Abstract
Bone marrow-derived endothelial progenitor cells (BM-EPCs) are stimulated by vascular endothelial growth factor-A (VEGF-A) and other potent proangiogenic factors. During angiogenesis, an increase in VEGF-A expression stimulates BM-EPCs to enhance endothelial tube formation and contribute to an increase in microvessel density. Hypoxia is known to produce an enhanced angiogenic response and heightened levels of VEGF-A have been seen in oxygen deprived epithelial and endothelial cells, yet the pathways for VEGF-A signaling in BM-EPCs have not been described. This study explores the influence of hypoxia on VEGF-A signaling in rat BM-EPCs utilizing a novel proteomic strategy to directly identify interacting downstream components of the combined VEGF receptor(s) signaling pathways, gene expression analysis, and functional phenotyping. VEGF-A signaling network analysis following liquid chromatographic separation and tandem mass spectrometry revealed proteins related to inositol/calcium signaling, nitric oxide signaling, cell survival, cell migration, and inflammatory responses. Alterations in BM-EPC expression of common angiogenic genes and tube formation in response to VEGF-A during hypoxia were measured and combined with the proteomic analysis to enhance and support the signaling pathways detected. BM-EPC tube formation assays in response to VEGF-A exhibited little tube formation; however, a cell projection/migratory phenotype supported the signaling data. Additionally, a novel assay measuring BM-EPC incorporation into preformed endothelial cell tubes indicated a significant increase of incorporated BM-EPCs after pretreatment with VEGF-A during hypoxia. This study verifies known VEGF-A pathway components and reveals several unidentified mechanisms of VEGF-A signaling in BM-EPCs during hypoxia that may be important for migration to sites of vascular regeneration.
Keywords: vascular endothelial growth factor, hypoxia, endothelial progenitor cells, mass spectrometry, signaling
the vascular endothelial growth factor (VEGF) gene family consists of several mammalian growth factors including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor (PlGF). VEGF-A is a diffusible mitogen derived from arteries, veins, and lymphatics that plays a critical role in angiogenesis and wound healing (12, 21, 23, 24, 52). VEGF-A has multiple isoforms, VEGF-A121, VEGF-A145, VEGF-A165, and VEGF-A189, each having different binding affinities to the VEGF receptors (VEGFRs). VEGFRs include VEGFR-1 (FLT-1), VEGFR-2 (FLK-1/KDR), and VEGFR-3 (FLT-4) (37). VEGF-A induces neovascularization and may be a valuable type of therapy for the treatment of peripheral vascular disease (22). During physiological angiogenesis, an increase in VEGF expression stimulates endothelial cell (EC) tube formation and increased microvessel density (3, 16, 39). Hypoxia is known to produce an angiogenic response, and heightened levels of VEGF-A have been seen in oxygen-deprived epithelial, endothelial, and bone marrow cells (13, 16, 40, 51, 57). Hypoxic conditions increase levels of hypoxia-inducible factor 1α (HIF-1α), which in turn upregulates VEGF-A expression by binding to a hypoxia response element (51, 57, 61). HIF-1α also induces VEGFR-1 but leaves VEGFR-2 unaffected or slightly suppressed (28).
Bone marrow-derived endothelial progenitor cells (BM-EPCs) are specific progenitor cells from a hematopoietic cell lineage found in the bone marrow that have the ability to develop endothelial-like features and are implicated in endothelial repair, angiogenesis, and vasculogenesis when stimulated by potent proangiogenic factors (4, 18, 53, 55, 59). Recruitment of BM-EPCs to the vascular lumen occurs after induction of cardiovascular stress (18), although the exact mechanism by which this occurs is not known. BM-EPCs are classified as early stage (2 days to 3 wk in culture after isolation from the bone marrow) at which point they resemble angiogenic monocytes or macrophages with low proliferation and capacity for enhancing tube formation or late stage (3–12 wk in culture after isolation from the bone marrow) with a highly proliferative nature and ability to form tubes (18, 54, 55, 59). However, BM-EPC identity and characterization are nontrivial and debated in the field (20). For instance, various combinations of VEGFR-2, CD34, cKIT, CD133, Ac-LDL, CD144, von Willebrand factor, endothelial nitric oxide synthase (eNOS), and CD14 have been used as markers to classify this cell population (16, 20). Since the proper markers to use for BM-EPCs are highly debated, it is very important to denote specific growth conditions, markers, and phenotypic characterizations used when classifying BM-EPCs. One hallmark of the CD34+/VEGFR-2+ population of BM-EPCs is that they have been associated with coronary artery disease (7, 18, 62). Once in the vasculature, the relationship of this population of BM-EPCs to neovascularization has been linked to enhanced anti-apoptotic function through VEGF-A stimulation of VEGFR-2, increased nitric oxide (NO) release, and enhanced PI3K/AKT signaling (18, 32, 68).
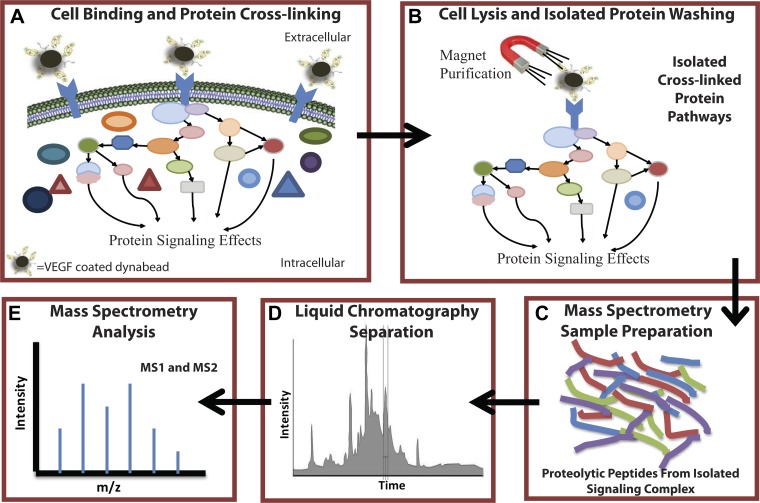
While it is known that BM-EPCs migrate to sites of injury and neovascularization (16, 18), the signaling mechanisms of this process are not well understood, particularly with regard to the influence of VEGF-A signaling. This study is the first to explore the direct influence of VEGF-A and hypoxia signaling in early stage BM-EPCs through large-scale signaling pathway analysis. To understand the mechanisms by which VEGF-A signals in BM-EPCs, we used an advanced high-throughput proteomic approach that utilized VEGF-A coupled to a magnetic epoxy resin, cell-permeable thiol-reducible cross-linker, subsequent purification, and tandem mass spectrometry (MS) analysis for signaling pathway analysis (Fig. 1). The use of various cross-linkers coupled with tandem MS analysis is becoming increasingly common in the study of protein-protein interactions and protein pathway analysis (26, 27, 35, 44). We hypothesized that hypoxic conditions, simulating cardiovascular stress, would enhance VEGF-A signaling in BM-EPCs compared with the normoxic state. Indeed, several unique pathways and proteins associated with VEGF-A signaling in hypoxic BM-EPCs were identified, along with numerous proteins already implicated in VEGF-A signaling pathways in other cell types. Additionally, changes in gene expression in response to hypoxia and VEGF-A were examined in conjunction with the pathway analysis, to supplement and verify particular pathways detected. The combination of gene expression data in tandem with the protein signaling analysis and functional tube formation assays provided an improved understanding of the effects of hypoxia and VEGF-A on BM-EPCs in the vasculature. At the same time a general high-throughput proteomic method adaptable and applicable to large-scale mapping of numerous cell signaling pathways was developed.
Fig. 1.
Experimental methods for large-scale proteomic signaling pathway analysis. VEGF-A signaling in bone marrow-derived endothelial progenitor cells (BM-EPCs) during hypoxia compared with normoxia. A: VEGF-A was coupled to a magnetic epoxy resin, bound to BM-EPCs (hypoxic and normoxic) via VEGF receptors (VEGFRs), allowing signal complexes to form. Complexes were cross-linked using a permeable thiol-reducible cross-linker (DSP) and selected with a magnet. B: cells were lysed and subjected to a series of washes while the bound complex was retained by the magnet, leaving behind the purified cross-linked signal pathway. C: pathway proteins were eluted with a reducing agent, alkylated, trypsinized, and peptides were desalted/concentrated. D and E: proteolytic peptides were separated by liquid chromatography on a hydrophobic resin and analyzed by tandem mass spectrometry (MS) sequencing. All peptide sequences detected were then matched against the rodent UniProt database.
EXPERIMENTAL PROCEDURES
BM-EPC isolation and culture.
All animal use protocols were approved through the Medical College of Wisconsin Institutional Animal Care and Use Committee. BM-EPCs were isolated from Sprague-Dawley (SD) rat (Harlan) hind limbs according to these approved protocols. Bone marrow isolation (50), BM-EPC culture in MCDB131 basal media (US Biologicals) plus 10% FBS (GIBCO by Invitrogen) and EGM-2 supplement packs (Lonza), and phenotypic verification were performed as previously described (16, 50). Using a set of accepted growth conditions (20), we plated approximately five million isolated primary bone marrow mononuclear cells (16) per bovine fibronectin-coated (10 μg/ml) 100 mm plate. After day 3 of culture, nonadherent cells were removed and new media were provided, with subsequent medium changes every 3 days until day 10–14 or when plated cells reached ∼60% confluence. Previous characterization of this BM-EPC population under the same isolation and growth conditions by our laboratory (data not shown) indicated that ∼90% of the cells are positive for a panel of markers including VEGFR2, CD34, CD133, cKIT, and Ac-LDL (16, 50). Additionally, throughout this study, we utilize conditions of standard cell culture normoxia (20% O2) and hypoxia (2% O2) shown to regulate VEGFRs at the cell surface (39), which can vary in vivo depending on the subset of vessels.
VEGF signaling pathway cross-linking and protein isolation.
