer and Burgoyne 1991; Wilhelm et al. 2005), indicating that XY gonadal cells can recruit neighboring XX gonadal cells to a testicular fate (Palmer and Burgoyne 1991). Subsequent experiments revealed that XY cells use prostaglandin D2 (PGD2) signaling to recruit neighboring XX cells to a Sertoli fate in these chimeras (Wilhelm et al. 2005). PGD2 is able to induce Sox9 expression in neighboring cells, and SOX9 is able to induce Pgds expression and hence secretion of PGD2 (Malki et al. 2005; Wilhelm et al. 2005, 2007a; Moniot et al. 2009). Extrapolating to normal XY gonadal development, it is likely that cells that express too little SRY to activate Sox9 directly can be persuaded to become Sox9-positive by other cells that do express levels of SRY sufficiently robust for Sox9 activation. This mechanism serves to canalize testis development in XY gonads given the lack of robustness of Sry (Polanco and Koopman 2007).
FGF9 may provide a second mechanism by which the number of Sox9-expressing supporting cells is maximized. FGF9 expression is activated downstream from Sry and Sox9 and plays a pivotal role in testis development, since deletion of Fgf9 or the receptor gene Fgfr2 in mice causes complete or partial male-to-female sex reversal, respectively (Colvin et al. 2001; Kim et al. 2007; Bagheri-Fam et al. 2008). It was initially proposed that FGF9 and SOX9 create a positive feedback loop to stabilize Sox9 expression (Kim et al. 2006), and clearly such a loop might also provide a recruitment mechanism similar to that described above for PGD2. More recent reanalysis suggests that the primary role of FGF9 is to repress pro-ovary genes such as Wnt4 (Jameson et al. 2012a); if that is the case, then the observed up-regulation of Sox9 by exogenous FGF9 (Hiramatsu et al. 2010) must be a secondary effect.
A third mechanism that helps to maximize the number of Sox9-positive pre-Sertoli cells is proliferation. A consequence of Sry expression is increased rate of cell division in this lineage (Schmahl et al. 2000), which would be expected to amplify the ratio of Sox9-positive cells relative to any Sox9-negative supporting cells that may be present in the XY genital ridge.
Despite the action of these mechanisms, it is commonly believed that in XX ↔ XY chimeras where the XY cell complement is below ∼20%, testis differentiation cannot proceed normally, and ovotestis or even ovarian development is observed (Palmer and Burgoyne 1991; Patek et al. 1991; Burgoyne et al. 1995). Thus, a threshold number of cells of the supporting cell lineage must be capable of cell-autonomous activation of Sox9 by SRY in order for PGD2-based and/or other secreted factor-based recruitment mechanisms to successfully convert all cells of that lineage to a Sertoli cell fate.
Together, the above observations place the spotlight on Sox9, not Sry, as the determinant and hallmark of Sertoli cell differentiation. In support of this view, Sox9 has the capacity to drive testis differentiation in the absence of Sry in transgenic XX embryos (Vidal et al. 2001). Although deleting Sox9 after testis cord formation has no detrimental effect on subsequent testis differentiation (Barrionuevo et al. 2009) and Sertoli cells can differentiate in the complete absence of Sox9 under some experimental conditions (Lavery et al. 2012), it is possible that one or both of the two closely related factors Sox8 and Sox10 can substitute for Sox9 in some contexts (Barrionuevo et al. 2009; Polanco et al. 2010).
Accelerated growth of the early testis
One of the hallmarks of the early testis is its very rapid growth rate compared with that of the early ovary (Schmahl et al. 2000). This phenomenon has been noted previously in several species (for review, see Mittwoch 1998) and had even been predicted to be a requirement for testis determination (Mittwoch et al. 1969), consistent with the requirement for maximal numbers of Sox9-positive pre-Sertoli cells as discussed above. It is likely that an increased rate of cell division stimulated by Sry expression is a major contributor to this accelerated growth (Schmahl et al. 2000). However, recent studies of Cbx2 mutant mouse gonads uncouple the Sry-dependent increased proliferation of pre-Sertoli cells from the observed increased growth rate of testes relative to ovaries, suggesting that other genes, mechanisms, and perhaps cell lineages contribute to gonadal size determination (Katoh-Fukui et al. 2012).
Aggregation of pre-Sertoli cells into primitive testis cords
The formation of testis cords—the future seminiferous tubules—is the defining event in testis differentiation. Testis cords perform several important roles. First, they segregate the two main functions of the testis; namely, spermatogenesis and androgen production. They also physically shield the gonocytes from retinoic acid present in the interstitium (Griswold et al. 2012) that otherwise would stimulate the germ cells to enter meiosis too soon. Finally, testis cords later come to play an important mechanical role in the export of mature sperm from the testis. How do these structures arise?
Shortly after Sox9 activation, when the genital ridges are still long and very thin, the pre-Sertoli cells start to aggregate around clusters of germ cells and form cords, at which point they are referred to as Sertoli cells. Their aggregation can be reproduced in vitro by culturing them on reconstituted basement membrane gels (Hadley et al. 1985) and is impeded by inhibitors of neurotrophic tyrosine kinase receptors (NTRKs) (Gassei et al. 2008). It is known that Ntrk3 is expressed by pre-Sertoli cells at the onset of testis cord formation (Russo et al. 1999; Cupp et al. 2000; Levine et al. 2000), as is the NTRK ligand NTF3, and both seem to be involved in forming adhesive cell contacts (Cupp et al. 2002; Gassei et al. 2008). However, knockout mice for both Ntrk1 and Ntrk3 show disruption, but not a complete absence, of seminiferous tubules (Cupp et al. 2002), and the story is far from complete.
Testis cord formation may also involve FGF9. When genital ridges are cut into three segments and cultured, testis cords do not form in the polar segments unless exogenous FGF9 is supplied, implicating rapid poleward diffusion of FGF9 in stimulating cord formation (Hiramatsu et al. 2010). Such a mechanism has been proposed to explain why cord formation occurs synchronously throughout the testis despite the wave of Sry and Sox9 expression that moves outward from the center of the genital ridge (Harikae et al. 2013).
Regardless of the molecular trigger mechanism, the process of cord formation must involve some form of tubularization, with attachment of both ends of each tube to a common junction complex adjacent to the mesonephros—a plexus known as the rete testis. It is clear that testis cord formation does not proceed via sprouting from any pre-existing tubular structure but instead happens de novo starting from the clusters described above. Furthermore, cords develop normally in XY gonads devoid of germ cells (Merchant 1975; McCoshen 1982, 1983; Escalante-Alcalde and Merchant-Larios 1992), indicating that the formation of Sertoli–germ cell aggregates is driven by Sertoli-to-Sertoli cell interactions and not by aggregation around germ cells as focal points. Thus, Sertoli cell aggregation evidently involves changes to their cell surface to make them more able to recognize and adhere to each other; yet, intriguingly, these aggregates develop into tubes rather than remaining as simple clumps, and furthermore, these tubes encase, rather than exclude, germ cells.
Somatic factors acting on XY germ cells
While there is currently no evidence that germ cells signal to initiate somatic testis differentiation or maintain testis architecture, it is abundantly clear that Sertoli cells are crucial for supporting both differentiation and survival of the germ cell lineage. Several of the molecules produced by Sertoli cells and influencing the germline have been identified (Fig. 7). The enzyme CYP26B1 acts to catabolyze retinoic acid that would otherwise promote entry of germ cells into meiosis, a step that is appropriate to ovarian but not testicular germ cells (Bowles et al. 2006; Koubova et al. 2006). Sertoli cell-derived FGF9 further acts to make germ cells less responsive to retinoic acid (Bowles et al. 2010) and stimulate the expression of the Nodal coreceptor Cripto (Bowles and Koopman 2010; Spiller et al. 2012), thus prolonging germ cell pluripotency and encouraging a spermatogenic fate. Activin A and transforming growth factor β (TGFβ) signaling have been implicated in the regulation of germ cell quiescence, as both Inhba and Tgfbr2 knockout mice display increased germ cell proliferation, concomitant with delayed G1/G0 arrest, in the fetal testis (Moreno et al. 2010; Mendis et al. 2011). Finally, platelet-derived growth factor (PDGF), estrogen, and TGFβ have been implicated in germ cell survival postnatally (Fig. 7; Li et al. 1997; Hasthorpe et al. 1999; Thuillier et al. 2003; La Sala et al. 2010). Given the intimate and dynamic relationship between Sertoli and germ cells, it is likely that future studies will reveal additional Sertoli cell-derived signaling factors and perhaps even evidence that the communication is not all one way.
Figure 7.

Effects of Sertoli cells on germ cell differentiation and survival. In the fetal testis, Sertoli cells influence XY germ cells via multiple signaling pathways. Degradation of retinoic acid (RA) by CYP26B1 expression together with FGF9 secretion allow gonocytes to avoid meiosis. FGF9 also stimulates expression of Cripto, which, together with Nodal, promotes maintenance of pluripotency. Factors inducing mitotic arrest are hypothesized to be produced by Sertoli cells and may include Activin A and TGFβ. Finally, several factors have been shown to influence the health and survival of germ cells postnatally, including PDGF, TGFβ, and estrogen (see the text for further details).
Testis cord partitioning and maturation
Combes et al. (2009a) referred to the primitive tubular structures as “proto-cords” and counted ∼13 of them per developing mouse testis at 12 dpc. Rapidly, within ∼24 h, the structure is resolved to generate about nine easily visualized “external” cords, each of which is toroid and somewhat uniform in thickness and lies perpendicular to the long axis of the testis (Combes et al. 2009a; Nel-Themaat et al. 2009). The testis cords initially resemble a stack of doughnuts joined together at a point on their circumference corresponding to the rete testis.
Several studies have revealed the involvement of vascular endothelial cells in cord formation (Coveney et al. 2008; Combes et al. 2009b; Cool et al. 2011). Evidently, streams of endothelial cells from the mesonephros partition the field of Sertoli and germ cells into proto-cords, the irregular size and spacing of which results from apparently stochastic spacing of the immigrant streams. Blocking the immigration of endothelial cells from the mesonephros disrupts cord formation (Coveney et al. 2008; Combes et al. 2009b). The Sertoli cells continue to proliferate and form stronger contacts around germ cell clusters, and the cords elongate (Nel-Themaat et al. 2011). They are remodeled to assume a relatively uniform thickness, probably influenced by mechanical factors such as the number of germ cells that need to be accommodated, the optimal number of Sertoli cells needed to form a stable tubular circumference, and the available space (that is, the length of the gonad). The observation that some cords are branched, fused, or blind-ended (Combes et al. 2009a; Nel-Themaat et al. 2009) most likely reflects the stochastic nature of the initial aggregation event.
