Abstract
The hypothesis that ovine luteal gene expression differs due to pregnancy status and day of estrous cycle was tested. RNA was isolated from corpora lutea (CL) on days 12 and 14 of the estrous cycle (NP) or pregnancy (P) and analyzed with the Affymetrix bovine microarray. RNA also was isolated from luteal cells on day 10 of estrous cycle that were cultured for 24 h with luteolytic hormones (OXT and PGF) and secretory products of the conceptus (IFNT and PGE2). Differential gene expression (>1.5-fold, P < 0.05) was confirmed using semiquantitative real-time PCR. Serum progesterone concentrations decreased from day 12 to day 15 in NP ewes (P < 0.05) reflecting luteolysis and remained >1.7 ng/ml in P ewes reflecting rescue of the CL. Early luteolysis (days 12–14) was associated with differential expression of 683 genes in the CL, including upregulation of SERPINE1 and THBS1. Pregnancy on day 12 (55 genes) and 14 (734 genes) also was associated with differential expression of genes in the CL, many of which were ISGs (i.e., ISG15, MX1) that were induced when culturing luteal cells with IFNT, but not PGE2. Finally, many genes, such as PTX3, IL6, VEGF, and LHR, were stabilized during pregnancy and downregulated during the estrous cycle and in response to culture of luteal cells with luteolytic hormones. In conclusion, pregnancy circumvents luteolytic pathways and activates or stabilizes genes associated with interferon, chemokine, cell adhesion, cytoskeletal, and angiogenic pathways in the CL.
Keywords: interferon, corpus luteum, progesterone, luteolysis, pregnancy
a better understanding of the mechanisms underlying establishment and loss of pregnancy may be applied to reduce the severe economic impact of embryo mortality on the agricultural community. For example, early embryonic mortality rates are as high as 28–43% in dairy cows, 33–38% in beef cows, and 12–26% in sheep (15). The consequences of embryo mortality in the beef cattle industry alone were estimated to be a loss of $1.2 billion dollars in 2005 (20). Causes of early embryo mortality may entail impaired signaling between the conceptus and mother. This “communication” is through conceptus secretory signals such as interferon tau (IFNT) that act directly on the endometrium and possibly through endocrine action on the corpus luteum (CL). The CL functions primarily to produce progesterone, which is critical in preparing the uterus for sustaining the early developing conceptus.
Prostaglandin F2 alpha (PGF) causes luteolysis, which is the structural and functional (loss of serum progesterone) demise of the CL (56). Binding of PGF to its receptor [prostaglandin F receptor (PTGFR)] on large luteal cells (LLC) induces several downstream apoptotic pathways in both LLC and small luteal cells (SLC). These include: 1) induction of a suicidal loop of PGF being produced by LLC through the prostaglandin-endoperoxide synthase 2 [prostaglandin G/H synthase/cyclooxygenase, (PTGS2)] pathway (55, 78); 2) induction of calcium influx into LLC; 3) activation of the protein kinase C (PKC) pathway, which blocks the synthesis of progesterone and causes the production of oxytocin (OXT) (55, 86); and 4) binding of OXT secreted by the LLC to the oxytocin receptor (OXTR) on the SLC, which causes an influx of calcium, activation of the PKC pathway, and lysis of the SLC (55, 85).
The sheep conceptus signals its presence by releasing IFNT. IFNT binds receptors in the endometrium and activates antiluteolytic responses, which permit continued production of progesterone from the CL. IFNT is released by the ovine conceptus on days 10 through 25, with the greatest concentrations released between days 14 and 16 (78). For a successful pregnancy to be recognized and maintained in the ewe the conceptus must be present from day 12 through day 17 (27, 51). The antiluteolytic actions of IFNT in the endometrium are mediated by silencing the upregulation of the estrogen receptor (ESR1), which normally occurs during the estrous cycle. Consequently, inhibition of ESR1 inhibits production of the endometrial OXTR, thereby disrupting pulsatile release of PGF (77–79). This paracrine action of conceptus-derived IFNT on the endometrium indirectly protects the CL of pregnancy.
A direct action of pregnancy on the ovine CL also has been suggested because the CL of pregnancy is more resistant to the lytic effects of PGF (34, 44, 63, 73). More recent evidence to support this concept is based on detection of IFNT in uterine vein blood and demonstration that IFNT has action on the CL through induction of IFN-stimulated genes (ISGs), such as IFN-stimulated gene 15 (ISG15) (6, 28, 59).
It is hypothesized herein that genes induced in the CL in response to early pregnancy counter the activation of genes involved in the demise of the CL in response to PGF. We aimed to test this hypothesis by screening mRNA isolated from CL on days 12 and 14 of the estrous cycle [nonpregnant (NP)] and pregnancy (P) in ewes using the bovine Affymetrix microarray, determining major activated pathways in response to pregnancy and early luteolysis and comparing luteal gene expression during the estrous cycle and early pregnancy with responses induced by PGF and OXT, as well as IFNT and PGE2 in cultured luteal cells.
MATERIALS AND METHODS
Animal care and collection of CL and blood samples.
All experiments using sheep were reviewed and approved by the Colorado State University Animal Care and Use Committee. Western range ewes purchased from a local producer were either exposed to a vasectomized ram (NP group, no semen exposure) or mated to a fertile ram (P group) to generate CL derived from the estrous cycle or pregnancy, respectively (day 0 = day of estrus). CL were collected during necropsy: day 12 (n = 4 NP and 4 P), day 13 (n = 5 NP and 5 P), day 14 (n = 5 NP and 6 P), day 15 (n = 6 NP and 10 P), and day 16 (n = 5 P). The CL had regressed by day 16 of the estrous cycle and for this reason was not examined. Presence of a conceptus was confirmed by visual identification following flushing the uterine lumen with sterile saline solution at necropsy.
Progesterone assay.
Blood samples were collected two times per day starting on day 12, processed to yield serum, and then analyzed for progesterone concentrations with radioimmunoassay (54). The sensitivity of the assay was 15 pg/ml. The mean intra-assay coefficient of variation (CV) was 5.83%. Three quality controls were examined in duplicate for each assay. The CVs were calculated for each standard used in the assay and presented as an average CV for the assay.
RNA isolation.
Total RNA was extracted from CL by TRIzol Reagent (MRC, Cincinnati, OH) protocol. RNase-free DNase and RNeasy MinElute Cleanup Kits (Qiagen) were used to digest DNA and purify RNA. RNA was quantified with a NanoDrop (NanoDrop Technologies, Valencia, CA). Purity of RNA was determined by A260/280 and A260/230 ratios. Proper ratios were between 1.75 and 2.0. RNA integrity was determined with an Agilent 2100 Bioanalyzer.
Analysis of gene microarray data.
Microarray analysis was completed at the Microarray Core Facility at the University of Nebraska Medical Center (Dr. Xiaoying Hou). The cDNA probes were synthesized from 200 ng of CL mRNA representing day 12 and 14 of the estrous cycle or pregnancy (n = 3 ewes for each day and pregnancy status) and were used to screen 24,000 targets by using the bovine microarray from Affymetrix (Santa Clara, CA). The microarray data were preprocessed with robust multiarray average algorithm for background correction, quartile normalization and gene-level probe set summation (35). Differential expression (P < 0.05) was determined by the LIMMA method (76). These data were further analyzed with the Metacore pathway analysis program from GeneGo (Carlsbad, CA) to identify signal transduction pathways and genes that are impacted by main effects of day and pregnancy status. Genes with a fold change >1.5, P < 0.05, and a control false discovery rate of 0.1 (from at least one comparison) were determined to be differentially expressed and included in this analysis.
Semiquantitative real-time RT-PCR.
Single-stranded cDNA was synthesized from 1 μg of RNA using the iScript cDNA synthesis kit (Bio-Rad Life Science, Hercules, CA). The synthesized cDNA was used as a template for RT-PCR using iQ SYBR Green Supermix (Bio-Rad Life Science). The cDNA samples were amplified in a 384-well plate with oligonucleotide primers specific to the targets (Table 1). Oligonucleotide primers were designed with an annealing temperature of 61°C, single-product melting curves, and consistent amplification efficiencies (Table 1). Amplification of PCR products was performed at 95°C for 30 s, 61°C for 30 s, and 72°C for 15 s and repeated over 40 cycles. Amplification of cDNA was normalized with the geometric mean of GAPDH, POLR2A, RPL19, and RN18s as internal standards. CT values were analyzed, whereas relative expression of RT-PCR products were plotted using mean 2−ΔCT; RT-PCR amplification efficiencies were between 1.8 and 2.2 (72). Amplicon size was verified through PCR amplification and gel electrophoresis; all amplicons were sequenced to confirm identity with targeted genes.
Table 1.
Oligonucleotide primer sequences and efficiency of amplification by using semi-quantitative RTPCR
| Gene Target | Forward Primer | Reverse Primer | RT-PCR Efficiency (1.8–2.2) |
|---|---|---|---|
| SERPINE1 | 5′TCATGCCCAACTTCTTCAGG3′ | 5′TTGACGATGAACCTGGCTCT3′ | 2.13 |
| THBS1 | 5′ACTGGGTTGTACGCCATCAG3′ | 5′CACGGCGTTAAATTCGTCAT3′ | 2.18 |
| ISG15 | 5′GGTATCCGAGCTGAAGCAGTT3′ | 5′ACCTCCCTGCTGTCAAGGT3′ | 1.96 |
| MX1 | 5′TCTGCAAATGGAGTGCTGTG3′ | 5′TTCACAAACCCTGGCAACTC3′ | 2.00 |
| IL6 | 5′CTGCAGTTCAGCCTGAGAG3′ | 5′CCCAGTGGACAGGTTTCTGA3′ | 2.04 |
| PTX3 | 5′TTGGGTCAAAGCCACAGAAG3′ | 5′CCACCCACCACAAGCATTAT3′ | 2.00 |
| VEGF | 5′TCTGCTCTCTTG GGTGCA3′ | 5′TCACTTCATGGGGTTTCTGC3′ | 2.06 |
| LHR | 5′GTGCAACCTCTCCTTTGCAG3′ | 5′CTGCCAGTCTATGGCATGGT3′ | 1.92 |
| GAPDH | 5′TGACCCCCTCATTGACCTTC3′ | 5′GGTTCTCTGCCTTGACTGTG3′ | 1.95 |
| POLR2A | 5′AGTCCAACATGCTGAAGGACATGA3′ | 5′AGCCAAGTGCCGGTAATTGACGTA3′ | 2.04 |
| RPL19 | 5′TCGCCGGAAGGGCAGGCATA3′ | 5′GGCTGTGATACATGTGGGGGTC3′ | 2.20 |
| RN18S | 5′GAGGCCCTGTAATTAGAATGAG3′ | 5′GCAGCAACTTTAATATACGCTATTGG3′ | 2.20 |
Culture of isolated SLC, LLC, and mixed luteal cells.
