Abstract
We previously described synaptic currents between baroreceptor fibers and second-order neurons in the nucleus tractus solitarius (NTS) that were larger in Syrian hamsters than in rats. This suggested that although electrical activity throughout the hamster brain decreased as brain temperature declined, the greater synaptic input to its NTS would support continued operation of cardiorespiratory reflexes at low body temperatures. Here, we focused on properties that would protect these neurons against potential damage from the larger synaptic inputs, testing the hypotheses that hamster NTS neurons exhibit: 1) intrinsic N-methyl-d-aspartate receptor (NMDAR) properties that limit Ca2+ influx to a greater degree than do rat NTS neurons and 2) properties that reduce gating signals to NMDARs to a greater degree than in rat NTS neurons. Whole cell patch-clamp recordings on anatomically identified second-order NTS baroreceptive neurons showed that NMDAR-mediated synaptic currents between sensory fibers and second-order NTS neurons were larger in hamsters than in rats at 33°C and 15°C, with no difference in their permeability to Ca2+. However, at 15°C, but not at 33°C, non-NMDAR currents evoked by glutamate released from baroreceptor fibers had significantly shorter durations in hamsters than in rats. Thus, hamster NMDARs did not exhibit lower Ca2+ influx than did rats (negating hypothesis 1), but they did exhibit significant differences in non-NMDAR neuronal properties at low temperature (consistent with hypothesis 2). The latter (shorter duration of non-NMDAR currents) would likely limit NMDAR coincidence gating and may help protect hamster NTS neurons, enabling them to contribute to signal processing at low body temperatures.
Keywords: baroreflex, AMPAR, NMDAR, cold
in syrian hamsters, cardiorespiratory brain stem neurocontrollers appropriately process sensory information over all stages of their hibernation bouts. That is, they are able to adjust perfusion pressure such that oxygen/metabolic supply is matched to demand, and all tissues/organ systems remain viable and function appropriately over the entire bout. Efficient control is not surprising because a selective advantage of hibernation is that adaptations allowing reduction of energy needs enable the animal to survive when food is scarce (5); i.e., if brain stem neurocontrollers did not optimize energy expenditure to reliably maintain homeostasis, “excess” expenditure would reduce the survival advantage of hibernation. Thus, although signal processing in several other brain regions of rodent hibernators appears to be significantly reduced, as illustrated by the flattening of their cortical EEG activity (17, 18, 30) and inactivation of hippocampal cellular mechanisms responsible for formation of new memories (1), brain stem circuits continue to operate effectively.
The finding that mammalian hibernating species, in contrast to nonhibernating species, can tolerate extreme winter environmental conditions without tissue damage (5) has prompted studies aimed at identifying protective adaptations/acclimations (6) that maintain cell viability in hibernators (6, 11–13, 26). Key to survival during hibernation are natural adaptations supporting the continued operation of brain stem cardiorespiratory and thermoregulatory reflexes over a hibernation bout. Notably, during the early stages of entry into hibernation (∼2 h in the Syrian hamster), telemetry recordings (19) showed that the baroreflex dynamically and effectively slowed heart rate, thereby ensuring that blood pressure closely tracked a steadily declining set-point. During torpor, blood pressure slowly rose, raising the possibility that baroreceptor signals encoding this blood pressure increase may be important in signaling the ascending arousal system to terminate torpor (19). During the late stages of arousal, the baroreflex returned overshoots in blood pressure and heart rate to prehibernation levels. However, while telemetry measurements delineated the overall role of the baroreflex during a hibernation bout (19), they did not identify protective cellular adaptations that may sustain signal processing over baroreflex pathways as core temperature declines.
The nucleus tractus solitarius (NTS) is the first brain stem nucleus that receives and integrates peripheral cardiovascular and pulmonary signals. These signals travel over first-order sensory neurons in the tractus solitarius (TS), enter the NTS, and synapse on second-order neurons. The latter play a pivotal role in homeostatic regulation because signal conditioning at these gateway neurons determines the magnitude, pattern, and duration of cardiovascular and respiratory reflex signals transmitted to other brain stem and central sites (27, 40). We previously showed that NTS second-order neurons in Syrian hamsters have specific adaptations, resulting in the amplitude of TS-evoked excitatory postsynaptic currents (EPSCs) being approximately twice those of rat NTS neurons at near euthermia (33°C), as well as at 15°C (37). Thus, even though decreased brain temperature of hibernators is accompanied by reduced neural activity throughout the CNS (17, 18, 22, 23, 30), the hamster NTS can still support cardiorespiratory signal processing at low temperatures (37).
Synaptic transmission between first-order sensory afferents and second-order NTS neurons is mediated by glutamate released from the sensory afferents. This neurotransmitter binds to N-methyl-d-aspartate receptors (NMDARs) and to non-NMDARs on the second-order NTS neurons (2, 4, 8). Depolarizing currents through non-NMDARs not only generate action potentials in second-order NTS neurons, they also affect the NMDAR gate that opens the NMDAR channel to Na+, K+, and Ca2+. Thus, it is possible that there is greater entry of Ca2+ through hamster vs. rat NMDARs. Although “excessive” calcium currents through NMDARs can trigger apoptosis and cell death (28, 35), the hamster neurons do not appear to be damaged, raising the possibility that they possess mechanisms preventing excessive Ca2+ entry (despite greater EPSCs) and/or Ca2+-induced damage, either of which could protect NMDAR signal processing during hibernation bouts.
The first possibility was evaluated in this study, using patch-clamp techniques to test the following two hypotheses: 1) that the second-order NTS baroreceptive neurons in hamsters have intrinsic NMDAR properties that limit Ca2+ influx to a greater degree than do rat NTS neurons; and 2) that these hamster neurons exhibit properties that reduce gating signals to NMDARs (and thus the amount of time the NMDAR channel is open) to a greater degree than do rat NTS neurons.
METHODS
All protocols were approved by the University of California Davis Institutional Animal Care and Use Committee in compliance with the Animal Welfare Act and Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Animals
Male adult Syrian hamsters (Mesocricetus auratus; n = 17; 117 ± 4 g; 14–31 wk of age) from our colony were bred from animals that had been selected over generations to more readily hibernate. They were all provided ad libitum access to food (Lab Diet 5001 Rodent Diet) and water, and 14 were continually housed at room temperature, 22 ± 2°C, on a 14:10 light-dark (LD) cycle. Three of the 17 hamsters were transferred to a 6 ± 1°C vivarium on an 8:16 LD photoperiod for 1 to 4 wk, during which time they entered hibernation. During the 3rd or 4th bout of hibernation (when body temperature was ∼8°C), these hamsters were used for slice experiments. Male adult Sprague-Dawley rats (n = 8; 474 ± 21 g; 15–20 wk from Charles River, Wilmington, MA) served as nonhibernating mammalian controls.
Labeling Terminal Endings of Aortic Depressor Nerve Fibers
To identify second-order NTS baroreceptive neurons, we anesthetized the animals with intramuscular injections of ketamine (100 mg/kg for hamsters; 50 mg/kg for rats) plus xylazine (10 mg/kg for both hamsters and rats). As previously described (7, 36, 37), the hamster was placed on a heating pad, and core temperature was monitored and maintained at 37°C, while a 4–5-mm segment of each aortic depressor nerve was isolated and placed on parafilm. The crystal form of the fluorescent tracer 1,1′-dilinoleyl-3,3,3′,3 tetra-methylindocarbo-cyanine, 4-chlorobenzene-sulfonate [FAST DiI solid; DiI Δ 9,12-C18 (7)] was placed on each nerve. The crystals and nerve segments were then coated with polyvinylsiloxane gel. The animals were allowed to recover for 2 wk before preparing slices for electrophysiological experiments or before transferring them to the cold vivarium. By 2 wk, DiI had diffused along the membrane of baroreceptor neurons (i.e., over their cell bodies in the nodose and jugular ganglia and their fibers that joined and then branched from the TS to form synapses with second-order NTS neurons). While DiI uniformly labels the baroreceptor neuron via lateral diffusion in the plasma membrane, it does not diffuse across the synapse and label second-order NTS neurons (29).
