Abstract
Chronic obstructive pulmonary disease (COPD) is associated with systemic oxidative stress and skeletal muscle dysfunction. The purpose of this study was to examine the impact of intravenous ascorbate administration (AO) on biological markers of antioxidant capacity and oxidative stress, and subsequently skeletal muscle function during dynamic, small muscle mass exercise in patients with COPD. Ten patients with spirometric evidence of COPD performed single-leg knee extensor (KE) trials matched for intensity and time (isotime) following intravenous ascorbate (2 g) or saline infusion (PL). Quadriceps fatigue was quantified by changes in force elicited by maximal voluntary contraction (MVC) and magnetic femoral nerve stimulation (Qtw,pot). AO administration significantly increased antioxidant capacity, as measured by the ferric-reducing ability of plasma (PL: 1 ± 0.1 vs. AO: 5 ± 0.2 mM), and significantly reduced malondialdehyde levels (PL: 1.16 ± 0.1 vs. AO: 0.97 ± 0.1 mmol). Additionally, resting blood pressure was significantly reduced (PL: 104 ± 4 vs. AO: 93 ± 6 mmHg) and resting femoral vascular conductance was significantly elevated after AO (PL: 2.4 ± 0.2 vs. AO: 3.6 ± 0.4 ml·min−1·mmHg−1). During isotime exercise, the AO significantly attenuated both the ventilatory and metabolic responses, and patients accumulated significantly less peripheral quadriceps fatigue, as illustrated by less of a fall in MVC (PL: −11 ± 2% vs. AO: −5 ± 1%) and Qtw,pot (PL: −37 ± 1% vs. AO: −30 ± 2%). These data demonstrate a beneficial role of AO administration on skeletal muscle fatigue in patients with COPD and further implicate systemic oxidative stress as a causative factor in the skeletal muscle dysfunction observed in this population.
Keywords: free radicals, peripheral fatigue, ascorbate
skeletal muscle dysfunction plays a prominent role in limiting exercise and activities of daily living in patients with chronic obstructive pulmonary disease (COPD) (21, 22). Numerous factors, including inactivity and skeletal muscle detraining (34), mitochondrial dysfunction (9), and oxidative stress (30) have all been implicated in the skeletal muscle dysfunction associated with COPD. Of these factors, the contribution of oxidative stress to reduced exercise capacity in patients with COPD has been well documented (11, 13, 24). Specifically, previous research has demonstrated an inverse correlation between exercise time to exhaustion and evidence of lipid peroxidation (12), as well as the favorable effects of preexercise antioxidant pretreatment with N-acetylcysteine (24) on performance in patients with COPD. Therefore, in patients with COPD, exercising skeletal muscle is a significant source of oxidative stress, the magnitude of oxidative stress likely impairs skeletal muscle function, and the modulation of redox state may enhance exercise capacity in this population.
Accordingly, our group previously utilized an acute, readily available, oral antioxidant cocktail (vitamins C, E, and α-lipoic acid), with documented efficacy (43), to examine the impact of oxidative stress on skeletal muscle function in COPD (32). The antioxidant cocktail decreased the electron paramagnetic resonance (EPR) spectroscopy free radical signal, but did not impact skeletal muscle fatigue measured after isotime knee extensor (KE) exercise in patients with COPD. However, the individual responses to the oral antioxidant cocktail were mixed, with only half of the patients exhibiting a substantially reduced EPR spectroscopy signal. Therefore, in this prior study (32), the role of free radicals on skeletal muscle fatigue in patients with COPD was not fully elucidated.
Likely due to free radical scavenging, the infusion of supraphysiological doses of the antioxidant ascorbate (AO) have previously been documented to restore vascular function in several pathophysiological conditions such as heart failure (19), hypertension (36), diabetes (38), as well in chronic smokers (18). In addition, high-dose AO infusion has been documented to improve resting (20) and exercising (14, 23) skeletal muscle blood flow in healthy older individuals. Improving limb blood flow, and possibly oxygen delivery, has the potential to attenuate the rate of development of peripheral muscle fatigue (3). Interestingly, intravenous AO administration also ameliorated the exaggerated exercise pressor reflex during plantar flexion exercise in patients with peripheral artery disease, which was attributed to a reduction in excessive group III/IV afferent stimulation under basal conditions (27). Decreasing the group III/IV afferent signal from the lower limbs has also been documented to extend exercise time to exhaustion in patients with COPD (17). Therefore, free radical scavenging by AO may confer beneficial vascular effects and dampen group III/IV afferent signaling, potentially translating into fatigue resistance in patients with COPD.