External factors, likely both molecular and physical, then help to mold the growing testis cords into toroid structures by ∼13 dpc. At this stage, the Sertoli cells mature from a fibroblast-like to an epithelial-like morphology (Nel-Themaat et al. 2011), and the testis cords develop an outer layer of peritubular myoid cells (PMCs) and an extracellular matrix (ECM). Elongation of each cord pushes the surface of the gonad outward, expanding its short axis to make the testis plumper (Combes et al. 2009a; Nel-Themaat et al. 2009). As space becomes limiting, each cord begins to buckle; further extension then yields the complex “spaghetti” of testis tubules seen in adult testes. It is interesting to note that the mature mouse testis is often mistakenly considered to have hundreds of tubules but in fact has only a dozen.
How each tubule comes to be plumbed into the rete testis at both ends, what remodels the proto-cords into definitive cords, and how the process can accommodate equally well the presence or absence of germ cells are issues that remain profoundly mysterious.
PMC origins and function
PMCs make up the outer cell layer of the seminiferous tubules and are in direct contact with the basal surface of the Sertoli cells (Fig. 1B; Skinner et al. 1985). In smaller rodents, the PMC sheet is only one cell layer thick, whereas in larger mammals, it typically consists of multiple layers separated by extracellular connective tissue (Maekawa et al. 1996). This boundary tissue—the lamina propria that helps separate tubules from interstitium and acts as contractile tissue to facilitate sperm export—typically consists of PMCs and ECM, although in humans, fibroblast cells (myofibroblasts) also occupy the outer layers of the lamina propria (Davidoff et al. 1990; Dym 1994; Holstein et al. 1996). The composition of ECM is contributed by both PMC and Sertoli cells (Tung et al. 1984; Skinner and Fritz 1985; Skinner et al. 1985; Georg et al. 2012).
During fetal testis differentiation, the PMCs emerge shortly after the formation of primitive testis cords and are morphologically recognizable at 13.5 dpc in mice (Fig. 1; Jeanes et al. 2005) or 12 wk of gestation in humans (Ostrer et al. 2007). PMCs were once thought to originate from the adjacent mesonephros as part of the cell population that migrates into the gonads (Buehr et al. 1993a; Merchant-Larios et al. 1993). However, more recent studies indicate that the migrating mesonephric cells do not contribute to the PMC lineage but rather are endothelial and contribute to testis vasculature (Cool et al. 2008; Combes et al. 2009b). Thus, the origins of PMCs remain unknown.
The main obstacle in delineating where and how the PMCs arise has been the lack of a PMC-specific molecular marker during early testis differentiation. Although morphologically distinguishable already at 13.5 dpc in the mouse testis, any molecular marker found to be expressed by PMCs at early stages is also expressed by additional interstitial cells (Jeanes et al. 2005). Therefore, based on current knowledge, it is likely that the PMCs originate from intragonadal cells (Fig. 5) or, as proposed by Combes et al. (2009b), the coelomic epithelium by epithelial-to-mesenchymal transformation. The latter possibility is consistent with the observation that some descendants of experimentally labeled surface cells were found to contribute to the interstitial cell population, although no specific cell types were definitively identified (Karl and Capel 1998).
Few animal models show PMC abnormalities during early testis development, but available data point to the involvement of Sertoli cells in normal PMC differentiation. Dhh is expressed in pre-Sertoli cells shortly after SRY (Bitgood et al. 1996; Park et al. 2007), and Dhh knockout mice display compromised PMC and fetal Leydig cell (FLC) differentiation in the developing testis (Clark et al. 2000; Pierucci-Alves et al. 2001; Yao et al. 2002). This dependence on normal testis cord differentiation upon DHH signaling has also been demonstrated in a marsupial (Chung et al. 2012). DHH is a secreted signaling factor that acts via the Patched receptor (PTCH1), which has been shown to be expressed by most interstitial cells at the time of PMC and FLC differentiation (Yao et al. 2002). Hence, Sertoli cells may induce PTCH1-positive precursor cells to further differentiate by secreting DHH. Interestingly, ectopic expression of DHH in developing XX gonads induced FLC differentiation but with no further masculinization effects on the ovary, including PMC development (Barsoum et al. 2009). Therefore, DHH/PTCH1 signaling is not the initial cue for PMC differentiation but is necessary for subsequent development of early PMCs.
Another mouse mutant model, the Dax1 knockout, also displays perturbed development of both FLCs and PMCs (Meeks et al. 2003), leading others to propose that FLCs and PMCs originate from a common interstitial precursor cell population (Wainwright and Wilhelm 2010). If so, the final cellular fate of the plastic precursor cells must depend on physical location within the testis, with close proximity to the immature Sertoli cells locally inducing differentiation into PMCs.
Establishing steroidogenic function: FLCs
Arguably the most important cell type for endocrine function of the testes is the Leydig cell, the source of steroid hormones that drive and maintain the secondary sexual features that essentially define what it is to be male. Decades of morphological and genetic observations have suggested that the Leydig cells found in fetal testes do not develop into those present in the adult but instead that the two cell types arise as two separate lineages with distinct functions and different cellular origins (Rosen-Runge and Anderson 1959; Lording and De Kretser 1972; Vergouwen et al. 1991; O'Shaughnessy et al. 2002; Chen et al. 2009). However, more recently, this dogma has been challenged, and three models are now being considered for the generation of FLC and adult Leydig cell (ALC) populations, as illustrated in Figure 8.
Figure 8.
Proposed models of FLC versus ALC origins. In most mammals, the FLC population appears to perish shortly after birth, to be replaced by a new ALC population. However, different hypotheses have been proposed to explain the origins of the two populations. (A) The most popular hypothesis: The FLCs and ALCs arise from two unique precursor cell populations unrelated to each other, with the FLCs disappearing completely (cross). (B) The least-regarded hypothesis: FLCs and ALCs are the same cell lineage; the FLC population almost disappears postnatally, but a few cells remain to divide and differentiate into ALCs. (C) Alternative hypothesis gaining momentum: FLCs diminish postnatally (cross), and the ALC population is established from the same precursor cells that gave rise to FLCs after lying dormant throughout prepubertal development.
FLC origin and differentiation
Surprisingly, although it is clear that the Leydig cells arise in two separate phases of development in eutherian mammals, the doctrine stipulating two distinct cell lineages (Fig. 8A) rests mainly on gene expression differences between the FLC and ALC populations and not on conclusive lineage-tracing data. Therefore, it remains formally possible that these cells share a common precursor (Fig. 8B,C; for review, see Griswold and Behringer 2009; Wainwright and Wilhelm 2010). Other testicular cell lineages, including Sertoli cells, also display distinct morphology and transcriptional output during different developmental stages. Therefore, the differences between fetal, immature, and mature Leydig cell populations may simply be attributable to the testicular macroenvironment, a consequence of stage-dependent functional requirements.
The origin of the FLC lineage has been vigorously debated, with several potential sources suggested (for review, see Griswold and Behringer 2009; Barsoum and Yao 2010; Svechnikov et al. 2010). In view of their function, it was speculated that FLCs stem from a shared precursor pool of cells destined for endocrine/steroidogenic cell fates. Hence, both neural crest cells (Middendorff et al. 1993; Mayerhofer et al. 1996b) and early adrenogonadal steroidogenic precursor cells (Hatano et al. 1996) were proposed to contribute to establishing the FLC population. However, analysis of early testis development in several strains of knockout mice carrying homozygous gene deletions suggests that neither is the case.
Deletion of Pdgfra (Pdgf receptor α) leads to a lack of FLCs, evidenced by the absence of a steroidogenic marker in the testis at 12.5 dpc despite being detected in the adrenal gland (Brennan et al. 2003). Furthermore, tissue recombination in ex vivo culture showed that cell migration from a wild-type mesonephros failed to rescue the block in Leydig cell differentiation in a Pdgfra-null testis, indicating that the FLC precursors arise from gonadal tissue and not from a migratory population (Brennan et al. 2003). However, this lack of FLC differentiation could also be a result of the mutant testis lacking a required signaling factor: The Pdgfra−/− testis also displays impaired proliferation of Sertoli cells and mesonephric cell migration, and it has been suggested that this Leydig cell phenotype is secondary to Sertoli cell function (Barsoum and Yao 2010).
As discussed previously, the early Sertoli cell marker Dhh has been proposed to act as a paracrine trigger for FLC differentiation, given that Leydig cell differentiation is initiated in the fetal ovary as a result of ectopic DHH expression (Barsoum et al. 2009; Huang and Yao 2010). Although not conclusive, these observations suggest that hedgehog signaling is responsible for inducing differentiation of multipotent precursor cells present in the gonad interstitium and common to nascent testes and ovaries. Accordingly, a recent study strongly supports the likelihood that NR5A1-positive precursor cells are the main contributor to FLCs and also potentially a source for ALCs later in life (Barsoum et al. 2013).
Notch signaling also influences FLC differentiation in mice, with loss and gain of function resulting in increased and reduced FLC numbers, respectively (Tang et al. 2008). Notably, Notch signaling does not appear to influence differentiated FLCs after 13.5 dpc but rather acts to maintain a progenitor cell lineage. These data, together with previous observations that FLCs proliferate by recruitment from a precursor pool of cells rather than by mitotic division of differentiated FLCs (Orth 1982; Kerr et al. 1988), support the hypothesis that FLCs originate from the same precursor pool of Nr5a1-positive cells as do Sertoli cells (Griswold and Behringer 2009; Barsoum and Yao 2010). In addition, a small proportion of FLCs appear to be recruited from a second pool of NR5A1-negative perivascular progenitor cells at the mesonephric–gonadal junction (Fig. 5; DeFalco et al. 2011, 2013). These cells express both the Notch ligand JAG1 and the androgen receptor, suggesting a means by which circulating testosterone may be involved in regulating the progenitor cell pool via Notch signaling (DeFalco et al. 2013).