VEGF-A165 (Shenandoah Biotechnology, cat. #300-31), the primary angiogenic isoform, was coupled to magnetic DynaBead M-450 epoxy resin (Invitrogen, cat. #14011) according to manufacturer protocol. After coupling was complete, the resin was washed according to protocol from the manufacturer and incubated with MCDB131 basal media. The ability of bead-coupled VEGF-A to bind and activate VEGFRs was demonstrated by an in vitro assay as previously described (39). After three washes with MCDB131 basal media to remove factors secreted from the cells, such as soluble VEGFR-1 (sFLT-1) that could act as an extravascular sink for VEGF, the cells were gently scraped from five enriched BM-EPC plates. BM-EPCs were then centrifuged at 300 g for 5 min and resuspended in 5 ml of MCDB131 basal media. VEGF-coupled Dynabeads (∼1,000 ng/ml VEGF-A) were then added to the cells, followed by a 10 min incubation at 37°C and magnet purification of bound BM-EPCs. We estimate the concentration of bead-bound VEGF-A presented to the cells was ∼100 ng/ml based on geometric constraints including steric hindrance, nonuniform binding of VEGF to the bead, and limited presentation of the bead surface to the cell. The pellet was then resuspended in 100 μl of 1 mM reducible cross-linker (see below) in Dulbecco's phosphate-buffered saline (DPBS, Invitrogen) and incubated at room temperature for 10 min. A pellet was isolated from the suspensions with a magnet and washed three times with DPBS, and BM-EPCs were lysed with 150 μl of mammalian protein extraction reagent (Bio-Rad) on ice for 30 min. Bound DynaBeads were pelleted and washed as before and resuspended in 250 μl of biotinylation kit elution buffer (Pierce). After 5 min 10 μl 1 M Tris was added to raise pH to ∼7.5–8.0, followed by addition of 10 mM dithiothreitol (DTT) to reduce cross-links, and this was incubated 1 h at 37°C. Dynabeads were pelleted with the magnet, supernatant was removed, and buffer swap into 20 mM ammonium bicarbonate was performed using Amicon Ultra-15 centrifugal units, MWCO 3000 (Millipore, Billerica, MA) with six buffer changes for 20 min at 3,500 g. Glycine-coated beads were used to assess specificity of the isolation procedure. Isolation performed with VEGF-A-coated Dynabeads under normoxia yielded ∼5 μg of protein compared with glycine-coated Dynabeads with protein yields virtually undetectable by EZQ protein quantification kit (Life Technologies, data not shown). Since the VEGF-A condition enhanced the protein isolated, we focused on this condition with hypoxic enhancement of VEGFR signaling (39) to minimize MS-driven dynamic range issues.
Optimization of cross-linking.
Dithiobis[succinimidyl propionate] (DSP, Pierce) at 100 mM in dimethyl sulfoxide stock solution was used for cross-linker dilutions. Optimization of cross-linking was performed using bovine insulin in DPBS at 5 μM and DSP at concentrations of 0, 0.25, 0.5, 1, 2, 5, 10, and 20 mM. The reactions were stopped with 1 M Tris·HCl, buffer swapped into 20 mM ammonium bicarbonate using Amicon Ultra Centrifugal Filter Units (Millipore), and dried with a vacuum centrifuge. The samples were reconstituted in DPBS, mixed with SDS loading buffer (Tris·HCl, pH 6.8, including SDS and glycerol), heated at 95°C for 5 min, and separated by polyacrylamide gel electrophoresis with a 15% precast gel (Bio-Rad). Kaleidoscope Precision Plus protein standards (Bio-Rad) were used as size determinants. Silver staining analysis was performed to visualize the protein bands. Optimum cross-linking occurred between 0.25 and 2 mM as determined by appearance of cross-links via SDS-PAGE silver staining and MALDI-MS (data not shown); 1 mM was chosen to be used for application to treatment groups.
MS protein sample preparation.
Eluted protein samples (∼5–10 μg) were dried with a speed vacuum centrifuge and resuspended in 100 μl 20 mM ammonium bicarbonate. Samples were incubated with 10 mM DTT at 56°C for 1 h and 20 mM iodoacetamide at room temperature in the dark for 30 min, followed by sequence grade-modified tryptic digestion (Promega) overnight at 37°C. Following proteolytic digestion, the peptide samples were acidified (∼pH 3–4) and desalted using Varian Omix C18 tips (Agilent Technologies, Santa Clara, CA). Desalted samples were then dried and resuspended in 6–10 μl 98% HPLC water/2% acetonitrile (ACN)/0.1% formic acid depending on protein concentration. Protein digests were then analyzed with a LTQ-Orbitrap Velos MS/MS (Thermo Scientific) or stored at −80°C until analysis.
Liquid chromatography and MS analysis.
Tryptic peptide mixtures (1.9 μl) were injected via a NanoAccuity UPLC system (Waters, Milford, MA) and passed over an in-house packed 5U C18 resin (Phenomenex, Torrance, CA) capillary column (10 cm long, 50 μm inner diameter). A gradual gradient from 98% HPLC water/2% ACN/0.1% formic acid to 98% ACN/2% HPLC water/0.1% formic acid over 240 min was applied to peptide mixtures. As peptides eluted they were analyzed with a LTQ-Orbitrap Velos mass spectrometer (Thermo Scientific) according to instrument method settings described in previous literature (30). Observed MS/MS spectra were then analyzed with both Sequest and Mascot compared with the UniProtKB Rodent database. All biological replicates for normoxia (n = 4, 12 runs total) and hypoxia (n = 4, 10 runs total) were combined separately and filtered. Stringent filters included removal of redundant proteins, removal of common contaminant proteins, presence in three of four biological replicates, scan count ≥ 7 for either condition, and a protein P ≥ 0.85 [equating to a false discovery rate (FDR) = 5%] and peptide P ≥ 0.80 to ensure removal of any nonspecific proteins and accuracy of the dataset. Relative quantification of protein abundance was performed using spectral counts as previously described (30). In-house Visualize software was utilized for the analysis of the protein lists and for statistical comparisons with multiple testing corrections to generate the P values, including normalization of total MS scan counts between groups, an FDR of 5%, and a G-score (G-test) (29, 30).
Real-time PCR analysis of VEGF-A-treated BM-EPCs.
VEGF-A-stimulated signaling in BM-EPCs was explored using the RT2 Profiler PCR Array PARN-091E-4 (QIAGEN) designed for profiling the expression of 84 key genes related to VEGF signaling during angiogenesis. Comparisons were made between VEGF-A165-stimulated and nonstimulated BM-EPCs under normoxic (17–20% O2) and hypoxic (2% O2) conditions to focus on VEGF-A signaling during induced neovascularization. BM-EPCs were isolated from SD rats and cultured as described previously (16) until ∼50–60% confluence (culture day 11–14). Cells were serum-starved in MCDB131 basal media (US Biologicals) plus 1% fetal bovine serum (FBS) for 24 h, and hypoxia-treated cells were subject to 2% O2 at this time. After incubation, BM-EPCs were left in MCDB131 basal media plus 1% FBS with one group receiving no treatment, one group receiving 100 ng/ml VEGF-A165 for 15 min, and one group receiving 100 ng/ml VEGF165 for 2 h. Plates were then scraped, groups of one or two 100 mm plates per treatment group were pooled if necessary, and RNA was isolated using the RNeasy Mini-Kit (QIAGEN, cat. #74104) according to manufacturer protocol. During RNA isolation, the RNase-Free DNase set (QIAGEN, cat. #79254) was used for spin column clean-up. Following RNA isolation, concentration was measured utilizing the NanoDrop system by absorbance. We then converted ∼400–700 ng of RNA to cDNA with the RT2 First Strand Kit (QIAGEN, cat. #330401). cDNA was then diluted according manufacturer protocol for the RT2 Profiler PCR Array PARN-091E-4 (QIAGEN). RT2 SYBR Green ROX (QIAGEN, cat. #330522) was used for the RT-PCR plate reaction. Samples were run in a 7900-HT Real-Time PCR Thermocycler (Invitrogen) according to the array manual and analyzed according to the QIAGEN online analysis software.
Angiogenic tube formation assay analysis.
Four-well slides (Nunc Lab-Tek) were coated with 250 μl of Growth Factor Reduced Matrigel (BD Biosciences) and allowed to solidify at room temperature for 2 h. Before the tube formation assay, BM-EPCs were washed once with DPBS, lifted with Enzymatic Free Cell Dissociation Buffer (Millipore) for 30 min at 37°C, and centrifuged at 300 g for 5 min, followed by two subsequent washes in DPBS. After the final wash, cells were resuspended in 1 ml MCDB131 basal media plus 10% FBS and counted on a cell countess (Invitrogen). BM-EPCs were then diluted accordingly, and 1 ml of media containing 40,000 cells was added to the appropriate well. Subsets were kept in normoxia (20% O2) and hypoxia (2% O2) plus or minus 100 ng/ml VEGF-A165. We took ×4 and ×10 magnification images on a Nikon TS-100 microscope with Flex camera (Nikon) at 24 and 48 h for analysis. Images were uploaded into Metamorph (Molecular Devices) for analysis of overall angiogenic tube length (microns) per field and number of cells with projections vs. those without projections. The results were analyzed in SigmaPlot (Systat Software) and averaged across four biological replicates with four images for each condition (16 total), and paired t-tests were performed between groups.
To further test the migratory phenotypes of the BM-EPCs, a novel cell incorporation tube formation assay was developed. Rat cardiac microvascular endothelial cells (RMVECs) (Cell Biologics, cat. #R1111) were grown according to protocol in MCDB131 basal media plus 10% FBS with an EGM-MV supplement pack (Lonza). Other than that, RMVECs were prepared for the assay as with the cultured BM-EPCs above. RMVECs were diluted for the addition of 20,000 cells per 1 ml media to each chamber of the four-chamber slides (Lab-Tek/Thermo Fisher Scientific) coated in matrigel as described earlier. RMVEC-containing matrigel slides were incubated in normoxia at 37°C for 48 h to allow tube-like structures to form. At the 24 h time-point, BM-EPCs cultured as mentioned earlier were started at a 24 h treatment in MCDB131 basal media plus 10% FBS under normoxia, normoxia plus 100 ng/ml VEGF-A165, hypoxia, or hypoxia plus 100 ng/ml VEGF-A165. After 24 h pretreatment, the BM-EPCs were isolated from culture plates, resuspended in 1 ml MCDB131 basal media (US Biologicals) with 10% FBS (GIBCO by Invitrogen) plus or minus 100 ng/ml VEGF-A165 for the appropriate conditions, and diluted appropriately as before. One hour prior to lifting the cells, we labeled BM-EPCs with 40 μg/ml 4′,6-diamidino-2-phenylindole (DAPI, Sigma Aldrich) for 1 h at 37°C. BM-EPCs (10,000 cells) in 1 ml medium were added to the preformed RMVEC tubes and allowed to incorporate for 16 h. Using the Nikon TS-100 microscope with Flex camera (Nikon) we acquired bright-field and fluorescent images at 2 and 16 h for four biological replicates with four sets of images from each. The fluorescent images were thresholded in MATLAB, and the number of unique objects was counted. Unique objects (BM-EPCs) were registered against the complimentary bright-field image, and the number of BM-EPCs detected through thresholding was separated for total cells detected and cells incorporated into preformed RMVEC tubes. A ratio of incorporated vs. total BM-EPCs was then generated and compared across groups by t-tests in Sigmaplot.
RESULTS
Total composition of BM-EPC VEGF-A signaling pathway analysis under normoxia and hypoxia.