Brain Stem Slice Preparation, Temperature Control, Identification of Second-Order NTS Neurons
As in our previous studies (7, 9, 36, 37), animals were decapitated, and the brain was rapidly submerged in cold (<4°C) high-sucrose artificial cerebrospinal fluid (aCSF) that contained (in mM final concentration): 3 KCl, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 220 sucrose, and 2 CaCl2 and was continuously aerated with 95% O2-5% CO2. Brain stem coronal slices (250 μm thick) containing the intermediate to caudal NTS (0 to 1,250 μm caudal to obex) were cut with a VT 1000S vibrating microtome (Leica, Richmond, IL). After sectioning, slices from rats and euthermic hamsters were incubated for 45 min at 37°C in the high-sucrose aCSF, and slices from hibernating hamsters were incubated under the same conditions except that the solution was at room temperature (23°C). After incubation, slices from hamsters and rats were placed in normal aCSF containing (in mM final concentration): 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose, and 2 CaCl2, continuously aerated with 95% O2-5% CO2, and maintained at room temperature.
For each experiment, a single slice was transferred from the incubation chamber to the recording chamber, held in place with a silk mesh, and continuously perfused with oxygenated normal aCSF at a rate of ∼4 ml/min. The temperature controller consisted of an inner tube that delivered oxygenated aCSF to the recording chamber and an outer tube ∼10 cm in length connected at each end via tubing that allowed switching to one of two circulating water baths. Each water bath was set at a fixed temperature. This concentric tube arrangement (inner tube for aCSF delivery and outer water-filled jacket for heating or cooling) was within 10 cm of the recording chamber. A temperature probe was positioned in the recording chamber to measure aCSF temperature and to ensure that recordings in each slice were obtained for a bath temperature of either 15 ± 1°C or 33 ± 1°C.
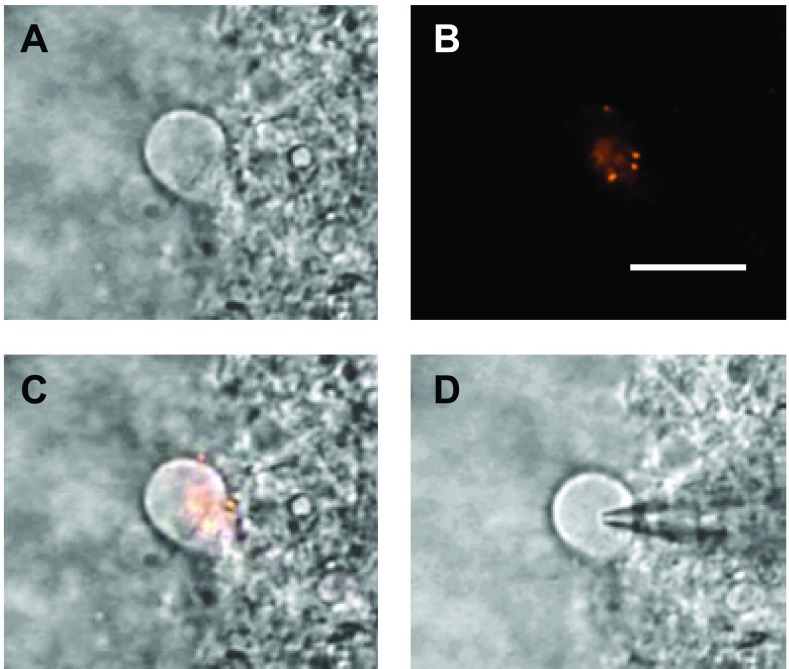
Neurons in the medial NTS were visualized using infrared differential interference contrast imaging with a charge-coupled device camera (OLY-105; Olympus America, Center Valley, PA) for display on a TV monitor and a video-encoding software (Winnov, Sunnyvale, CA) for storage in a PC computer. The second-order NTS baroreceptive neurons were identified by detecting fluorescent boutons on them as visualized with an optical filter set for DiI (U-MWIGA 3, Olympus) and an image-integrating system (InstaGater; Dage-MTI, Michigan City, IN). An example of a labeled second-order NTS hamster neuron is shown in Fig. 1. All recordings in this study were made in this class of visualized second-order NTS baroreceptive neurons.
Fig. 1.
Example of anatomical identification of a second-order nucleus tractus solitarius (NTS) baroreceptive neuron from a hibernating hamster. A: NTS neuron viewed under infrared differential interference contrast imaging. B: same neuron viewed under fluorescence to visualize presynaptic baroreceptor afferent fiber terminal boutons. C: overlay of A and B. D: patch electrode attached to the same cell for whole cell recording. Scale bar = 10 μm.
Once visualized, whole cell currents were recorded using borosilicate glass pipettes (2.2–5.0 MΩ; 4.1 ± 0.1 MΩ) filled with a cesium fluoride-based solution. The seal resistance was >1 GΩ, and the series resistance was <34 MΩ (17.1 ± 0.6 MΩ) and was compensated by 50–70%. Recordings were made with the MultiClamp 700B amplifier (Axon Instruments, Foster City, CA). Signals were filtered at 2 kHz, digitized at 10 kHz with the DigiData 1322A interface (Axon Instruments), and stored in an IBM-compatible computer. Data were analyzed off-line (including construction of I-V plots, and determination of decay constants of TS-evoked EPSCs) using pCLAMP9.2 software (Axon Instruments).
Protocols
After establishing the whole cell configuration, the membrane potential was held at −60 mV while determining the whole cell input resistance from hyperpolarizing current injections (200 ms, 10–30 mV).
Ca2+/Cs+ permeability ratios of NMDA receptors.
To determine Ca2+/Cs+ permeability ratios, the pipette solution contained (in mM final concentration): 145 CsF, 1 MgCl2, 3 Mg-ATP, 0.2 Na-GTP, 10 EGTA, and 10 HEPES; pH was adjusted to 7.4 with CsOH. Normal aCSF extracellular solution was replaced with a Na+- and K+-free extracellular solution containing (in mM final concentration): 104 Tris·HCl, 52 N-methylglucamine, 1 MgCl2, 10 HEPES, 10 glucose, and 2 CaCl2. By omitting K+ and reducing Na+ to a minimum (0.2 Na-GTP to maintain cell viability), transmembrane currents through NMDARs were primarily Ca2+ and Cs+ currents.