FLC function
FLCs produce androgens necessary for several aspects of secondary sexual development in the fetus, a function first appreciated after the pioneering work of Jost (1947, 1953). They include development of the Wolffian ducts and external genitalia, testicular descent, and potentially also sex-specific brain patterning (Vilain and McCabe 1998; Klonisch et al. 2004; Griswold and Behringer 2009; Scott et al. 2009); androgen and INSL3 deficiency during early development can lead to feminization of the external genitalia and cryptorchidism and increase the risk of testicular pathologies in later life (Klonisch et al. 2004; Hutson and Hasthorpe 2005; Sharpe and Skakkebaek 2008; Griswold and Behringer 2009; Wohlfahrt-Veje et al. 2009). These functions of FLCs are schematized in Figure 9. Also of note is that the early phase of fetal androgen production by FLCs is independent of pituitary-derived gonadotropins, thus initiating masculinization prior to the establishment of the hypothalamic–pituitary–gonadal signaling axis (Huhtaniemi 1989; O'Shaughnessy and Fowler 2011).
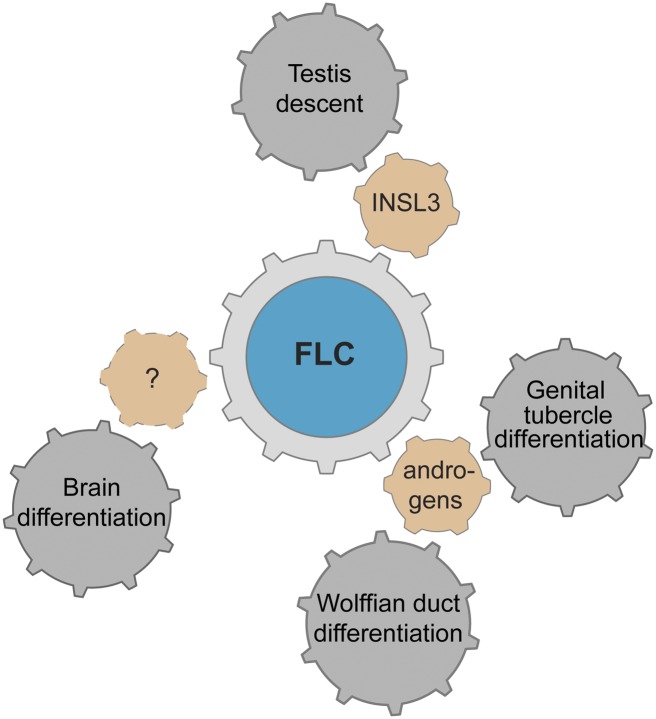
Figure 9.
Factors produced by FLCs affecting male sexual development. The Leydig cells are steroidogenic cells, and both the FLCs and ALCs are responsible for producing androgens. During fetal life, androgens induce the Wolffian ducts to form epididymides, vasa deferentia, and seminal vesicles. Androgens further stimulate the elongation of the genital tubercle to form the penis and tissue fusion to form the penile shaft and scrotum. FLCs also produce factors necessary for testicular descent, including INSL3. Evidence strongly suggests that sex hormones influence differences in brain development between the sexes, but the factors involved remain unknown.
The function of the FLCs could also potentially shed light on their early differentiation and maturation. Testosterone production requires the precursor cholesterol, but testosterone is present in the male fetus prior to cholesterol being detected in the testis (Büdefeld et al. 2009). Hence, initial testosterone synthesis must depend on cholesterol from the blood vasculature (for review, see Azhar et al. 2003). In turn, this could indicate that early FLCs develop near established vasculature (such as the coelomic blood vessel) or the vascular plexus at the mesonephric border, consistent with the model proposed by DeFalco and colleagues (2011) (Fig. 5).
Macrophages
Macrophages may represent as much as 25% of the interstitial cell population in adult mice and rats (Niemi et al. 1986; Hutson 1992; Giannessi et al. 2005) but have remained an underappreciated contributor to testicular development and function during the fetal period. They are known to play important protective and functional roles during reproductive life (Hutson 2006; Pérez et al. 2013). When they first populate the testis is not clear: In rats, testicular macrophages were first reported to arise just before 19 dpc (Hutson 1990), but a subsequent study reported their presence ∼3 d earlier (Livera et al. 2006). It is commonly assumed that they arise from circulating blood monocytes (Hutson 2006). One study reported the presence of macrophages in fetal testes removed from the circulatory system, but these may have arisen from a small number of progenitors or founders that had migrated into the testis early during development (Livera et al. 2006). It would be of interest to revisit this question to clarify when monocytes first migrate into the interstitium to become testicular macrophages and to study any putative early function during gonadal development.
Testicular macrophages have a distinctive phenotype, associating with Leydig cells to form junctional complexes (Christensen and Gillim 1969; Miller et al. 1983; Hutson 1990). In the mature testis, macrophages influence steroidogenesis in Leydig cells, secreting 25-hydroxycholesterol, which is acquired by ALCs and used in testosterone synthesis (Lukyanenko et al. 2000, 2001; Nes et al. 2000). It is not clear whether macrophages play similar or additional roles during fetal testis development or whether FLCs and/or ALCs play a reciprocal role in regulating macrophage differentiation, proliferation, or function. However, studies of Leydig cell function in testis explant or xenograft models, where macrophage numbers might vary significantly from normal, should take into account the potential influence of macrophages on Leydig cells.
Testis vascularization
All developing organs need a vasculature to deliver oxygen, hormones, and nutrients and remove waste; endocrine organs require also a means of secreting their hormonal products to the circulation. During fetal development, the arterial blood vasculature of the testis is established early and is likely to be necessary for testis morphogenesis in addition to simply supplying blood. Following arterialization, the venous network is established to export blood from the testis and also help regulate temperature at reproductive age (Harrison 1949; Harrison and Weiner 1949). Testis lymphangiogenesis appears to be one of the last events during fetal development (Fig. 1), suggesting that lymphatic vasculature is only required during late gestation or postnatally for testis function and fluid homeostasis.
Blood vasculature
Initially, a prominent vascular plexus can be observed in the mesonephroi of both XX and XY embryos, with microvessels extending into the gonadal tissue. As the gonads continue to grow, the blood vasculature of the ovary is thought to develop by angiogenesis (Coveney et al. 2008), whereas in the testis, the first blood vessels develop by a nonangiogenic process involving rearrangement of migrating endothelial cells emanating from the pre-existing mesonephric vascular plexus (Brennan et al. 2002; Coveney et al. 2008). Detailed imaging experiments involving transgenic mouse models coupled with confocal microscopy have shown that endothelial cells first detach from the arteries of the mesonephric plexus, become motile, and actively migrate through the testis to the anti-mesonephric region, where they rearrange to form the main testis artery (the coelomic vessel) (Fig. 1) subjacent to the coelomic endothelium (Coveney et al. 2008). Vascular endothelial growth factor (VEGF) isoforms have been shown to be required for this process (Bott et al. 2008; Cool et al. 2011). From the coelomic vessel, new branches extend toward the tunica albuginea and also into the testis interstitium, ultimately reconnecting at the rete testis. In addition to this testis-specific arterialization, endothelial cell migration is also involved in testis morphogenesis by influencing testis cord partitioning (see “Testis Cord Partitioning and Maturation,” above). Several mouse mutant models display both testis cord and vascular abnormalities (Brennan et al. 2003; Bott et al. 2006; Yao et al. 2006), confirming that the two processes are closely intertwined.
Compared with arterialization, little is known about the mechanisms of venous development during testis organogenesis. Nevertheless, it appears that the venous vasculature arises from the mesonephros—in the rete testis—before sprouting into the testis, probably following an already established arterial network (Brennan et al. 2002).
In the early mouse gonads (∼11.5 dpc), both the arterial marker Efnb2 and the venous marker Ephb4 have been identified in presumptive microvessels in both sexes (Brennan et al. 2002). However, Ephb4 expression was observed to be rapidly down-regulated in the XY gonads, and endothelial cells contributing to the coelomic (arterial) vessel were all Ephb4-negative (Brennan et al. 2002). Interestingly, a venous-specific upstream regulator of Ephb4 has been identified as Nr2f2 (also known as COUP-TFII), which is responsive to retinoic acid (You et al. 2005; Kruse et al. 2008). Thus, clearance of retinoic acid due to up-regulation of CYP26B1 shortly after 11.5 dpc (MacLean et al. 2001; Kashimada et al. 2011), a prerequisite for protecting testicular germ cells from entering meiosis (Bowles et al. 2006; MacLean et al. 2007), may down-regulate Nr2f2, suppressing Ephb4 expression and temporarily suspending venous development in the testis. However, further studies are required to test these possibilities.
Lymphatic vasculature
Although hormone circulation is essential for male embryonic differentiation and virilization, it remains unclear whether testicular lymphatic vessels play a role in this process in addition to the blood circulatory system. Lymphatic vessels have been observed within the adult testis in diverse mammalian species (Fawcett et al. 1969; Fawcett et al. 1973; Clark 1976; Robaire and Bayly 1989; Sonne et al. 2006; Heinzelbecker et al. 2013), and it has been assumed that these vessels develop by lymphangiogenesis. However, when and how lymphangiogenesis occurs in the developing testis has only recently been studied.
First insights in mice came from analysis of heterozygous Prox1+/lacZ lymphatic reporter embryos (Brennan et al. 2002); lacZ-positive vessels were observed at the gonad–mesonephric border from 13.5 dpc, but surprisingly, no lymphatic vessels were observed to invade the testis by 18.5 dpc. Use of the lymphatic marker Ccl21 (chemokine, C-C motif, and ligand 21; commonly called SLC) confirmed this observation, with Ccl21+ lymphatic vessels seen on the gonad surface from 17.5 dpc (Brennan et al. 2002).
Recently, a Prox1-EGFP reporter mouse was used to detail the spatiotemporal development of gonadal lymphangiogenesis (Svingen et al. 2012). In the testis, lymphangiogenesis was found to be initiated at ∼17 dpc, originating from a rich lymphatic network already established along the vasa deferentia and epididymis (Svingen et al. 2012). Interestingly, as new vessels reach the gonad at the rete testis, they sprout to cover the testis cap but never grow further than just under the tunica albuginea, supporting observations from adult rodent testes (Hirai et al. 2012). This patterning is in contrast to larger mammals, and the reason for (and, indeed, the mechanism underlying) the limited range of lymphangiogenesis in the mouse testis remains unknown.
