Abstract
We have previously observed that many of the renal and hemodynamic adaptations seen in normal pregnancy can be induced in virgin female rats by chronic systemic vasodilation. Fourteen-day vasodilation with sodium nitrite or nifedipine (NIF) produced plasma volume expansion (PVE), hemodilution, and increased renal medullary phosphodiesterase 5A (PDE5A) protein. The present study examined the role of the renin-angiotensin-aldosterone system (RAAS) in this mechanism. Virgin females were treated for 14 days with NIF (10 mg·kg−1·day−1 via diet), NIF with spironolactone [SPR; mineralocorticoid receptor (MR) blocker, 200–300 mg·kg−1·day−1 via diet], NIF with losartan [LOS; angiotensin type 1 (AT1) receptor blocker, 20 mg·kg−1·day−1 via diet], enalapril (ENAL; angiotensin-converting enzyme inhibitor, 62.5 mg/l via water), or vehicle (CON). Mean arterial pressure (MAP) was reduced 7.4 ± 0.5% with NIF, 6.33 ± 0.5% with NIF + SPR, 13.3 ± 0.9% with NIF + LOS, and 12.0 ± 0.4% with ENAL vs. baseline MAP. Compared with CON (3.6 ± 0.3%), plasma volume factored for body weight was increased by NIF (5.2 ± 0.4%) treatment but not by NIF + SPR (4.3 ± 0.3%), NIF + LOS (3.6 ± 0.1%), or ENAL (4.0 ± 0.3%). NIF increased PDE5A protein abundance in the renal inner medulla, and SPR did not prevent this increase (188 ± 16 and 204 ± 22% of CON, respectively). NIF increased the α-subunit of the epithelial sodium channel (α-ENaC) protein in renal outer (365 ± 44%) and inner (526 ± 83%) medulla, and SPR prevented these changes. There was no change in either PDE5A or α-ENaC abundance vs. CON in rats treated with NIF + LOS or ENAL. These data indicate that the PVE and renal medullary adaptations in response to chronic vasodilation result from RAAS signaling, with increases in PDE5A mediated through AT1 receptor and α-ENaC through the MR.
Keywords: α-subunit of the epithelial sodium channel, arterial underfilling, phosphodiesterase 5A
renal sodium and water retention are required to support the plasma volume expansion (PVE) of a healthy pregnancy (2). In women, plasma volume (PV) expands ∼40% and is necessary for perfusion of the growing uterus, placenta, and fetus (4). Inadequate volume expansion is associated with pregnancies compromised by fetal growth restriction (3, 31). Furthermore, prevention of PVE through restriction of either sodium or water intake during late pregnancy in the rat results in growth-restricted pups (28, 30).
Although the mechanisms of increased sodium reabsorption in pregnancy are still largely unknown, recent work highlights the importance of the collecting duct. We have reported increased abundance and activity of phosphodiesterase 5A (PDE5A) in the inner medulla of the normal pregnant kidney (24). Increased PDE5A activity in the inner medulla blunts the cGMP-mediated natriuretic actions of atrial natriuretic peptide (ANP) and nitric oxide (NO) in the pregnant rat (15, 24, 33). Furthermore, chronic inhibition of PDE5A with sildenafil in the pregnant rat attenuates renal sodium retention and PVE (32). This local increase of PDE5A in the renal medulla permits selective loss of the natriuretic responses to ANP and NO, whereas the cortical vasodilatory actions are maintained.
In addition, we recently examined the protein profile of the major sodium transporters/channels along the renal tubule during pregnancy and found that the only protein consistently upregulated throughout gestation was the α-subunit of the epithelial sodium channel (α-ENaC) (38). ENaC is a heteromultimeric channel composed of three homologous subunits (α, β, γ), with α-ENaC being the rate-limiting subunit for channel formation (21). We also found that ENaC blockade with benzamil abolished the PVE in late pregnancy (38). These data taken together emphasize the importance of collecting duct adaptations in mediating the renal sodium retention and volume expansion of normal pregnancy.