Luteal cells were isolated from CL collected from adult western range ewes on day 10 of the estrous cycle; SLC and LLC were separated by elutriation (17). Cells were cultured in six-well plates at the following concentrations: SLC, 2 × 106/ml; LLC, 5 × 105/ml, and mixed luteal cells (MLC), 1 × 106/ml luteal cells. Isolated luteal cell populations were cultured for 24 h at 37°C and 5% CO2 in M199 medium supplemented with 10% FBS and 1% penicillin-streptomycin. After 24 h incubation, the medium was replaced with serum-free medium, and luteal cells were not treated (control) or treated with 1) recombinant ovine (ro) IFNT (1 ng/ml, 108 U/mg; from Dr. Fuller Bazer, Texas A&M University) or 2) prostaglandin E2 (PGE2, 3.5 ng/ml; Sigma Aldrich, Milwaukee, WI). In addition to roIFNT and PGE2, SLC were also treated with OXT [10 μg/ml; Sigma Aldrich, St. Louis, MO (11)], and LLC were treated with PGF (11) (3.5 ng/ml; Fisher Scientific, Houston, TX). All cells were treated with IFNT and PGE2 to study genes that were upregulated based on microarray in CL from pregnant ewes. PGE2 was tested in these experiments because it has been described as a luteotrophic agent (29, 43, 63, 69). PGF binds receptors on LLC and OXT binds receptors on SLC to induce luteolysis (55), thus these luteolytic hormones were tested so that genes regulated in response to luteolysis could be examined. Luteal cell mRNA was isolated following 24 h culture with treatments with Trizol reagent. Concentrations of OXT, PGE2, and PGF were selected based on previous reports of effectiveness in inducing an in vitro response (11). Amount of roIFNT (1 ng/ml) added to luteal cells was determined by a concentration-dependent induction of ISG15 described by Antoniazzi et al. (1) and represented the lowest concentration required to induce a maximal ISG15 response.
Data analysis of RT-PCR.
Analysis of RT-PCR data was completed on gene targets reflecting major affected signal transduction pathways by use of two-way ANOVA for unequal sample size with day (12–15) and pregnancy status as main effects with SAS software version 9.3 (SAS Institute, Cary, NC). Type I error within the two-way ANOVA was corrected by a Tukey adjustment. CL collected on day 16 of pregnancy were compared with CL collected on other days of pregnancy by ANOVA rather than being included in two-way ANOVA because of lack of a viable CL for comparison on day 16 of the estrous cycle. Note that ewe sample size was larger for analysis of CL by RT-PCR: day 12 (n = 4 NP and 4 P), day 13 (n = 5 NP and 5 P), day 14 (n = 5 NP and 6 P), day 15 (n = 6 NP and 10 P), and day 16 (n = 5 P) compared with microarray analysis. Differences between treatments in cell culture were tested by ANOVA with a Tukey adjustment.
RESULTS
Serum progesterone concentrations.
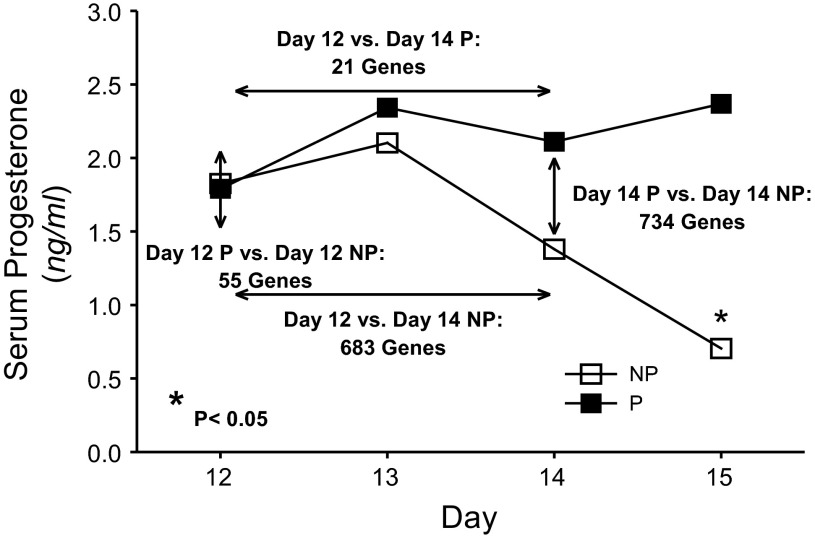
Serum progesterone concentrations did not differ in day 12 or 13 NP and P ewes (Fig. 1). In NP ewes, serum progesterone concentrations started to decline on day 14 and then continued to decline by day 15 to levels <1 ng/ml, indicating that the CL was regressing in these ewes. In contrast, serum progesterone concentrations remained unchanged (∼1.7 ng/ml) from days 12–15 in P ewes.
Fig. 1.
Serum progesterone concentrations and differential gene expression in corpora lutea (CL) collected on days 12 and 14 of pregnancy (P) and the estrous cycle (NP). Serum progesterone was maintained from day 12 to 15 of pregnancy, whereas it declined in a manner consistent with luteolysis from day 12 to 15 of the estrous cycle. Number of genes differentially expressed (>1.5-fold, P < 0.05) was determined via microarray and is shown in context of serum progesterone profiles as a function of day or pregnancy status. *Significant difference (P < 0.05) between serum progesterone from nonpregnant and pregnant ewes on day 15. Values represent means.
CL microarray analysis.
Numbers of genes differentially expressed 1.5-fold or greater (P < 0.05) following microarray analysis are presented in Fig. 1 in context of serum progesterone profiles in day 12 or 14 NP and P ewes. On day 12, 55 genes were differentially expressed in CL collected from NP compared with P ewes. As the estrous cycle progressed from day 12 to 14, which also corresponded with the onset of luteolysis in response to endogenous PGF, there were 683 differentially expressed genes. As pregnancy progressed from day 12 to 14, there were 21 differentially expressed genes in P ewes. On day 14, there were 734 differentially expressed genes in CL from P compared with NP ewes.
Pathway analysis.
Because there were only 55 differentially expressed genes on day 12 in NP compared with P ewes and 21 differentially expressed genes as pregnancy progressed from day 12 to 14, pathway analysis was limited but implicated pregnancy-associated immune response and IFN alpha/beta signaling, as well as steroid biosynthesis and cytoskeletal remodeling in the CL (Tables 2 and 3). Key pathways identified in CL from day 14 P compared with NP ewes were: cell adhesion, chemokines [interleukin 8 (IL-8)], cytoskeletal remodeling and transforming growth factor (TGF) beta signaling (P < 0.0001, 8–21 genes; Table 4). Genes differentially expressed as the CL entered early stages of luteolysis from day 12 to 14 NP belonged to cell cycle, adhesion, chemokine (IL-8), TGF-β, and cytoskeleton pathways (P < 0.0001, 10–21 genes; Table 5).
Table 2.
The top pathways in CL on day 12 of NP compared with P in ewes
| Pathway 12NP/12P | P Value | Genes in Pathway |
|---|---|---|
| 1. Δ508-CFTR traffic/ER to Golgi in CF | 0.02 | 2/13 |
| 2. Normal wtCFTR traffic/ER to Golgi | 0.02 | 2/13 |
| 3. Cell cycle initiation of mitosis | 0.07 | 2/25 |
| 4. Cytoskelton remodeling fibronectin-binding integrins in cell motility | 0.08 | 2/28 |
CL, corpus luteum; NP, estrous cycle (nonpregnant); P, pregnant; CFTR, cystic fibrosis transmembrane conductance regulator; CF, cystic fibrosis.
Table 3.
The top pathways in CL from days 12 to 14 of P in ewes
| Pathway 14P/12P | P Value | Genes in Pathway |
|---|---|---|
| 1. Androstendedione and testosterone biosynthesis and metabolism p.2 | 0.03 | 2/17 |
| 2. Androstendedione and testosterone biosynthesis and metabolism p.2 rodent version | 0.03 | 2/18 |
Table 4.
The top 10 pathways in CL on day 14 of the NP compared with P in ewes
| Pathway 14NP/14P | P Value | Genes in Pathway |
|---|---|---|
| 1. Cell adhesion chemokines and adhesion | 1 × 10−6 | 20/93 |
| 2. Cytoskeleton remodeling | 2 × 10−6 | 20/96 |
| 3. TGF, WNT, and cytoskeletal remodeling | 4 × 10−6 | 21/107 |
| 4. Development TGF-β-dependent induction of EMT via MAPK | 4 × 10−6 | 13/46 |
| 5. Cell adhesion plasmin signaling | 6 × 10−6 | 11/34 |
| 6. Development TGF-β-dependent induction of EMT via Smads | 5 × 10−5 | 10/35 |
| 7. Cell adhesion ECM remodeling | 8 × 10−5 | 12/51 |
| 8. Development role of IL-8 in angiogenesis | 2 × 10−4 | 11/47 |
| 9. Development regulation of EMT | 2 × 10−4 | 13/63 |
| 10. Cytoskeleton remodeling, fibronectin-binding integrins in cell motility | 3 × 10−4 | 8/28 |
TGF, transforming growth factor; WNT, wingless-type MMTV integration site family; EMT, epithelial-to-mesenchymal transition.