The tunica albuginea
The mammalian testes are surrounded by a protective capsule of fibrous tissue, of which the tunica albuginea constitutes the main component (Fig. 1B). The thickness of the tunica varies between species, and in larger mammals, it also contains numerous smooth muscle (Setchell et al. 1994) and contractile cells (Middendorff et al. 2002). Apart from serving as a protective capsule, it is also involved in regulating blood flow, intertesticular pressure, and sperm movement through rhythmic contractions (Setchell et al. 1994). Despite having been well characterized in the postnatal testis, fetal development of the tunica albuginea has received little attention.
Studies from various mammalian species have shown that the tunica albuginea forms early during fetal testis differentiation, shortly after the primitive testis cords (Waters and Trainer 1996; Karl and Capel 1998; Carmona et al. 2009). The process involves the formation of a fibrous basement membrane just beneath the coelomic epithelium and is suggested to involve both cell movement and differentiation, as cells need to undergo changes in shape and location and are known to express migratory markers (Carmona et al. 2009). It is still unclear exactly what cells contribute to this membrane, and both the coelomic epithelium and testis interstitium have been proposed (Karl and Capel 1998; Carmona et al. 2009). Also, as discussed, extragonadal cell migration contributes to key morphological events of early testis differentiation, but after the formation of the tunica albuginea, this cell migration ceases (Karl and Capel 1998). Since the tunica albuginea contains numerous smooth muscle cells, at least in larger mammals, it would be interesting to delineate both the precursor cells and the genetic triggers of this cell lineage. Such studies could potentially also help illuminate the origin of the PMCs.
Testis innervation
It is well known that physical trauma to the testes can cause severe pain for their owners, suggesting that testes contain abundant and highly functional sensory neurons. However, more than a century ago, it was also suggested that the sensory neurons might localize to the surrounding tissues and structures rather than to the testes themselves (Cushing 1900). Many subsequent studies have addressed neuronal activity in testes subjected to pressure, stretching, and general trauma (Woollard and Carmichael 1933; Moore 1938; Oettle 1954; Peterson and Brown 1973; Yoshioka et al. 2010).
The testes have a neuronal network that depends on two main nerve supplies: the superior and inferior spermatic nerves. Denervation experiments have established that these nerves are important for adult testis function, including endocrine regulation and spermatogenesis (Frankel and Ryan 1981; Nagai et al. 1982; Campos et al. 1993; Chiocchio et al. 1999; Chow et al. 2000; Lee et al. 2002; Gong et al. 2009). However, very little is known regarding the involvement of the nervous system in fetal testis development. Also, the developmental timing of testis innervation in various species is poorly delineated, and thus a potential function during fetal development is difficult to envisage.
Nerve fibers have been noted in the human fetal testis as early as week 16 of gestation, with increasing numbers observed from week 17 and a clear localization between the seminiferous cords from 20 wk (Otulakowski 1992). It is assumed that these are extrinsic nerves entering the gonad by innervation, and the existence of intrinsic neurons in the fetal testis has not been clearly established. Nevertheless, intrinsic neurons have been identified in postnatal testes of primates (Mayerhofer et al. 1996a; Mayerhofer et al. 1999; Frungieri et al. 2000) and also in fetal ovaries of both humans and pigs (Anesetti et al. 2001; Dees et al. 2006). In rodent ovaries, there appear to be few or no intrinsic neurons (D'Albora and Barcia 1996; D'Albora et al. 2000). However, there are clear sexual differences in the neuronal network of adult rodent gonads (Allen et al. 1989), suggesting that fetal testes may not possess intrinsic neurons despite the fetal ovaries doing so.
Interestingly, studies performed to delineate factors that promote or inhibit neurogenesis elsewhere in the embryo have revealed spatial pro- and anti-neurogenic zones involving retinoic acid and FGF signaling (Diez del Corral et al. 2003; Maden 2007). An antagonistic relationship between the same signaling molecules is also pivotal for gonadal sex differentiation; therefore, although speculative, these overlapping molecular pathways could help explain the sexually dimorphic neuronal network that develops in the gonads.
Concluding remarks
The embryo resembles a set of Russian dolls: Open it and you will find in each organ the same issues of structural organization and cell fate specification as occur in the embryo itself but on a smaller scale. Open each organ and most likely you will find smaller, repeated structural units—alveoli, glomeruli, and crypts—in which the same issues are played out once more on a smaller scale still. In recent decades, the availability of molecular tools, imaging systems, and mouse models has provided the opportunity to shed light on these processes in the developing testis.
Clearly, Sertoli cells stand out as the central drivers of testis differentiation, the testicular equivalent of an embryonic organizing center. Having one cell lineage direct the fate of the others avoids the problem that would otherwise occur if different transcriptional cascades were flowing from a single master regulator gene in different cell types. It also allows the behavior of several lineages to be coordinated rather than independently specified. In that regard, it is curious that no mechanism appears to exist that ties the onset of Sry expression in the supporting cell lineage to the arrival of the germ cells, given how tightly these two events need to dovetail.
Unlike other organ systems, at least three cell lineages (supporting cells, steroidogenic cells, and germ cells) are bipotential and can differentiate into testicular or ovarian counterparts (Fig. 2). Having the Sertoli cells coordinate testis development also provides a means of canalizing gonadal fate: Where expression of Sry is weak or delayed, recruitment mechanisms are in play to encourage all supporting cells to follow the Sertoli cell path, maximizing the signals that induce Leydig cell differentiation and promote male germ cell fate and minimizing the possibility of mixed-sex cell types in the gonad.
It is especially noteworthy that Sertoli (or granulosa) cell fate, once specified, is not permanent but instead needs to be constantly reinforced. Loss of FOXL2 function in the adult ovary allows granulosa cells to transdifferentiate into Sertoli cells (Uhlenhaut et al. 2009), while loss of DMRT1 in Sertoli cells of the adult testis allows their transformation into granulosa cells (Matson et al. 2011). Perhaps this plasticity is an evolutionary vestige of mechanisms that allow some fish species to change sex during adult life in response to social cues.
Considering their importance for endocrine function and hence the intensity with which Leydig cells have been studied, it is surprising that their origins and the nature of the difference between FLCs and ALCs have not been resolved. Comparative studies involving marsupials, which appear to have only one population of Leydig cells, may be informative, and detailed lineage-tracing studies using appropriate mouse marker strains will be required to settle the issue conclusively.
Some mechanisms used during testis organogenesis are completely novel. Testis cord formation proceeds via a mechanism that is completely distinct from other modes of tube formation in the embryo, such as the branching morphogenesis seen in the kidneys and lungs and the sprouting of existing tubes under the guidance of tip cells, as occurs during angiogenesis and pancreatic duct outgrowth (for review, see Cleveland et al. 2012; Tung et al. 2012). In the testis, dispersed pre-Sertoli cells coalesce into primitive tubes that are then remodeled in close association with the testis vasculature. Clearly, this mechanism deserves detailed real-time imaging in three dimensions and molecular study; it would also be surprising if this mechanism were not used elsewhere in the embryo.
Similarly, testis vascularization appears to be mechanistically unique. Rather than sprouting from pre-existing arteries by angiogenesis, the coelomic blood vessel that runs the length of the testis forms from endothelial cells that originate from a vascular plexus in the adjacent mesonephros, migrate across the width of the gonad, and reassemble on its opposite face. Although it is not known how or indeed why this occurs, this mechanism is completely distinct from known modes of vasculogenesis and angiogenesis.
Unlike the situation with Sertoli, Leydig, and germ cells, where a bipotential lineage is induced to adopt one of two alternative fates, the vasculature and neuronal components of the developing testes arise from external cell populations exposed to immigration cues that differ between the testis and ovary. While these cues are currently not well understood, analysis of ovotestis development may prove instructive. Ovotestes provide a unique situation in which both testicular and ovarian tissues are present in the one organ, allowing for detailed analyses of the interplay between different cell populations in instructing formation and patterning of immigrant cell networks. Detailed study of cell behavior at the border of testis and ovary in these organs will allow juxtacrine, paracrine, and autocrine signaling to be distinguished and can also help distinguish between the presence of attractive or repulsive cues. Further study of ovotestis development may also shed light on the origin and sexual dimorphism of other cell types of the gonad about which less is known.
Despite tremendous advances, many questions pertaining to testis development and function remain. The basic determinants of gonadal shape and size remain unresolved. Some components such as the PMC lineage and the tunica albuginea remain very poorly characterized. Furthermore, the tools that have been so thoroughly exploited in the mouse system have not been widely used in comparative studies to date. Understanding the detailed processes involved in building a testis will guide efforts to identify the genes and pathways that drive them, which in turn will fuel the search for genetic lesions that underpin the many XY disorders of sex development whose causes remain unknown. Similarly, understanding how somatic cells and cord structures physically and molecularly regulate the proliferation, meiosis, maturation, and export of germ cells is likely to reveal new causes for infertility and germ cell cancers.
Acknowledgments
We thank Dr. Dagmar Wilhelm and Dr. Cassy Spiller for insightful discussions, and Dr. Yoshiakira Kanai for helpful comments on the manuscript. This work was supported by grants from the Australian Research Council and the National Health and Medical Research Council of Australia and a Vice-Chancellor's Senior Research Fellowship from the University of Queensland.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.228080.113.