We have previously observed that many of the renal and hemodynamic adaptations that occur in normal pregnancy can also be induced in virgin female rats by chronic systemic vasodilation. Chronic (14-day) systemic vasodilation with the calcium channel blocker nifedipine (NIF) and the NO donor NaNO2 produced PVE and hemodilution and increased medullary PDE5A (10). One aim of the present study was to analyze the response of the distal nephron apical sodium transporters, α-ENaC in particular, during chronic systemic vasodilation. Furthermore, because sodium retention in the collecting duct is under tight regulation by angiotensin II (ANG II) and aldosterone, an additional aim of this study was to determine if the vasodilation-mediated changes required an intact renin-angiotensin-aldosterone system (RAAS).
METHODS
Animal experiments.
Animal experiments were carried out using virgin female Sprague-Dawley rats (Harlan Laboratories) at the University of Florida in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Protocols were reviewed and approved by the University of Florida Institutional Animal Care and Use Committee. Rats were anesthetized with isoflurane (IsoFlo; Abbott Laboratories, North Chicago, IL), and telemetry transmitters (Data Sciences, St. Paul, MN) were implanted in the left femoral artery. Rats were allowed 7–10 days to recover from surgery before data acquisition. Mean arterial pressure (MAP) was recorded continuously for 5 min every 30 min as described (32). After baseline blood pressure measurements were obtained, rats were treated for 14 days with NIF (calcium channel blocker, 10 mg·kg−1·day−1 via diet; Sigma) (10), NIF with spironolactone [SPR; mineralocorticoid receptor (MR) blocker, 200–300 mg·kg−1·day−1 via diet; Sigma] (25, 27), NIF with losartan [LOS; angiotensin type 1 (AT1) receptor blocker, 20 mg·kg−1·day−1 via diet; TCI, Portland, OR], enalapril [ENAL; angiotensin-converting enzyme (ACE) inhibitor, 62.5 mg/l via drinking water; Sigma], or vehicle. All rats received a gelled diet containing water (56.02%), agar (1.06%; Becton-Dickenson), and normal rodent chow (42.91%; Harlan) as well as water ad libitum.
Plasma measurements.
On day 14 of treatment, rats were anesthetized with isoflurane, and the right femoral artery and vein were cannulated. A baseline arterial blood sample was taken for determination of hematocrit, and a “blank” was taken for the Evan's blue measurement. Following baseline collection, 250 μl of Evan's blue dye (0.3 mg/ml; Sigma) were injected in the venous line. The line was then flushed with 200 μl of isotonic saline. Arterial blood collections (300 μl) were taken at 5 and 10 min after dye infusion and used to calculate PV as described previously (10). At the end of the experiment, the kidneys were removed, separated into cortex (CTX), outer medulla (OM), and inner medulla (IM), snap-frozen in liquid nitrogen, and stored at −80°C.
Western blot analysis.
Protein abundances were detected using Western blotting as previously described (39). Briefly, 200 μg of kidney CTX and 100 μg of IM or OM were loaded for PDE5A; and 100 μg of CTX and 75 μg of OM or IM were loaded for the thiazide-sensitive sodium-chloride cotransporter (NCC) and the three subunits of the amiloride-sensitive ENaC (α-, β-, and γ-subunits of ENaC). Samples were loaded on 7.5% polyacrylamide gels and separated by electrophoresis. We used rabbit polyclonal antibodies against NCC and ENaC subunits produced in this laboratory using the sequence and protocol as previously described (20). Membranes were incubated overnight with a specific antibody for PDE5A (1:500 dilution; sc-32884, Santa Cruz Biotechnology, Santa Cruz, CA) and for two nights for the antibodies NCC (1:1,000 dilution), α-ENaC (1:1,000 dilution), β-ENaC (1:500 dilution), and γ-ENaC (1:500 dilution). Blots were then incubated with a goat anti-rabbit IgG-HRP secondary antibody (1:8,000 dilution; sc-2004; Santa Cruz Biotechnology). Bands of interest were visualized using enhanced chemiluminescence reagent (Supersignal West Pico; Thermo Scientific, Rockford, IL) and quantified by densitometry (VersaDoc imaging system and Quantity One Analysis software; Bio-Rad). Densitometry was normalized to Ponceau staining (Sigma) and controls, with the mean for the control group being defined as 100%. Densitometric analysis for NCC and ENaC subunits was additionally normalized to kidney CTX positive control.
Statistical analysis.
Data are given as means ± SE. An unpaired t-test, one-way ANOVA, or repeated-measures one-way ANOVA with Tukey's post hoc was performed. The null hypothesis was rejected at P < 0.05.