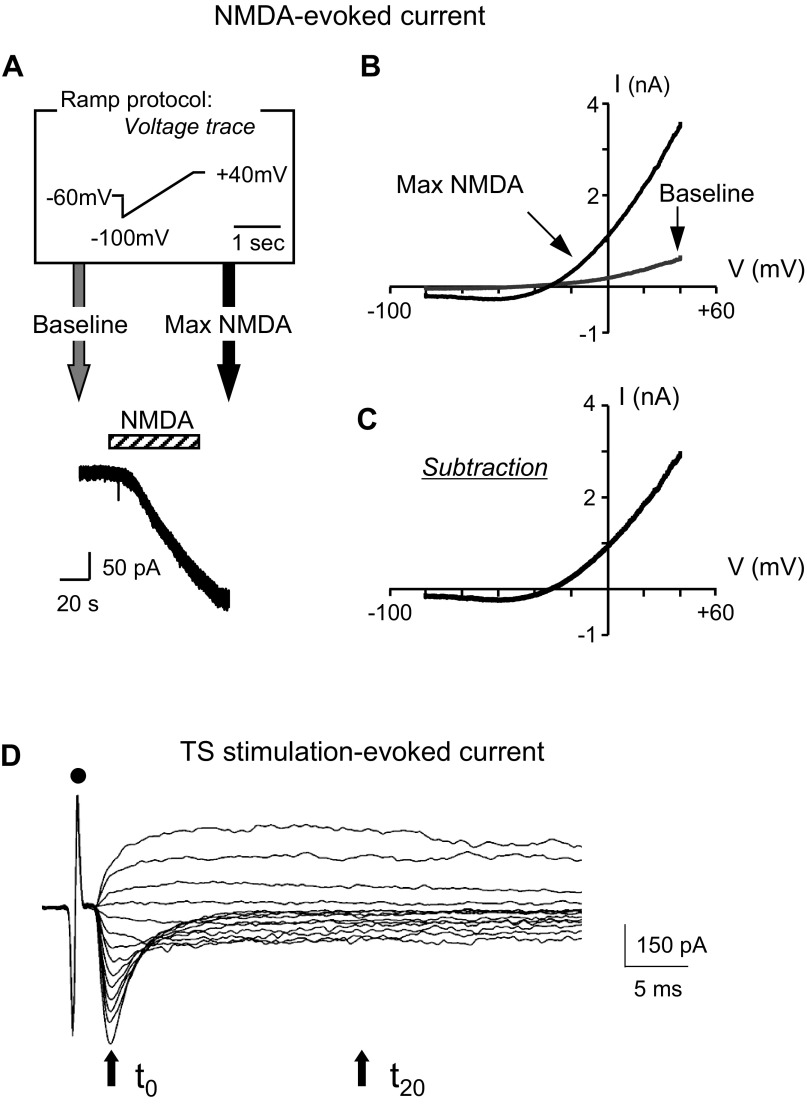
To determine Ca2+/Cs+ ratios, a voltage ramp was applied before and during slice perfusion with NMDA, an NMDAR agonist (Fig. 2A). Specifically, after perfusion with the Na+- and K+-free extracellular solution for at least 3 min, a baseline I-V plot was obtained using a 1.5-s voltage ramp from an initial potential of −100 mV to a final potential of +40 mV (Fig. 2A). This baseline I-V plot (Fig. 2B) was obtained immediately prior to perfusion of the slice with NMDA (Fig. 2A). The membrane voltage was then clamped at −60 mV, and the slice was perfused for 1 min (cross-hatched bar in Fig. 2A) with 0.3 mM NMDA added to the extracellular solution to induce an inward NMDAR current. Once the inward current had reached a maximum and became level (Fig. 2A), a second ramp-generated I–V plot was obtained (denoted Max NMDA I-V plot in Fig. 2B). The voltage dependence of an NMDA-induced current was determined by subtracting the baseline I-V plot (recorded in the absence of NMDA) from the max NMDA I-V plot (recorded in the presence of NMDA) (Fig. 2C). To estimate the permeability of NMDAR channels to Ca2+, the constant-field equation (20) was used as a first approximation: PCa/PCs = ([Cs+]i/[Ca2+]o) exp(EF/RT)[exp(EF/RT) + 1]/4, where E is the reversal potential [the voltage at which NMDAR current is zero, a value measured at the intersection of the I-V curve with the voltage axis on I-V plots (Fig. 2C)], F is Faraday's constant, R is the gas constant, and T is the absolute temperature. PCa and PCs represent the permeability coefficients of Ca2+ and Cs+, respectively.
Fig. 2.
N-methyl-d-aspartate (NMDA) perfusion and TS stimulation protocols used to construct I-V plots. A, top: ramp protocol (in box). Bottom: example trace of a whole cell recording at a −60-mV holding potential. The ramp protocol was used just before (gray arrow) NMDA application (0.3 mM, 1 min) and again at the maximum NMDA response (black arrow). The bar above the current trace indicates NMDA application. NMDA evoked a slowly increasing inward (plotted downward) current that leveled off, at which time a second ramp voltage was applied (black arrow). The bath solution was Na+- and K+-free. B: using the ramp protocol sketched in A, a control I-V plot before NMDA application (gray line, baseline) and one at the maximum NMDA response (black line, max NMDA) are shown. C: subtraction current from traces in B. At membrane potentials more negative than −40 mV, currents were inward and relatively constant. At depolarizing potentials more positive than −30 mV, currents were outward and increased with membrane voltage depolarization. D: example traces of TS-evoked excitatory postsynaptic currents (EPSCs) at various voltages (−90 mV to +40 mV) from an NTS neuron of an euthermic hamster. From the tractus solitarius-evoked responses, current-voltage (I-V) plots were constructed at two times: at the peak (t0) and 20 ms after the peak (t20). Dot indicates stimulus artifact.
TS-evoked NTS responses and I-V plots.
The pipette solution contained (in mM final concentration): 145 CsF, 5 NaCl, 1 MgCl2, 3 Mg-ATP, 0.2 Na-GTP, 10 EGTA, and 10 HEPES; pH was adjusted to 7.4 with CsOH at room temperature (23°C). Stimulating voltages (7–20 V, 0.1-ms square wave pulses) were delivered through bipolar tungsten electrodes (1-μm tips separated by 80 μm; FHC, Bowdoin, ME) to the TS ipsilateral to the recording site with a microelectrode stimulator (SD9; Grass Instruments, Quincy, MA). Stimulus intensity was just above the lowest amplitude for evoking maximal currents. TS-evoked responses were measured with the membrane potential clamped at 10-mV increments from a voltage range of −90 mV to +40 mV (Fig. 2D). From the TS-evoked responses, current-voltage (I-V) plots were constructed at two times: 1) at the peak of the large inward current obtained with the voltage clamped at −90 mV (the peak denoted by the arrow at time t0 in Fig. 2D) and 2) 20 ms after this peak (denoted by the arrow at time t20 in Fig. 2D). These times were chosen because previous studies on rat NTS neurons at 22°C showed that the peak component was due primarily to current through non-NMDAR channels, while at 20 ms after the peak, the current was due primarily to NMDAR currents (2). At 22°C, this separation of the net current into non-NMDAR and NMDAR components (with minimal overlap of components by t20) was validated for rat NTS neurons by the use of ionotropic glutamate receptor blockers (2). Moreover, the I-V plot constructed at time t0 had the linear shape consistent with non-NMDARs, while the I-V plot constructed at time t20 had the rectified shape characteristic of NMDARs [reflecting an ionic block of the NMDAR channel at hyperpolarizing voltages (2, 31)].
Data analysis.
Data are expressed as means ± SE. A two-way repeated-measures ANOVA (RM-ANOVA) was used for comparing I-V relationships with species as the between factor and voltage as the within factor. A two-way ANOVA was used to compare reversal potential, calcium permeability, and decay time constant of non-NMDAR-mediated currents with species as one factor and temperature as the other factor. Fisher's least significant difference post hoc test was performed when appropriate. Significance was set at P < 0.05.
Drugs.