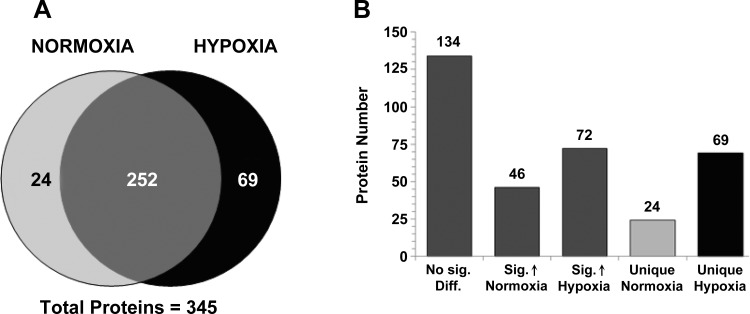
Using the stringent MS analysis criteria described in the experimental procedures, 276 proteins were detected in VEGF-A signaling pathway elutions under normoxic conditions and 321 proteins in samples under hypoxic conditions. Relative quantitation of normalized spectral counts through comparison of the normoxic vs. hypoxic condition samples resulted in a list of 345 total proteins of which 252 were detected under both conditions (Fig. 2A and Supplemental Table S1).1 Comparisons of the normalized scan counts for the 252 common proteins detected revealed that 134 were not significantly different between conditions, 46 were significantly increased in normoxic samples, and 72 were significantly increased in hypoxia samples (Fig. 2B and Supplemental Table S1). Of the remaining proteins, 24 were unique to elution samples from BM-EPCs under normoxic conditions and 69 unique to hypoxic conditions (Fig. 2B and Supplemental Table S1). Analysis of the total protein list in Ingenuity Pathway Analysis identified biological processes including inflammation/response to injury (21 proteins), NO signaling (20 proteins), growth factor and G protein signaling (19 proteins), cell motility and migration (19 proteins), inositol/calcium signaling (17 proteins), and endo/exocytosis (16 proteins) (Table 1, Supplemental Table S1). Additionally, VEGF and HIF-1α/hypoxia signaling proteins were highly represented (Table 1, Supplemental Table S1).
Fig. 2.
Large-scale proteomic analysis of VEGF-A signaling in BM-EPCs. A: total protein comparisons between isolated VEGF-A signaling pathways from BM-EPCs under normoxic and hypoxic conditions after application of stringent filters. B: differences in protein abundance in the comparison data were separated into those proteins not significantly different (No Sig. Diff.), significantly increased (Sig.) in either condition, and proteins unique to normoxic or hypoxic BM-EPC VEGF-A pathways isolated (A). The bars in B are shade matched with A.
Table 1.
Comparison of important signaling targets isolated during the proteomic analysis of VEGF-A signaling pathways in BM-EPCs under hypoxic vs. normoxic conditions
| Normoxia |
Hypoxia |
|||||
|---|---|---|---|---|---|---|
| Annotated Protein | Peptide Count | Scan Count | Peptide Count | Scan Count | Hypoxia Fold Change (norm. P value) | Biological Process Involvement |
| AKT1 kinase | 1 | 1 | 1 | 7 | 5.22 (0.38) | cell survival, cell projection, angiogenesis |
| Amyotrophic lateral sclerosis protein 2 homolog | 5 | 5 | 8 | 9 | 2.3 (0.41) | cell projection, response to oxidative stress, G protein signaling |
| Annexin-1 (Phospholipase A2 inhibitory protein) | 20 | 56 | 6 | 7 | −6.38 (1.4E-12) | inflammation (anti) |
| Apoptosis-related protein 3 | 1 | 5 | 2 | 20 | 3.62 (0.0054) | cell differentiation regulation |
| Attractin | 7 | 8 | 6 | 7 | −1.76 (0.60) | inflammation, response to oxidative stress |
| Basement membrane-specific heparan sulfate proteoglycan | 10 | 11 | 2 | 2 | −5.3 (0.0044) | angiogenesis |
| Breast cancer type 1 susceptibility protein (BRCA1) | 3 | 5 | 7 | 13 | 2.36 (0.10) | cell cycle/DNA repair |
| Breast cancer type 2 susceptibility protein homolog | 10 | 11 | 13 | 17 | 1.86 (0.43) | cell cycle/DNA repair |
| Butyrate response factor 1 | 0 | 0 | 2 | 7 | unique (0.0030) | vasculogenesis, response to growth factors |
| Calmodulin (CaM) | 9 | 41 | 6 | 44 | −1.18 (0.77) | calcium signaling, inflammation |
| Calnexin | 6 | 9 | 2 | 6 | −1.54 (0.30) | ATP/calcium signaling |
| cAMP-specific 3′,5′-cyclic phosphodiesterase 4B | 0 | 0 | 3 | 8 | unique (0.0015) | second messanger in response to extracellular stimuli |
| Calmodulin-binding protein 1 | 5 | 5 | 6 | 12 | 2.14 (0.14) | calcium signaling |
| Caspase-12 | 0 | 0 | 3 | 10 | unique (3.9E-4) | apoptosis |
| Citron Rho-interacting kinase | 4 | 6 | 4 | 29 | 4.14 (2.4E-4) | cell cycle |
| Death effector domain-containing protein | 0 | 0 | 4 | 11 | unique (2.0E-4) | apoptosis |
| Delta-like protein 1 | 0 | 0 | 4 | 7 | unique (0.0030) | cell-cell communication, stem cell maintenance |
| Extracellular calcium-sensing receptor (CaSR) | 1 | 1 | 2 | 9 | 5.94 (0.012) | G protein-coupled calcium-sensing receptor |
| Fibrinogen silencer binding protein | 2 | 3 | 1 | 18 | 4.78 (0.0016) | transcription repressor |
| Fibronectin | 38 | 76 | 64 | 276 | 3.34 (1.5E-22) | angiogenesis, cell adhesion |
| G2/mitotic-specific cyclin-B1 | 0 | 0 | 3 | 7 | unique (0.0030) | cell cycle |
| G2/mitotic-specific cyclin-B3 | 6 | 6 | 4 | 10 | 2.08 (0.45) | cell cycle |
| Glucosidase 2 subunit beta (Protein kinase C substrate) | 17 | 36 | 2 | 3 | −7.54 (9.1E-10) | negative regulation of neuronal cell projections |
| Guanine nucleotide exchange factor DBS | 0 | 0 | 2 | 7 | unique (0.0030) | RAC1, RHOA and CDC42 signaling |
| Inositol 1,4,5-trisphosphate receptor type 2 | 0 | 0 | 4 | 11 | unique (2.0E-4) | inositol and calcium signaling |
| Inositol 1,4,5-trisphosphate receptor type 3 | 3 | 5 | 5 | 12 | 2.14 (0.15) | inositol and calcium signaling |
| Interferon-gamma-inducing factor | 0 | 0 | 3 | 11 | unique (2E-4) | inflammation, angiogenesis |
| Janus kinase 1 (JAK-1) | 3 | 6 | 6 | 16 | 2.44 (0.062) | inflammation |
| Low-density lipoprotein receptor-related protein 1B | 7 | 11 | 10 | 14 | 1.31 (0.79) | endocytosis (bind receptors for internalization) |
| MAPK/ERK kinase kinase kinase 4 | 4 | 4 | 3 | 9 | 1.94 (0.24) | response to stress |
| Matrilin-2 | 4 | 4 | 5 | 18 | 3.95 (0.0050) | cell migration, response to injury |
| Metabotropic glutamate receptor 4 | 3 | 3 | 3 | 15 | 4.25 (0.0073) | apotosis (anti), cell projections |
| Myristolated alanine-rich C-kinase substrate (MARCKS) | 8 | 24 | 8 | 26 | −1.14 (0.85) | PKC substrate, calcium signaling |
| Netrin G1 precursor | 0 | 0 | 3 | 10 | unique (3.9E-4) | promotes neurite outgrowth |
| Neuronal apoptosis inhibitory protein 1 | 3 | 6 | 4 | 8 | 1.44 (0.77) | apoptosis (anti) |
| Nitric oxide synthase, inducible | 1 | 1 | 3 | 10 | 6.24 (0.0066) | hypoxic response, inflammation |
| Nucleolar transcription factor 1 | 1 | 3 | 7 | 14 | 4.04 (0.012) | PI3K transcriptional regulation |
| Oxygen-regulated protein 1 | 3 | 3 | 8 | 11 | 2.36 (0.049) | hypoxic response, JNK signaling |
| Peroxiredoxin-1 | 14 | 35 | 2 | 8 | 4.65 (2.6E-6) | cell redox homeostasis |
| Peroxiredoxin-5 | 0 | 0 | 2 | 9 | unique (7.7E-4) | cell redox homeostasis |
| Phosphoinositide 3-kinase-C2-gamma (PI3K-C2 gamma) | 2 | 8 | 3 | 4 | −2.38 (0.16) | cell proliferation, survival, and migration signaling |
| Phosphatidylinositol-4-phosphate 5-kinase type II beta | 3 | 3 | 3 | 7 | 2.058 (0.28) | inositol and calcium signaling |
| Phosphatidylinositol-4,5-bisphosphate 5-phosphatase | 2 | 2 | 2 | 7 | 3.22 (0.13) | IP3/DAG pathway |
| Pleckstrin homology-like domain family B member 1 | 2 | 3 | 6 | 15 | 4.26 (0.0073) | inositol signaling |
| Probable G protein-coupled receptor 158 | 2 | 2 | 6 | 11 | 4.53 (0.017) | regulation of GPCR signaling |
| Programmed cell death protein 2 | 0 | 0 | 4 | 8 | unique (0.0015) | apoptosis |
| Protein kinase NTK | 0 | 0 | 1 | 10 | unique (3.9E-4) | hematopoetic cell regulation |
| S100 calcium-binding protein A4 | 13 | 149 | 11 | 133 | −1.70 (0.038) | NF-κB signaling |
| Proteoglycan-4 (Megakaryocyte stimulating factor) | 4 | 4 | 7 | 8 | 1.62 (0.35) | immune response |
| Rab6-interacting protein 1 | 0 | 0 | 7 | 13 | unique (5.3E-5) | vesicle trafficking |
| Rap1-interacting factor 1 homolog | 5 | 5 | 5 | 21 | 3.76 (0.0034) | cell survival |
| RB1-inducible coiled-coil protein 1 | 0 | 0 | 4 | 7 | unique (0.0030) | apoptosis (anti) |
| Receptor of activated protein kinase C 1 (RACK1) | 5 | 8 | 2 | 2 | −4.38 (0.030) | PKC signaling, apoptosis (anti) cell migration |
| Selenocysteine insertion sequence binding protein 2 | 4 | 6 | 2 | 15 | 2.24 (0.090) | cell redox homeostasis |
| Serine-protein kinase ATM | 4 | 4 | 7 | 7 | 1.2 (0.49) | cell survival |
| Ser/thr-protein kinase DCAMKL2 | 3 | 4 | 10 | 20 | 4.24 (0.0019) | MAPK/JNK sgnaling |
| Ser/thr-protein kinase MARK2 | 2 | 2 | 6 | 8 | 3.61 (0.079) | cell migration, cell projections |
| Ser/thr-protein kinase Nek11 | 0 | 0 | 2 | 9 | unique (7.7E-4) | cell survival |
| Ser/thr-protein kinase PLK4 | 0 | 0 | 5 | 14 | unique (2.7E-05) | cell survival |
| Slit homolog 2 protein | 3 | 7 | 2 | 29 | 3.45 (6.4E-04) | angiogenesis, cell migration |
| Stabilin-1 | 3 | 4 | 5 | 7 | 2.22 (0.49) | inflammation, angiogenesis (-) |
| Talin-1 | 7 | 8 | 9 | 17 | 1.78 (0.14) | cell projections, cell adhesion |
| Thioredoxin | 7 | 39 | 6 | 32 | 1.94 (0.16) | cell redox homeostasis |
| Toll-like receptor 8 | 0 | 0 | 5 | 9 | unique (7.7E-4) | inflammation, NF-κB signaling |
| Transient receptor potential-PLC-interacting kinase | 2 | 2 | 5 | 9 | 3.95 (0.047) | calcium signaling, apoptosis |
| Unc-13 homolog C | 5 | 6 | 3 | 17 | 2.62 (0.043) | eocytosis (DAG regulated) |
| Vascular endothelial growth factor (VEGF)-A | 7 | 30 | 3 | 8 | −4.2 (4E-05) | angiogenesis,cell migration, apoptosis (anti) |
| VEGF receptor 1 | 1 | 1 | 6 | 10 | 6.26 (2E-06) | angiogenesis, cell survival, cell migration |
| VEGF receptor 2 | 0 | 0 | 9 | 18 | unique (4E-05) | angiogenesis, cell survival, cell migration |
| Villin-1 | 0 | 0 | 4 | 19 | unique (1.0E-6) | cell migration, cell morphogenesis |
| Von Willebrand factor | 0 | 0 | 5 | 7 | unique (0.0030) | cell adhesion, homeostasis |
| Xanthine dehydrogenase/oxidase | 3 | 3 | 6 | 7 | 2.06 (0.28) | cell redox homeostasis, inhibits vasculogenesis |
| 78 kDa glucose-regulated protein | 36 | 96 | 19 | 38 | −3.06 (4.6E-09) | apoptosis (anti), TGF-β inhibitor |
All P values are normalized (norm.) to actual/expected scan counts; boldface indicates a significant fold change in hypoxia (P < 0.05). Biological processes include but are not limited to those listed. BM-EPC, bone marrow-derived endothelial progenitor cell.