RESULTS
MAP.
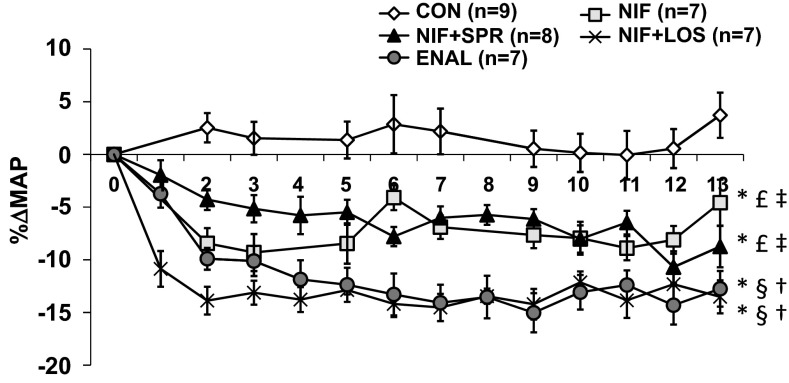
In control (CON) animals, MAP remained constant throughout the 14 days (Fig. 1). MAP was reduced to the same extent in NIF-treated animals with and without SPR, whereas rats treated with NIF and LOS and rats treated with ENAL had greater reductions in MAP (Fig. 1). The mean decreases in MAP over the 14 days were 7.4 ± 0.5% in NIF, 6.33 ± 0.5% in NIF with SPR, 13.3 ± 0.9% in NIF with LOS, and 12.0 ± 0.4% in ENAL-treated animals vs. baseline MAP levels.
Fig. 1.
Percent change in mean arterial pressure (MAP) as measured by telemetry in control (CON)-, nifedipine (NIF)-, nifedipine in combination with spironolactone (NIF + SPR)-, nifedipine in combination with losartan (NIF + LOS)-, and enalapril (ENAL)-treated virgin female rats. A one-way repeated-measures ANOVA was performed. Values are presented as means ± SE. P < 0.05 vs. CON (*), NIF (§), NIF + SPR (†), NIF + LOS (£), and ENAL (‡).
Plasma measurements.
PV was increased by NIF treatment (as also reported earlier by us) (10), but not by NIF in combination with SPR, NIF in combination with LOS, or with ENAL treatment (Table 1). As also shown in Table 1, hematocrit was reduced in the NIF- and ENAL-treated groups but not when NIF was combined with SPR or with LOS.
Table 1.
Plasma measurements
| CON (n = 10) | NIF (n = 9) | NIF + SPR (n = 10) | NIF + LOS (n = 7) | ENAL (n = 8) | |
|---|---|---|---|---|---|
| PV/body wt, % | 3.63 ± 0.3 | 5.21 ± 0.4*‡£ | 4.30 ± 0.3 | 3.62 ± 0.1 | 3.96 ± 0.3 |
| Hct, % | 43.7 ± 0.7 | 41.1 ± 0.7* | 43.6 ± 0.6 | 43.2 ± 0.4 | 41.1 ± 0.5* |
Values are means ± SE; n, no. of rats. CON, control; NIF, nifedipine; SPR, spironolactone; LOS, losartan, ENAL, enalapril; PV, plasma volume; Hct, hematocrit. A one-way ANOVA with Tukey's post hoc was performed. P < 0.05 vs. CON (
), NIF + LOS (
), and ENAL (
).
Renal PDE5A protein expression.
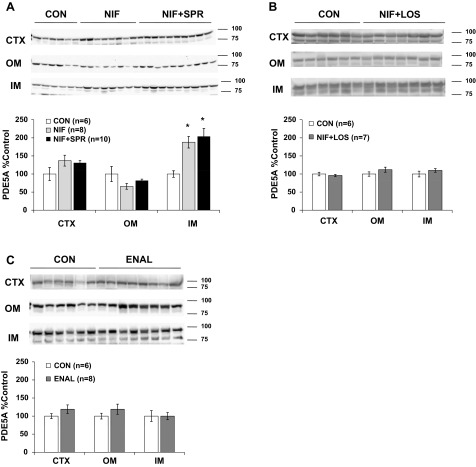
NIF increased PDE5A protein abundance in the renal IM (as reported previously) (10), and this increase persisted when SPR was also administered. No changes were observed in PDE5A protein abundance in the OM or CTX of NIF or NIF + SPR-treated animals (Fig. 2A). PDE5A protein abundance was unchanged from control in rats treated with NIF and LOS and in rats treated with ENAL in all kidney regions (Fig. 2, B and C).