Table 5.
The top 10 pathways in CL during early luteolysis on days 12–14 of NP in ewes
| Pathway14NP/12NP | P Value | Genes in Pathway |
|---|---|---|
| 1. Cell cycle spindle assembly and chromosome seperation | 3 × 10−6 | 11/32 |
| 2. Cell adhesion chemokines and adhesion | 5 × 10−6 | 19/93 |
| 3. Development TGF-β-dependent induction of EMT via SMADs | 7 × 10−6 | 11/35 |
| 4. Cytoskeleton remodeling | 8 × 10−6 | 19/96 |
| 5. TGF, WNT, and cytoskeletal remodeling | 1 × 10−6 | 20/107 |
| 6. Cell cycle chromosome condensation in prometaphase | 2 × 10−5 | 8/20 |
| 7. Development TGF-β-dependent induction of EMT via MAPK | 2 × 10−5 | 12/46 |
| 8. Development role of IL-8 in angiogenesis | 3 × 10−5 | 12/47 |
| 9. Cytoskeleton remodeling fibronectin-binding integrins in cell motility | 4 × 10−5 | 9/28 |
| 10. Development regulation of EMT | 1 × 10−4 | 13/63 |
Selection of the differentially expressed genes for further study was based on representation in the pathway analysis, but also on significance and fold change from the microarray analysis data (Table 6). The eight genes selected for further analysis were: serpine peptidase inhibitor (SERPINE1), thrombospondin 1 (THBS1), ISG15, myxovirus (influenza virus) resistance 1 (MX1), IL6, pentraxin 3 long (PTX3), vascular endothelial growth factor A (VEGF), and luteinizing hormone/choriogonadotropin receptor (LHR). We also examined genes that have been implicated previously in the CL in processes such as [steroidogenesis steroid acute regulatory protein (STAR), peripheral benzodiazepine receptor (PBR), P450 side-chain cleavage (CYP11A1), and 3β-hydroxysteroid dehydrogenase (3βHSD)], and prostaglandin biosynthesis and action [hydroxyprostaglandin dehydrogenase 15-(NAD) (HPGD), PTGS2, prostaglandin F synthase (PGFS), and prostaglandin E synthase (PTGES)] to determine if they were differentially expressed in the CL in response to pregnancy or luteolysis based on microarray analysis (Table 6). STAR, CYP11A1, and 3βHSD mRNA concentrations were downregulated reflecting decline in production of progesterone from days 12 to 14 of the estrous cycle. These steroidogenic proteins also were downregulated on day 14 of the estrous cycle compared with pregnancy (Table 6). PBR and the prostaglandin biosynthesis enzyme mRNA concentrations did not change during the estrous cycle or pregnancy.
Table 6.
Genes selected from microarray analysis that were differentially expressed in the CL in response to day or in response to pregnancy status
|
Day 12 P vs. NP |
Day 14 P vs. 12 P |
Day 14 NP vs. 12 NP |
Day 14 P vs. NP |
|||||
|---|---|---|---|---|---|---|---|---|
| Gene Targets | Fold | P Value | Fold | P Value | Fold | P Value | Fold | P Value |
| SERPINE1 | NS | NS | 21.3 | 5.4 × 10−11 | −16.8 | 1 × 10−10 | ||
| THBS1 | NS | NS | 4.09 | 2 × 10−6 | −4.06 | 3 × 10−6 | ||
| ISG15 | NS | 2.3 | 0.022 | NS | 3.1 | 0.004 | ||
| MX1 | 1.5 | 0.035 | 1.35 | 0.096 | NS | 2.2 | 0.0005 | |
| IL-6 | NS | NS | −6.8 | 0.0007 | 4.4 | 0.0045 | ||
| PTX3 | NS | NS | −4.1 | 0.0008 | 3.6 | 0.0016 | ||
| VEGF | NS | NS | −2.7 | 3 × 10−5 | 3.4 | 4 × 10−6 | ||
| LHCGR | NS | NS | −2.2 | 0.0002 | 2.1 | 0.0004 | ||
| STAR | NS | NS | −1.62 | 0.0001 | 1.7 | 5 × 10−5 | ||
| PBR | NA | NA | NA | NA | NA | NA | NA | NA |
| CYP11A1 | NS | NS | −1.38 | 0.015 | 1.4 | 0.001 | ||
| HSD3B | NS | NS | −1.7 | 0.0009 | 1.7 | 0.001 | ||
| HPGD | NA | NA | NA | NA | NA | NA | NA | NA |
| PTGS2 | NA | NA | NA | NA | NA | NA | NA | NA |
| PGFS | NA | NA | NA | NA | NA | NA | NA | NA |
| PTGES | NA | NA | NA | NA | NA | NA | NA | NA |
| TGFB | NS | NS | 1.69 | 4 × 10−5 | −1.68 | 4 × 10−5 | ||
| IL8 | −1.27 | 0.013 | NS | 1.19 | 0.06 | −1.48 | 0.0004 | |
NA, genes that have a role in CL function but are nonapplicable because they were not shown to be differentially expressed in the microarrary data; NS, nonsignificant microarray genes within noted comparison that were different within other comparisons.
Microarray and RT-PCR validation of gene targets.
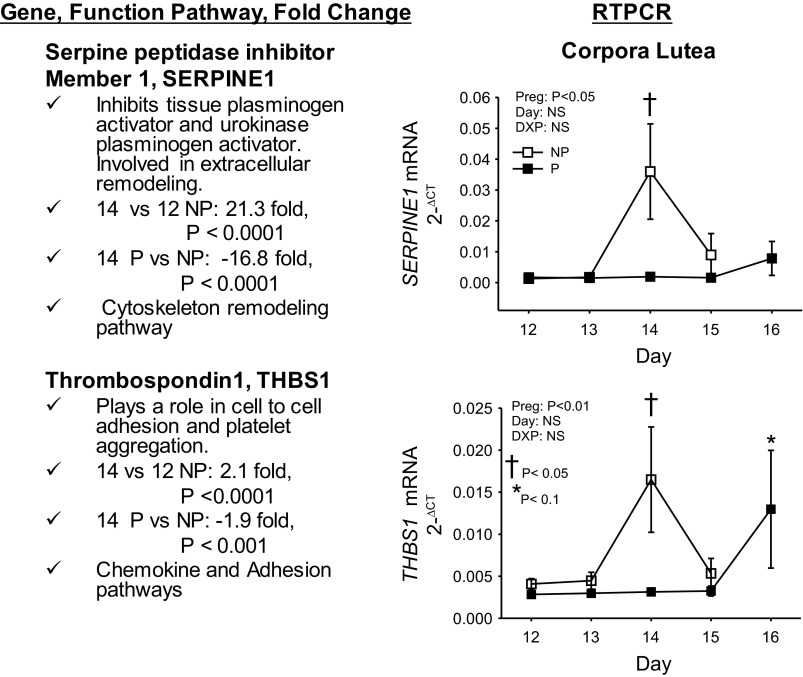
Genes upregulated in the CL during early stages of luteolysis based on microarray data were SERPINE and THBS1 (Fig. 2). SERPINE1 (P < 0.05) and THBS1 (P < 0.01) were affected by pregnancy in the model and increased in CL from NP compared with P ewes on day 14. Neither SERPINE1 nor THBS1 mRNA concentrations changed very much during days 12–15 of pregnancy. However, after day 15, THBS1 mRNA concentrations tended (P < 0.10) to increase.
Fig. 2.
Genes transiently upregulated in response to luteolysis based on microarray and RT-PCR. Two genes, SERPINE1 and THBS1, out of 683 genes differentially expressed in response to luteolysis, were more extensively examined in the CL. General function, fold changes in SERPINE1, and THBS1 mRNA concentrations based on microarray analysis and implicated pathways are described at left; factorial analysis of RT-PCR for these mRNAs over days of the NP and P are shown at right. †Differences across pregnancy status on the same day; P < 0.05. *Tendency for difference across pregnancy status on the same day; P < 0.10. Values represent means ± SE.
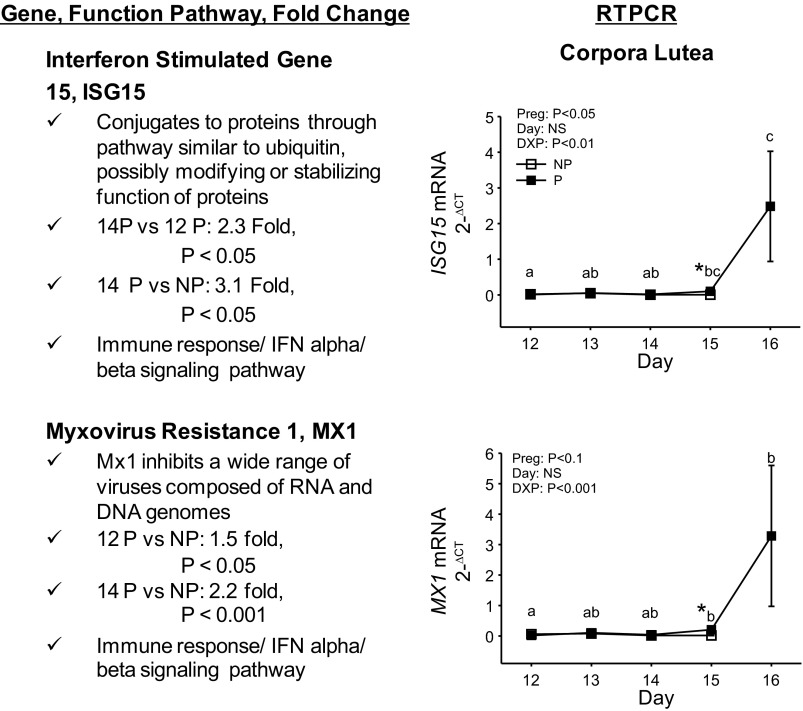
Genes upregulated in response to pregnancy based on microarray analysis were ISG15 and MX1 (Fig. 3). ISG15 and MX1 mRNA concentrations did not change during the estrous cycle. However, by day 15 of pregnancy, mRNA concentrations increased and continued to increase through day 16 of pregnancy.