References
- Albrecht KH, Eicher EM 2001. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol 240: 92–107 [DOI] [PubMed] [Google Scholar]
- Allen LG, Wilson FJ, Macdonald GJ 1989. Neuropeptide Y-containing nerves in the rat gonads: Sex difference and development. Biol Reprod 40: 371–378 [DOI] [PubMed] [Google Scholar]
- Anderson R, Copeland TK, Schöler H, Heasman J, Wylie C 2000. The onset of germ cell migration in the mouse embryo. Mech Dev 91: 61–68 [DOI] [PubMed] [Google Scholar]
- Anesetti G, Lombide P, D'Albora H, Ojeda SR 2001. Intrinsic neurons in the human ovary. Cell Tissue Res 306: 231–237 [DOI] [PubMed] [Google Scholar]
- Azhar S, Leers-Sucheta S, Reaven E 2003. Cholesterol uptake in adrenal and gonadal tissues: The SR-BI and ‘selective’ pathway connection. Front Biosci 8: s998–s1029 [DOI] [PubMed] [Google Scholar]
- Bagheri-Fam S, Sim H, Bernard P, Jayakody I, Taketo MM, Scherer G, Harley VR 2008. Loss of Fgfr2 leads to partial XY sex reversal. Dev Biol 314: 71–83 [DOI] [PubMed] [Google Scholar]
- Baillie AH 1964. The histochemistry and ultrastructure of the gonocyte. J Anat 98: 641–645 [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G 2006. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod 74: 195–201 [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Georg I, Scherthan H, Lecureuil C, Guillou F, Wegner M, Scherer G 2009. Testis cord differentiation after the sex determining stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol 327: 301–312 [DOI] [PubMed] [Google Scholar]
- Barsoum IB, Yao HH 2010. Fetal Leydig cells: Progenitor cell maintenance and differentiation. J Androl 31: 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HH 2009. Activation of the Hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev Biol 329: 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum IB, Kaur J, Ge RS, Cooke PS, Yao HH 2013. Dynamic changes in fetal Leydig cell populations influence adult Leydig cell populations in mice. FASEB J 27: 2657–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg JA, Parker KL, et al. 2000. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 403: 909–913 [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP 1996. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol 6: 298–304 [DOI] [PubMed] [Google Scholar]
- Bott RC, McFee RM, Clopton DT, Toombs C, Cupp AS 2006. Vascular endothelial growth factor and kinase domain region receptor are involved in both seminiferous cord formation and vascular development during testis morphogenesis in the rat. Biol Reprod 75: 56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott RC, Clopton DT, Cupp AS 2008. A proposed role for VEGF isoforms in sex-specific vasculature development in the gonad. Reprod Domest Anim 43: 310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Koopman P 2010. Sex determination in mammalian germ cells: Extrinsic versus intrinsic factors. Reproduction 139: 943–958 [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, et al. 2006. Retinoid signaling determines germ cell fate in mice. Science 312: 596–600 [DOI] [PubMed] [Google Scholar]
- Bowles J, Feng CW, Spiller CM, Davidson TL, Jackson A, Koopman P 2010. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell 19: 440–449 [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B 2004. One tissue, two fates: Molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 5: 509–521 [DOI] [PubMed] [Google Scholar]
- Brennan J, Karl J, Capel B 2002. Divergent vascular mechanisms downstream of Sry establish the arterial system in the XY gonad. Dev Biol 244: 418–428 [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B 2003. Pdgfr-α mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev 17: 800–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büdefeld T, Jezek D, Rozman D, Majdic G 2009. Initiation of steroidogenesis precedes expression of cholesterologenic enzymes in the fetal mouse testes. Anat Histol Embryol 38: 461–466 [DOI] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A 1993a. Mesonephric contribution to testis differentiation in the fetal mouse. Development 117: 273–281 [DOI] [PubMed] [Google Scholar]
- Buehr M, McLaren A, Bartley A, Darling S 1993b. Proliferation and migration of primordial germ cells in We/We mouse embryos. Dev Dyn 198: 182–189 [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P 2001. Spatially dynamic expression of Sry in mouse genital ridges. Dev Dyn 221: 201–205 [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P 2005. Delayed Sry and Sox9 expression in developing mouse gonads underlies B6-Y(DOM) sex reversal. Dev Biol 278: 473–481 [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP 1995. The genetic basis of XX–XY differences present before gonadal sex differentiation in the mouse. Philos Trans R Soc Lond B Biol Sci 350: 253–260 [DOI] [PubMed] [Google Scholar]
- Campos MB, Chiocchio SR, Calandra RS, Ritta MN 1993. Effect of bilateral denervation of the immature rat testis on testicular gonadotropin receptors and in vitro androgen production. Neuroendocrinology 57: 189–194 [DOI] [PubMed] [Google Scholar]
- Carmona FD, Lupiáñez DG, Martin JE, Burgos M, Jiménez R, Zurita F 2009. The spatio-temporal pattern of testis organogenesis in mammals—insights from the mole. Int J Dev Biol 53: 1035–1044 [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A 2004. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131: 1891–1901 [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, Svingen T, Ng ET, Epp T, Spiller CM, Clark C, Cooper H, Koopman P 2012. Loss of Wnt5a disrupts primordial germ cell migration and male sexual development in mice. Biol Reprod 86: 1–12 [DOI] [PubMed] [Google Scholar]
- Chen H, Ge RS, Zirkin BR 2009. Leydig cells: From stem cells to aging. Mol Cell Endocrinol 306: 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocchio SR, Suburo AM, Vladucic E, Zhu BC, Charreau E, Décima EE, Tramezzani JH 1999. Differential effects of superior and inferior spermatic nerves on testosterone secretion and spermatic blood flow in cats. Endocrinology 140: 1036–1043 [DOI] [PubMed] [Google Scholar]
- Chow SH, Giglio W, Anesetti R, Ottenweller JE, Pogach LM, Huang HF 2000. The effects of testicular denervation on spermatogenesis in the Sprague-Dawley rat. Neuroendocrinology 72: 37–45 [DOI] [PubMed] [Google Scholar]
- Christensen AK, Gillim SW 1969. The correlation of fine structure and function in steroid-secreting cells, with emphasis on those of the gonads. In The gonads (ed. McKerns KW), 415–488. Appleton-Century-Crofts, New York [Google Scholar]
- Chung JW, Pask AJ, Renfree MB 2012. Seminiferous cord formation is regulated by Hedgehog signaling in the marsupial. Biol Reprod 86: 80. [DOI] [PubMed] [Google Scholar]
- Clark RV 1976. Three-dimensional organization of testicular interstitial tissue and lymphatic space in the rat. Anat Rec 184: 203–225 [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD 2000. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod 63: 1825–1838 [DOI] [PubMed] [Google Scholar]
- Cleveland MH, Sawyer JM, Afelik S, Jensen J, Leach SD 2012. Exocrine ontogenies: On the development of pancreatic acinar, ductual and centroacinar cells. Semin Cell Dev Biol 23: 711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM 2001. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell 104: 875–889 [DOI] [PubMed] [Google Scholar]
- Combes AN, Lesieur E, Harley VR, Sinclair AH, Little MH, Wilhelm D, Koopman P 2009a. Three-dimensional visualization of testis cord morphogenesis, a novel tubulogenic mechanism in development. Dev Dyn 238: 1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes AN, Wilhelm D, Davidson TL, Dejana E, Harley V, Sinclair A, Koopman P 2009b. Endothelial cell migration directs testis cord formation. Dev Biol 326: 112–120 [DOI] [PubMed] [Google Scholar]
- Cool J, Carmona FD, Szucsik JC, Capel B 2008. Peritubular myoid cells are not the migrating population required for testis cord formation in the XY gonad. Sex Dev 2: 128–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool J, DeFalco T, Capel B 2011. Vascular–mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci 108: 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coveney D, Cool J, Oliver T, Capel B 2008. Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci 105: 7212–7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp AS, Kim GH, Skinner MK 2000. Expression and action of neurotropin-3 and nerve growth factor in embryonic and early postnatal rat testis development. Biol Reprod 63: 1617–1628 [DOI] [PubMed] [Google Scholar]
- Cupp AS, Tessarollo L, Skinner MK 2002. Testis developmental phenotypes in neurotropin receptor trkA and trkC null mutations: Role in formation of seminiferous cords and germ cell survival. Biol Reprod 66: 1838–1845 [DOI] [PubMed] [Google Scholar]
- Cushing H 1900. The employment of local anaesthesia in the radical cure of certain cases of hernia, with a note upon the nervous anatomy of the inguinal region. Ann Surg 31: 1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Albora H, Barcia JJ 1996. Intrinsic neuronal cell bodies in the rat ovary. Neurosci Lett 205: 65–67 [DOI] [PubMed] [Google Scholar]
- D'Albora H, Lombide P, Ojeda SR 2000. Intrinsic neurons in the rat ovary: An immunohistochemical study. Cell Tissue Res 300: 47–56 [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Breucker H, Holstein AF, Seidl K 1990. Cellular architecture of the lamina propria of human seminiferous tubules. Cell Tissue Res 262: 253–261 [DOI] [PubMed] [Google Scholar]
- Dees WL, Hiney JK, McArthur NH, Johnson GA, Dissen GA, Ojeda SR 2006. Origin and ontogeny of mammalian ovarian neurons. Endocrinology 147: 3789–3796 [DOI] [PubMed] [Google Scholar]
- DeFalco T, Takahashi S, Capel B 2011. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol 352: 14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco T, Saraswathula A, Briot A, Iruela-Arispe ML, Capel B 2013. Testosterone levels influence mouse fetal Leydig cell progenitors through Notch signaling. Biol Reprod 88: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K 2003. Opposing FGF and retinoid pathways control ventral pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40: 65–79 [DOI] [PubMed] [Google Scholar]
- Donovan PJ, Stott D, Cairns LA, Heasman J, Wylie C 1986. Migratory and postmigratory mouse primordial germ cells behave differently in culture. Cell 44: 831–838 [DOI] [PubMed] [Google Scholar]