Fig. 2.
Phosphodiesterase 5A (PDE5A) protein abundance in kidney cortex (CTX) and outer (OM) and inner (IM) medulla of CON, NIF-, and NIF + SPR-treated (A), CON and NIF + LOS-treated (B), and CON and ENAL-treated (C) virgin female rats after 14-day treatment. Densitometry was normalized to virgin controls with controls set at 100% and summarized as bar graphs. A one-way ANOVA with Tukey's post hoc was run in A, and an unpaired t-test was performed in B and C. Data are presented as means ± SE. *P < 0.05 vs. CON.
Apical sodium transporter protein expression in the distal nephron.
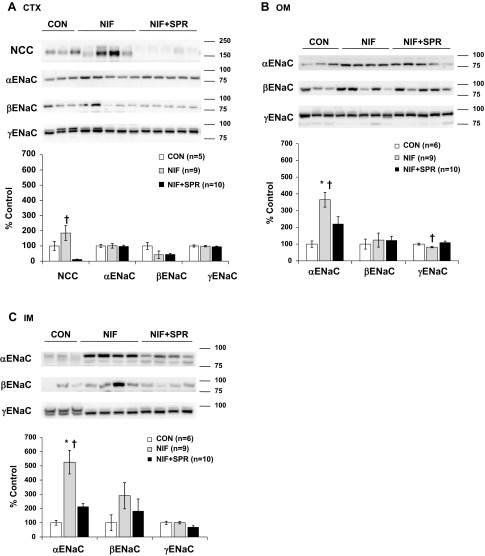
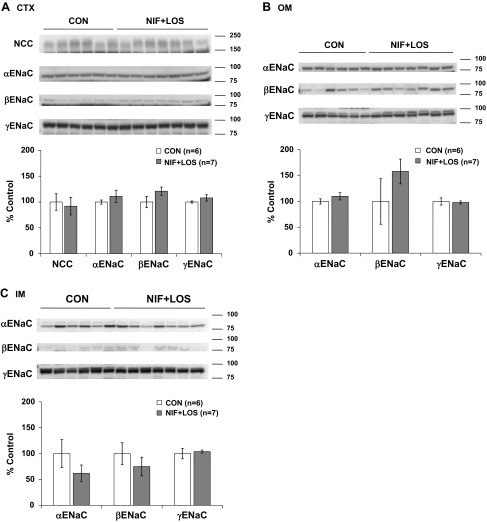
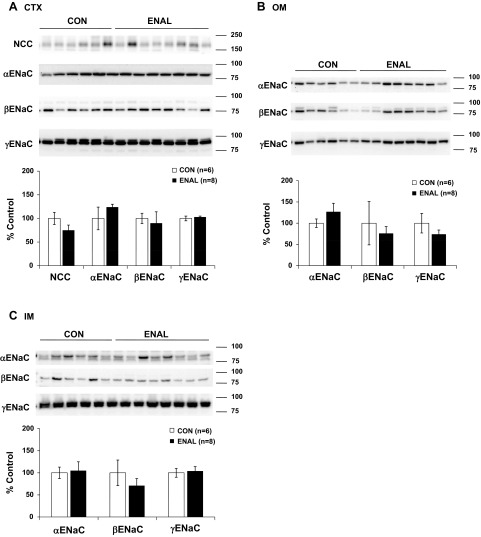
NIF had no effect on NCC or α-, β-, or γ-ENaC protein expression in the renal CTX, but selective increases in α-ENaC protein abundance were observed in the renal OM and IM. When SPR was also present, the NIF-induced increase in medullary α-ENaC protein abundance was prevented. The addition of SPR decreased cortical NCC abundance, but had no effect on the ENaC subunits in the renal CTX (Fig. 3, A–C). NIF treatment with LOS had no effect on NCC or α-, β-, or γ-ENaC protein expression in any region of the kidney (Fig. 4, A–C). Chronic ENAL-induced vasodilation did not result in any changes in NCC or α-, β-, or γ-ENaC protein abundance in any region of the kidney (Fig. 5, A–C).