Thus the purpose of this study was to examine the impact of intravenous AO administration, a potent water-soluble antioxidant with no known side effects, on oxidative stress and skeletal muscle fatigue during dynamic KE exercise in patients with COPD. In addition, this study sought to comprehensively evaluate the impact of reducing oxidative stress with AO on the physiological responses to KE exercise in patients with COPD. We tested the hypotheses that in patients with COPD intravenous AO administration would 1) improve antioxidant capacity and decrease oxidative stress and, 2) decrease the magnitude of peripheral quadriceps fatigue induced by KE exercise matched for intensity and duration (isotime).
METHODS
Subjects.
Written, informed consent was obtained from all participants before their inclusion in this study, and the Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center approved all protocols. Ten patients with COPD were enrolled based on spirometric evidence of moderate to severe airflow obstruction [FEV1/FVC ≤ 0.7 (10)], as assessed by standard pulmonary function tests performed during an initial visit to the laboratory. General anthropometric characteristics, including thigh volume, which was used to estimate quadriceps muscle mass (16), were also determined during this visit. Resting arterial blood analyses, collected in a parallel study in which the current subjects took part, are also presented here to better characterize the patients.
Exercise protocols and general procedures.
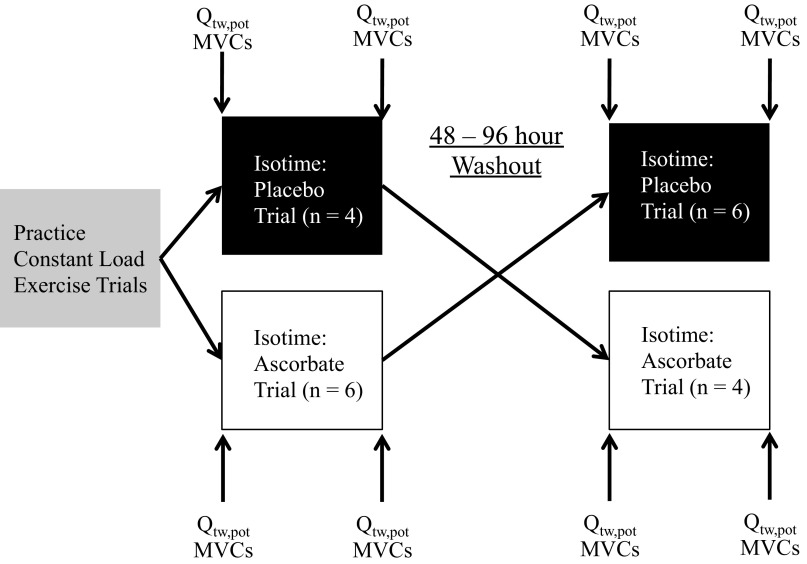
All subjects were familiarized with single-leg KE exercise, which was performed at a cadence of 60 rpm, during two preliminary visits to the laboratory. Subsequently, peak KE work rate was determined with subject-specific protocols designed to reach exhaustion within 8–12 min, consisting of 2–5 W/min increases. The experimental protocol is depicted in Fig. 1. After the peak work rate tests, subjects performed at least two practice constant-load exercise trials at 80% of maximal workload to the limit of tolerance to determine a target exercise intensity and duration for the subsequent isotime trials. The intensity of the practice trials was adjusted until subjects could maintain the intensity, at a cadence of 60 rpm, for ∼6 to 8 min before their cadence dropped below 50 rpm and the trial was terminated. Once these criteria were met, the trial time was adopted as the target time for the subsequent isotime trials. Next, in a repeated-measures design, isotime trials, separated by 48–96 h, were performed following either a bolus infusion of AO (100 mg/ml AO dissolved in normal saline, infused at 1 ml/min for 20 min) or saline (PL: 0.9% NaCl infused at 1 ml/min for 20 min) via an intravenous catheter in the arm (Fig. 1). The patient and all members of the research team, except for the individual administering the AO or PL, were blinded to the experimental condition.
Fig. 1.
Study protocol schematic. MVC, maximal voluntary contraction. Qtw,pot, potentiated twitch force.
Neuromuscular function tests were performed before infusion, after infusion (but before exercise), and 10 min after the isotime trials. In addition, venous blood samples were taken before and immediately after the isotime trials to determine pro- and antioxidant status, and for spin trapping and EPR spectroscopy to directly assess free radical concentration. Before each exercise bout, 1 min of resting data were collected and subjects performed 1 min of unloaded warmup KE exercise. Ventilation, gas exchange, heart rate (HR), mean arterial pressure (MAP), ratings of perceived exertion and breathlessness, arterial oxygen saturation, femoral blood flow, and quadriceps electromyograms (EMG) were measured during the isotime trials.