- Dym M 1994. Basement membrane regulation of Sertoli cells. Endocr Rev 15: 102–115 [DOI] [PubMed] [Google Scholar]
- Eggers S, Sinclair A 2012. Mammalian sex determination—insights from humans and mice. Chromosome Res 20: 215–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL, Whitney JB III, Morrow KE 1982. Mus poschiavinus Y chromosome in the C57BL/6J murine genome causes sex reversal. Science 217: 535–537 [DOI] [PubMed] [Google Scholar]
- Escalante-Alcalde D, Merchant-Larios H 1992. Somatic and germ cell interactions during histogenetic aggregation of mouse fetal testes. Exp Cell Res 198: 150–158 [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Heidger PM, Leak LV 1969. Lymph vasculature system of the interstitial tissue of the testis as revealed by electron microscopy. J Reprod Fertil 19: 109–119 [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Neaves WB, Flores MN 1973. Comparative observations on intertubular lymphatics and the organization of the interstitial tissue of the mammalian testis. Biol Reprod 9: 500–532 [DOI] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, et al. 1994. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372: 525–530 [DOI] [PubMed] [Google Scholar]
- Frankel AI, Ryan EL 1981. Testicular innervation is necessary for the response of plasma testosterone levels to acute stress. Biol Reprod 124: 491–495 [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Urbanski HF, Höhne-Zell B, Mayerhofer A 2000. Neuronal elements in the testis of the rhesus monkey: Ontogeny, characterization and relationship to testicular cells. Neuroendocrinology 71: 43–50 [DOI] [PubMed] [Google Scholar]
- Fujimoto Y, Tanaka SS, Yamaguchi YL, Kobayashi H, Kuroki S, Tachibana M, Shinomura M, Kanai Y, Morohashi K, Kawakami K, et al. 2013. Homeoproteins six1 and six4 regulate male sex determination and mouse gonadal development. Dev Cell 26: 416–430 [DOI] [PubMed] [Google Scholar]
- Gassei K, Ehmcke J, Schlatt S 2008. Initiation of testicular tubulogenesis is controlled by neurotrophic tyrosine receptor kinases in a three-dimensional Sertoli cell aggregation assay. Reproduction 136: 459–469 [DOI] [PubMed] [Google Scholar]
- Georg I, Barrionuevo F, Wiech T, Scherer G 2012. Sox9 and Sox8 are required for basal lamina integrity of testis cords and for suppression of FOXL2 during embryonic testis development in mice. Biol Reprod 87: 99. [DOI] [PubMed] [Google Scholar]
- Giannessi F, Giambelluca MA, Scavuzzo MC, Ruffoli R 2005. Ultrastructure of testicular macrophages in aging mice. J Morphol 263: 39–46 [DOI] [PubMed] [Google Scholar]
- Gong YG, Wang YQ, Gu M, Feng MM, Zhang W, Ge RS 2009. Deprival of testicular innervation induces apoptosis of Leydig cells via caspase-8-dependent signaling: A novel survival pathway revealed. Biochem Biophys Res Commun 382: 165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold SL, Behringer RR 2009. Fetal Leydig cell origin and development. Sex Dev 3: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD, Hogarth CA, Bowles J, Koopman P 2012. Initiating meiosis: The case for retinoic acid. Biol Reprod 86: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbay J, Koopman P, Collignon J, Burgoyne P, Lovell-Badge R 1990. Normal structure and expression of Zfy genes in XY female mice mutant in Tdy. Development 109: 647–653 [DOI] [PubMed] [Google Scholar]
- Hadley MA, Byers SW, Suárez-Quian CA, Kleinman HK, Dym M 1985. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol 101: 1511–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Kanai-Azuma M, Uemura M, Shitara H, Taya C, Yonekawa H, Kawakami H, Tsunekawa N, Kurohmaru M, Kanai Y 2009. Evidence for crucial role of hindgut expansion in directing proper migration of primordial germ cells in mouse early embryogenesis. Dev Biol 330: 427–439 [DOI] [PubMed] [Google Scholar]
- Harikae K, Miura K, Kanai Y 2013. Early gonadogenesis in mammals: Significance of long and narrow gonadal structures. Dev Dyn 242: 330–338 [DOI] [PubMed] [Google Scholar]
- Harrison RG 1949. The comparative anatomy of the blood-supply of the mammalian testis. Proc Zool Soc Lond 119: 325–344 [Google Scholar]
- Harrison RG, Weiner JS 1949. Vascular patterns of the mammalian testis and their functional significance. J Exp Biol 26: 304–316 [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Kubokawa R, Yamazaki K, Noguchi M, Kato Y 1990. Germ cell deficiency causes testis cord differentiation in reconstituted mouse fetal ovaries. J Exp Zool 253: 61–70 [DOI] [PubMed] [Google Scholar]
- Hasthorpe S, Barbic S, Farmer PJ, Hutson JM 1999. Neonatal mouse gonocyte proliferation assayed by an in vitro clonogenic method. J Reprod Fertil 116: 335–344 [DOI] [PubMed] [Google Scholar]
- Hatano O, Takakusu A, Nomura M, Morohashi K 1996. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1: 663–671 [DOI] [PubMed] [Google Scholar]
- Heinzelbecker J, Kempf KM, Kurz K, Steidler A, Weiss C, Jackson DG, Bolenz C, Haecker A, Trojan L 2013. Lymph vessel density in seminomatous testicular cancer assessed with the specific lymphatic endothelium cell markers D2-40 and LYVE-1: Correlation with pathologic parameters and clinical outcome. Urol Oncol 31: 1386–1394 [DOI] [PubMed] [Google Scholar]
- Hirai S, Naito M, Terayama H, Qu N, Kuerban M, Musha M, Ikeda A, Miura M, Itoh M 2012. The origin of lymphatic capillaries in murine testes. J Androl 33: 745–751 [DOI] [PubMed] [Google Scholar]
- Hiramatsu R, Matoba S, Kanai-Azuma M, Tsunekawa N, Katoh-Fukui Y, Kurohmaru M, Morohashi K, Wilhelm D, Koopman P, Kanai Y 2009. A critical time window of Sry action in gonadal sex determination in mice. Development 136: 129–138 [DOI] [PubMed] [Google Scholar]
- Hiramatsu R, Harikae K, Tsunekawa N, Kurohmaru M, Matsuo I, Kanai Y 2010. FGF signaling directs a center-to-pole expansion of tubulogenesis in mouse testis differentiation. Development 137: 303–312 [DOI] [PubMed] [Google Scholar]
- Holstein AF, Maekawa M, Nagano T, Davidoff MS 1996. Myofibroblasts in the lamina propria of human seminiferous tubules are dynamic structures of heterogenous phenotype. Arch Histol Cytol 59: 109–125 [DOI] [PubMed] [Google Scholar]
- Huang CC, Yao HH 2010. Diverse functions of Hedgehog signaling in formation and physiology of steroidogenic organs. Mol Reprod Dev 77: 489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Wang S, Ning Y, Lamb A, Bartley J 1999. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet 87: 349–353 [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I 1989. Endocrine function and regulation of the fetal and neonatal testis. Int J Dev Biol 33: 117–123 [PubMed] [Google Scholar]
- Hutson JC 1990. Changes in the concentration and size of testicular macrophages during development. Biol Reprod 43: 885–890 [DOI] [PubMed] [Google Scholar]
- Hutson JC 1992. Development of cytoplasmic digitations between Leydig cells and testicular macrophages of the rat. Cell Tissue Res 267: 385–389 [DOI] [PubMed] [Google Scholar]
- Hutson JC 2006. Physiological interactions between macrophages and Leydig cells. Exp Biol Med (Maywood) 231: 1–7 [DOI] [PubMed] [Google Scholar]
- Hutson JM, Hasthorpe S 2005. Abnormalities of testicular descent. Cell Tissue Res 322: 155–158 [DOI] [PubMed] [Google Scholar]
- Jameson SA, Lin YT, Capel B 2012a. Testis development requires the repression of Wnt4 by Fgf9 signaling. Dev Biol 370: 24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SA, Natarajan A, Cool J, DeFalco T, Maatouk DM, Mork L, Munger SC, Capel B 2012b. Temporal transcriptional profiling of somatic and germ cells reveals biased lineage priming of sexual fate in the fetal mouse gonad. PLoS Genet 8: e1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes A, Wilhelm D, Wilson MJ, Bowles J, McClive PJ, Sinclair AH, Koopman P 2005. Evaluation of candidate markers for the peritubular myoid cell lineage in the developing mouse testis. Reproduction 130: 509–516 [DOI] [PubMed] [Google Scholar]
- Jost A 1947. Recherches sur la differenciation sexuelle de l'embryon de lapin. Arch Anat Microsc Morphol Exp 36: 271–315 [Google Scholar]
- Jost A 1953. Problems of fetal endocrinology: The gonadal and hypophyseal hormones. Recent Prog Horm Res 8: 379–418 [DOI] [PubMed] [Google Scholar]
- Karl J, Capel B 1998. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol 203: 323–333 [DOI] [PubMed] [Google Scholar]
- Kashimada K, Svingen T, Feng CW, Pelosi E, Bagheri-Fam S, Harley VR, Schlessinger D, Bowles J, Koopman P 2011. Antagonistic regulation of Cyp26b1 by transcription factor SOX9/SF1 and FOXL2 during gonadal development in mice. FASEB J 25: 3561–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Fukui Y, Tsuchiya R, Shiroishi T, Nakahara Y, Hashimoto N, Noguchi K, Higashinakagawa T 1998. Male-to-female sex reversal in M33 mutant mice. Nature 393: 688–692 [DOI] [PubMed] [Google Scholar]
- Katoh-Fukui Y, Miyabayashi K, Komatsu T, Owaki A, Baba T, Shima Y, Kidokoro T, Kanai Y, Schedl A, Wilhelm D, et al. 2012. Cbx2, a polycomb group gene, is required for Sry gene expression in mice. Endocrinology 153: 913–924 [DOI] [PubMed] [Google Scholar]
- Kerr JB, Risbridger GP, Knell CM 1988. Stimulation of interstitial cell growth after selective destruction of foetal Leydig cells in the testis of postnatal rats. Cell Tissue Res 252: 89–98 [DOI] [PubMed] [Google Scholar]
- Kidokoro T, Matoba S, Hiramatsu R, Fujisawa M, Kanai-Azuma M, Taya C, Kurohmaru M, Kawakami H, Hayashi Y, Kanai Y, et al. 2005. Influence on spatiotemporal patterns of a male-specific Sox9 activation by ectopic Sry expression during early phases of testis differentiation in mice. Dev Biol 278: 511–525 [DOI] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B 2006. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol 4: e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, Capel B 2007. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli cell differentiation during male sex determination. Proc Natl Acad Sci 104: 16558–16563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonisch T, Fowler PA, Hombach-Klonisch S 2004. Molecular and genetic regulation of testis descent and external genitalia development. Dev Biol 270: 1–18 [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R 1991. Male development of chromosomally female mice transgenic for Sry. Nature 351: 117–121 [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC 2006. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci 103: 2474–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R 1993. WT-1 is required for early kidney development. Cell 74: 679–691 [DOI] [PubMed] [Google Scholar]
- Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, Vonrhein C, Xu Y, Wang L, Tsai SY, Tsai MJ, et al. 2008. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol 6: e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DJ, Altshuler-Keylin S, Kissner MD, Zhou X, Anderson KV 2011. Ror2 enhances polarity and directional migration of primordial germ cells. PLoS Genet 7: e1002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Sala G, Farini D, De Felici M 2010. Rapid estrogen signalling in mouse primordial germ cells. Exp Cell Res 316: 1716–1727 [DOI] [PubMed] [Google Scholar]
- Lavery R, Chassot AA, Pauper E, Gregoire EP, Klopfenstein M, de Rooij DG, Mark M, Schedl A, Ghyselinck NB, Chaboissier MC 2012. Testicular differentiation occurs in absence of Rspondin-1 and Sox9 in mouse sex reversals. PLoS Genet 8: e1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Hage WJ 1994. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found Symp 182: 68–84 [DOI] [PubMed] [Google Scholar]
- Lee S, Miselis R, Rivier C 2002. Anatomical and functional evidence for a neural hypothalamic–testicular pathway that is independent of the pituitary. Endocrinology 143: 4447–4454 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Pazin DE, Kahlon RS, Correa SM, Albrecht KH 2009. Novel markers of early ovarian pre-granulosa cells are expressed in an Sry-like pattern. Dev Dyn 238: 812–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine E, Cupp AS, Skinner MK 2000. Role of neurotropins in rat embryonic testis morphogenesis (cord formation). Biol Reprod 62: 132–142 [DOI] [PubMed] [Google Scholar]
- Li H, Papadopoulos V, Vidic B, Dym M, Culty M 1997. Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: Identification of signaling mechanisms involved. Endocrinology 138: 1289–1298 [DOI] [PubMed] [Google Scholar]
- Livera G, Delbes G, Pairault C, Rouiller-Fabre V, Habert R 2006. Organotypic culture, a powerful model for studying rat and mouse fetal testis development. Cell Tissue Res 324: 507–521 [DOI] [PubMed] [Google Scholar]
- Lording DW, De Kretser DM 1972. Comparative ultrastructural and histochemical studies of the interstitial cells of the rat testis during fetal and postnatal development. J Reprod Fertil 29: 261–269 [DOI] [PubMed] [Google Scholar]
- Lukyanenko YO, Carpenter AM, Boone MM, Baker CR, McGunegle DE, Hutson JC 2000. Specificity of a new lipid mediator produced by testicular and peritoneal macrophages on steroidogenesis. Int J Androl 23: 258–265 [DOI] [PubMed] [Google Scholar]
- Lukyanenko YO, Chen JJ, Hutson JC 2001. Production of 25-hydroxycholesterol by testicular macrophages and its effects on Leydig cells. Biol Reprod 64: 790–796 [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77: 481–490 [DOI] [PubMed] [Google Scholar]
- MacLean G, Abu-Abed S, Dolle P, Tahayato A, Chambon P, Petkovich M 2001. Cloning of a novel retinoic-acid metabolizing cytochrome P450, Cyp26B1, and comparative expression analysis with Cyp26A1 during early murine development. Mech Dev 107: 195–201 [DOI] [PubMed] [Google Scholar]
- MacLean G, Li H, Metzger D, Chambon P, Petkovich M 2007. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 148: 4560–4567 [DOI] [PubMed] [Google Scholar]
- Maden M 2007. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci 8: 755–765 [DOI] [PubMed] [Google Scholar]
- Maekawa M, Kamimura K, Nagano T 1996. Peritubular myoid cells in the testis: Their structure and function. Arch Histol Cytol 59: 1–13 [DOI] [PubMed] [Google Scholar]
- Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Méjean C, Berta P, Poulat F, Boizet-Bonhoure B 2005. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J 24: 1798–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D 2011. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476: 101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiske D, Kume T, Hogan BL 2006. The mouse forkhead gene Foxc1 is required for primordial germ cell migration and antral follicle development. Dev Biol 290: 447–458 [DOI] [PubMed] [Google Scholar]
- Mauduit C, Hamamah S, Benahmed M 1999. Stem cell factor/c-kit system in spermatogenesis. Hum Reprod Update 5: 535–545 [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Danilchik M, Pau KY, Lara HE, Russell LD, Ojeda SR 1996a. Testis of prepubertal rhesus monkeys receives a dual catecholaminergic input provided by the extrinsic innervation and an intragonadal source of catecholamines. Biol Reprod 55: 509–518 [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Lahr G, Seidl K, Eusterschulte B, Christoph A, Gratzl M 1996b. The neural cell adhesion molecule (NCAM) provides clues to the development of testicular Leydig cells. J Androl 17: 223–230 [PubMed] [Google Scholar]
- Mayerhofer A, Frungieri MB, Fritz S, Bulling A, Jessberger B, Vogt HJ 1999. Evidence for catecholaminergic, neuronlike cells in the adult human testis: Changes associated with testicular pathologies. J Androl 20: 341–347 [PubMed] [Google Scholar]
- McCoshen JA 1982. In vitro sex differentiation of congeneic germinal cell aplastic gonads. Am J Obstet Gynecol 142: 83–88 [DOI] [PubMed] [Google Scholar]
- McCoshen JA 1983. Quantification of sex chromosomal influence(s) on the somatic growth of fetal gonads in vivo. Am J Obstet Gynecol 145: 469–473 [DOI] [PubMed] [Google Scholar]
- Meeks JJ, Crawford SE, Russell TA, Morohashi K, Weiss J, Jameson JL 2003. Dax1 regulates testis cord organization during gonadal differentiation. Development 130: 1029–1036 [DOI] [PubMed] [Google Scholar]
- Mendis SH, Meachem SJ, Sarraj MA, Loveland KL 2011. Activin A balances Sertoli and germ cell proliferation in the fetal mouse testis. Biol Reprod 84: 379–391 [DOI] [PubMed] [Google Scholar]
- Merchant H 1975. Rat gonadal and ovarian organogenesis with and without germ cells. An ultrastructural study. Dev Biol 44: 1–21 [DOI] [PubMed] [Google Scholar]
- Merchant-Larios H, Moreno-Mendoza N, Buehr M 1993. The role of the mesonephros in cell differentiation and morphogenesis of the mouse fetal testis. Int J Dev Biol 37: 407–415 [PubMed] [Google Scholar]
- Middendorff R, Davidoff M, Holstein AF 1993. Neuroendocrine marker substance in human Leydig cells—changes by disturbance of testicular function. Andrologia 25: 257–262 [DOI] [PubMed] [Google Scholar]
- Middendorff R, Müller D, Mewe M, Mukhopadhyay AK, Holstein AF, Davidoff MS 2002. The tunica albuginea of the human testis is characterized by complex contraction and relaxation activities regulated by cyclic GMP. J Clin Endocrinol Metab 87: 3486–3499 [DOI] [PubMed] [Google Scholar]
- Miller SC, Bowman BM, Rowland HG 1983. Structure, cytochemistry, endocytic activity, and immunoglobulin (Fc) receptors of rat testicular interstitial-tissue macrophages. Am J Anat 168: 1–13 [DOI] [PubMed] [Google Scholar]
- Mittwoch U 1998. Phenotypic manifestations during the development of the dominant and default gonads in mammals and birds. J Exp Zool 281: 466–471 [PubMed] [Google Scholar]
- Mittwoch U, Delhanty JD, Beck F 1969. Growth of differentiating testes and ovaries. Nature 221: 446–448 [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S 1997. Defects of urogenital development in mice lacking Emx2. Development 124: 1653–1664 [DOI] [PubMed] [Google Scholar]
- Møllgård K, Jespersen A, Lutterodt MC, Yding Andersen C, Høyer PE, Byskov AG 2010. Human primordial germ cells migrate along nerve fibers and Schwann cells from the dorsal hind gut mesentery to the gonadal ridge. Mol Hum Reprod 16: 621–631 [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Stallock J, Schaible K, Wylie C 2001. Time-lapse analysis of living mouse germ cell migration. Dev Biol 240: 488–498 [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O'Brien W, Raz E, Littman D, Wylie C, et al. 2003. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 130: 4279–4286 [DOI] [PubMed] [Google Scholar]
- Moniot B, Declosmenil F, Barrionuevo F, Scherer G, Aritake K, Malki S, Marzi L, Cohen-Solal A, Georg I, Klattig J, et al. 2009. The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation. Development 136: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RM 1938. Some experimental observations relating to visceral pain. Surgery 3: 534–555 [Google Scholar]
- Moreno SG, Attali M, Allemand I, Messiaen S, Fouchet P, Coffigny H, Romeo PH, Habert R 2010. TGFβ signaling in male germ cells regulates gonocyte quiescence and fertility in mice. Dev Biol 342: 74–84 [DOI] [PubMed] [Google Scholar]
- Nagai K, Murano S, Minokoshi Y, Okuda H, Kinutani M 1982. Effects of denervation and local 6-hydroxydopamine injection on testicular growth in rats. Experientia 38: 592–594 [DOI] [PubMed] [Google Scholar]
- Nef S, Verma-Kurvari S, Merenmies J, Vassalli JD, Efstratiadis A, Accili D, Parada LF 2003. Testis determination requires insulin receptor family function in mice. Nature 426: 291–295 [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, et al. 2005. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol 287: 361–377 [DOI] [PubMed] [Google Scholar]
- Nel-Themaat L, Vadakkan TJ, Wang Y, Dickinson ME, Akiyama H, Behringer RR 2009. Morphometric analysis of testis cord formation in Sox9-EGFP mice. Dev Dyn 238: 1100–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel-Themaat L, Jang CW, Stewart MD, Akiyama H, Viger RS, Behringer RR 2011. Sertoli cell behaviors in developing testis cords and postnatal seminiferous tubules of the mouse. Biol Reprod 84: 342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes WD, Lukyanenko YO, Jia ZH, Quideau S, Howald WN, Pratum TK, West RR, Hutson JC 2000. Identification of the lipophilic factor produced by macrophages that stimulates steroidogenesis. Endocrinology 141: 953–958 [DOI] [PubMed] [Google Scholar]
- Niemi M, Sharpe RM, Brown WR 1986. Macrophages in the interstitial tissue of the rat testis. Cell Tissue Res 243: 337–344 [DOI] [PubMed] [Google Scholar]
- Oettle AG 1954. The technique of testicular biopsy with a note on testicular pain. S Afr J Clin Sci 5: 45–62 [PubMed] [Google Scholar]
- Orth JM 1982. Proliferation of Sertoli cells in fetal and postnatal rats: A quantitative autoradiographic study. Anat Rec 203: 485–492 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Fowler PA 2011. Endocrinology of the mammalian fetal testis. Reproduction 141: 37–46 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Johnston H, Willerton L, Baker PJ 2002. Failure of normal adult Leydig cell development in androgen-receptor-deficient mice. J Cell Sci 115: 3491–3496 [DOI] [PubMed] [Google Scholar]