Fig. 3.
Protein abundance of the thiazide-sensitive cotransporter [sodium-chloride cotransporter (NCC)] and the three subunits of ENaC (α, β, and γ) in CTX (A), OM (B), and IM (C) in CON, NIF-, and NIF + SPR-treated virgin female rats. Band densities were normalized to virgin controls with virgins set at 100% and summarized as bar graphs. A one-way ANOVA with Tukey's post hoc was performed. Data are presented as means ± SE. P < 0.05 vs. CON (*), and NIF + SPR (†).
Fig. 4.
Protein abundance of the thiazide-sensitive cotransporter (NCC) and the three subunits of ENaC (α, β, and γ) in CTX (A), OM (B), and IM (C) in CON and NIF + LOS-treated virgin female rats. Band densities were normalized to virgin controls with virgins set at 100% and summarized as bar graphs. An unpaired t-test was performed. Data are presented as means ± SE.
Fig. 5.
Protein abundance of the thiazide-sensitive cotransporter (NCC) and the three subunits of ENaC (α, β, and γ) in CTX (A), OM (B), and IM (C) in CON and ENAL-treated virgin female rats. Band densities were normalized to virgin controls with virgins set at 100% and summarized as bar graphs. An unpaired t-test was performed. Data are presented as means ± SE.
DISCUSSION
The main novel findings of this study are: 1) the apical sodium transporter profile of the distal nephron with chronic NIF-induced vasodilation is similar to the previously defined profile in pregnancy (38), with a selective increase in α-ENaC abundance in the medulla. 2). The PVE and renal medullary adaptations (increased α-ENaC and PDE5A) in response to chronic vasodilation in virgin rats requires an intact RAAS. Interestingly, these individual modifications were signaled through independent pathways. The increased medullary α-ENaC was inhibited by MR blockade, whereas the increased medullary PDE5A was inhibited by AT1 receptor blockade.
Schrier and colleagues have long postulated that normal pregnancy is an “underfilled” state with renal sodium retention being driven by a reduction in effective circulating volume, secondary to chronic vasodilation (34, 35). Because renal sodium retention and PVE begin very early, within the first 6 wk of normal human pregnancy (5), the initiating signal is presumably a fall in maternal peripheral resistance, since the “fetoplacental shunt” does not open until later in gestation. We previously tested Schrier's underfill hypothesis in virgin female rats and showed that 14 days of chronic systemic vasodilation with either NIF or NaNO2 led to PVE or “refilling” of the vasculature, as well as increased PDE5A protein abundance in medulla (10). The similarity in medullary responses to these two structurally and mechanistically diverse vasodilators suggests that it is vasodilation “per se” that initiates these renal changes. In the present study, chronic vasodilation of the virgin rat with NIF again resulted in PVE and increases in medullary PDE5A and also increases in medullary α-ENaC protein abundance. Although the α-ENaC is distributed throughout the entire collecting duct, we found no difference in the protein abundance of α-ENaC in the renal CTX in underfilled virgins. Differential regulation of α-ENaC in the kidney CTX and medulla has been previously reported in streptozotocin-induced diabetic rats, rats receiving the AT1 receptor blocker candesartan, rats receiving E prostanoid-1 receptor agonists, and in nucleotide receptor P2Y2 knockout mice (11, 14, 41). We found no change in renal cortical NCC (an aldosterone responsive sodium transporter in the distal tubule) with NIF-induced chronic vasodilation in the present study, although Wang et al. did report increased NCC using a much higher dose of NIF (37). However, in normal pregnancy, we also observed that cortical NCC was unchanged (38); thus, the chronic systemic vasodilation in virgin female rats in the present study recapitulates the changes seen in normal pregnancy (24, 38).
The renal sodium retention of normal pregnancy is accompanied by early and sustained activation of the RAAS (5, 17, 34), a physiological response to an “underfill” signal. Here we investigated the importance of the RAAS in mediating the collecting duct changes that facilitate renal sodium retention in response to chronic vasodilation in nonpregnant rats. In the first series, we found that antagonism of the MR with SPR did not prevent the increased medullary PDE5A; however, AT1 receptor blockade with LOS did prevent the increase, suggesting that ANG II is mediating the increased PDE5A. NIF + SPR did prevent the increased PVE and medullary α-ENaC protein abundance, suggesting that aldosterone mediates the increased ENaC abundance in medulla (as anticipated), which plays a primary role in the sodium retention leading to PVE. Although ENaC is responsible for only ∼2% of sodium reabsorption, the collecting duct is the principal site of determination of net sodium balance (16, 18). Furthermore, we recently demonstrated that ENaC activity is necessary for maintenance of PVE in the late pregnant rat (38).