Evaluation of the VEGFR family of proteins in BM-EPCs under hypoxia and normoxia.
For verification that the VEGF-A signaling pathway was properly identified by this high-throughput signaling pathway identification technique, the VEGFR family of proteins was examined. We hypothesized that stimulation under hypoxia would allow increased isolation of VEGF signaling pathway components due to an increase in cell surface exposure of receptors (39). Under normoxic conditions, VEGR1 (peptides = 1, scans = 1) and VEGFR2 (peptides = 0, scans = 0) were virtually undetectable in the complex mixture of proteins. This result was expected since VEGFRs are of lower signaling capacity under normoxic conditions and likely below the dynamic range of MS detection for lower abundant proteins in the complex protein mixture. Additionally, this method is dependent on VEGF-Dynabeads binding surface accessible receptors, hence fewer VEGFRs at the surface would result in a lower yield of bound protein. In hypoxia-treated BM-EPC VEGF-A pathway isolations we saw a significant increase in both VEGFR1 (peptides = 6, scans = 22, P = 5.6E-6) and VEGFR2 (peptides = 9, scans = 18, P = 0.0004) isolated vs. normoxia, suggesting an increase in VEGF-A-mediated VEGFR signaling during hypoxia (Table 1, Supplemental Table S1). VEGF-A, most likely from elution off the epoxy resin, was detected under both normoxic (peptides = 7, scans = 30) and hypoxic (peptides = 4, scans = 9) conditions. VEGF-A was expected since the elution is a sequential twofold process where 1) cross-linking pathway molecules are eluted off the Dynabeads utilizing a reducing agent to break the thiol bond in the linker of the cross-linker arm and 2) harsh elution with an acidic catalyst to break the VEGF epoxy bond releasing any remaining signaling molecule left on the resin. VEGF-A was detected at higher abundance in normoxic vs. hypoxic elutions (P = 3.1E-6), possibly because of the significant increase in surface-accessible VEGFRs in hypoxia samples acting as a buffer for free VEGF-A and increasing sample complexity affecting the MS dynamic range of detection.
Interestingly, various regulators of growth factor and G protein-coupled receptor endo/exocytotic translocation were detected to be significantly increased in hypoxic BM-EPC pathway samples, such as the UNC-13 homolog C (1.31-fold, P = 0.042), vacuolar protein sorting-associated protein 45 (2.47-fold, P = 9.5E-4), and clathrin light chain B (1.68-fold, P = 0.05). UNC-13 homolog C is also a link from phospholipase and inositol/calcium signaling to exocytosis through binding of SNAP-25. SNAP-25-interacting protein was detected in both samples but only passed stringent filters in the hypoxic samples. The probable G protein-coupled receptor 158, controlling ability of receptors to signal through directing localization to the cell membrane, was also significantly increased (2.27-fold, P = 0.017) in the VEGF-A pathways isolated from hypoxic BM-EPCs.
Comparison of VEGF-signaling proteomic pathways detected in hypoxia- vs. normoxia-exposed BM-EPCs.
VEGF-A signaling pathway molecules previously detected in other cell types were expected to be found in this study (19, 36, 47), along with unknown effector pathways specific for BM-EPCs. VEGF-A signal pathway isolation from BM-EPCs under both normoxic and hypoxic conditions revealed proteins from canonical proangiogenic pathways (Table 1, Supplemental Table S1), such as inositol and calcium signaling, NO signaling, and growth factor signaling pathways leading to cell proliferation, survival, and migration. Many components of the signaling pathways beyond the primary receptors, VEGFR1 and VEGFR2, were significantly increased in BM-EPCs exposed to a hypoxic environment (Table 1, Supplemental Table S1). Ingenuity pathway analysis of the proteins present in VEGF-A signaling complex isolations from hypoxic BM-EPCS identified 10 known direct VEGF-A signaling molecules, 11 proteins involved in NO signaling, 15 in actin cytoskeleton signaling, and 10 signal molecules involved in Rho family GTPase signaling (Table 1, Supplemental Table S1). All of these pathways were detected at increasing amounts in hypoxic vs. normoxic BM-EPCs. Interestingly, the Src substrate cortactin (−3.84-fold, P = 2.8E-07) and RACK1 (−2.19-fold, P = 0.03) were both significantly less abundant in hypoxic vs. normoxic VEGF-A signaling pathways isolated. Protein kinase NTK, only found in hypoxic pathway isolations, is also suggested to negatively regulate the Src family of proteins. On the other hand, RhoA and RAC1 signal effectors, such as citron Rho-interacting kinase (2.08-fold, P = 2.4E-4), huntingtin-associated protein-interacting protein (2.13-fold, P = 0.028), and guanine nucleotide exchange factor DBS (hypoxia only, P = 0.0030), were significantly increased in detection of hypoxic samples vs. the normoxic counterpart.
VEGF-A signaling pathways in hypoxia-treated BM-EPCs also displayed a significant increase in numerous apoptosis and cell cycle regulation/repair proteins, along with many proteins involved in the inflammatory response, 14 proteins for each. Key regulatory proteins in hematopoiesis and epithelial morphogenesis through growth factor signaling, such as protein kinase NTK (hypoxia only, P = 3.9E-4), serine/threonine-protein kinase MARK2 (3.61-fold, P = 0.079), butyrate response factor 1 (hypoxia only, P = 3.9E-4), and amyotrophic lateral sclerosis protein 2 homolog (2.3-fold, P = 0.41), were also highly prevalent or unique to VEGF-A pathways isolated in hypoxia-treated BM-EPCs. Other protein regulators important for maturation of progenitor or stem cells detected at significantly increased amounts in hypoxia samples were the transcription factor SOX-6 (3.66-fold, P = 6.8E-07) and SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily C (5.16-fold, P = 4E-11).
Regulation of BM-EPC redox homeostasis by VEGF-A signaling during hypoxic stress.
NO signaling is a key regulatory pathway of VEGF-A signaling response, and pathway components were upregulated in hypoxia-treated BM-EPCs (Table 1, Supplemental Table S1). Thioredoxin and xanthine oxidase were not significantly different between conditions; however, NO synthase 2 (NOS2) or inducible NOS was significantly increased in samples from hypoxia-treated cells (6.24-fold, P = 0.0066). Peroxiredoxin-5 was unique to hypoxia-treated cell pathway samples at significance (hypoxia only, P = 0.00077), while the SEBP2 protein, crucial for glutathione peroxidase function, was approaching significance (2.24-fold, P = 0.090). Altogether, it appears that the key regulation of VEGF-A-induced NO signaling during hypoxia stems from NOS2 and the later step of regulation in the free radical scavenging pathway. In addition to the direct response to hypoxia via redox pathway regulation, a significant increase in response to stress was seen with interleukin-12 beta chain (IL-12B; hypoxia only, P = 3.9E-4), interferon-gamma-inducing factor (IL-18; hypoxia only, P = 2.0E-4), matrilin-2 (3.95-fold, P = 0.0050), and toll-like receptor 8 (hypoxia only, P = 7.7E-4). Annexin-1, a phospholipase A2 inhibitory protein, was also detected at significantly lower amounts (−6.38-fold, P = 1.4E-12) in VEGF-A pathway isolations from hypoxic vs. normoxic samples. Phospholipase A2 is an important mediator of inflammation, prostaglandins, and leukotrienes.
A distinct set of VEGF-A-isolated signal complex proteins were increased under hypoxic conditions vs. the normoxic counterpart. AKT1 kinase, crucial for cell survival, was present at a 5.22-fold (P = 0.38) increase in hypoxic BM-EPC VEGF-A-isolated signaling complexes. Cell cycle regulatory proteins isolated from hypoxic conditions vs. normoxic conditions included citron Rho-interacting kinase (4.14-fold, P = 2.4E-4), G2/mitotic-specific cyclin-B1 (hypoxic only, P = 0.0030), metabotropic glutamate receptor 4 (4.25-fold, P = 0.0073), RAP1-interacting factor 1 homolog (3.76-fold, P = 0.0034), RB1-inducible coiled coil protein 1 (hypoxic only, P = 0.0030), and serine/threonine kinases Nek11 (hypoxic only, P = 7.7E-4) and PLK4 (hypoxia only, P = 2.7E-5), among others (Table 1, Supplemental Table 1). While apoptotic factors increased in response to hypoxic stress, numerous apoptotic inhibitors, such as the cell cycle regulator DCAMKL2 (4.24-fold, P = 0.0019), the metabotropic glutamate receptor 4, and the RB1-inducible coiled coil protein 1, were significantly increased. DCAMKL2 causes a decrease in JNK activation in response to exocitotic stress causing antiapoptotic or protective effects.
Analysis of gene expression in VEGF-A-treated BM-EPCs under normoxia and hypoxia.