Fig. 3.
Genes upregulated in the CL during pregnancy. Two genes, ISG15 and MX1, out of 21 differentially expressed in response to pregnancy on day 12, 21 genes from day 12 to 14 of pregnancy and 734 genes in response to pregnancy on day 14, were more extensively examined. General function, fold changes in ISG15, and MX1 mRNA concentrations based on microarray analysis and implicated pathways are described at left. Factorial analysis of RT-PCR for these mRNAs over days of the NP and P are shown at right. Means marked with different letters differ across days of pregnancy (P < 0.05). *Differences across pregnancy status on the same day; P < 0.05. Values represent means ± SE.
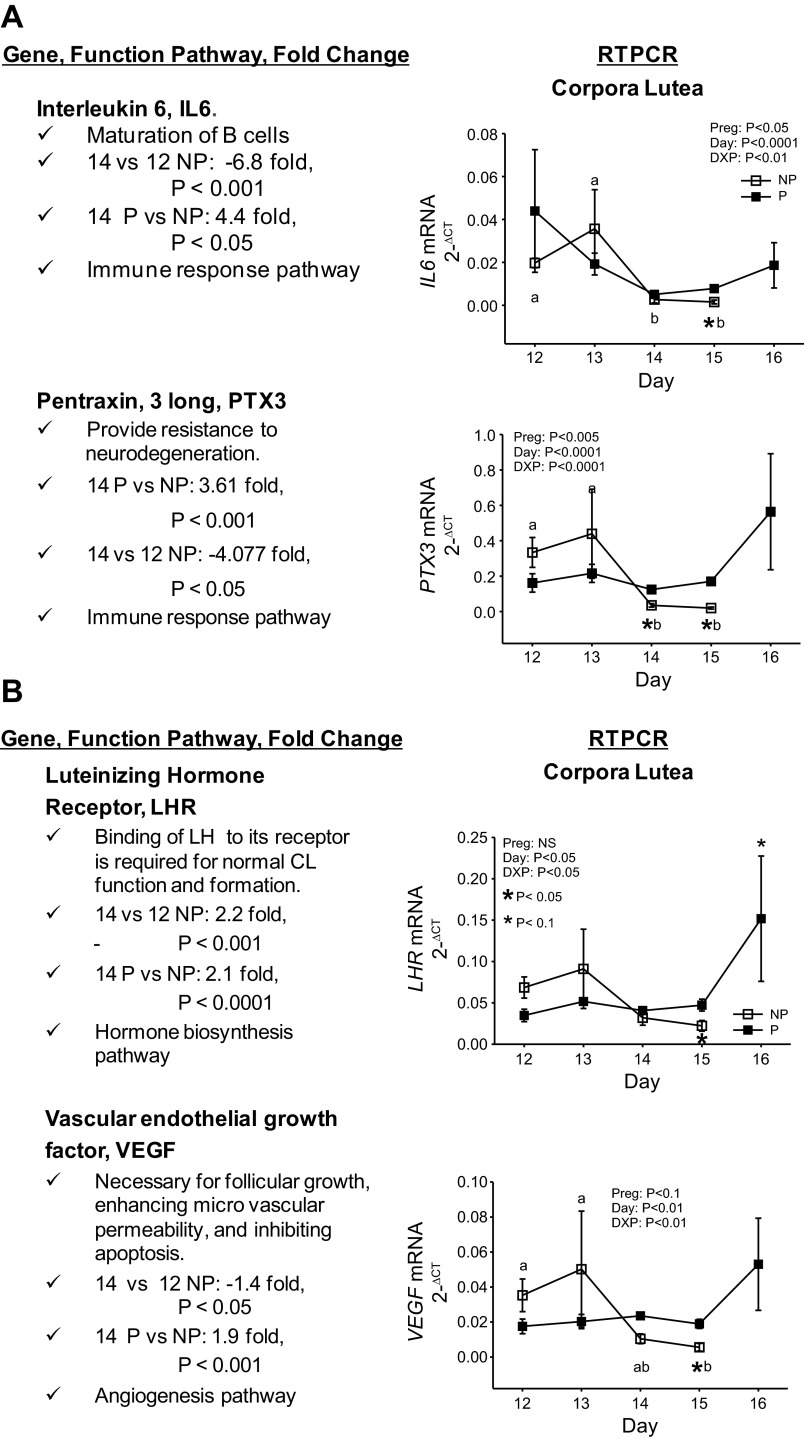
Genes stabilized during pregnancy and downregulated in response to luteolysis were IL6 (P < 0.05), PTX3 (P < 0.001), LHR (P = 0.05), and VEGF (P < 0.05; Fig. 4, A and B). IL6 and PTX3 mRNA concentrations were downregulated as early as day 14 and remained downregulated through day 15 of the estrous cycle. LHR and VEGF were downregulated by day 15 of the estrous cycle. All of these genes were stabilized during pregnancy, and in one case, LHR, there was a tendency (P < 0.10) for upregulation in mRNA concentrations by day 16 of pregnancy.
Fig. 4.
Genes stabilized during pregnancy and down-regulated in the CL during luteolysis. Selected genes out of 734 differentially expressed in CL on day 14 of P compared with the NP included IL6 and PTX3 (A) and LHR and VEGF (B). General function, fold changes in mRNA concentrations based on microarray analysis, and implicated pathways are described at left. Factorial analysis of RT-PCR for these mRNAs over days of the NP and P are shown at right. Means represented with different letters differ across days of the estrous cycle (P < 0.05). *Differences (P < 0.05) across pregnancy status on the same day. *Tendency (P < 0.10) in differences across pregnancy status on the same day. Values represent means ± SE.
Culture of SLC, LLC, and MLC.
SERPINE1 mRNA concentrations increase transiently during luteolysis (Fig. 2), and for this reason it was examined in cultured luteal cells (Fig. 5A). Isolated SLC and LLC had similar SERPINE1 mRNA concentrations regardless of treatments in vitro. While IFNT had no effect, PGE2, PGF, and OXT tended (P < 0.10) to decrease SERPINE1 mRNA concentrations when cultured with MLC.
Fig. 5.
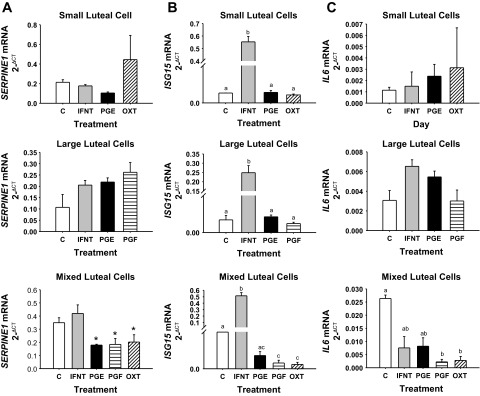
SERPINE1 (A), ISG15 (B), and IL6 (C) mRNA concentration in cultured small, large, or mixed luteal cells. Luteal cells were cultured for 24 h with either 0 or 1 ng/ml IFNT, 3.5 ng/ml PGE2 (PGE), 3.5 ng/ml PGF, or 10 μM OXT. Means marked with different superscript letters differ (P < 0.05). *Tendency (P < 0.10). Values represent means ± SE.
Culture of SLC, LLC, and MLC with IFNT caused massive induction of ISG15 (Fig. 5B), which was consistent with the response of the CL to pregnancy based on microarray and RT-PCR data. Culture of luteal cells with PGE2 had no impact on ISG15 mRNA concentrations. Likewise, culture of SLC and LLC with PGF and OXT had no effect on ISG15 mRNA concentrations. In contrast, culture of MLC luteal cells with PGF and OXT caused downregulation of ISG15 mRNA concentrations compared with control cultures.
IL6 mRNA concentrations did not change in cultured isolated SLC and LLC (Fig. 5C). In MLC, PGF and OXT caused downregulation of IL6, whereas culture with IFNT and PGF had no effect. Interestingly, this same general trend in downregulation by culture with PGF and OXT in MLC with no effect in isolated SLC and LLC was the same for PTX3 (Fig. 6A) and VEGF (Fig. 6C) mRNA concentrations. A tendency (P < 0.10) for downregulation of LHR mRNA concentrations following culture of SLC with OXT, and LLC with PGF was supported by significant (P < 0.05) downregulation of LHR mRNA concentrations following culture of MLC with PGF and OXT (Fig. 6B). Interestingly, culture of only LLC with IFNT caused an upregulation of LHR and a tendency (P < 0.08) for upregulation of VEGF mRNA concentrations, whereas there was no effect of IFNT in cultured SLC or MLC.
Fig. 6.
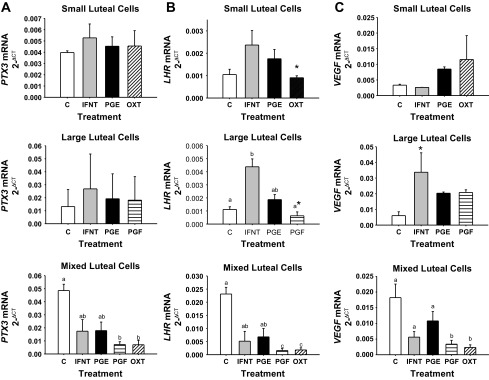
PTX3 (A), LHR (B), and VEGF (C) mRNA concentration in cultured small, large, or mixed luteal cells. Luteal cells were cultured for 24 h with either 0 or 1 ng/ml IFNT, 3.5 ng/ml PGE2 (PGE), 3.5 ng/ml PGF, or 10 μM OXT. Means marked with different superscript letters differ (P < 0.05). *Tendency (P < 0.10). Values represent means ± SE.