We purchased DiI from Molecular Probes (Eugene, OR), ketamine and xylazine from Vedco (St. Joseph, MO), polyvinylsiloxane gel from Charles Laboratories (Rockville Center, NY), and CsF, Mg-ATP, Na-GTP, EGTA, HEPES, and CaCl2 from Sigma (St. Louis, MO). All other chemicals were obtained from Fisher (Fair Lawn, NJ).
RESULTS
Whole-cell recordings were obtained from 158 neurons identified by fluorescent attached boutons (Fig. 1). Input resistance at 33°C averaged 935 ± 131 MΩ in euthermic hamsters (n = 35) and did not differ from that of rats (698 ± 141 MΩ; n = 33) (t-test, P > 0.05). At 15°C, the input resistance averaged 879 ± 154 MΩ in euthermic hamsters (n = 48), 1,050 ± 167 MΩ in hibernating hamsters (n = 13), and 950 ± 146 MΩ in rats (n = 55). There was no significant difference among these values (one-way ANOVA, P > 0.05).
Intrinsic properties of NMDARs (Ca2+/Cs+ permeability ratio and coincidence gate).
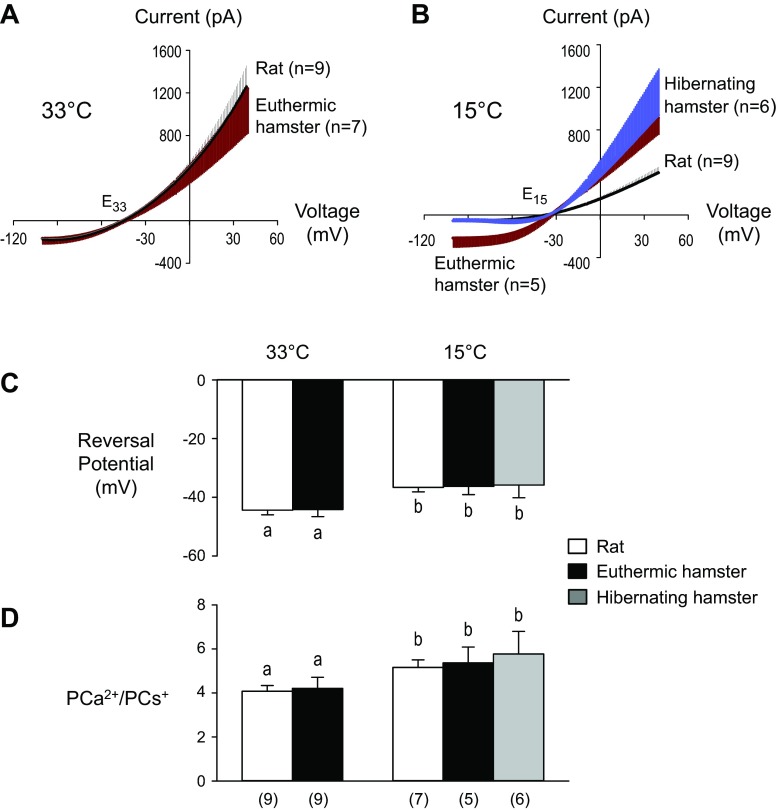
To examine the intrinsic properties of NMDARs without the confounding effects of non-NMDAR channel currents, we obtained data for pairs of I-V plots before and after NMDA perfusion (Fig. 2B). The difference between the pair of I-V plots for a given neuron (Fig. 2C) was taken as the I-V plot for NMDARs activated by the exogenous NMDA added to the slice. Group data for rats and hamsters (Figs. 3, A and B) show that I-V plots retained their rectified shape when temperature was lowered from 33°C to 15°C. Specifically, these nonlinear I-V plots indicate that at both temperatures (33°C and 15°C), NMDAR channel conductance in hamster and rat neurons was relatively low when the neuron was hyperpolarized and relatively high when it was depolarized. At 33°C, the I-V relationship of the NMDA-induced current of the hamster neurons did not differ from that of the rats (Fig. 3A). But when temperature was lowered from 33°C to 15°C, these currents were dramatically reduced in the rat neurons (by ∼69%) and to a lesser extent (by ∼21%) in the hamster neurons (Fig. 3B), indicating that hamster NMDAR channels can continue to conduct substantial ionic currents at the lower temperature. Moreover, intrinsic gating properties of NMDARs failed to display protective adaptations/acclimations in the hamster that were not present in the rat, as evidenced by the fact that hamster I-V plots showed that the NMDAR coincidence gate in hamster and rat slices responded similarly to external signals (glutamate and depolarization), opening NMDAR channels at 15°C and at 33°C. Thus, intrinsic properties of the hamster NMDAR do not appear to have adaptations that limit either the gating of its channel or the net current through the channel.
Fig. 3.
I-V plots and their reversal potentials used to calculate NMDA receptor (NMDAR) PCa2+/PCs+ ratios. A: group data showing I-V relationships of NMDA-induced current at 33°C. Vertical lines are standard errors and are plotted on the upper side of the I-V plot for rats and the lower side for hamsters. Reversal potentials for both rat and hamster I-V plots were near E33. There was no difference in the I-V plots of euthermic hamsters and rats [two-way repeated-measures (RM)-ANOVA, species P > 0.05, voltage P < 0.001, interaction P > 0.05]. B: group data showing I-V relationships of NMDA-induced current at 15°C. Vertical lines are standard errors. Reversal potentials of I-V plots were near E15. NMDA-induced currents were larger in hamsters (both hibernating and euthermic) vs. rats at membrane voltages greater than 7 mV (two-way RM-ANOVA, species P > 0.05, voltage P < 0.001, interaction P < 0.001). C: comparison of reversal potentials. Although the reversal potential shifted to a more depolarizing potential at 15°C vs. 33°C, there was no species difference at either temperature (two-way ANOVA, species P > 0.05, temperature, P = 0.002, interaction P > 0.05). Bars sharing the same letter do not significantly differ [Fisher's least significant difference (LSD) test]. D: Ca2+/Cs+ ratios calculated from reversal potentials of I-V plot for NMDA-induced currents. NMDAR permeability to Ca2+ was significantly higher at 15°C than at 33°C, but there were no species differences at either temperature (two-way ANOVA, species P > 0.05, temperature P = 0.041, interaction P > 0.05). Bars sharing the same letter do not significantly differ (Fisher's LSD test). Numbers in parentheses indicate sample size for the cells in C and D (same cells).
The NMDA-evoked responses at 15°C in slices from hibernating hamsters were compared with slices from nonhibernating hamsters (Fig. 3B). Positive to the reversal potential (E15 for all I-V plots in Fig. 3B), there was no difference between hibernating and nonhibernating hamster I-V plots, although currents were larger for both hamster groups than for the rats.
To determine whether hamster NMDAR channels, once opened, were less permeable to Ca2+ ions than those of rats, we first measured reversal potentials (i.e., the intersections of the I-V curves for rats and hamsters, as they crossed the voltage axis near E33 and E15 in Fig. 3, A and B, respectively) and plotted them in a bar graph (Fig. 3C). At 33°C and 15°C, I-V plots in Fig. 3, A and B that had reversal potentials near E33 and E15, respectively, did not significantly differ, although they were more negative at 33°C vs. 15°C (Fig. 3C). For each reversal potential, a permeability ratio PCa/PCs was calculated using the constant field equation, and our statistical analysis identified no significant species differences at either 33°C or at 15°C (Fig. 3D). The failure to see a greater decrease in the Ca2+/Cs+ permeability ratio in hamsters than in rats at the lower temperature (Fig. 3D) and the larger currents in hamsters than in rats at 15°C (Fig. 3B) indicate that hamsters do not have protective adaptations/acclimations that specifically limit Ca2+ influx through their NMDAR channels.