For further verification of proteomic VEGF-A-induced signaling pathway changes detected in BM-EPCs under normoxic or hypoxic conditions, RT-PCR was performed on a subset of 84 common cell signaling effector molecules related to VEGF-stimulated angiogenesis. Real-time PCR analysis of these genes was run using serum-starvation (1% FBS) during comparison of BM-EPCs under normoxia or hypoxia for 24 h. Several genes were upregulated in BM-EPCs under hypoxia vs. normoxia, including Vegf-a (2.05-fold, P = 0.014) and placental growth factor (Pgf; 1.57-fold, P = 0.0067), which is a member of the VEGF family. Flt-1 (VEGFR1) and Kdr (VEGFR2) gene expression were not significantly altered (Table 2). Expression of Hras was significantly downregulated (−1.5-fold, P = 0.0052), whereas Rac2 was upregulated (3.89-fold, P = 0.14) by hypoxia. Additionally, Map2k2 (−1.47-fold, P = 0.041), Mapk1 (−1.4-fold, P = 0.038), and Mapk3 (−1.54-fold, P = 0.028) gene expression was significantly downregulated. Lastly, the phospholipase C gamma 2 gene, Plcg2, was significantly upregulated (2.14-fold, P = 0.019), and numerous related proteins involved in phosphatidylinositol signaling were up- or downregulated and approaching significance (P < 0.15) (Table 2), supporting proteomic data. Interestingly, in a comparison of the 84 genes analyzed in this study with the same genes studied in response to hypoxia in Dahl SS rat lung tissue (GEO dataset GSE8078), only two of the 84 genes exhibited a significant concordant gene expression change: Pgf (P < 0.05) and Pla2g2d (P < 0.06) increased. This comparison, along with the functional phenotype of BM-EPCs in Fig. 2 differing from typical EC response, suggests potential differences in signaling vs. other cell types.
Table 2.
Expression changes among common angiogenic genes in BM-EPCs under hypoxic vs. normoxic conditions
| Gene | Protein Annotation | Fold Change (Hypoxia) | P Value | Biologic Process Involvement |
|---|---|---|---|---|
| Vegfa | vascular endothelial growth factor (VEGF)-A | 2.05 | 0.014 | angiogenesis, cell migration, cell proliferation, response to hypoxia |
| Flt-1 | vascular endothelial growth factor receptor 1 (VEGFR1) | −1.09 | 0.60 | angiogenesis, cell migration, response to hypoxia |
| Kdr | vascular endothelial growth factor receptor 2 (VEGFR2) | 1.12 | 0.77 | angiogenesis, cell migration, cell proliferation, response to hypoxia |
| Flt-4 | vascular endothelial growth factor receptor 3 (VEGFR3) | −6.61 | 0.14 | angiogenesis, cell migration, cell proliferation |
| Hras | GTPase H-Ras | −1.50 | 0.0052 | angiogenesis, response to hypoxia, cell migration |
| Map2k2 | mitogen activated protein kinase kinase 2 | −1.47 | 0.041 | cell motility, stress-activated signaling |
| Mapk1 | mitogen activated protein kinase 1 | −1.40 | 0.038 | cell migration, cell proliferation, stress-activated signaling |
| Mapk3 | mitogen activated protein kinase 3 | −1.54 | 0.028 | stress-activated signaling |
| Nfatc2 | nuclear factor of activated T-cells, cytoplasmic 2 | 2.05 | 0.13 | immune response, cell migration |
| Nos3 | endothelial nitric oxide synthase | −2.77 | 0.10 | angiogenesis, cell migration, response to hypoxia |
| Pgf | placental growth factor | 1.57 | 0.0067 | angiogenesis, cell differentiation, cell proliferation, response to hypoxia |
| Pik3cd | phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit delta | 2.34 | 0.12 | inositol signaling |
| Pik3r3 | phosphoinositide-3-kinase, subunit 3 | −3.00 | 0.078 | insulin receptor signaling |
| Pla2g12b | phospholipase A2, group 12B | −3.63 | 0.11 | inflammation, prostaglandin production |
| Pla2g2d | phospholipase A2, group 2D | 5.70 | 0.060 | inflammation, prostaglandin production |
| Plcg2 | phospholipase C, gamma 2 | 2.14 | 0.019 | inflammation, prostaglandin production |
| Prkcg | protein kinase C, gamma | −3.09 | 0.066 | cell survival |
| Rac2 | Rho family, small GTP binding Protein Rac2 | 3.89 | 0.14 | cell proliferation, cell projection assembly |
Boldface indicates significant fold change in gene expression (P ≤ 0.05). Genes with altered expression with a tendency toward significance (P ≤ 0.15) are also indicated (exception if Vegf-A and the family of receptors). Biologic processes include but are not limited to those above (n = 4).
BM-EPCs subject to a hypoxic environment for 24 h, followed by 2 h incubation with 100 ng/ml VEGF-A, resulted in a downregulation of Vegf-a (−1.66-fold, P = 0.17), however, not at significance, vs. BM-EPCS in hypoxia minus VEGF-A (Table 3). As seen in the hypoxic vs. normoxic nontreated BM-EPC gene expression comparisons, the VEGFR genes were not significantly altered. Among those genes with significantly altered expression in the VEGF-A-treated hypoxic BM-EPCs vs. the hypoxic nontreated condition were Nfatc1 (−2.86-fold, P = 0.012), Pik3r3 (3.5-fold, P = 0.061), Pla2g2d (−4.0-fold, P = 0.031), and Pla2g5 (4.04-fold, P = 0.0036) (Table 3). Of particular interest was the significant increase in the calcium-dependent phospholipase A2 (Pla2g5) expression due to the high prevalence of protein signaling components in relation to calcium and inositol signaling in the proteomic results. Also, annexin-1, a phospholipase A2 inhibitor, was significantly decreased in hypoxic BM-EPC pathway isolations. Prostaglandin G/H synthase 2 (Ptgs2) was also increased in expression (2.37-fold, P = 0.15).
Table 3.
Expression changes among common angiogenic genes in BM-EPCs treated with VEGF-A vs. those minus VEGF-A under hypoxic conditions
| Gene | Protein Annotation | Fold Change (+VEGF) | P Value | Biologic Process Involvement |
|---|---|---|---|---|
| Vegfa | vascular endothelial growth factor A (VEGF-A) | −1.66 | 0.17 | angiogenesis, cell migration, cell proliferation, response to hypoxia |
| Flt-1 | vascular endothelial growth factor receptor 1 (VEGFR1) | 1.10 | 0.50 | angiogenesis, cell migration, response to hypoxia |
| Kdr | vascular endothelial growth factor receptor 2 (VEGFR2) | 4.01 | 0.26 | angiogenesis, cell migration, cell proliferation, response to hypoxia |
| Nfatc1 | nuclear factor of activated T-cells, cytoplasmic 1 | −2.86 | 0.012 | immune response, cell migration, stem cell quinesence |
| Pik3cg | phosphatidylinositol 3-kinase gamma | −2.17 | 0.13 | angiogenesis, endocytosis |
| Pik3r3 | phosphatidylinositol 3-kinase regulatory subunit gamma | 3.50 | 0.061 | insulin receptor signaling |
| Pla2g2a | phospholipase A2, membrane associated | −21.58 | 0.11 | inflammation |
| Pla2g2d | phospholipase A2, group 2D | −4.00 | 0.031 | inflammation |
| Ppp3r2 | calcineurin subunit B type 2 | 3.86 | 0.15 | inositol and calcium signaling |
| Pla2g5 | calcium-dependent phospholipase A2 | 4.04 | 0.0036 | inflammation, prostaglandin production |
| Prkca | protein kinase C, alpha | 2.67 | 0.15 | angiogenesis, cell migration, cell proliferation, inflammation |
| Ptgs2 | prostaglandin G/H synthase 2 | 2.37 | 0.15 | angiogenesis, response to hypoxia, cell migration |
Boldface indicates significant fold change in gene expression (P ≤ 0.05). Genes with altered expression with a tendency toward significance (P ≤ 0.15) are also indicated (exception if Vegf-A and the family of receptors). Biologic processes include but are not limited to those above (n = 4).
Physiological studies of VEGF-A-induced BM-EPC sprouting angiogenesis and cell migration pathway regulation during hypoxia.
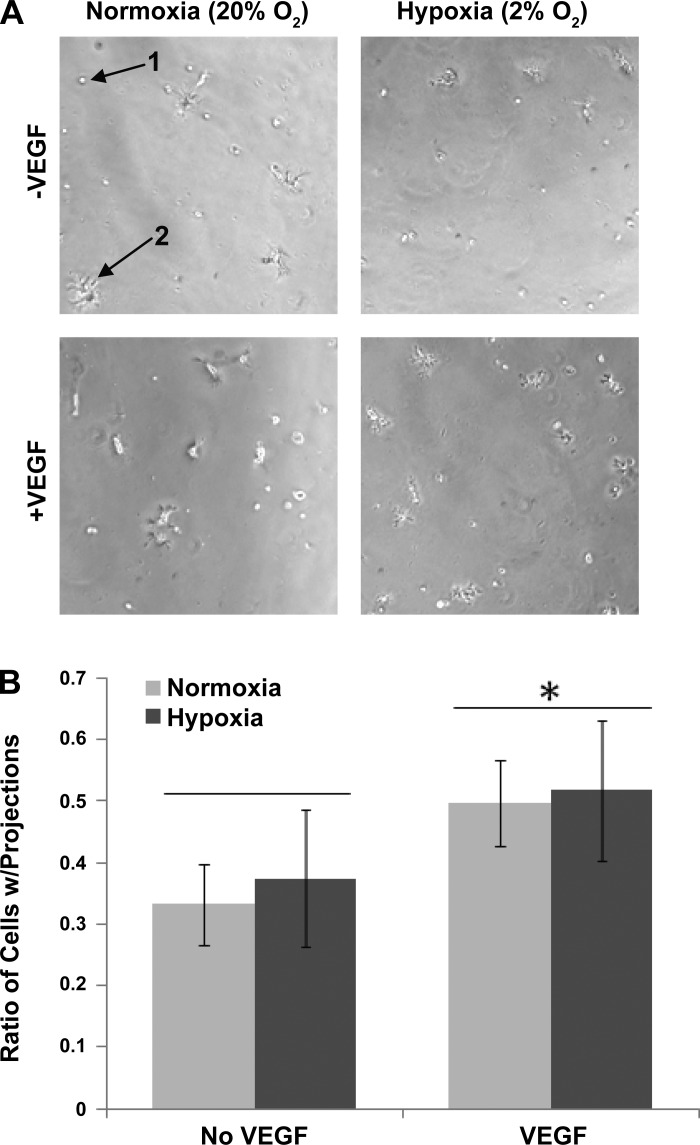
One of the more consistent trends of BM-EPC biological processes regulated by the VEGF-A under hypoxic conditions were proteins annotated for involvement in sprouting angiogenesis, cell projection formation, and cell migration (Table 1). Matrilin-2 (3.95-fold, P = 0.005), metabotropic glutamate receptor 4 (4.25-fold, P = 0.0073), netrin G1 precursor (hypoxia only, P = 3.9E-4), serine/threonine-protein kinase MARK2 (3.61-fold, P = 0.079), slit 2 homolog (3.45-fold, P = 6.4E-4), microtubule-actin crosslinking factor 1 (2.81-fold, P = 6.7E-5), and villin-1 (hypoxia only, P = 1E-6), among others, were present (Table 1 and Supplemental Table S1). Additionally, a negative regulator of cell projections, glucosidase 2 subunit beta, was significantly decreased in hypoxic VEGF-A signaling pathways isolated (−7.45-fold, P = 9.1E-10). BM-EPCs have been shown to have the ability to enhance EC tube-like structures in matrigel (16), hence this assay was used to directly determine both the phenotypic effects of hypoxia and VEGF-A on BM-EPCs in the absence of ECs. BM-EPC tube formation was assessed during normoxia and hypoxia, plus or minus 100 ng/ml VEGF-A. Minimal tube formation was seen in the normoxia- or hypoxia-treated early-stage BM-EPCs, so no quantification was performed on tube length per area. A distinct and significant increase in the number of BM-EPCs with a cell projection phenotype was observed following VEGF-A treatment (P < 0.001); however, this effect was not different between normoxia and hypoxia (Fig. 3).