DISCUSSION
Establishment of early pregnancy in sheep is mediated through conceptus-derived IFNT and paracrine inhibition of upregulation of ESR1 and OXTR that occurs in the endometrium during the estrous cycle (77). In addition to this well-described paracrine action, an endocrine role for IFNT has been suggested based on detection of antiviral activity in uterine vein blood (59). Confirmation that IFNT is present in uterine vein blood was based on inhibition of antiviral activity following preadsorption with antibody against IFNT (6) and detection of IFNT by radioimmunoassay (1) and mass spectroscopy approaches (Romero JJ and Hansen TR, unpublished results). Indirect evidence to support endocrine action of IFNT provided herein and reported previously (6, 59) is based on upregulation of ISGs in the CL in response to pregnancy and IFNT. Likewise, systemic infusion of roIFNT for 24 h protects the CL against a subluteolytic challenge with PGF (1) when administered on day 10 of the estrous cycle. However, to our knowledge, IFNT has never been detected in systemic blood during pregnancy in ruminants. The present experiments studied systemic impact of early pregnancy in sheep on the CL and further examined the hypothesis that pregnancy induces genes, including ISGs that contribute to survival of the CL and resistance of the CL to luteolysis. Differential CL gene expression in response to early stages of luteolysis also was examined.
Validation of animal model.
Serum progesterone concentrations were the same regardless of pregnancy status on days 12 and 13. For this reason, collection and analysis of CL on these days provides an excellent reference point in context of representing a viable CL that is producing progesterone. By day 14 of the estrous cycle, serum progesterone concentrations were declining, and by day 15 of the estrous cycle serum progesterone had reached concentrations that were significantly lower and representative of luteolysis compared with days 12 and 13 of the estrous cycle and days 14–15 of pregnancy. Rather than focusing on day 15, which represented endpoint responses of the CL to luteolysis, we collected CL on day 14 of the estrous cycle and pregnancy to focus on early stages of luteolysis and maternal recognition of pregnancy. However, by also implementing RT-PCR, a larger sample (ewe) size and days 12, 13, 14, and 15 of the estrous cycle and pregnancy, a more temporal representation of gene expression in the CL was possible.
Early mediators of luteolysis.
One of the proteins that is believed to be involved with the extensive extracellular matrix remodeling of the CL during its formation and regression is SERPINE1 (PAI-1) (74). SERPINE1 mRNA concentrations have not been shown to change in CL during the ovine estrous cycle on days 3, 7, 10, 13, and 16 (74). However, in the present studies, SERPINE1 mRNA concentrations increased transiently from days 13 to 14 and then declined by day 15. This transient increase in SERPINE1 also was described by others in the ovine CL within 6 h following in vivo treatment with PGF (74). Exactly why SERPINE1 mRNA concentrations increase transiently during the late estrous cycle and in response to PGF in vivo is unknown but might be related to preparing the extracellular matrix of the CL for luteolysis. A balance of remodeling of extracellular matrix may be achieved through this transient increase in this inhibitor of plasminogen activator. For example, in the bovine CL, SERPINE1 and other plasminogen activators such as urokinase-type plasminogen activator (uPA), uPA receptor, and tissue-type PA have been shown to be upregulated in response to PGF-mediated luteolysis (41). Through regulation of extracellular matrix, SERPINE and PA may regulate invasion of immune cells as well as inhibition of the synthesis of progesterone. The transient increase in SERPINE1 mRNA concentrations during the estrous cycle was not observed during pregnancy.
When examined in cultured luteal cells, SERPINE1 mRNA concentrations did not change in isolated SLC and LLC. However, in MLC there was a tendency for downregulation of SERPINE1 mRNA concentrations following 24 h culture with PGE2, PGF, and OXT. Smith and coworkers (74) described a transient increase in SERPINE1 mRNA concentrations within 6 h following in vivo treatment with PGF. Whether this early rapid increase in SERPINE1 mRNA concentrations also occurs following treatment of MLC with PGF in vitro remains to be determined. Also, the reason for tendency in SERPINE1 mRNA concentrations to be suppressed following treatments in vitro, while concentrations are transiently increased on day 14 of the estrous cycle, remains to be determined.
This transient increase in CL SERPINE1 mRNA concentrations on day 14 of the estrous cycle was very similar to the profile of THBS1 mRNA concentrations in the CL during the estrous cycle. THBS1 increased as early luteolysis progressed in CL from day 12 to 14 of the estrous cycle. THBS1 is a secreted extracellular matrix glycoprotein that has been shown to be involved in platelet activation, cell adhesion, cell-to-cell and cell-to-matrix communication, promotion, and inhibition of angiogenesis and tissue healing (16, 42, 47). THBS1 induces TGFB (68), which also was upregulated 1.7-fold (P < 005) in the CL during the estrous cycle (see supplemental microarray files).1 Previous studies in the cow demonstrate that PGF induces luteal expression of TGFB (32, 50), which may contribute to functional and structural regression of the CL (46). TGFB induces SERPINE1 gene expression (90). Thus, THBS1 may cause upregulation of SERPINE1 through the TGFB pathway.
The antiangiogenic properties of THBS1 have been shown in several studies (42, 49). Zalman et al. (88) demonstrated that endothelial and steroidogenic cells of the CL express abundant concentrations of THBS1 mRNA. THBS1 has been shown to bind as well as sequester proangiogenic factors such as VEGF, which was stabilized in the present studies in CL during pregnancy and might be associated with luteal resistance (25, 45). THBS1 promotes the internalization of VEGF by low-density lipoprotein receptor-related protein-1 in nonendothelial cells and partially suppresses VEGF expression in those cells (23). The effects of THBS1 on endothelial cells result in cell cycle arrest, repressed motility, chemotaxis, and increased apoptosis (2, 24, 36, 68). In the bovine CL, Zalman and coworkers (88) also demonstrated that PGF induces THBS1 in MLC and suggested that it may be involved in luteolysis. THBS1 has been shown to cause apoptosis in endothelial cells; thus it may act in a similar manner in the CL.
THBS1 may cause apoptosis by activation of the CD36/p59fyn/caspase-3/p38MAPK cascade in endothelial cells. Antibodies against THBS1 can block apoptotic activation through neutralizing THBS1 or preventing access of THBS1 to CD36 (36). THBS1 action can also be blocked by compounds that inhibit p38MAPK or caspase-3-like proteases (36). THBS1 upregulates the cytokines FasL and TNF (66) and Bax (58), which are known mediators of apoptosis. In this regard, TNF induces apoptosis of bovine endothelial cells (30, 64). Because THBS1 is upregulated in CL on day 14 of the estrous cycle, is induced by PGF, and has antiangiogenic and proapoptotic properties, it may have a critical role in the demise of the CL.
Upregulation of pregnancy-associated genes in the CL: ISGs.
Pregnancy induces several ISGs in the endometrium and CL (28). Microarray analysis demonstrated upregulation of several of these ISGs in the CL such as: ISG15 (Fig. 3), MX1 (Fig. 3), MX2, IRF6, IRF9, CCL2, CCL8, IFI44, and OAS in response to pregnancy. ISG15 has been shown to be induced by IFNT in several reproductive tissues, as well as peripheral blood cells (3, 4, 21, 26, 37, 80, 87) and in luteal cells cultured with roIFNT (Fig. 5). While the exact functions of ISG15 in the CL are not known, ISG15 is able to conjugate to and regulate proteins through an enzymatic pathway similar to that described for ubiquitin utilizing the ubiquitin-activating enzyme 1-like protein (67). This IFNT-induced regulation of intracellular proteins by ISG15 may help provide resistance of the CL to lysis by PGF and is the focus of future experiments.
Culture of SLC, LLC, and MLC with roIFNT caused a massive upregulation of ISG15 mRNA concentrations, which is consistent with earlier reports (1, 6, 28, 59). The addition of PGE2 had no impact on ISG15 mRNA concentrations in cultured luteal cells. This is interpreted to suggest that luteotrophic action of PGE2 is not mediated through the ISGs. The suppression of ISG15 mRNA concentrations in MLC after culture with PGF or OXT might reflect damaging effects of these lytic hormones.
MX1 gene expression is induced in the endometrium by pregnancy, progesterone, and IFNs (α, β, and τ) (22, 62). MX1 is upregulated in the ovine glandular epithelium in response to IFNT released from the conceptus during early pregnancy in ruminants and following in vitro culture of endometrial cells with IFNT (8, 83, 84). MX1 protein concentrations have also been shown to increase in uterine flushings from pregnant ewes after day 15 (83, 84).
In the present experiments, MX1 mRNA concentrations were upregulated in the CL in response to pregnancy as early as day 12 and remained upregulated through day 14 of pregnancy based on the microarray analysis. RT-PCR demonstrated that MX1 mRNA concentrations were significantly greater in the CL on days 15 and 16 in P ewes. Thus, MX1 mRNA concentrations remained elevated in the CL up to and possibly beyond day 16 of pregnancy. MX1 is a GTPase that mediates antiviral responses (31) through a functional GTP binding motif (18). MX1 may also facilitate “nontraditional” secretion of proteins (53, 83) that are distinct from known classical secretion mechanisms via the endoplasmic reticulum and Golgi (61). The function of MX1 in the CL during early pregnancy is unknown but might entail mediating acute immune responses and intracellular GTP-driven mechanisms such as nontraditional release of proteins.
Pregnancy stabilizes genes that are downregulated in the CL during the estrous cycle.