Oxidative stress, antioxidant assays, and direct measurement of free radicals.
Plasma samples were stored at −80°C until analysis. Lipid peroxidation, a marker of oxidant damage, was assessed by plasma malondialdehyde levels (Bioxytech LPO-586, Foster City, CA). Total antioxidant capacity was evaluated by determining the ferric-reducing ability of plasma (FRAP), using the method described by Benzie and Strain (6). The efficacy of the AO specific to plasma ascorbate levels was assayed as previously described (8) (CosmoBio, Carlsbad, CA). Free radical scavenging, assessed by superoxide dismutase and catalase activity, was also assayed in the plasma (42) (Cayman Chemical, Ann Arbor, MI). EPR spectroscopy was performed on pre- and postexercise blood samples to directly assess the ability of the AO to reduce the concentration of free radicals with an EMX X-band spectrometer (Bruker, MA), as previously described (31, 32).
Pulmonary and cardiovascular responses.
Ventilation and pulmonary gas exchange were measured at rest and during exercise using an open-circuit system (ParvoMedics, Sandy, UT). HR, determined from the R-R interval of a three-lead electrocardiogram, and arterial oxygen saturation (SaO2), estimated using a pulse oximeter (Nellcor N-595, Pleasanton, CA) with adhesive forehead sensors, were also acquired during these trials at 200 Hz using a data acquisition system (AcqKnowledge; Biopac Systems, Goleta, CA). MAP was determined with a finometer (Finapres Medical Systems, The Netherlands) at heart level. Patients were asked how hard their leg was working (rating of perceived exertion, RPE) and how labored was their breathing (dyspnea) every minute during the exercise trials using Borg's CR10 scale (7).
Leg blood flow.
Measurements of femoral artery blood velocity and vessel diameter in the leg being studied were performed at rest and throughout isotime exercise, using a Logic 7 ultrasound system (General Electric Medical Systems) as previously described (39). Blood flow in the femoral artery was calculated as the following: blood flow = (mean velocity)π(vessel diameter/2)2 × 60.
Quadriceps electromyograms.
Quadriceps EMGs were recorded from the vastus lateralis muscle during exercise from electrodes placed in a bipolar configuration with an interelectrode distance of 20 mm over the middle of the muscle belly, with the active electrodes placed over the motor point of the muscle and the reference electrode in an electrically neutral site (4). To ensure similar electrode placement between trials, the electrode location was marked with indelible ink. Raw EMG signals were filtered with a bandpass filter (with a low-pass cut-off frequency of 15 Hz and a high-pass cut-off frequency of 650 Hz) and after visual inspection of the filtered signal; a threshold voltage was set to identify the onset of EMG activity (AcqKnowledge; Biopac Systems). For data analysis, the integral of each EMG burst (integrated EMG) was calculated to determine the percent increase in integrated EMG from the first minute of exercise (4), an index of the development of peripheral fatigue during exercise. The EMG electrodes were also used to record magnetically evoked compound action potentials (M-waves, area and peak-to-peak amplitude) to evaluate changes in membrane excitability from pre- to postexercise during potentiated twitch force (Qtw,pot) assessments.
Neuromuscular function assessment.
The magnitude of peripheral quadriceps fatigue was quantified by pre- to postinfusion, and pre- to postexercise changes in quadriceps maximal voluntary contraction (MVC) and Qtw,pot evoked by supramaximal magnetic stimulation of the femoral nerve (4, 29) with a magnetic stimulator (Magstim 200, The Magstim, Wales, UK) connected to a double 70-mm coil (26). Specifically, while laying semirecumbent with a knee joint angle of 90 degrees, subjects performed a series of six MVCs separated by 30 s, with Qtw,pot assessments interspersed 5 s after each MVC. Patients viewed a computer monitor displaying real-time visual feedback to ensure maximal effort during all MVCs. The neuromuscular function assessment procedure (6 MVCs and 6 Qtw,pot maneuvers) was performed before the AO or PL infusion, after the infusion (but before exercise), and 10 min after exercise. In addition, to quantify voluntary activation of the quadriceps during the MVCs, the additional force generated by a single twitch superimposed on the MVC was compared with the force produced by the potentiated twitch immediately following the MVC to determine the percent voluntary muscle activation (4). Force was obtained from a calibrated load cell (Transducer Techniques, Temecula, CA) connected to a noncompliant strap placed around the subject's ankle and acquired at 200 Hz with a data acquisition system (AcqKnowledge; Biopac Systems). On a separate visit, to ensure supramaximality of stimulation during magnetic stimulation of the femoral nerve, the plateau in evoked force following serial twitch forces, obtained every 30 s, at 70, 80, 85, 90, 95, and 100% of maximal stimulator output, was also evaluated.