The Ca2+/Cs+ permeability ratios were greater at 15°C vs. 33°C (Fig. 3D). The small temperature-induced increase in Ca2+/Cs+ permeability ratio was associated with a small shift in reversal potential toward a more depolarized membrane potential in both hamsters and rats (Fig. 3D). Taken together, Fig. 3 shows that, compared with rat NMDARs, hamster NMDARs did not have any intrinsic adaptations/acclimations limiting gating, markedly reducing net channel current, or becoming more impermeable to Ca2+ when temperature was lowered from 33°C to 15°C.
NMDAR and non-NMDAR Currents Evoked by TS Stimulation
Experiments with bath application of NMDA and ramp depolarization resulted in activation only of NMDARs, but TS stimulation results in release of glutamate and activation of both NMDARs and non-NMDARs. In experiments described above (Fig. 3), the exogenous application of NMDA activated NMDARs over the entire cell, including extrasynaptic NMDARs on the plasma membrane and NMDARs at synapses not excited by TS stimulation. To counteract the possibility that these experiments might fail to detect localized changes affecting available NMDARs or changes in non-NMDARs at synapses between baroreceptor fibers and second-order NTS neurons, we recorded TS-evoked responses (the sum of currents through both NMDAR and non-NMDAR channels) in voltage-clamp configuration.
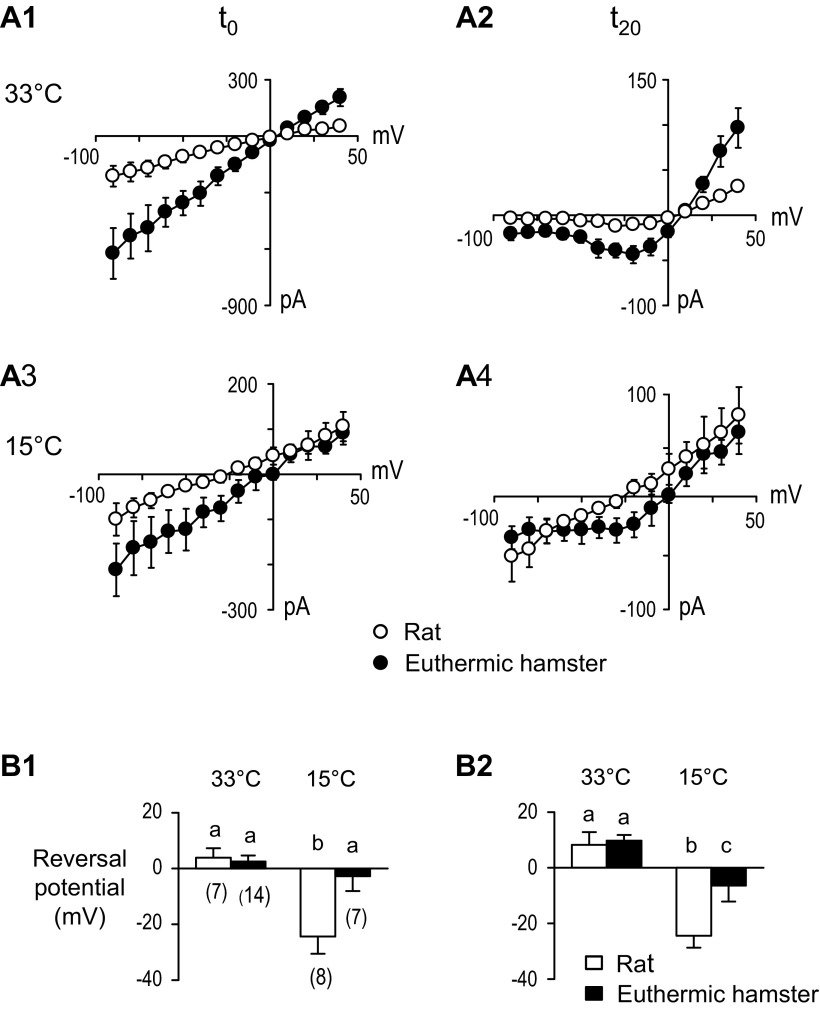
The extent to which NMDAR and non-NMDAR currents contributed to the net current at the synapse is illustrated in Fig. 4. At 33°C, I-V plots constructed at t0 (Fig. 4, A1) and t20 (Fig. 4, A2) had significantly larger currents in hamster than in rat neurons. In both species, the I-V plots at t0 were linear, while the I-V plots at t20 were nonlinear. Because the exogenous NMDA-induced currents through NMDARs always had rectified I-V plots in both species at 33°C and 15°C (Fig. 3), we associated the nonlinear, rectified I-V plots at t20 with NMDARs, and the linear I-V plots at t0 with non-NMDARs. These findings suggest that adaptations did not reduce the number of NMDARs available for activation at the hamster synapse. Comparing the earlier (t0) I-V plot in A1 with the later (t20) I-V plot in A2 along the upper row in Fig. 4 shows that the net current was composed of component currents with different temporal properties: that is, following TS stimulation, non-NMDARs opened first, reached a peak at t0, and then closed, while NMDARs opened more slowly and were still conducting appreciable current 20 ms later at t20.
Fig. 4.
TS-evoked EPSCs and reversal potentials at t0 (left column A1, A3, and B1) and at t20 (right column A2, A4, and B2). A1: Current-voltage (I-V) plots at peak current (t0) at 33°C show greater current amplitudes for hamsters than rats (two-way RM-ANOVA, P < 0.001 for both voltage and interaction). A2: I-V plots at 20 ms after peak (t20) at 33°C show greater current amplitudes for hamsters than rats (two-way RM-ANOVA, P < 0.001 for both voltage and interaction). A3: I-V plots t0 at 15°C show greater inward (downward) current amplitudes for hamsters than rats (two-way RM-ANOVA, P < 0.001 for voltage and P < 0.05 for species). A4: I-V plots at t20 at 15°C show no clear outward rectification for rats (two-way RM-ANOVA, P < 0.01 for voltage). B1: reversal potentials for t0 show a shift to more negative membrane voltages in rats than in hamsters at 15°C (two-way ANOVA, species P = 0.022, temperature P < 0.001, interaction P = 0.010). Bars sharing the same letter do not significantly differ [Fisher's least significant difference (LSD) test]; numbers in parenthesis indicate sample size. B2: in the same cells as B1, reversal potentials for t20 show a greater shift to more negative membrane voltages in rats than hamsters at 15°C (two-way ANOVA, species P = 0.018, temperature P < 0.001, interaction P = 0.043). Bars sharing the same letter do not significantly differ (Fisher's LSD test).
In experiments at 15°C, plots A3 and A4 on the lower row of Fig. 4A show that TS-evoked responses were significantly larger in hamster than in rat neurons at t0, and the linear shape of the plots (Fig. 4A3) indicates that non-NMDARs were again the first to open following TS stimulation. Later, in time, at t20 (Fig. 4A4), the hamster I-V plot had a nonlinear, rectified waveform of NMDARs. In contrast, the rat I-V plot at t20 (Fig. 4A4) was linear, indicating that 20 ms after its initial peak, the non-NMDAR channel had still not closed. The data suggest that the temporal response of non-NMDARs to glutamate at the synapse was more prolonged in the rat than in the hamster neuron, degrading signal processing at 15°C.