IL6, PTX3, LHR, and VEGF mRNA concentrations were stabilized in the CL over days 12, 13, 14, 15, and 16 of pregnancy, with a tendency for an increase in LHR mRNA concentrations on day 16 of pregnancy. All of these genes were downregulated by day 14 (IL6 and PTX3) or 15 (LHR and VEGF) of the estrous cycle, which corresponds to the decline in serum progesterone concentrations and luteolysis. In cultured MLC, PGF and OXT caused downregulation of each of these genes compared with controls. This is consistent with in vivo data showing downregulation of these genes in the CL as the late estrous cycle progressed. Culture of SLC and MLC with IFNT had no effect on mRNA concentrations for these genes. Interestingly, the MLC model tended to provide greater responses to treatments applied in vitro. This might be explained through interactions in MLC cultures between SLC and LLC, but also with cells in the CL such as endothelial and immune cells. However, in LLC, IFNT upregulated LHR and tended to upregulate VEGF mRNA concentrations. These data are interpreted to mean that pregnancy (i.e., IFNT) stabilizes expression of these genes, which would otherwise become downregulated in response to luteolysis during the estrous cycle. Stabilization of these genes during early pregnancy may contribute to resistance of the CL to luteolysis.
IL6, also known as IFN beta 2, is pro- and anti-inflammatory and pyrogenic and activates B- and T-cells (48). Overexpression of IL6 is associated with several diseases such as rheumatoid arthritis and postmenopausal osteoporosis (52, 81). IL6 has been described in day 15 cultured luteal cells; however, treatment of these cells with progesterone silenced expression of IL6 (82), and IL6 gene expression appears to be silenced in the CL during pregnancy. Pregnancy-associated signals, such as IFNT, may help stabilize IL6 expression in the ovine CL, if progesterone does indeed have inhibitory action. In MLC described herein, culture with PGF and OXT reduced IL6 mRNA concentrations. Stabilization of basal levels of IL6 might be necessary in the CL during early pregnancy. One benefit for continued action of IL6 in the CL is the indirect induction of angiogenesis through inducing VEGF expression (10). VEGF may have a functional role in luteal resistance; therefore IL6 expression could be functioning in luteal resistance and maintenance of the CL by inducing VEGF (see later discussion). It also could be acting synergistically with IFNT to modulate immune responses in the CL during early pregnancy.
Pentraxins are a superfamily of multifunctional proteins that are highly conserved from arthropods to mammals and expressed by several cell types (7, 12). PTX3 has been shown to be present in follicular fluid and plasma (19). PTX3 expression is upregulated in human stromal cells by progesterone and by trophoblast conditioned medium or trophoblast explants (19). It also is upregulated in follicular theca and granulosa cells in response to LH (9). PTX3 expression has been shown to provide resistance to neurodegeneration possibly rescuing neurons from irreversible damage (12, 65). PTX3 double-knockout mice have increased myocardial damage suggesting that PTX3 plays a cardioprotective role (71). These two findings indicate that PTX3 may play a protective role in cells undergoing stress and may contribute to the maintenance of pregnancy by protecting the CL. Stabilization of PTX3 in the CL during pregnancy may be relevant in context of cell survival responses designed for protection against apoptosis and autoimmune responses such as those mounted against cell remnants from antigen-presenting cells (70). The idea that PTX3 is stabilized in the CL of pregnancy is further supported by the fact that PTX3 is downregulated when MLC are cultured with PGF and OXT for 24 h. These data are different than those described by Zalman et al. (88), where PTX3 mRNA concentrations were shown to increase following 4 h culture of MLC with 100 ng/ml PGF. Two primary differences in the design of these experiments were a longer culture period (24 h) and use of lower concentrations of PGF (3.5 ng/ml) in the present in vitro experiments. The enclosed experiments also examine CL at several stages of the late estrous cycle.
LH is required for normal CL function and formation and for the maintenance of the mature CL (38). Infusion of LH has been shown to prolong luteal life (39). Ovine luteal weight, luteal concentration of progesterone, total number of LH receptors, and the number of receptors occupied by LH do not change 7.5 h after injection of PGF (13). However, all of these parameters were affected negatively by 22 h following injection of PGF, which is consistent with decline in LHR mRNA concentrations by day 15 of the estrous cycle in the present studies. The number of occupied, unoccupied and affinity of LHR also do not change on days 12, 16, and 20 of pregnancy. This was later confirmed by Zelinski and coworkers (89) when comparing days 13 to 16 of the estrous cycle to pregnancy (14). However, Smith and coworkers (75) demonstrated that LHR mRNA concentrations were greater during the midluteal phase on days 10–13 compared with earlier or later days of the estrous cycle in sheep CL. A decrease in LHR within 6 h after injection of PGF also was described. This concurs with the decline in LHR mRNA concentrations in CL during the late estrous cycle when analyzed herein by microarray and RT-PCR approaches. It also is consistent with the downregulation of LHR mRNA concentrations when MLC were cultured herein for 24 h with PGF and OXT.
The primary difference in the present studies compared with those previously reported for LHR in ovine CL during pregnancy is the tendency for upregulation of LHR mRNA concentrations on day 16 of pregnancy and the apparent increase in LHR mRNA concentrations in response to culture of MLC with roIFNT. An explanation for this discrepancy might be provided by slight differences in actual day of pregnancy and degree of development of the conceptus across these studies. For example, in the present experiments, day 16 of pregnancy might represent a slightly more advanced conceptus and secretory protein (i.e., IFNT) producing capacity.
IFNT has been implicated in lymphangiogenesis in bovine CL through induction of VEGF (57). VEGF is a multifunctional cytokine that is necessary for follicular growth, enhancing microvascular permeability, angiogenesis, ovulation, and development and function of the CL (5, 60) VEGF protein concentrations steadily decrease from early to late stages of luteolysis (5, 60). This is probably caused through direct action of PGF because day 11 luteal cells cultured with PGF have decreased VEGF mRNA concentrations compared with control, untreated cells (88). This also is consistent with enclosed downregulation of VEGF mRNA concentrations following culture of MLC with luteolytic PGF and OXT. In microarray and RT-PCR results described herein, VEGF mRNA concentrations decreased on day 14 and 15 during the estrous cycle, suggesting that PGF causes a decrease in VEGF mRNA concentrations. VEGF gene expression is tightly associated with other genes that were identified in our microarray such as IL6 and THBS1. IL6 mRNA concentrations were associated with pregnancy and correlated with upregulation of VEGF in the CL. THBS1 was associated with luteolysis and with a downregulation in VEGF. VEGF mRNA concentrations in the ovine CL appear to be associated with pregnancy status, are stabilized in the CL during early pregnancy, and tend to be induced, at least in LLC, following culture with IFNT.
Conclusions
In the absence of a conceptus, spontaneous regression of the CL occurs as a consequence of differential expression of at least 683 genes that include cell adhesion, chemokines, cytoskeletal remodeling, and apoptotic pathways. Two of these genes, SERPINE1 and THBS1, were selected for further study and found to be transiently upregulated during the latter part of the estrous cycle and during early luteolysis. These genes may play a role in regulation of the extracellular matrix (33, 40) to facilitate invasion of immune cells and inhibition of the synthesis of progesterone. Future experiments may focus on the TGFB pathway, which had 19 genes differentially expressed during luteolysis and may provide a link between SERPINE1 and THBS1 action in the early regressing CL.
Until recently, early pregnancy was described to be maintained through exclusive paracrine action of the conceptus on the endometrium in ruminants. Based on enclosed microarray data describing differential expression of 55 genes in CL between P and NP ewes on day 12, 21 genes between days 12 and 14 of pregnancy, and 734 genes between P and NP ewes on day 14, it is concluded that pregnancy-associated gene expression occurs in the CL. Several conceptus secretory products might be driving this differential gene expression in the CL, but a primary candidate based on induction of ISGs by pregnancy and through culture of isolated luteal cells is IFNT. This conclusion is supported by upregulation of several ISGs in the CL based on microarray data, two of which, ISG15 and MX1 were more extensively examined in the CL by RT-PCR approaches. Neither of these ISGs was affected by culture of SLC, LLC, or MLC with PGE2, which is interpreted to suggest that luteotrophic action of PGE2 is not mediated via ISGs.
The maintenance of a healthy conceptus is a complicated process that involves cell proliferation, differentiation and continued activation of the steroidogenic pathway for continued production of progesterone (IL6, VEGFA, and LHR), as well as regulation of immune responses and activation of interferon signaling (PTX3, ISG15, and MX1). Pregnancy stabilizes ISG15, MX1, IL6, PTX3, LHR, and VEGFA, whereas luteolysis causes downregulation of these genes. Conceptus-derived IFNT maintains/induces IL6, VEGFA, LHR, ISG15, MX1, and PTX3 in vivo. In vitro, PGF and OXT suppressed all of these genes in MLC. In addition to providing protection or resistance of the CL to lytic action of PGF, the endocrine actions of IFNT may prime the maternal innate immune system for more rapid and robust antiviral responses to protect the embryo and early developing fetus from disease or infection.