Statistical analysis.
Two-way repeated measures ANOVA were used to compare the effect of antioxidant treatment on physiological parameters during exercise, with a Tukey post hoc analysis if a significant main effect was found. Student's paired t-tests were used to compare the effect of AO in terms of antioxidant efficacy and indices of peripheral fatigue. Statistical significance was set at α = 0.05 for all tests. All group data are expressed as means ± SE.
RESULTS
Subject characteristics.
Subject characteristics are documented in Table 1. One patient was a current smoker, who refrained from the use of tobacco products for 12 h before all data collection. Two patients qualified for supplemental oxygen; these patients, however, at the time of data collection, only used the supplemental oxygen while sleeping. No patients reported any side effects of AO or PL administration and were therefore successfully blinded to the experimental condition. Supramaximality of magnetic nerve stimulation was demonstrated in all patients by evidence of a plateau in evoked force with increasing stimulus intensity.
Table 1.
Subject characteristics
| Age, yr | 62 ± 3 |
| Height, m | 1.73 ± 0.03 |
| Weight, kg | 84 ± 7 |
| BMI, kg/m2 | 28 ± 2 |
| Quadriceps muscle mass, kg | 1.7 ± 0.2 |
| Peak knee-extensor work rate, W | 28 ± 3 |
| Male/Female | 7/3 |
| Pulmonary function | |
| FVC, l (% predicted) | 3.6 ± 0.2 (86 ± 5) |
| FEV in 1 s, l/s (% predicted) | 1.8 ± 0.2 (57 ± 5) |
| FEV1/FVC, % | 51 ± 5 |
| Resting arterial blood gases | |
| Hemoglobin concentratio, g/dl | 14 ± 1 |
| Oxyhemoglobin, % | 92 ± 1 |
| Partial pressure of oxygen, mmHg | 70 ± 2 |
| Partial pressure of carbon dioxide, mmHg | 32 ± 2 |
| Bicarbonate, mmol/l | 22 ± 1 |
| pH | 7.45 ± 0.01 |
Values expressed as means ± SE. FEV1/FVC, forced expiratory volume in 1 s relative to forced vital capacity; BMI, body mass index.
Antioxidant efficacy.
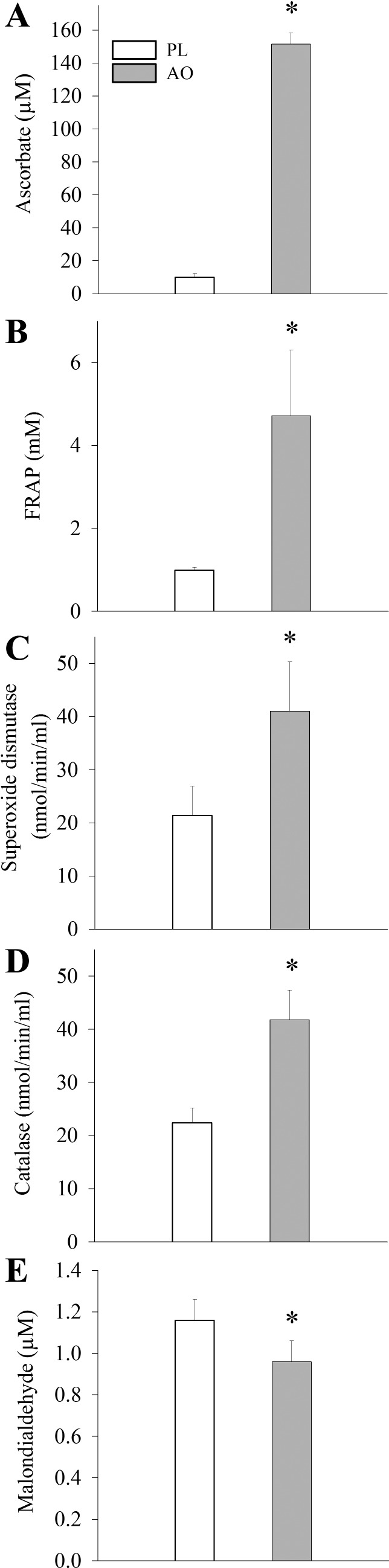
Before exercise, AO caused an ∼10-fold elevation in plasma ascorbate levels (Fig. 2A). AO infusion also increased endogenous antioxidant capacity, as measured by FRAP, and resulted in greater free radical scavenging, as evidenced by increased superoxide dismutase enzymatic catalase activities (Fig. 2, B–D). Consequently, resting malondialdehyde levels, a marker of lipid peroxidation and oxidative stress, were decreased following AO infusion (Fig. 2E). In contrast, and somewhat surprisingly, there was no detectable difference in plasma free radical levels, directly measured by EPR spectroscopy, between conditions (AO: 10.9 ± 3.1 AU vs. PL: 11.6 ± 3.7 AU, P > 0.05).