In the second series, we used ENAL as a different method of chronic vasodilation. ENAL decreases blood pressure by reducing total peripheral resistance (29, 36). The mechanism of this vasodilation is in part the result of the increased concentration of bradykinin, since ACE inhibitors also block kininase II, which degrades bradykinin (36). The purpose of using the ENAL alone was to use an agent that induced peripheral vasodilation (decreased total peripheral resistance) but interrupted the RAAS by ACE inhibition. As with NIF, MAP fell during the 14-day treatment period, but after ∼day 5 the decline was greater with ENAL than with NIF. There was no PVE with ENAL, which probably led to the greater eventual fall in blood pressure because of a failure to “refill” the expanded vasculature. Despite no evidence of PVE, there was a fall in hematocrit, which may seem counterintuitive. However, ANG II (via the AT1 receptor) stimulates renal erythropoetin release and thus erythropoesis, and both ACE inhibitors and AT1 receptor antagonists lower hematocrit because of decreased erythropoeisis (7, 12, 19).
With both ENAL-induced chronic vasodilation and NIF + LOS there was no increase in either medullary α-ENaC or PDE5A protein abundance. Because upregulation of PDE5A was prevented by ACE inhibition and AT1 receptor blockade, but not MR blockade, this suggests that ANG II signaling in the renal medulla is directly regulating PDE5A expression. This relationship has already been reported in tissues from nonpregnant animals. For example, in rat aortic vascular smooth muscle cells, ANG II increases PDE5A expression via the AT1 receptor, ERK1/2 pathway (13). Also, ANG II increases PDE5A mRNA, protein, and enzyme activity in the left cardiac ventricle of rats (22). All of the components of RAAS are present in the kidney, and RT-PCR of microdissected tubules has identified the AT1 receptor in the cortical, outer medullary, and inner medullary collecting duct (23). However, there are conflicting reports regarding the regulation of angiotensin receptors in pregnancy. One group has found unchanged renal cortical AT1 receptor protein levels, increased AT2 receptor, and increased AT1 receptor/AT2 receptor heterodimerization in the late pregnant rat kidney (1). Another group found no change in whole kidney mRNA or protein expression of AT1 receptor or AT2 receptor throughout gestation in the rat (8). Expression and abundance of angiotensin receptors along the tubule have yet to be investigated in pregnancy. Although ANG II sensitivity to the renal circulation is reduced in pregnancy (6, 9) there are conflicting reports regarding tubular sensitivity (26, 40). More studies are needed to establish the direct effects of ANG II on the renal tubule during pregnancy. Since the current and previous work highlights the regional specificity of modifications occurring in the kidney, it will be important to characterize the intrarenal RAAS during pregnancy.
Perspectives and Significance
In summary, this study gives further support for the underfill hypothesis as a mechanism driving renal sodium retention and volume expansion and highlights the importance of the RAAS in mediating the protein changes that facilitate the PVE. In addition to pregnancy, this work has implications for edematous disorders such as cirrhosis, heart failure, and nephrotic syndrome. These patients are thought to have decreased effective blood volumes, and therefore the pathological sodium and water retention occurring in these patients could be the result of arterial underfilling. Insight into these mechanisms could provide potential molecular targets for therapy.
GRANTS
This work was supported by National Institutes of Health Grants R01-HD-041571 and R01-DK-56843 to C. Baylis.
DISCLOSURES
The authors declare no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
Author contributions: C.A.W., S.S., J.M.S., S.M.M., and C.B. conception and design of research; C.A.W., S.S., J.M.S., A.F., T.A., and M.W.C. performed experiments; C.A.W., S.S., and J.M.S. analyzed data; C.A.W., S.S., J.M.S., and C.B. interpreted results of experiments; C.A.W. prepared figures; C.A.W. and C.B. drafted manuscript; C.A.W., S.M.M., and C.B. edited and revised manuscript; C.A.W., S.S., J.M.S., A.F., T.A., M.W.C., S.M.M., and C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bruce Cunningham and Harold Snellen for expert technical assistance.