GRANTS
This project was supported by the Agriculture and Food Research Initiative Competitive Grant 2011-67015-20067 and National Needs Fellowship Grant 2010-38420-20397. The University of Nebraska Microarray core receives partial support from the National Center for Research Resources (5P20RR-016469, RR-018788-08) and the National Institute for General Medical Science (NIGMS) (8P20GM-103471, GM-103471-09). This publication's contents are the sole responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or NIGMS.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.J.R., A.Q.A., J.S.D., and T.R.H. conception and design of research; J.J.R., A.Q.A., N.P.S., B.T.W., F.Y., and T.R.H. performed experiments; J.J.R., A.Q.A., and F.Y. analyzed data; J.J.R., A.Q.A., J.S.D., and T.R.H. interpreted results of experiments; J.J.R. and T.R.H. prepared figures; J.J.R. drafted manuscript; J.J.R., A.Q.A., N.P.S., B.T.W., F.Y., J.S.D., and T.R.H. edited and revised manuscript; J.J.R., A.Q.A., N.P.S., B.T.W., F.Y., J.S.D., and T.R.H. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We express our gratitude to Dr. Hans Mayan for conducting elutriation of luteal cells over the years and to the staff and students at the Animal Reproduction and Biotechnology Laboratory for kind assistance with animal husbandry and surgical and necropsy procedures.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Antoniazzi AQ, Webb BT, Romero JJ, Ashley RL, Smirnova NP, Henkes LE, Bott RC, Oliveira JF, Niswender GD, Bazer FW, Hansen TR. Endocrine delivery of interferon tau protects the corpus luteum from prostaglandin F2 alpha-induced luteolysis in ewes. Biol Reprod 88: 144, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong LC, Bjorkblom B, Hankenson KD, Siadak AW, Stiles CE, Bornstein P. Thrombospondin 2 inhibits microvascular endothelial cell proliferation by a caspase-independent mechanism. Mol Biol Cell 13: 1893–1905, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin KJ, Carr AL, Pru JK, Hearne CE, George EL, Belden EL, Hansen TR. Localization of ISG15 and conjugated proteins in bovine endometrium using immunohistochemistry and electron microscopy. Endocrinology 145: 967–975, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Austin KJ, Ward SK, Teixeira MG, Dean VC, Moore DW, Hansen TR. Ubiquitin cross-reactive protein is released by the bovine uterus in response to interferon during early pregnancy. Biol Reprod 54: 600–606, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Berisha B, Schams D, Kosmann M, Amselgruber W, Einspanier R. Expression and tissue concentration of vascular endothelial growth factor, its receptors, and localization in the bovine corpus luteum during estrous cycle and pregnancy. Biol Reprod 63: 1106–1114, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bott RC, Ashley RL, Henkes LE, Antoniazzi AQ, Bruemmer JE, Niswender GD, Bazer FW, Spencer TE, Smirnova NP, Anthony RV, Hansen TR. Uterine vein infusion of interferon tau (IFNT) extends luteal life span in ewes. Biol Reprod 82: 725–735, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Braunschweig A, Jozsi M. Human pentraxin 3 binds to the complement regulator c4b-binding protein. PLoS One 6: e23991, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charleston B, Stewart HJ. An interferon-induced Mx protein: cDNA sequence and high-level expression in the endometrium of pregnant sheep. Gene 137: 327–331, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Christenson LK, Gunewardena S, Hong X, Spitschak M, Baufeld A, Vanselow J. Research resource: preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol Endocrinol 27: 1153–1171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 271: 736–741, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Davis TL, Bott RC, Slough TL, Bruemmer JE, Niswender GD. Progesterone inhibits oxytocin- and prostaglandin F2alpha-stimulated increases in intracellular calcium concentrations in small and large ovine luteal cells. Biol Reprod 82: 282–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deban L, Jaillon S, Garlanda C, Bottazzi B, Mantovani A. Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res 343: 237–249, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Diekman MA, O'Callaghan PO, Nett TM, Niswender GD. Effect of prostaglandin F2alpha on the number of LH receptors in ovine corpora lutea. Biol Reprod 19: 1010–1013, 1978 [DOI] [PubMed] [Google Scholar]
- 14.Diekman MA, O'Callaghan PO, Nett TM, Niswender GD. Validation of methods and quantification of luteal receptors for LH throughout the estrous cycle and early pregnancy in ewes. Biol Reprod 19: 999–1009, 1978 [DOI] [PubMed] [Google Scholar]
- 15.Diskin MG, Morris DG. Embryonic and early foetal losses in cattle and other ruminants. Reprod Domest Anim 43, Suppl 2: 260–267, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Esemuede N, Lee T, Pierre-Paul D, Sumpio BE, Gahtan V. The role of thrombospondin-1 in human disease. J Surg Res 122: 135–142, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Fitz TA, Mayan MH, Sawyer HR, Niswender GD. Characterization of two steroidogenic cell types in the ovine corpus luteum. Biol Reprod 27: 703–711, 1982 [DOI] [PubMed] [Google Scholar]
- 18.Flohr F, Schneider-Schaulies S, Haller O, Kochs G. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett 463: 24–28, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Garlanda C, Maina V, Martinez de la Torre Y, Nebuloni M, Locati M. Inflammatory reaction and implantation: the new entries PTX3 and D6. Placenta 29, Suppl B: 129–134, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Geary TW. Management strategies to reduce embryonic loss. Proc Range Beef Cow Symposium XIX October 3rd: 78–87, 2005 [Google Scholar]
- 21.Gifford CA, Racicot K, Clark DS, Austin KJ, Hansen TR, Lucy MC, Davies CJ, Ott TL. Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. J Dairy Sci 90: 274–280, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Gray CA, Abbey CA, Beremand PD, Choi Y, Farmer JL, Adelson DL, Thomas TL, Bazer FW, Spencer TE. Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biol Reprod 74: 383–394, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1). J Cell Physiol 210: 807–818, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo N, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res 57: 1735–1742, 1997 [PubMed] [Google Scholar]
- 25.Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis 3: 147–158, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Han H, Austin KJ, Rempel LA, Hansen TR. Low blood ISG15 mRNA and progesterone levels are predictive of non-pregnant dairy cows. J Endocrinol 191: 505–512, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Hansen PJ, Anthony RV, Bazer FW, Baumbach GA, Roberts RM. In vitro synthesis and secretion of ovine trophoblast protein-1 during the period of maternal recognition of pregnancy. Endocrinology 117: 1424–1430, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Hansen TR, Henkes LK, Ashley RL, Bott RC, Antoniazzi AQ, Han H. Endocrine actions of interferon-tau in ruminants. Soc Reprod Fertil Suppl 67: 325–340, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Henderson KM, Scaramuzzi RJ, Baird DT. Simultaneous infusion of prostaglandin E2 antagonizes the luteolytic action of prostaglandin F2alpha in vivo. J Endocrinol 72: 379–383, 1977 [DOI] [PubMed] [Google Scholar]
- 30.Henkes LE, Sullivan BT, Lynch MP, Kolesnick R, Arsenault D, Puder M, Davis JS, Rueda BR. Acid sphingomyelinase involvement in tumor necrosis factor alpha-regulated vascular and steroid disruption during luteolysis in vivo. Proc Natl Acad Sci USA 105: 7670–7675, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horisberger MA, Staeheli P, Haller O. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc Natl Acad Sci USA 80: 1910–1914, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou X, Arvisais EW, Jiang C, Chen DB, Roy SK, Pate JL, Hansen TR, Rueda BR, Davis JS. Prostaglandin F2alpha stimulates the expression and secretion of transforming growth factor B1 via induction of the early growth response 1 gene (EGR1) in the bovine corpus luteum. Mol Endocrinol 22: 403–414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Border WA, Lawrence DA, Noble NA. Noninhibitory PAI-1 enhances plasmin-mediated matrix degradation both in vitro and in experimental nephritis. Kidney Int 70: 515–522, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Inskeep EK, Smutny WJ, Butcher RL, Pexton JE. Effects of intrafollicular injections of prostaglandins in non-pregnant and pregnant ewes. J Anim Sci 41: 1098–1104, 1975 [DOI] [PubMed] [Google Scholar]
- 35.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 6: 41–48, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Johnson GA, Spencer TE, Hansen TR, Austin KJ, Burghardt RC, Bazer FW. Expression of the interferon tau inducible ubiquitin cross-reactive protein in the ovine uterus. Biol Reprod 61: 312–318, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Kaltenbach CC, Graber JW, Niswender GD, Nalbandov AV. Effect of hypophysectomy on the formation and maintenance of corpora lutea in the ewe. Endocrinology 82: 753–759, 1968 [DOI] [PubMed] [Google Scholar]
- 39.Karsch FJ, Roche JF, Noveroske JW, Foster DL, Norton HW, Nalbandov AV. Prolonged maintenance of the corpus luteum of the ewe by continuous infusion of luteinizing hormone. Biol Reprod 4: 129–136, 1971 [DOI] [PubMed] [Google Scholar]
- 40.Khan KM, Falcone DJ. Role of laminin in matrix induction of macrophage urokinase-type plasminogen activator and 92-kDa metalloproteinase expression. J Biol Chem 272: 8270–8275, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Kliem H, Welter H, Kraetzl WD, Steffl M, Meyer HH, Schams D, Berisha B. Expression and localisation of extracellular matrix degrading proteases and their inhibitors during the oestrous cycle and after induced luteolysis in the bovine corpus luteum. Reproduction 134: 535–547, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol 12: 634–640, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Magness RR, Huie JM, Hoyer GL, Huecksteadt TP, Reynolds LP, Seperich GJ, Whysong G, Weems CW. Effect of chronic ipsilateral or contralateral intrauterine infusion of prostaglandin E2 (PGE2) on luteal function of unilaterally ovariectomized ewes. Prostaglandins Med 6: 389–401, 1981 [DOI] [PubMed] [Google Scholar]
- 44.Mapletoft RJ, Del Campo MR, Ginther OJ. Local venoarterial pathway for uterine-induced luteolysis in cows. Proc Soc Exp Biol Med 153: 289–294, 1976 [DOI] [PubMed] [Google Scholar]