Fig. 3.
Tube formation assays assessing angiogenic response of BM-EPCs alone in the presence or absence of VEGF-A under normoxic and hypoxic conditions. A: little tube formation was seen in BM-EPCs (40,000) plus or minus VEGF-A; however, a sprouting/projection phenotype was observed. Arrows indicate an example of a cell with no projections (1) and projections (2) at ×10 magnification. B: quantification of cells with projections as a ratio of cells with projections per total cell population (n = 3, each with four images). Cell counts were normalized to total cells per condition. VEGF-A induced a significant (P < 0.001) increase in the number of BM-EPCs displaying a sprouting/projection phenotype that was not influenced by hypoxia.
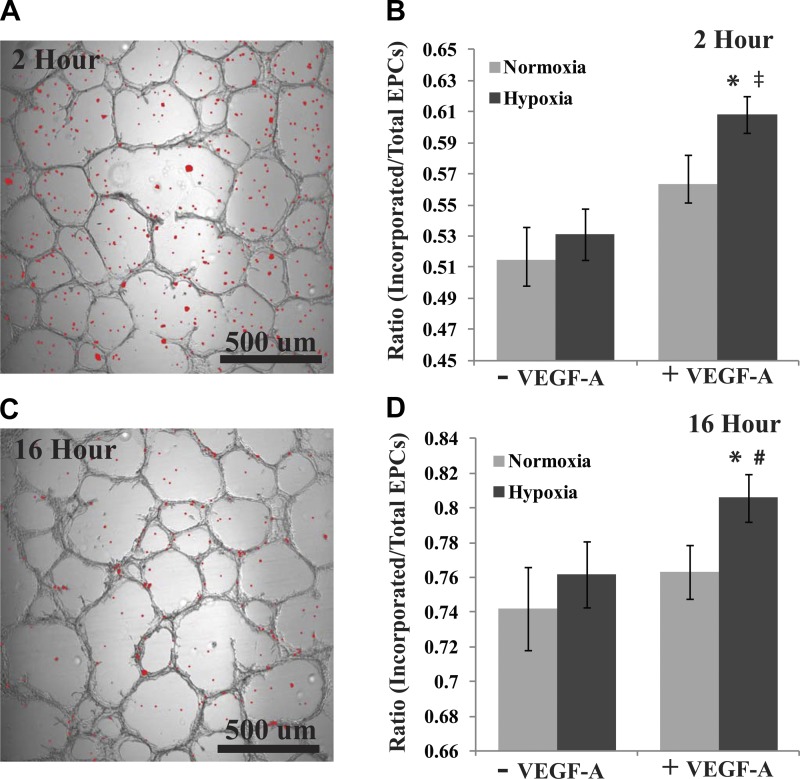
To further validate the cell projection/migratory phenotype of rat BM-EPCs treated with VEGF-A under hypoxic conditions, we developed a novel assay to measure the incorporation of BM-EPCs into preformed RMVEC tubes in matrigel. All RMVEC tubes were preformed for 48 h in normoxic conditions before addition of 20,000 DAPI-stained BM-EPCs pretreated with normoxia, normoxia plus VEGF-A, hypoxia, or hypoxia plus VEGF-A for 24 h (Fig. 4). At 2 h postaddition of pretreated BM-EPCs to the preformed RMVEC tubes only the VEGF-A hypoxia pretreatment displayed a significant increase in BM-EPC incorporation vs. normoxic pretreatment (P < 0.001). Hypoxia was approaching significance vs. normoxia, but it was not reached (P = 0.102). The VEGF-A hypoxia pretreatment of BM-EPCs also displayed a significant increase of incorporation into RMVEC tubes vs. normoxia plus VEGF-A (P < 0.001) and was approaching significance vs. hypoxia alone (P = 0.06). After incubation for 16 h, a similar result was seen with VEGF-A hypoxia pretreatment, resulting in a significant increase of incorporation vs. normoxia (P = 0.028) and hypoxia (P = 0.048), while approaching significance vs. normoxia plus VEGF-A (P = 0.072). Together, these assays suggest a migratory phenotype of BM-EPCs that is enhanced by VEGF-A during hypoxia.
Fig. 4.
A novel tube formation assay assessing the ability of BM-EPCs to incorporate into preformed rat cardiac microvascular endothelial cell (RMVEC) tubes in matrigel was developed to explore the BM-EPC migratory phenotype further. RMVEC tubes were preformed for 48 h, followed by the addition of BM-EPCs pretreated with normoxia, normoxia plus 100 ng/ml VEGF-A, hypoxia, or hypoxia plus 100 ng/ml for 24 h. A: displayed is a representative image portraying a bright-field capture of RMVEC tube formation overlayed with 4′,6-diamidino-2-phenylindole (DAPI)-stained BM-EPCs from hypoxia plus VEGF-A pretreatment group after 2 h incubation. DAPI staining was thresholded and converted to red for display in MATLAB. B: overlay images were quantified, and a ratio was generated for BM-EPCs incorporated into preformed RMVEC tubes vs. total number detected. At 2 h post-BM-EPC addition, the VEGF-A hypoxia-pretreated BM-EPCs displayed a significant increase of incorporation vs. the normoxia (*P < 0.001) and normoxia plus VEGF-A (‡P < 0.001) pretreatments, while approaching significance vs. hypoxia (P = 0.06). C: representation of overlay as in A with the same condition at 16 h incorporation. D: at 16 h post-BM-EPC addition, the VEGF-A hypoxia-pretreated BM-EPCs displayed a significant increase of incorporation vs. the normoxia (*P < 0.028) and hypoxia (#P < 0.048) pretreatments, while near significance vs. normoxia plus VEGF-A (P = 0.072).
DISCUSSION
VEGF-A is a proangiogenic growth factor that stimulates BM-EPCs, a cell type of shown to improve angiogenesis phenotypes in vitro and in vivo through EC tube formation and microvessel density assays (15, 16, 20). However, the VEGF-A signaling mechanisms in BM-EPCs, a hematopoietic cell line, are relatively unexplored. We explored VEGF-A signaling pathways and the functional phenotype of BM-EPCs during hypoxic stress and VEGF-A stimulation. Due to limitations of traditional pathway analysis tools, we developed a high-throughput proteomic approach utilizing a cell-permeable thiol-reducible cross-linker (DSP), VEGF-A coupled to a magnetic resin, and complex tandem MS analysis to decipher the VEGF-A signaling pathway in BM-EPCs. The main focus of this study was to explore VEGF-A signaling in BM-EPCs. We tested the hypothesis that VEGF-A signaling in BM-EPCs would share similar pathways to those described in other cell types but might also involve alternative proteins because of the unique phenotype of the BM-EPCs. Experiments were carried out under a variety of different stimuli with measurements of proteins, RNA, and functional phenotype. To do this we developed a novel method for the discovery and analysis of protein signaling pathways. Using this method we were able to demonstrate a set of known and unique signaling pathways in BM-EPCs that exhibited increased signaling during hypoxia and could contribute to the angiogenic enhancement in the endothelium and migratory attributes.
To understand signaling pathways, traditional methods have focused on nodal targets hypothesized to be parts of specific pathways. This approach results in a step-wise approach with a single end-point for each hypothesis. While valuable, these approaches are not conducive to large-scale systems biology studies requiring high-throughput techniques for global signaling pathway analysis (1, 64). The emergence of microarray technologies leading to high-throughput discovery of novel gene expression changes has been beneficial in building signaling pathways (42); however, the measurement of RNA expression is still a step removed from protein pathways critical for signaling. To overcome this caveat, proteomics has advanced over recent years to allow development of high-throughput discovery studies through utilization of advanced technologies in exploring cascades of signaling events by analyzing cellular phosphoproteome, protein-protein interactions, DNA-protein interactions, and quantitative protein abundance under varying conditions or disease models (11, 26, 27, 35, 41, 44, 46, 48). Methods such as immunoisolation and protein biotin labeling to purify protein signaling complexes, followed by MS analysis have been commonly used to explore signaling pathways (26, 27, 41); however, these methods are not ideal in all conditions. While useful, immunoisolation is dependent on reliable antibodies and has limitations in depth of the signaling complex. Preferential NH2-terminal biotin labeling of a bait protein, avoiding multiple labels potentially blocking binding, has been shown to work efficiently in previous studies (26, 27, 41); however, a free NH2 terminus is required. VEGF-A does not contain a free NH2 terminus, hence is not amenable for NH2-terminal biotin labeling; thus a VEGF-A-coupled metallic epoxy resin was used in this study with cell-permeable cross-linkers to compensate (26, 27, 35, 44). However, even with the ability to cross-link protein signaling complexes there is still great difficulty in efficiently and effectively isolating these cross-linked complexes from the tissue or cell sample. The strategy presented in this study (Fig. 1), using a ligand-coupled epoxy magnetic resin, allowed for efficient capture of bound BM-EPCs and subsequent isolation of VEGF-A cross-linked protein signaling complexes.
While methods that rely on affinity capture for analysis of receptor complexes are powerful, there are several shortcomings to be considered. First, the Dynabead immobilization of VEGF or other signaling molecules could alter signaling (10, 65). Immobilization may affect the geometry of the bait molecule, resulting in changes in receptor affinity. In our studies, we verified binding of immobilized VEGF to VEGFRs but were not able to prove that activation of EPCs by soluble and immobilized VEGF was equivalent. Second, determining the proper control experiment may be challenging. In the current studies, we used glycine-coated beads to control for nonspecific binding; however, because the process of complex isolation required VEGF-A coupled to beads, it was not possible to perform a “no VEGF” control isolation. Third, the use of immobilized ligands, followed by cross-linking and isolation of the assembled complex and proteomic analysis, primarily identifies those parts of the signaling complex that are physically close to the receptor. Distal events, such as endosomal signaling, will be limited in capture by our technique. For these reasons, we supplemented the proteomic analysis with examination of VEGF-A stimulated changes in transcription to reveal more distal portions of the overall signaling pathway.