Part of this study has appeared in abstract form FASEB J 25: 836.4.
REFERENCES
- 1.Anguiano-Robledo L, Reyes-Melchos PA, Bobadilla-Lugo RA, Perez-Alvarez VM, Lopez-Sanchez P. Renal angiotensin-II receptors expression changes in a model of preeclampsia. Hypertens Pregnancy 26: 151–161, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Baylis C. Glomerular filtration and volume regulation in gravid animal models. Baillieres Clin Obstet Gynaecol 8: 235–264, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Bedard S, Sicotte B, St-Louis J, Brochu M. Modulation of body fluids and angiotensin II receptors in a rat model of intra-uterine growth restriction. J Physiol 562: 937–950, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown MA, Gallery ED. Volume homeostasis in normal pregnancy and pre-eclampsia: physiology and clinical implications. Baillieres Clin Obstet Gynaecol 8: 287–310, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 54: 2056–2063, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Chu ZM, Beilin LJ. Demonstration of the existence of nitric oxide-independent as well as nitric oxide-dependent vasodilator mechanisms in the in situ renal circulation in near term pregnant rats. Br J Pharmacol 122: 307–315, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole J, Ertoy D, Lin H, Sutliff RL, Ezan E, Guyene TT, Capecchi M, Corvol P, Bernstein KE. Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice. J Clin Invest 106: 1391–1398, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornock R, Langley-Evans SC, Mobasheri A, McMullen S. The impact of maternal protein restriction during rat pregnancy upon renal expression of angiotensin receptors and vasopressin-related aquaporins (Abstract). Reprod Biol Endocrinol 8: 105, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invesy 96: 482–490, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fekete A, Sasser J, Baylis C. Chronic vasodilation produces plasma volume expansion and hemodilution in rats: consequences of decreased effective arterial blood volume. Am J Physiol Renal Physiol 300: F113–F118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez AA, Cespedes C, Villanueva S, Michea L, Vio CPE. Prostanoid-1 receptor regulates renal medullary alphaENaC in rats infused with angiotensin II. Biochem Biophys Res Commun 389: 372–377, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Gossmann J, Burkhardt R, Harder S, Lenz T, Sedlmeyer A, Klinkhardt U, Geiger H, Scheuermann EH. Angiotensin II infusion increases plasma erythropoietin levels via an angiotensin II type 1 receptor-dependent pathway. Kidney Int 60: 83–86, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Aizawa T, Wei H, Pi X, Rybalkin SD, Berk BC, Yan C. Angiotension II increases phosphodiesterase 5A expression in vascular smooth muscle cells: a mechanism by which angiotensin II antagonizes cGMP signaling. J Mol Cell Cardiol 38: 175–184, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein JD, Rash A, Sands JM, Ecelbarger CM, Tiwari S. Candesartan differentially regulates epithelial sodium channel in cortex versus medulla of streptozotocin-induced diabetic rats (Abstract). J Epithel Biol Pharmacol 2: 23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight S, Snellen H, Humphreys M, Baylis C. Increased renal phosphodiesterase-5 activity mediates the blunted natriuretic response to ANP in the pregnant rat. Am J Physiol Renal Physiol 292: F655–F659, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lifton R, Gharavi A, Geller D. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Lindheimer MD, August P. Aldosterone, maternal volume status and healthy pregnancies: a cycle of differing views. Nephrol Dial Transplant 24: 1712–1714, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Loffing J, Schild L. Functional domains of the epithelial sodium channel. J Am Soc Nephrol 16: 3175–3181, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Marathias KP, Agroyannis B, Mavromoustakos T, Matsoukas J, Vlahakos DV. Hematocrit-lowering effect following inactivation of renin-angiotensin system with angiotensin converting enzyme inhibitors and angiotensin receptor blockers. Curr Top Med Chem 4: 483–486, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.May A, Puoti A, Gaeggeler HP, Horisberger JD, Rossier BC. Early effect of aldosterone on the rate of synthesis of the epithelial sodium channel alpha subunit in A6 renal cells. J Am Soc Nephrol 8: 1813–1822, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Mokni W, Keravis T, Etienne-Selloum N, Walter A, Kane M, Schini-Kerth V, Lugnier C. Concerted regulation of cGMP and cAMP phosphodiesterases in early cardiac hypertrophy induced by angiotensin II. PLoS ONE 5: e14227. 