There was a significantly greater shift in the rat reversal potential to a more hyperpolarized membrane potential when temperature decreased from 33°C to 15°C (Fig. 4, B1 and B2). Since at both t0 and t20, rat I-V plots were linear, the shift in reversal potential suggests ion permeability changes in non-NMDAR channels.
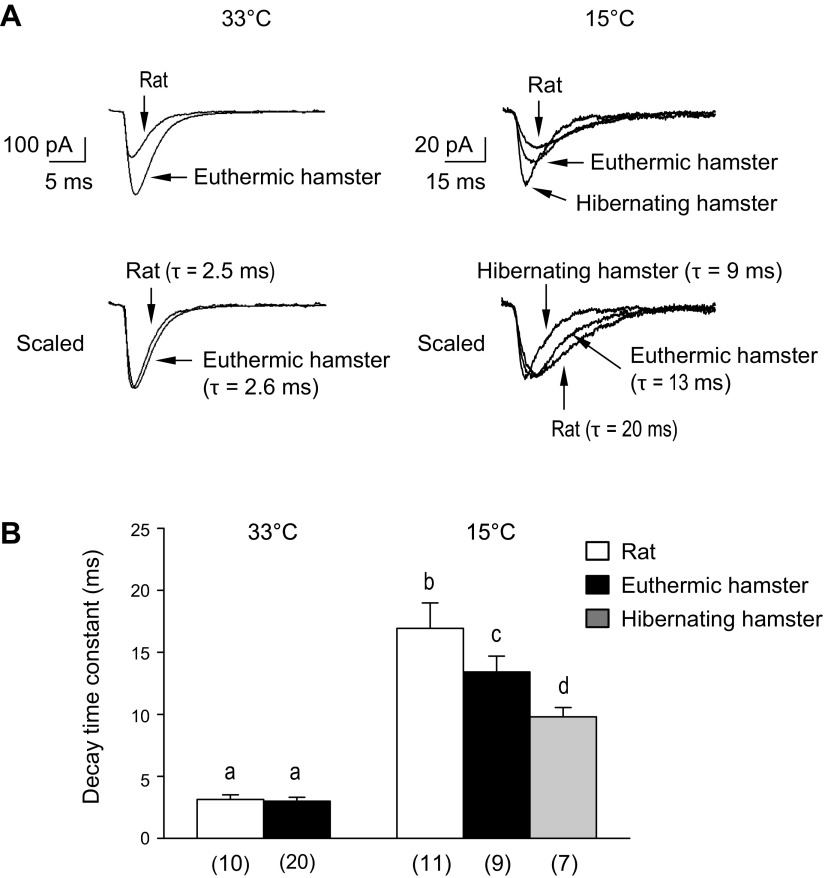
Decay Rates of TS-Evoked non-NMDAR EPSCs
I-V plots (Fig. 4A4) imply that at 15°C, non-NMDARs remained open longer in rats than in hamsters following single-shock TS stimulation. That this was the case is shown by example traces (Fig. 5A) and group data (Fig. 5B). When temperature decreased from 33°C to 15°C, all sample traces (Fig. 5A) exhibited decreased amplitude and prolonged time constant for the decay of current, consistent with the general effect at low temperatures, i.e., slower kinetics. At 33°C, rats had smaller non-NMDAR currents than did euthermic hamsters, and, as shown by normalizing currents to facilitate comparison of decay constants (scaling peak amplitudes to 1), the time constants were not different. At 15°C, the decay time constant of non-NMDAR EPSCs in rats was significantly longer than that in euthermic and hibernating hamsters (Fig. 5A). As summarized by group data in Fig. 5B, there was no difference in the decay time constants of non-NMDAR EPSCs of hamsters vs. rats at 33°C, but at 15°C, the decay time constant of non-NMDAR EPSCs in rats was significantly longer than that in both euthermic and hibernating hamsters. Because inward currents through non-NMDARs lead to postsynaptic depolarization under in vivo conditions, the shorter decay would reduce postsynaptic depolarization and limit NMDAR gating.
Fig. 5.
TS-evoked non-NMDAR currents at holding potential of −60 mV. A: traces showing inward (downward) non-NMDAR current following TS stimulation. For each temperature, the amplitude of the peak current in the bottom traces was normalized to 1.0. The recovery phase of the current was fitted with a single exponential curve, from which its decay time constant (τ) was obtained. B: group data of TS-evoked decay time constants. At 33°C, there were no differences between rats and euthermic hamsters, but at 15°C, the decay time constant was significantly larger in rats compared with both euthermic and hibernating hamsters (two-way ANOVA, species P = 0.002, temperature P < 0.001, interaction P > 0.05). Bars sharing the same letter do not significantly differ (Fisher's LSD test). Numbers in parenthesis indicate sample size.
DISCUSSION
In this study, we evaluated two mechanisms that could protect hamster NTS second-order neurons with their large synaptic inputs (compared to those of rats) against potential Ca2+ induced damage—namely, adaptations of hamster NMDARs that would limit Ca2+ influx (i.e., adaptations intrinsic to the NMDARs and consistent with hypothesis 1) and/or adaptations in the signaling from non-NMDARs to the coincidence gate in the NMDAR, which could also limit Ca2+ influx (i.e., adaptations extrinsic to the NMDAR and consistent with hypothesis 2). With respect to hypothesis 1, we found no significant differences in Ca2+ permeability of NMDARs in the hamster vs. rat neurons at either 33°C or 15°C. Nor did hamster NMDARs exhibit any adaptations in their gate that limited Ca2+ influx. That is, no intrinsic adaptations in NMDARs themselves were identified in support of hypothesis 1. In contrast, there was a significant reduction in the duration of non-NMDAR-mediated current in response to TS stimulation at 15°C in the hamster compared with the rat. This adaptation, extrinsic to the NMDAR, would reduce the duration of NMDAR gating, limiting Ca2+ influx, and protecting the NTS neurons, a finding consistent with hypothesis 2. Moreover, although at 15°C, the TS-evoked response of non-NMDARs had a shortened duration, the depolarizing response was sufficient to reliably generate action potentials in hamster NTS neurons (37) and enable support of cardiovascular reflexes. Taken together, our data show that when temperature decreased from 33°C to 15°C, both NMDARs and non-NMDARs in hamster NTS neurons functioned well, remaining responsive to afferent signals.
Ca2+ Influx Through NMDARs
Our data showing that, at 33°C and 15°C, hamster NMDARs are functional [TS-evoked responses in Fig. 4 show rectified I-V plots (2, 31)] are consistent with the presence of NR1 subunits in the NTS of nonhibernating Arctic ground squirrels that imply NMDARs are functional (41). Further, our finding that there are no species differences in the Ca2+/Cs+ permeability ratios of euthermic hamster vs. rat NMDARs in NTS neurons at 33°C or 15°C (Fig. 3) shows that NMDARs remain permeable to Ca2+ at both temperatures.