The approach utilized for the current study revealed a significant increase of both VEGFR1 and VEGFR2 isolated by VEGF-A coupled-Dynabeads in hypoxic vs. normoxic conditions (Table 1), suggesting an increase of VEGFRs on the surface of the hypoxic BM-EPCS. Lower signaling capacity of VEGFRs on BM-EPCs under normoxic conditions were expected because of low surface exposure as seen in previous literature (28, 39), and this, coupled with dynamic range of detection limitations for low abundant proteins in a complex mixture by tandem MS analysis, likely led to an inability to detect VEGFRs in the normoxic elution samples. Previous literature has shown an increase in VEGF receptors on the cell surface due to hypoxia (28, 39), suggesting either increased expression or translocation to the cell surface. Hypoxia has been shown to induce VEGFR-1 and leave VEGFR-2 unaffected or slightly suppressed in ECs (28); however, we saw significant increases of VEGFR-1 and VEGFR2 in VEGF-A protein pathway isolations from BM-EPCs under hypoxic vs. normoxic conditions, meaning there was an increase of receptors isolated at the cell surface (Table 1). Additionally, the gene expression analysis of BM-EPCs shows that the VEGFR family of proteins did not exhibit a significant increase in expression following hypoxia or VEGF-A treatment. These data suggest the increase of isolated VEGFR1 and VEGFR2 from the cell surface of BM-EPCS during exposure to a hypoxic environment likely resulted from translocation to the cell surface, which is further supported by the proteomic data demonstrating an increase in receptor endo-/exocytosis regulatory proteins detected in hypoxic pathway isolations (Table 1, Supplemental Table S1). A distinct and complex regulation of VEGF signaling through VEGFR1 and VEGFR2 during vascularization has been displayed in previous studies (19, 25, 36, 47, 60), which along with data presented here suggests a complex balance of VEGFRs regulating BM-EPCs in the vasculature. Furthermore, the ability to isolate VEGFRs via VEGF-A coupled to Dynabeads, cell-permeable cross-linking, subsequent pathway purification, and large-scale tandem MS identification validates this technique as an extremely useful resource for monitoring ligand-dependent cell surface receptors, as well as for signaling pathway identification.
Through proteomic analysis and comparison of VEGF-A signaling pathways in BM-EPCs to those known in other cell types, both traditional and nontraditional VEGF-A signaling was identified and found to vary between cells exposed to a normoxic and hypoxic environment. Under both sets of conditions proteins were detected from the NOS pathway, inositol and calcium signaling, G protein signaling, inflammation, and phospholipase signaling (Fig. 5). NO signaling is essential for vessel homeostasis, and components were detected in abundance during VEGF-A pathway isolations of hypoxic BM-EPCs. This is suggestive that BM-EPCs entering a hypoxic environment at sites of vascular regeneration could produce NO for paracrine function. Additionally, the inflammatory response was altered in VEGF-A pathways during hypoxia, which the data suggest involved IL-12B-induced production of IFN-γ resulting in AKT1 signaling and NOS2 production (Table 1). There also appeared to be a shift in the G protein signaling, typically Src for VEGFRs, involved in signaling. We observed a shift toward RAC and RHO in the hypoxic BM-EPCs, which are important for cytoskeletal rearrangement and cell migration (Fig. 5). Altogether, the proteomic VEGF-A signaling pathways data during hypoxia in BM-EPCs suggest an important role for canonical VEGF-A signaling, regulation of redox homeostasis, cell survival, cell migration, and potentially enhancement of angiogenesis or vascular regeneration.
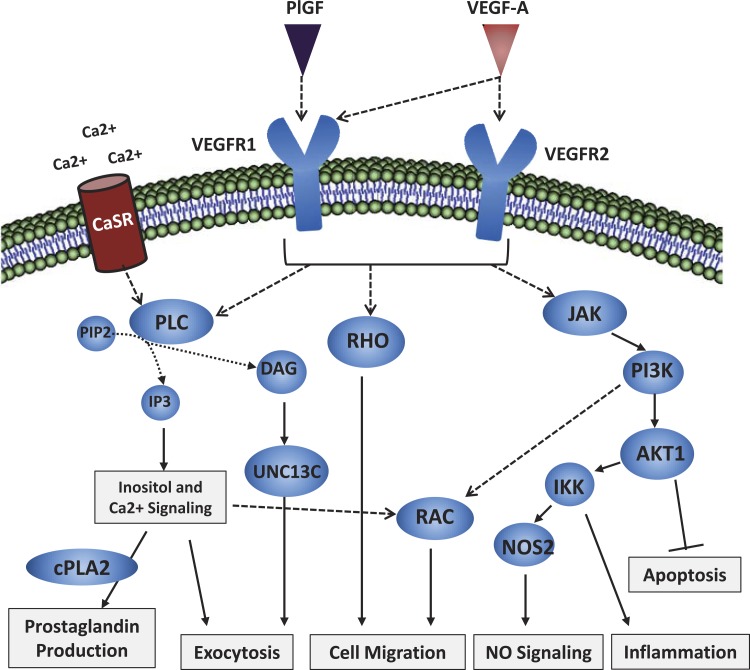
Fig. 5.
The proposed signaling pathway model for VEGF-A signaling in hypoxia-stressed BM-EPCs compiled from the proteomic and gene expression analysis is highlighted here. All of the signaling cascade members in the generated pathways depicted above were observed as significantly increased or near significance in the VEGF-A hypoxia conditions during proteomic signaling pathway analysis or in the RT-PCR signaling data. The outcomes of this pathway model are also supported by functional data in this study. Based on the results in this study, we hypothesize that the influence of VEGF-A stimulation of BM-EPCs in a hypoxic environment induces cell migration of this cell type to sites of vascular regeneration through a variety of pathways, including inositol/calcium, NO, inflammatory, cell survival, and G protein signaling processes, among others.
For comparison and expansion of the proteomic signaling pathway analysis we used a gene expression array specifically designed for examining VEGF signaling pathways critical for neovascularization. As would be expected (13, 51, 58), BM-EPCs in hypoxic vs. normoxic conditions (Table 2) showed a significant increase in Vegf-a (2.05-fold, P = 0.014). Interestingly, placental growth factor gene (Pgf), another member of the VEGF family, also had significantly increased expression (1.57-fold, P = 0.0067). PlGF selectively binds VEGFR1 with high affinity and potentiates the action of VEGF in vitro and in vivo (6, 49, 58). Vegf-b, Vegf-c, and Vegf-d did not show significant hypoxia-induced gene expression changes, suggesting VEGF-A and PlGF are the main members of this protein family regulated by hypoxia in BM-EPCs and act through a balance of VEGFR1 and VEGFR2. In this study the VEGFR gene family did not exhibit significant changes in expression in BM-EPCs as a result of hypoxia, whereas an increase of VEGFR1 but not VEGFR2 expression in response to hypoxia has previously been shown in ECs (28). Potential increases in VEGF-A or PlGF could be more important for paracrine regulation of the endothelium and/or VEGFRs in BM-EPCs are regulated through translocation to the surface as the proteomic pathway data suggests. Previous literature suggests both VEGF-A paracrine regulation on the endothelium by BM-EPCs (8, 63, 66), as seen with neurophils and platelets (38), and that VEGF-A serum levels increase in patients with hypoxic stress (17, 56). Additionally, translocation of VEGFRs to the surface of ECs following hypoxia has been observed (39). Hypoxia also regulated gene expression in numerous protein signaling pathways isolated by VEGF-A-coupled Dynabeads, such as G protein, MAPK/ERK, phospholipase, and phosphatidylinositol/calcium signaling (Table 2). Upon introduction of VEGF-A to the BM-EPCs under hypoxic conditions, a downregulation of Vegf-a was observed, which most likely is the result of a negative feedback loop due to addition of VEGF-A165. Phospholipase and phosphatidylinositol signaling was also significantly altered (Tables 2 and 3), which is consistent with signaling pathways identified in the protein datasets (Table 1). The proteomic datasets and the gene expression array suggest a connection between calcium-dependent phospholipase A2 (Pla2g5), calcium and inositol signaling, and the decrease of annexin-1, an inhibitor of phospholipase A2, in hypoxic BM-EPC VEGF-A signaling pathways. These pathways also stimulate exocytosis and potential regulation of cell surface receptors. Nfatc1 was also significantly downregulated after addition of VEGF-A, and downregulation of this gene is important in activation of adult stem cells from a quiescence state to proliferative phenotype (31).
EC lineages have been shown to exhibit tube formation in a matrigel assay, and BM-EPCs associate with these tubes (16); however, the direct ability of BM-EPCs to form tubes themselves has not been explored. VEGF-A signaling pathways isolated in the current experiment indicate a general role in cell survival, cell migration, inflammation, and angiogenic signaling in BM-EPCs. Therefore, growth factor-depleted matrigel tube formation assays were performed with BM-EPCs minus and plus VEGF-A under hypoxic and normoxic conditions. As the proteomic signaling pathway and gene expression array explored direct effects of VEGF-A and hypoxia on BM-EPCs, this experiment relates those direct signaling pathways to the functional potential of BM-EPCs in neovascularization. BM-EPCs alone did not display a strong angiogenic phenotype plus or minus VEGF-A as was hypothesized; while some tubes were observed it was minimal. However, during tube formation assays under hypoxia or normoxia, VEGF-A induced a significant increase in BM-EPCs with cell projections, indicating a sprouting or migratory phenotype (Fig. 3). This migratory functional phenotype was validated further during the cellular incorporation assay in which BM-EPCs pretreated with VEGF-A during hypoxia displayed a significant increase of incorporation into preformed RMVEC tubes at various time points (Fig. 4). Previously, VEGF-A expression has been shown to correlate with mobilization of BM-EPCs in patients with coronary artery disease (33, 34, 62) and during neovascularization (4, 5, 9). HIF-1α-induced VEGF-A increase under hypoxic stress has also been shown to increase EC and BM-EPC migration (40, 67). These data, along with the previous literature, suggest that VEGF-A stimulation of BM-EPCs may be an important factor in migration to sites of neovascularization where other factors may contribute to endothelium incorporation.
In conclusion, this study verified numerous known VEGF-A signaling pathway components observed in endothelial cell lineages, as well as several previously unidentified VEGF-A signaling mechanisms in BM-EPCs during hypoxia that may be important for cell survival and migration to sites of neovascularization. Additionally, this high-throughput signaling pathway network study utilizing proteomic, genomic, and functional experimentation allows for a more systematic understanding of VEGF-A signaling in hypoxic and normoxic BM-EPCs. Systematic studies, such as these, are valuable with the emergence of advanced computational biology geared toward understanding cell signaling networks (2, 14, 43, 45). Interestingly, computational modeling has been used to predict outcomes of ECs during angiogenesis and suggested that VEGF-A stimulation would induce a survival and sprouting phenotype (14) observed in BM-EPCs. The caveat is that this computational approach is dependent on prior knowledge from high-throughput proteomic, transcriptional profiling, and functional outcomes; however, the capability of a combined experimental and computational approach allows for a deep understanding of complex datasets than has been previously available.