10.1371/journal.pone.0014227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyata N, Park F, Li XF, Cowley AW. Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol 276: F437–F446, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Ni XP, Safai M, Rishi R, Baylis C, Humphreys MH. Increased activity of cGMP-specific phosphodiesterase (PDE5) contributes to resistance to atrial natriuretic peptide natriuresis in the pregnant rat. J Am Soc Nephrol 15: 1254–1260, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen J, Kwon T, Masilamani S, Beutler K, Hager H, Nielsen S, Knepper MA. Sodium transporter abundance profiling in kidney: effect of spironoloactone. Am J Physiol Renal Physiol 283: F923–F933, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Novak J, Reckelhoff J, Bumgarner L, Cockrell K, Kassab S, Granger JP. Reduced sensitivity of the renal circulation to angiotensin II in pregnant rats. Hypertension 30: 580–584, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Pechanova O, Matuskova J, Capikova D, Jendekova L, Paulis L, Simko F. Effect of spironolactone and captopril on nitric oxide and S-nitrosothiol formation in kidney of L-NAME-treated rats. Kidney Int 70: 170–176, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Pike RL. Sodium requirement of the rat during pregnancy. In: Hypertension in Pregnancy, edited by Lindheimer MD, Katz AI, Zuspan FP. New York, NY: Wiley, 1976, p. 207–216 [PubMed] [Google Scholar]
- 29.Richer C, Doussau MP, Giudicelli JF. Differential systemic and regional hemodynamic profiles of four angiotensin-I converting-enzyme inhibitors in the rat. Cardiovasc Drugs Ther 3: 865–872, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Salas SP, Giacaman A, Vio CP. Renal and hormonal effects of water deprivation in late-term pregnant rats. Hypertension 44: 334–339, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 47: 203–208, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Sasser JM, Baylis C. Effects of sildenafil on maternal hemodynamics and fetal growth in normal rat pregnancy. Am J Physiol Regul Integr Comp Physiol 298: R433–R438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasser J, Ni X, Humphreys M, Baylis C. Increased renal phosphodiesterase-5 activity mediates the blunted natriuretic response to a nitric oxide donor in the pregnant rat. Am J Physiol Renal Physiol 299: F810–F814, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrier RW, Dürr JA. Pregnancy: an overfill or underfill state. Am J Kidney Dis 9: 284–289, 1987 [DOI] [PubMed] [Google Scholar]
- 35.Schrier RW, Ohara M. Dilemmas in human and rat pregnancy: proposed mechanisms relating to arterial vasodilation. J Neuroendocrinol 22: 400–406, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Tio RA, Heiligers JP, de Langen CD, van Gilst WH, Konig W, Saxena PR, Wesseling H. The heamodynamic effects of two angiotensin converting enzyme inhibitors, enalaprilat and zofenoprilat, in the rat: evidence for the involvement of bradykinin. J Hypertens Suppl 7: S296–S297, 1989 [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Kwon T, Li C, Flyvbjerg A, Knepper M, Frokiaer J, Nielsen S. Altered expression of renal aquaporins and Na+ transporters in rats treated with L-type calcium blocker. Am J Physiol Regul Integr Comp Physiol 280: R1632–R1641, 2001 [DOI] [PubMed] [Google Scholar]
- 38.West C, Zhang Z, Ecker G, Masilamani S. Increased renal α epithelial sodium channel (ENaC) protein and increased ENaC activity in normal pregnancy. Am J Physiol Regul Integr Comp Physiol 299: R1326–R1332, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao S, Erdely A, Wagner L, Baylis C. Uremic levels of BUN do not cause nitric oxide deficiency in rats with normal renal function. Am J Physiol Renal Physiol 280: F996–F1000, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Yu T, Khraibi AA. Renal interstitial hydrostatic pressure and natriuretic response to high doses of angiotensin II in pregnant rats. AJH 19: 300–305, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Listhrop R, Ecelbarger CM, Kishore BK. Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion. Am J Physiol Renal Physiol 300: F657–F668, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]