- Ostrer H, Huang HY, Masch RJ, Shapiro E 2007. A cellular study of human testis development. Sex Dev 1: 286–292 [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D 2007. Loss of Wnt4a and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet 16: 2795–2804 [DOI] [PubMed] [Google Scholar]
- Otulakowski B 1992. Innervation of the testis in human fetuses from 16th to 22nd week. Folia Morphol (Warsz) 51: 81–85 [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne PS 1991. In situ analysis of fetal prepubertal and adult XX–XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development 112: 265–268 [DOI] [PubMed] [Google Scholar]
- Park SY, Tong M, Jameson JL 2007. Distinct roles for steroidogenic factor 1 and desert hedgehog pathways in fetal and adult Leydig cell development. Endocrinology 148: 3704–3710 [DOI] [PubMed] [Google Scholar]
- Patek CE, Kerr JB, Gosden RG, Jones KW, Hardy K, Muggleton-Harris AL, Handyside AH, Whittingham DG, Hooper ML 1991. Sex chimaerism, fertility and sex determination in the mouse. Development 113: 311–325 [DOI] [PubMed] [Google Scholar]
- Pellas TC, Ramachandran B, Duncan M, Pan SS, Marone M, Chada K 1991. Germ-cell deficient (gcd), an insertional mutation manifested as infertility in transgenic mice. Proc Natl Acad Sci 88: 8787–8791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelliniemi LJ 1975. Ultrastructure of gonadal ridge in male and female pig embryos. Anat Embryol (Berl) 147: 20–34 [PubMed] [Google Scholar]
- Pérez CV, Theas MS, Jacobo PV, Jarazo-Dietrich S, Guazzone VA, Lustig L 2013. Dual role of immune cells in the testis: Protective or pathogenic for germ cells? Spermatogenesis 3: e23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DF, Brown AM 1973. Functional afferent innervation of testis. J Neurophysiol 36: 425–433 [DOI] [PubMed] [Google Scholar]
- Pierucci-Alves F, Clark AM, Russell LD 2001. A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod 65: 1392–1402 [DOI] [PubMed] [Google Scholar]
- Polanco JC, Koopman P 2007. Sry and the hesitant beginnings of male development. Dev Biol 302: 13–24 [DOI] [PubMed] [Google Scholar]
- Polanco JC, Wilhelm D, Davidson TL, Knight D, Koopman P 2010. Sox10 gain-of-function causes XX sex reversal in mice: Implications for human 22q-linked disorders of sex development. Hum Mol Genet 19: 506–516 [DOI] [PubMed] [Google Scholar]
- Quinn A, Koopman P 2012. The molecular genetics of sex determination and sex reversal in mammals. Semin Reprod Med 30: 351–363 [DOI] [PubMed] [Google Scholar]
- Richardson BE, Lehmann R 2010. Mechanisms guiding primordial germ cell migration: Strategies from different organisms. Nat Rev Mol Cell Biol 11: 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire B, Bayly SF 1989. Testicular signaling: Incoming and outgoing messages. Ann N Y Acad Sci 564: 250–260 [DOI] [PubMed] [Google Scholar]
- Rodemer-Lenz E 1989. On cell contribution to gonadal soma formation in quail-chick chimeras during the indifferent stage of gonadal development. Anat Embryol (Berl) 179: 237–242 [DOI] [PubMed] [Google Scholar]
- Rosen-Runge EC, Anderson D 1959. The development of the interstitial cells in the testis of the albino rat. Acta Anat (Basel) 37: 125–137 [DOI] [PubMed] [Google Scholar]
- Russo MA, Giustizieri ML, Favale A, Fantini MC, Campagnolo L, Konda D, Germano F, Farini D, Manna C, Siracusa G 1999. Spatiotemporal patterns of expression of neurotrophins and neurotrophin receptor in mice suggests functional roles in testicular and epididymal morphogenesis. Biol Reprod 61: 1123–1132 [DOI] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B 2000. Sry induces cell proliferation in the mouse gonad. Development 127: 65–73 [DOI] [PubMed] [Google Scholar]
- Scott HM, Mason JI, Sharpe RM 2009. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 30: 883–925 [DOI] [PubMed] [Google Scholar]
- Setchell BP, Maddocks S, Brooks DE 1994. Anatomy, vasculature, innervation and fluids of the male reproductive tract. In The physiology of reproduction (ed. Knobil E, Neill JD), pp. 1063–1175. Raven Press, New York [Google Scholar]
- Sharpe RM, Skakkebaek NE 2008. Testicular dysgenesis syndrome: Mechanistic insights and potential new downstream effects. Fertil Steril 89: e33–e38 [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN 1990. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346: 240–244 [DOI] [PubMed] [Google Scholar]
- Skinner MK, Fritz IB 1985. Structural characterization of proteoglycans produced by testicular peritubular cells and Sertoli cells. J Biol Chem 260: 11874–11883 [PubMed] [Google Scholar]
- Skinner MK, Tung PS, Fritz IB 1985. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol 100: 1941–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne SB, Herlihy AS, Hoei-Hansen CE, Nielsen JE, Almstrup K, Skakkebaek NE, Marks A, Leffers H, Rajpert-De Meyts E 2006. Identity of M2A (D2-40) antigen and gp36 (Aggrus, T1A-2), podoplanin in human developing testis, testicular carcinoma in situ and germ-cell tumours. Virchows Arch 449: 200–206 [DOI] [PubMed] [Google Scholar]
- Spiller CM, Feng CW, Jackson A, Gillis AJ, Rolland AD, Looijenga LH, Koopman P, Bowles J 2012. Endogenous Nodal signaling regulates germ cell potency during mammalian testis development. Development 139: 4123–4132 [DOI] [PubMed] [Google Scholar]
- Svechnikov K, Landreh L, Weisser J, Izzo G, Colón E, Svechnikova I, Söder O 2010. Origin, development and regulation of human Leydig cells. Horm Res Paediatr 73: 93–101 [DOI] [PubMed] [Google Scholar]
- Svingen T, François M, Wilhelm D, Koopman P 2012. Three-dimensional imaging of Prox1-EGFP transgenic mouse gonads reveals divergent modes of lymphangiogenesis in the testis and ovary. PLoS ONE 7: e52620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SS, Yamaguchi YL, Steiner KA, Nakano T, Nishinakamura R, Kwan KM, Behringer RR, Tam PP 2010. Loss of Lhx1 activity impacts on the localization of primordial germ cells in the mouse. Dev Dyn 239: 2851–2859 [DOI] [PubMed] [Google Scholar]
- Tang H, Brennan J, Karl J, Hamada Y, Raetzman L, Capel B 2008. Notch signaling maintains Leydig progenitor cells in the mouse testis. Development 135: 3745–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuillier R, Wang Y, Culty M 2003. Prenatal exposure to estrogenic compounds alters the expression pattern of platelet-derived growth factor receptor α and β in neonatal rat testis: Identification of gonocytes as targets of estrogen exposure. Biol Reprod 68: 867–880 [DOI] [PubMed] [Google Scholar]
- Tung PS, Skinner MK, Fritz IB 1984. Cooperativity between Sertoli cells and peritubular myoid cells in the formation of the basal lamina in the seminiferous tubule. Ann N Y Acad Sci 438: 435–446 [DOI] [PubMed] [Google Scholar]
- Tung JJ, Tattersall IW, Kitajewski J 2012. Tips, stalks, tubes: Notch-mediated cell fate determination and mechanisms of tubulogenesis during angiogenesis. Cold Spring Harb Perspect Med 2: a006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, et al. 2009. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139: 1130–1142 [DOI] [PubMed] [Google Scholar]
- Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG 1991. Proliferation activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil 93: 233–243 [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de Rooij DG, Schedl A 2001. Sox9 induces testis development in XX transgenic mice. Nat Genet 28: 216–217 [DOI] [PubMed] [Google Scholar]
- Vilain E, McCabe ER 1998. Mammalian sex determination: From gonads to brain. Mol Genet Metab 65: 74–84 [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, et al. 1994. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79: 1111–1120 [DOI] [PubMed] [Google Scholar]
- Wainwright EN, Wilhelm D 2010. The game plan: Cellular and molecular mechanisms of mammalian testis development. Curr Top Dev Biol 90C: 231–262 [DOI] [PubMed] [Google Scholar]
- Warr N, Greenfield A 2012. The molecular and cellular basis of gonadal sex reversal in mice and humans. Wiley Interdiscip Rev Dev Biol 1: 559–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters BL, Trainer TD 1996. Development of the human fetal testis. Pediatr Pathol Lab Med 16: 9–23 [PubMed] [Google Scholar]
- Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P 2005. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol 287: 111–124 [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Hiramatsu R, Mizusaki H, Widjaja L, Combes AN, Kanai Y, Koopman P 2007a. SOX9 regulates prostaglandin D synthase gene transcription in vivo to ensure testis development. J Biol Chem 282: 10553–10560 [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P 2007b. Sex determination and gonadal development in mammals. Physiol Rev 87: 1–28 [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Washburn LL, Truong V, Fellous M, Eicher EM, Koopman P 2009. Antagonism of the testis- and ovary-determining pathways during ovotestis development in mice. Mech Dev 126: 324–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfahrt-Veje C, Main KM, Skakkebaek NE 2009. Testicular dysgenesis syndrome: Foetal origin of adult reproductive problems. Clin Endocrinol (Oxf) 71: 459–465 [DOI] [PubMed] [Google Scholar]
- Woollard HH, Carmichael EA 1933. The testis and referred pain. Brain 56: 293–303 [Google Scholar]
- Yao HH, Whoriskey W, Capel B 2002. Desert hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 16: 1433–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Aardema J, Holthusen K 2006. Sexually dimorphic regulation of inhibin β B in establishing gonadal vasculature in mice. Biol Reprod 74: 978–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ 2001. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol 232: 484–492 [DOI] [PubMed] [Google Scholar]
- Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ 2000. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol Endocrinol 14: 1053–1063 [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Tanahashi M, Uchida W 2010. Behavioral and urological evaluation of a testicular pain model. Urology 75: 943–948 [DOI] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY 2005. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435: 98–104 [DOI] [PubMed] [Google Scholar]