Because NMDA-induced currents reflect NMDARs distributed over the cell membrane and at a variety of synapses, we more closely examined the synapse between first-order sensory fibers and second-order NTS neurons. Previous studies have demonstrated that the synaptic strength at this synapse was at least twice as large in Syrian hamsters (37) than in several nonhibernating species, including rats (2, 37), primates (38), guinea pigs (36), and mice (32). Several mechanisms may contribute to the localized enhanced synaptic strength (large TS-evoked EPSCs) at this synapse, including increased presynaptic glutamate release, increased number of synaptic contacts, decreased glutamate reuptake, and/or receptor redistribution from extrasynaptic to synaptic loci. [NMDAR redistribution is associated with neuroprotection in rat hippocampal neurons (15); i.e., activation of synaptic NMDARs upregulated hippocampal brain-derived neurotrophic factor (BDNF) and promoted cell survival, while activation of extrasynaptic NMDA receptors downregulated hippocampal BDNF]. The alternative that neuroprotection in hamster NTS neurons is achieved simply by a large reduction in the number of available NMDARs at the synapse and that non-NMDARs take over the whole task of responding to baroreceptor signals proved not to be the case (Fig. 4). Although at 15°C, TS-evoked currents in NMDARs were attenuated compared with those at 33°C, they were still appreciable (Fig. 4), and NMDAR channels were still permeable to Ca2+ (Fig. 3). Thus, when body temperature of the hamsters dropped, protection was not achieved simply by removal of most NMDARs from the synapse via internalization from the plasma membrane (34) or by lateral translocation to extra-synaptic sites on the plasma membrane. Our finding that synaptic NMDAR currents, albeit attenuated, were observed in Syrian hamsters at 15°C is consistent with studies on nonhibernating species showing that some level of NMDAR activity is neuroprotective (14, 15).
Further, our single-cell experiments showing hamster NMDARs remained functional at 15°C are consistent with in vivo results in other hibernating species at even lower temperatures. For example, blockage of NMDARs by intraperitoneal injection of the NMDAR antagonist MK-801 induced arousal in both golden-mantled ground squirrels (16) and Arctic ground squirrels (21) suggesting that NMDARs are associated with neural networks maintaining the animals in torpor.
Depolarizing non-NMDAR Currents and Their Relationship to NMDAR Currents at 15°C (Near Torpor)
The NMDAR coincidence gate of second-order NTS neurons opens in response to depolarization acting in concert with glutamate released from first-order neurons binding non-NMDARs as well as NMDARs. Under in vivo conditions, non-NMDARs are closely associated with NMDAR gating (3) because non-NMDAR-depolarizing currents alter the transmembrane potential at nearby NMDARs. Schurr (35) has reviewed studies in nonhibernating species of the association of non-NMDARs (primarily AMPARs) and NMDARs and effects on one of the first few steps in the apoptotic cascade—i.e., reduced Ca2+ influx as a result of limiting non-NMDAR depolarizing currents could profoundly alter later steps in the cascade and reduce cell death. More recently, Besancon et al. (3) considered how minimizing other types of depolarizing currents could also affect NMDAR gating.
Our data show that at 15°C, the hamster has evolved a potential protective adaptation that limits the duration of cell depolarization by non-NMDARs. In in vivo conditions, this novel non-NMDAR adaptation would alter gating at nearby NMDARs and provide an added margin of safety for the second-order neurons by limiting Ca2+ influx and lowering the risk of initiating apoptosis. While lowering temperature prolonged the decay time of non-NMDAR-depolarizing currents in both rats and hamsters (Fig. 5), at 15°C, the decay time constant of the non-NMDAR-evoked response to TS stimulation was longer in rats than in hamsters, and euthermic hamsters had longer time constants than did hibernating hamsters (Fig. 5). The species difference likely reflects a natural adaptation that becomes evident at low temperature, while the differences between neurons from euthermic vs. hibernating hamsters indicates that preparation for hibernation (short-photoperiod and cold exposure) and/or torpor itself is associated with additional shortening of the decay time. Slowing of channel kinetics at low temperatures is a common finding [e.g., it has been shown to occur in AMPARs in the rat calyx of Held (33)]. In a previous study (37), we found that while lowering temperature lengthened the duration of action potentials, as expected, the action potentials were significantly shorter in hamsters than in rats, another hamster NTS neuron adaptation that shortens the duration of a depolarizing current. Thus, at 15°C, the hamster NTS neuron actually has two adaptations, one affecting non-NMDAR depolarizing currents and a second resulting in more compact action potentials. Together, these would reduce the net time that NTS neurons are depolarized following synaptic excitation, thereby limiting NMDAR gating.
The physiological role of the linkage between non-NMDA and NMDA cell depolarization has been most thoroughly studied as it relates to hippocampal neuroplasticity (10) in hibernating, as well as in nonhibernating species. The observation that robust long-term potentiation can be evoked in Syrian hamsters by tetanus of Schaffer collaterals at temperatures above, but not below, ∼20°C has been attributed to decreased non-NMDAR depolarizing currents and a failure to open a sufficient number of NMDAR coincidence gates (1, 24, 25, 39). That NMDARs themselves remain functional is suggested by the finding that increasing extracellular Ca2+ concentration lowers the temperature threshold for evoking long-term potentiation (24). This same situation appears to prevail in the hamster NTS.
Rat non-NMDAR depolarizing currents are longer and drawn out at 15°C, as evidenced by decay constants (Fig. 5). The left-shifted reversal potentials for non-NMDARs in the rat to a more negative voltage indicates a decrease in inward currents or an increase in outward currents, or both, when temperature decreased from 33°C to 15°C. Although these long non-NMDAR rat currents and shift in reversal potentials prolong depolarizing currents and degrade signal transmission, the temperatures at which this occurs are well below the rat's physiological temperature range.
In contrast, 15°C is well within the physiological range of a hamster entering or arousing from hibernation. Despite their brevity, action potentials in hamster second-order NTS neurons are reliably generated by TS stimulation at 15°C (37). Moreover, TS stimulation evokes larger EPSCs in both non-NMDAR and NMDAR EPSCs in hamsters than in rats at 15°C (as well as at 33°C) (Fig. 4). Thus, as temperature declines and the hamster enters deeper into hibernation, TS signals may continue to evoke non-NMDAR and NMDAR EPSCs, albeit with decreased amplitudes at lower temperatures.
Perspectives and Significance
Previous telemetry studies of successive hibernation bouts showed that the Syrian hamster baroreflex operates over a wide range of body temperatures (19), and this study extends these systemic findings to the cellular level by examining a key synapse on the baroreflex pathway. Signal transmission at the glutamatergic synapse between hamster baroreceptors and second-order NTS neurons was supported by enhanced non-NMDAR and NMDAR responses at both 33°C and 15°C. In addition, we identified an adaptation that shortened the duration of non-NMDAR-depolarizing currents at the synapse and thus could provide an added margin of safety (by limiting NMDAR gating), while supporting signal fidelity and conserving energy (since shorter-duration currents reduce the metabolic energy required to maintain ionic homeostasis). Further studies are needed to determine whether 1) the adaptations at this synapse occur at other glutamatergic synapses in the chain of neurons sustaining cardiorespiratory reflexes and 2) if second-order NTS neurons in hibernating species have additional protective adaptations [such as those found in hippocampal neurons (11–13, 26)] that increase their tolerance to oxygen and oxygen/glucose deprivation.
GRANTS
This study was supported by National Institutes of Health Heart, Lung and Blood Institute Grant R01 HL-091763.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.-i.S., B.A.H., J.M.H., and C.-Y.C. conception and design of research; S.-i.S. performed experiments; S.-i.S., J.M.H., and C.-Y.C. analyzed data; S.-i.S., B.A.H., J.M.H., and C.-Y.C. interpreted results of experiments; S.-i.S. and C.-Y.C. prepared figures; S.-i.S., J.M.H., and C.-Y.C. drafted manuscript; S.-i.S., B.A.H., J.M.H., and C.-Y.C. edited and revised manuscript; S.-i.S., B.A.H., J.M.H., and C.-Y.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The initial breeders for the hamster colony used in this study were generous gifts from Dr. John R. Willis when he was at the University of Illinois. The authors thank Jock Hamilton and Sat Chau for their technical assistance.