- 45.Margosio B, Marchetti D, Vergani V, Giavazzi R, Rusnati M, Presta M, Taraboletti G. Thrombospondin 1 as a scavenger for matrix-associated fibroblast growth factor 2. Blood 102: 4399–4406, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Maroni D, Davis JS. TGFB1 disrupts the angiogenic potential of microvascular endothelial cells of the corpus luteum. J Cell Sci 124: 2501–2510, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaughlin JN, Mazzoni MR, Cleator JH, Earls L, Perdigoto AL, Brooks JD, Muldowney JA, 3rd, Vaughan DE, Hamm HE. Thrombin modulates the expression of a set of genes including thrombospondin-1 in human microvascular endothelial cells. J Biol Chem 280: 22172–22180, 2005 [DOI] [PubMed] [Google Scholar]
- 48.McWaters P, Hurst L, Chaplin PJ, Collins RA, Wood PR, Scheerlinck JP. Characterisation of monoclonal antibodies to ovine interleukin-6 and the development of a sensitive capture ELISA. Vet Immunol Immunopathol 73: 155–165, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Mirochnik Y, Kwiatek A, Volpert OV. Thrombospondin and apoptosis: molecular mechanisms and use for design of complementation treatments. Curr Drug Targ 9: 851–862, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mondal M, Schilling B, Folger J, Steibel JP, Buchnick H, Zalman Y, Ireland JJ, Meidan R, Smith GW. Deciphering the luteal transcriptome: potential mechanisms mediating stage-specific luteolytic response of the corpus luteum to prostaglandin F2α. Physiol Genomics 43: 447–456, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Moor RM, Rowson LE. The corpus luteum of the sheep: effect of the removal of embryos on luteal function. J Endocrinol 34: 497–502, 1966 [DOI] [PubMed] [Google Scholar]
- 52.Muller-Newen G, Kuster A, Hemmann U, Keul R, Horsten U, Martens A, Graeve L, Wijdenes J, Heinrich PC. Soluble IL-6 receptor potentiates the antagonistic activity of soluble gp130 on IL-6 responses. J Immunol 161: 6347–6355, 1998 [PubMed] [Google Scholar]
- 53.Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem 270: 2109–2119, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Niswender GD. Influence of the site of conjugation on the specificity of antibodies to progesterone. Steroids 22: 413–424, 1973 [DOI] [PubMed] [Google Scholar]
- 55.Niswender GD, Davis TL, Griffith RJ, Bogan RL, Monser K, Bott RC, Bruemmer JE, Nett TM. Judge, jury and executioner: the auto-regulation of luteal function. Soc Reprod Fertil Suppl 64: 191–206, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev 80: 1–29, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Nitta A, Shirasuna K, Haneda S, Matsui M, Shimizu T, Matsuyama S, Kimura K, Bollwein H, Miyamoto A. Possible involvement of IFNT in lymphangiogenesis in the corpus luteum during the maternal recognition period in the cow. Reproduction 142: 879–892, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Nor JE, Mitra RS, Sutorik MM, Mooney DJ, Castle VP, Polverini PJ. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J Vasc Res 37: 209–218, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Oliveira JF, Henkes LE, Ashley RL, Purcell SH, Smirnova NP, Veeramachaneni DN, Anthony RV, Hansen TR. Expression of interferon (IFN)-stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-tau release from the uterine vein. Endocrinology 149: 1252–1259, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Otani N, Minami S, Yamoto M, Shikone T, Otani H, Nishiyama R, Otani T, Nakano R. The vascular endothelial growth factor/fms-like tyrosine kinase system in human ovary during the menstrual cycle and early pregnancy. J Clin Endocrinol Metab 84: 3845–3851, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Palade G. Intracellular aspects of the process of protein synthesis. Science 189: 347–358, 1975 [DOI] [PubMed] [Google Scholar]
- 62.Pletneva LM, Haller O, Porter DD, Prince GA, Blanco JC. Interferon-inducible Mx gene expression in cotton rats: cloning, characterization, and expression during influenza viral infection. J Interferon Cytokine Res 26: 914–921, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Pratt BR, Butcher RL, Inskeep EK. Antiluteolytic effect of the conceptus and of PGE2 in ewes. J Anim Sci 45: 784–791, 1977 [DOI] [PubMed] [Google Scholar]
- 64.Pru JK, Lynch MP, Davis JS, Rueda BR. Signaling mechanisms in tumor necrosis factor alpha-induced death of microvascular endothelial cells of the corpus luteum. Reprod Biol Endocrinol 1: 17, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ravizza T, Moneta D, Bottazzi B, Peri G, Garlanda C, Hirsch E, Richards GJ, Mantovani A, Vezzani A. Dynamic induction of the long pentraxin PTX3 in the CNS after limbic seizures: evidence for a protective role in seizure-induced neurodegeneration. Neuroscience 105: 43–53, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Rege TA, Stewart J, Jr, Dranka B, Benveniste EN, Silverstein RL, Gladson CL. Thrombospondin-1-induced apoptosis of brain microvascular endothelial cells can be mediated by TNF-R1. J Cell Physiol 218: 94–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rempel LA, Francis BR, Austin KJ, Hansen TR. Isolation and sequence of an interferon-tau-inducible, pregnancy- and bovine interferon-stimulated gene product 15 (ISG15)-specific, bovine ubiquitin-activating E1-like (UBE1L) enzyme. Biol Reprod 72: 365–372, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Ren B, Yee KO, Lawler J, Khosravi-Far R. Regulation of tumor angiogenesis by thrombospondin-1. Biochim Biophys Acta 1765: 178–188, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Reynolds LP, Stigler J, Hoyer GL, Magness RR, Huie JM, Huecksteadt TP, Whysong GL, Behrman HR, Weems CW. Effect of PGE1 on PGF2 alpha-induced luteolysis in nonbred ewes. Prostaglandins 21: 957–972, 1981 [DOI] [PubMed] [Google Scholar]
- 70.Rovere P, Peri G, Fazzini F, Bottazzi B, Doni A, Bondanza A, Zimmermann VS, Garlanda C, Fascio U, Sabbadini MG, Rugarli C, Mantovani A, Manfredi AA. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood 96: 4300–4306, 2000 [PubMed] [Google Scholar]
- 71.Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, Pasqualini F, Latini R, Garlanda C, Mantovani A. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 117: 1055–1064, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Silvia WJ, Niswender GD. Maintenance of the corpus luteum of early pregnancy in the ewe. III. Differences between pregnant and nonpregnant ewes in luteal responsiveness to prostaglandin F2 alpha. J Anim Sci 59: 746–753, 1984 [DOI] [PubMed] [Google Scholar]
- 74.Smith GW, Gentry PC, Bao B, Long DK, Roberts RM, Smith MF. Control of extracellular matrix remodelling within ovarian tissues: localization and regulation of gene expression of plasminogen activator inhibitor type-1 within the ovine corpus luteum. J Reprod Fertil 110: 107–114, 1997 [DOI] [PubMed] [Google Scholar]
- 75.Smith GW, Gentry PC, Roberts RM, Smith MF. Ontogeny and regulation of luteinizing hormone receptor messenger ribonucleic acid within the ovine corpus luteum. Biol Reprod 54: 76–83, 1996 [DOI] [PubMed] [Google Scholar]
- 76.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Spencer TE, Becker WC, George P, Mirando MA, Ogle TF, Bazer FW. Ovine interferon-tau inhibits estrogen receptor up-regulation and estrogen-induced luteolysis in cyclic ewes. Endocrinology 136: 4932–4944, 1995 [DOI] [PubMed] [Google Scholar]
- 78.Spencer TE, Burghardt RC, Johnson GA, Bazer FW. Conceptus signals for establishment and maintenance of pregnancy. Anim Reprod Sci 82–83: 537–550, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Spencer TE, Ing NH, Ott TL, Mayes JS, Becker WC, Watson GH, Mirando MA, Brazer FW. Intrauterine injection of ovine interferon-tau alters oestrogen receptor and oxytocin receptor expression in the endometrium of cyclic ewes. J Mol Endocrinol 15: 203–220, 1995 [DOI] [PubMed] [Google Scholar]
- 80.Spencer TE, Stagg AG, Ott TL, Johnson GA, Ramsey WS, Bazer FW. Differential effects of intrauterine and subcutaneous administration of recombinant ovine interferon tau on the endometrium of cyclic ewes. Biol Reprod 61: 464–470, 1999 [DOI] [PubMed] [Google Scholar]
- 81.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol 15: 4971–4979, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Telleria CM, Ou J, Sugino N, Ferguson S, Gibori G. The expression of interleukin-6 in the pregnant rat corpus luteum and its regulation by progesterone and glucocorticoid. Endocrinology 139: 3597–3605, 1998 [DOI] [PubMed] [Google Scholar]
- 83.Toyokawa K, Carling SJ, Ott TL. Cellular localization and function of the antiviral protein, ovine Mx1 (oMx1): I. Ovine Mx1 is secreted by endometrial epithelial cells via an ‘unconventional’ secretory pathway. Am J Reprod Immunol 57: 13–22, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Toyokawa K, Leite F, Ott TL. Cellular localization and function of the antiviral protein, ovine Mx1 (oMx1): II. The oMx1 protein is a regulator of secretion in an ovine glandular epithelial cell line. Am J Reprod Immunol 57: 23–33, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Vinatier D, Dufour P, Subtil D. Apoptosis: a programmed cell death involved in ovarian and uterine physiology. Eur J Obstet Gynecol Reprod Biol 67: 85–102, 1996 [DOI] [PubMed] [Google Scholar]
- 86.Wiltbank MC, Guthrie PB, Mattson MP, Kater SB, Niswender GD. Hormonal regulation of free intracellular calcium concentrations in small and large ovine luteal cells. Biol Reprod 41: 771–778, 1989 [DOI] [PubMed] [Google Scholar]
- 87.Yankey SJ, Hicks BA, Carnahan KG, Assiri AM, Sinor SJ, Kodali K, Stellflug JN, Ott TL. Expression of the antiviral protein Mx in peripheral blood mononuclear cells of pregnant and bred, non-pregnant ewes. J Endocrinol 170: R7–R11, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Zalman Y, Klipper E, Farberov S, Mondal M, Wee G, Folger JK, Smith GW, Meidan R. Regulation of angiogenesis-related prostaglandin f2alpha-induced genes in the bovine corpus luteum. Biol Reprod 86: 92, 2012 [DOI] [PubMed] [Google Scholar]
- 89.Zelinski MB, Selivonchick DP, Stormshak F. Characterization of plasma membrane lipids and luteinizing hormone receptors of ovine corpora lutea during luteolysis and early pregnancy. Biol Reprod 38: 768–779, 1988 [DOI] [PubMed] [Google Scholar]
- 90.Zhu Y, Yin WL, Ba YF, Tian L, Gu ZQ, Zhang MS, Zhong CN. Transforming growth factor-1 promotes the transcriptional activation of plasminogen activator inhibitor type 1 in carcinoma-associated fibroblasts. Mol Med Rep 6: 1001–1005, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.