GRANTS
This work was supported in part by the Biotechnology and Bioengineering and Innovation Centers at the Medical College of Wisconsin, as well as National Heart, Lung, and Blood Institute (NHLBI) Grant HL-082798 to A. S. Greene. B. R. Hoffmann was supported by NHLBI Grant T32HL-094273. Further support was provided by a generous donation from Drs. Robert D. and Patricia E. Kern.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.R.H., A.J., and A.S.G. conception and design of research; B.R.H., J.R.W., A.R.P., and A.J. performed experiments; B.R.H., J.R.W., and A.R.P. analyzed data; B.R.H., J.R.W., A.R.P., and A.S.G. interpreted results of experiments; B.R.H. prepared figures; B.R.H. and J.R.W. drafted manuscript; B.R.H., J.R.W., A.R.P., A.J., and A.S.G. edited and revised manuscript; B.R.H., J.R.W., A.R.P., A.J., and A.S.G. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
While all laboratory work was performed at the Medical College of Wisconsin Biotechnology and Bioengineering Center, Agnieszka Janiak was a visiting scientist with affiliation to the Milwaukee School of Engineering.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem 102: 580–592, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulos LG, Saez-Rodriguez J, Cosgrove BD, Lauffenburger DA, Sorger PK. Networks inferred from biochemical data reveal profound differences in toll-like receptor and inflammatory signaling between normal and transformed hepatocytes. Mol Cell Proteom 9: 1849–1865, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral SL, Maier KG, Schippers DN, Roman RJ, Greene AS. CYP4A metabolites of arachidonic acid and VEGF are mediators of skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol 284: H1528–H1535, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18: 3964–3972, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Shibuya M, Collen D, Conway EM, Carmeliet P. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med 9: 936–943, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Briguori C, Testa U, Riccioni R, Colombo A, Petrucci E, Condorelli G, Mariani G, D'Andrea D, De Micco F, Rivera NV, Puca AA, Peschle C. Correlations between progression of coronary artery disease and circulating endothelial progenitor cells. FASEB J 24: 1981–1988, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Brogi E, Schatteman G, Wu T, Kim EA, Varticovski L, Keyt B, Isner JM. Hypoxia-induced paracrine regulation of vascular endothelial growth factor receptor expression. J Clin Invest 97: 469–476, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, Tan Z, Dong CY, Li X, Xie Y, Wu Y, Chen X, Guo S. Combination of VEGF(165)/Angiopoietin-1 gene and endothelial progenitor cells for therapeutic neovascularization. Eur J Pharmacol 568: 222–230, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chen TT, Luque A, Lee S, Anderson SM, Segura T, Iruela-Arispe ML. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol 188: 595–609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciaccio MF, Wagner JP, Chuu CP, Lauffenburger DA, Jones RB. Systems analysis of EGF receptor signaling dynamics with microwestern arrays. Nat Meth 7: 148–155, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conn G, Bayne ML, Soderman DD, Kwok PW, Sullivan KA, Palisi TM, Hope DA, Thomas KA. Amino acid and cDNA sequences of a vascular endothelial cell mitogen that is homologous to platelet-derived growth factor. Proc Natl Acad Sci USA 87: 2628–2632, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1α induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol 42: 1036–1044, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das A, Lauffenburger D, Asada H, Kamm RD. A hybrid continuum-discrete modelling approach to predict and control angiogenesis: analysis of combinatorial growth factor and matrix effects on vessel-sprouting morphology. Philos Transact A Math Phys Eng Sci 368: 2937–2960, 2010 [DOI] [PubMed] [Google Scholar]
- 15.de Resende MM, Greene AS. Effect of ANG II on endothelial cell apoptosis and survival and its impact on skeletal muscle angiogenesis after electrical stimulation. Am J Physiol Heart Circ Physiol 294: H2814–H2821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Resende MM, Stodola TJ, Greene AS. Role of the renin angiotensin system on bone marrow-derived stem cell function and its impact on skeletal muscle angiogenesis. Physiol Genomics 42: 437–444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Raimondo F, Azzaro MP, Palumbo GA, Bagnato S, Stagno F, Giustolisi GM, Cacciola E, Sortino G, Guglielmo P, Giustolisi R. Elevated vascular endothelial growth factor (VEGF) serum levels in idiopathic myelofibrosis. Leukemia 15: 976–980, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Durik M, Seva Pessoa B, Roks AJ. The renin-angiotensin system, bone marrow and progenitor cells. Clin Sci (Lond) 123: 205–223, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol 24: 188–193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 110: 624–637, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 280: C1358–C1366, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Ferrara N, Bunting S. Vascular endothelial growth factor, a specific regulator of angiogenesis. Curr Opin Nephrol Hypertens 5: 35–44, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 18: 4–25, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Ferrara N, Keyt B. Vascular endothelial growth factor: basic biology and clinical implications. EXS 79: 209–232, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66–70, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Freed JK, Greene AS. Proteomic analysis of shear stress-mediated protection from TNF-alpha in endothelial cells. Microcirculation 17: 259–270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freed JK, Smith JR, Li P, Greene AS. Isolation of signal transduction complexes using biotin and crosslinking methodologies. Proteomics 7: 2371–2374, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes: Flt-1, BUT NOT Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem 272: 23659–23667, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Halligan BD, Greene AS. Visualize: a free and open source multifunction tool for proteomics data analysis. Proteomics 11: 1058–1063, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann BR, El-Mansy MF, Sem DS, Greene AS. Chemical proteomics-based analysis of off-target binding profiles for rosiglitazone and pioglitazone: clues for assessing potential for cardiotoxicity. J Med Chem 55: 8260–8271, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132: 299–310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imanishi T, Hano T, Nishio I. Angiotensin II potentiates vascular endothelial growth factor-induced proliferation and network formation of endothelial progenitor cells. Hypertens Res 27: 101–108, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Kalka C, Tehrani H, Laudenberg B, Vale PR, Isner JM, Asahara T, Symes JF. VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann Thorac Surg 70: 829–834, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Kang L, Chen Q, Wang L, Gao L, Meng K, Chen J, Ferro A, Xu B. Decreased mobilization of endothelial progenitor cells contributes to impaired neovascularization in diabetes. Clin Exp Pharmacol Physiol 36: e47–e56, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Kao A, Chiu CL, Vellucci D, Yang Y, Patel VR, Guan S, Randall A, Baldi P, Rychnovsky SD, Huang L. Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes. Mol Cell Proteomics 10: M110.002212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2: a006502, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J 437: 169–183, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Koehne P, Willam C, Strauss E, Schindler R, Eckardt KU, Buhrer C. Lack of hypoxic stimulation of VEGF secretion from neutrophils and platelets. Am J Physiol Heart Circ Physiol 279: H817–H824, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Lee JE, Didier DN, Lockett MR, Scalf M, Greene AS, Olivier M, Smith LM. Characterization of vascular endothelial growth factor receptors on the endothelial cell surface during hypoxia using whole cell binding arrays. Anal Biochem 369: 241–247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Pan L, Zhuo Y, Gong Q, Rose P, Zhu Y. Hypoxia-inducible factor-1alpha is involved in the pro-angiogenic effect of hydrogen sulfide under hypoxic stress. Biol Pharm Bull 33: 1550–1554, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Mann M, Hendrickson RC, Pandey A. Analysis of proteins and proteomes by mass spectrometry. Annu Rev Biochem 70: 437–473, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Moffat J, Sabatini DM. Building mammalian signalling pathways with RNAi screens. Nat Rev Mol Cell Biol 7: 177–187, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Morris MK, Saez-Rodriguez J, Clarke DC, Sorger PK, Lauffenburger DA. Training signaling pathway maps to biochemical data with constrained fuzzy logic: quantitative analysis of liver cell responses to inflammatory stimuli. PLoS Comput Biol 7: e1001099, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller MQ, Dreiocker F, Ihling CH, Schafer M, Sinz A. Cleavable cross-linker for protein structure analysis: reliable identification of cross-linking products by tandem MS. Anal Chem 82: 6958–6968, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Narayanan T, Gersten M, Subramaniam S, Grama A. Modularity detection in protein-protein interaction networks. BMC Res Notes 4: 569, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olive DM. Quantitative methods for the analysis of protein phosphorylation in drug development. Expert Rev Proteom 1: 327–341, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 7: 359–371, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Pandey A, Andersen JS, Mann M. Use of mass spectrometry to study signaling pathways. Sci STKE 2000: pl1, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269: 25646–25654, 1994 [PubMed] [Google Scholar]
- 50.Parker SJ, Didier DN, Karcher JR, Stodola TJ, Endres B, Greene AS. Bone marrow mononuclear cells induce beneficial remodeling and reduce diastolic dysfunction in the left ventricle of hypertensive SS/MCWi rats. Physiol Genomics 44: 925–933, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pham I, Uchida T, Planes C, Ware LB, Kaner R, Matthay MA, Clerici C. Hypoxia upregulates VEGF expression in alveolar epithelial cells in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 283: L1133–L1142, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Plouet J, Schilling J, Gospodarowicz D. Isolation and characterization of a newly identified endothelial cell mitogen produced by AtT-20 cells. EMBO J 8: 3801–3806, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qian C, Schoemaker RG, van Gilst WH, Roks AJ. The role of the renin-angiotensin-aldosterone system in cardiovascular progenitor cell function. Clin Sci (Lond) 116: 301–314, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Richardson MR, Yoder MC. Endothelial progenitor cells: quo vadis? J Mol Cell Cardiol 50: 266–272, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roks AJ, Rodgers K, Walther T. Effects of the renin angiotensin system on vasculogenesis-related progenitor cells. Curr Opin Pharmacol 11: 162–174, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med 165: 67–70, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Semenza G, Prabhakar N. The role of hypoxia-inducible factors in oxygen sensing by the carotid body. Adv Exp Med Biol 758: 1–5, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semenza GL. Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. J Clin Invest 108: 39–40, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sirker AA, Astroulakis ZM, Hill JM. Vascular progenitor cells and translational research: the role of endothelial and smooth muscle progenitor cells in endogenous arterial remodelling in the adult. Clin Sci (Lond) 116: 283–299, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Stefanini MO, Wu FT, Mac Gabhann F, Popel AS. The presence of VEGF receptors on the luminal surface of endothelial cells affects VEGF distribution and VEGF signaling. PLoS Comput Biol 5: e1000622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuzuki Y, Fukumura D, Oosthuyse B, Koike C, Carmeliet P, Jain RK. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1α → hypoxia response element → VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res 60: 6248–6252, 2000 [PubMed] [Google Scholar]
- 62.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89: E1–E7, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Villegas G, Lange-Sperandio B, Tufro A. Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int 67: 449–457, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Dong Y, Jiwani AJ, Zou Y, Pastor J, Kuro OM, Habib AA, Ruan M, Boothman DA, Yang CR. Improved protein arrays for quantitative systems analysis of the dynamics of signaling pathway interactions. Proteome Sci 9: 53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wijelath ES, Murray J, Rahman S, Patel Y, Ishida A, Strand K, Aziz S, Cardona C, Hammond WP, Savidge GF, Rafii S, Sobel M. Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ Res 91: 25–31, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol 42: 441–448, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye L, Haider HK, Jiang SJ, Sim EK. Therapeutic angiogenesis using vascular endothelial growth factor. Asian Cardiovasc Thorac Ann 12: 173–181, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Yin T, Ma X, Zhao L, Cheng K, Wang H. Angiotensin II promotes NO production, inhibits apoptosis and enhances adhesion potential of bone marrow-derived endothelial progenitor cells. Cell Res 18: 792–799, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.