REFERENCES
- 1.Arant RJ, Goo MS, Gill PD, Nguyen Y, Watson KD, Hamilton JS, Horowitz JM, Horwitz BA. Decreasing temperature shifts hippocampal function from memory formation to modulation of hibernation bout duration in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 301: R438–R447, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Aylwin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. J Neurophysiol 77: 2539–2548, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci 29: 268–275, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bonham AC, Chen CY. Glutamatergic neural transmission in the nucleus tractus solitarius: N-methyl-d-aspartate receptors. Clin Exp Pharmacol Physiol 29: 497–502, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Carey HV, Martin SL, Horwitz BA, Yan L, Bailey SM, Podrabsky J, Storz JF, Ortiz RM, Wong RP, Lathrop DA. Elucidating nature's solutions to heart, lung, and blood diseases and sleep disorders. Circ Res 110: 915–921, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CY, Bechtold AG, Tabor J, Bonham AC. Exercise reduces GABA synaptic input onto nucleus tractus solitarii baroreceptor second-order neurons via NK1 receptor internalization in spontaneously hypertensive rats. J Neurosci 29: 2754–2761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CY, Bonham AC. Non-NMDA and NMDA receptors transmit area postrema input to aortic baroreceptor neurons in NTS. Am J Physiol Heart Circ Physiol 275: H1695–H1706, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Chen CY, Horowitz JM, Bonham AC. A presynaptic mechanism contributes to depression of autonomic signal transmission in NTS. Am J Physiol Heart Circ Physiol 277: H1350–H1360, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Collingridge GL, Randall AD, Davies CH, Alford S. The synaptic activation of NMDA receptors and Ca2+ signalling in neurons. Ciba Found Symp 164: 162–171, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem 102: 1713–1726, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drew KL, Harris MB, LaManna JC, Smith MA, Zhu XW, Ma YL. Hypoxia tolerance in mammalian heterotherms. J Exp Biol 207: 3155–3162, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab 18: 168–175, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci 26: 81–89, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5: 405–414, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Harris MB, Milsom WK. Is hibernation facilitated by an inhibition of arousal? In: Life in the Cold, edited byHeldmaier G, Klingenspor M. Berlin: Springer-Verlag, 2000, p. 241–250 [Google Scholar]
- 17.Heller HC. Hibernation: neural aspects. Annu Rev Physiol 41: 305–321, 1979 [DOI] [PubMed] [Google Scholar]
- 18.Horowitz JM, Horrigan DJ. Hibernation in mammals:central nervous system fuctions. In: Handbook of Physiology, Environmental Physiology, sect 4: vol. 1, edited by Freglly MJ, Blatteis CM. Bethesda MD: Am Physiol Soc, 1996, p. 533–539 [Google Scholar]
- 19.Horwitz BA, Chau SM, Hamilton JS, Song C, Gorgone J, Saenz M, Horowitz JM, Chen CY. Temporal relationships of blood pressure, heart rate, baroreflex function, and body temperature change over a hibernation bout in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 305: R759–R768, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iino M, Ozawa S, Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol 424: 151–165, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jinka TR, Rasley BT, Drew KL. Inhibition of NMDA-type glutamate receptors induces arousal from torpor in hibernating arctic ground squirrels (Urocitellus parryii). J Neurochem 122: 934–940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilduff TS, Miller JD, Radeke CM, Sharp FR, Heller HC. 14C-2-deoxyglucose uptake in the ground squirrel brain during entrance to and arousal from hibernation. J Neurosci 10: 2463–2475, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilduff TS, Sharp FR, Heller HC. [14C]2-deoxyglucose uptake in ground squirrel brain during hibernation. J Neurosci 2: 143–157, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krelstein MS, Horowitz JM. Tetanus during a high extracellular calcium pulse overrides the block of long-term potentiation seen at 20°C in the hamster hippocampal slice. Brain Res 536: 105–113, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Krelstein MS, Thomas MP, Horowitz JM. Thermal effects on long-term potentiation in the hamster hippocampus. Brain Res 520: 115–122, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Lewis CJ, Becker JJ, Manis AD, Hamilton JS, Horowitz JM, Horwitz BA. Neuroprotection supports signal processing in the hippocampus of Syrian hamsters, a facultative hibernator. Neurosci Lett 520: 20–25, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Loewy AD. Central autonomic pathways. In: Central Regulation of Autonomic Functions, edited by Loewy AD, Spyer KM. New York: Oxford University Press, 1990, p. 88–103 [Google Scholar]
- 28.Lutz PL. Mechanisms for anoxic survival in the vertebrate brain. Annu Rev Physiol 54: 601–618, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labeled aortic baroreceptor terminals on neurons from the nucleus tractus solitarius. Brain Res 581: 339–343, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Mihailovic LT. Cortical and subcortical electrical activity in hibernation and hypothermia. In: Hibernation and Hypothermia, Perpectives and Challenges, edited by South FE, Hannon J, Willis JR, Pengelley ET, Alpert NR. Amsterdam: Elsevier, 1972, p. 487–532 [Google Scholar]
- 31.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307: 462–465, 1984 [DOI] [PubMed] [Google Scholar]
- 32.Ohi Y, Ishii Y, Haji A, Noguchi S, Sasaoka T, Fujimori T, Nabeshima Y, Sasahara M, Hattori Y. Platelet-derived growth factor (PDGF)-BB inhibits AMPA receptor-mediated synaptic transmission via PDGF receptor-β in murine nucleus tractus solitarius. Brain Res 1159: 77–85, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Postlethwaite M, Hennig MH, Steinert JR, Graham BP, Forsythe ID. Acceleration of AMPA receptor kinetics underlies temperature-dependent changes in synaptic strength at the rat calyx of Held. J Physiol 579: 69–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci 4: 794–802, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Schurr A. Neuroprotection against ischemic/hypoxic brain damage: blockers of ionotropic glutamate receptor and voltage sensitive calcium channels. Curr Drug Targets 5: 603–618, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Sekizawa S, Bechtold AG, Tham RC, Bonham AC. A novel postsynaptic group II metabotropic glutamate receptor role in modulating baroreceptor signal transmission. J Neurosci 29: 11807–11816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekizawa S, Horowitz JM, Horwitz BA, Chen CY. Realignment of signal processing within a sensory brainstem nucleus as brain temperature declines in the Syrian hamster, a hibernating species. J Comp Physiol A 198: 267–282, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekizawa S, Joad JP, Pinkerton KE, Bonham AC. Secondhand tobacco smoke exposure differentially alters nucleus tractus solitarius neurons at two different ages in developing non-human primates. Toxicol Appl Pharmacol 242: 199–208, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Spangenberger H, Nikmanesh FG, Igelmund P. Long-term potentiation at low temperature is stronger in hippocampal slices from hibernating Turkish hamsters compared to warm-acclimated hamsters and rats. Neurosci Lett 194: 127–129, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Spyer KM. The central nervous organization of reflex circulatory control. In: Central Regulation of Autonomic Functions, edited by Loewy AD, Spyer KM. New York: Oxford University Press, 1990, p. 168–188 [Google Scholar]
- 41.Zhao HW, Christian SL, Castillo MR, Bult-Ito A, Drew KL. Distribution of NMDA receptor subunit NR1 in arctic ground squirrel central nervous system. J Chem Neuroanat 32: 196–207, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]