Fig. 2.
Quantitative assessment of antioxidants and markers of oxidative stress after intravenous saline (PL) or ascorbate (AO) administration. FRAP, ferric-reducing ability of plasma. Values are presented as means ± SE. *Significantly different from the PL condition.
After exercise, AO and FRAP remained elevated over PL values in the AO condition (AO: 107.6 ± 8.1 μg/ml vs. 12.9 μg/ml, P < 0.05; FRAP: 1.5 ± 0.08 mM vs. PL: 0.97 ± 0.08, P < 0.05 exercise, for AO and PL, respectively). In addition, MDA was decreased to a similar extent as before exercise (AO: 0.94 ± 0.1 μM vs. PL: 1.2 ± 0.1 μM, P < 0.05). Postexercise, there were no differences between conditions in terms of antioxidant enzyme (superoxide dismutase or catalase) activity or plasma free radical levels, assessed by EPR spectroscopy.
Isotime trials.
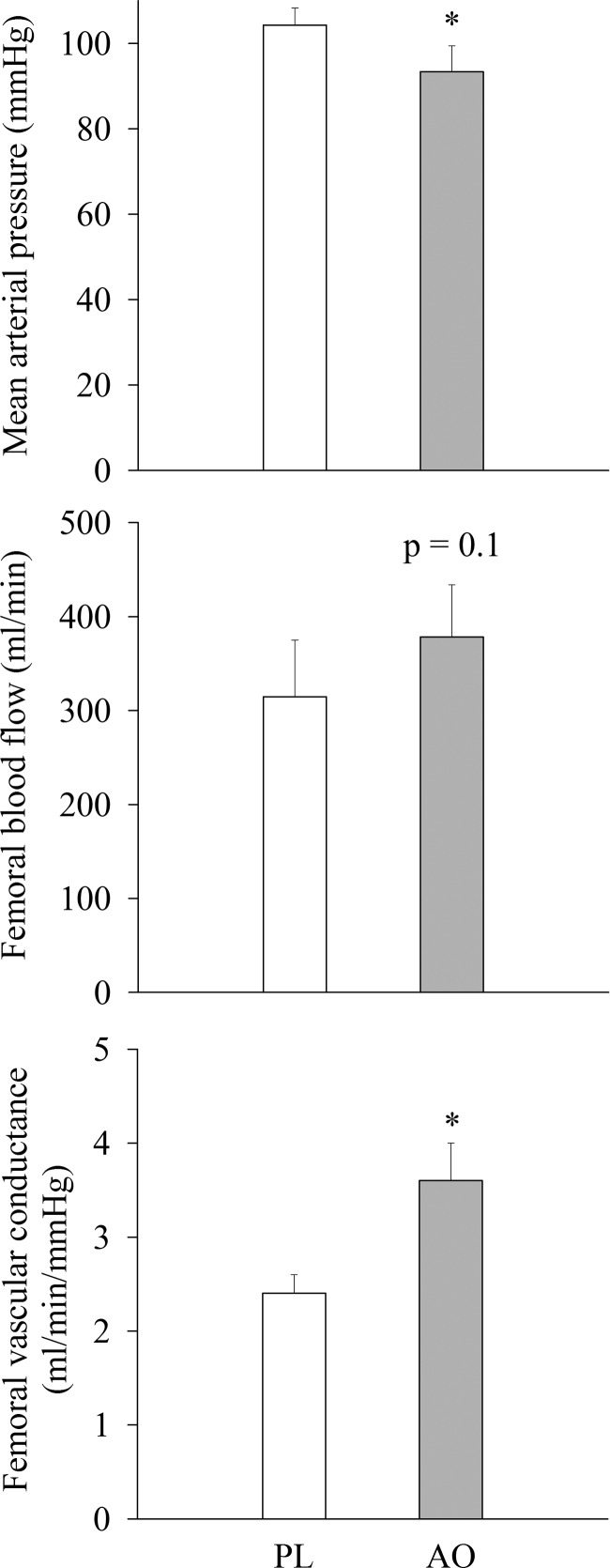
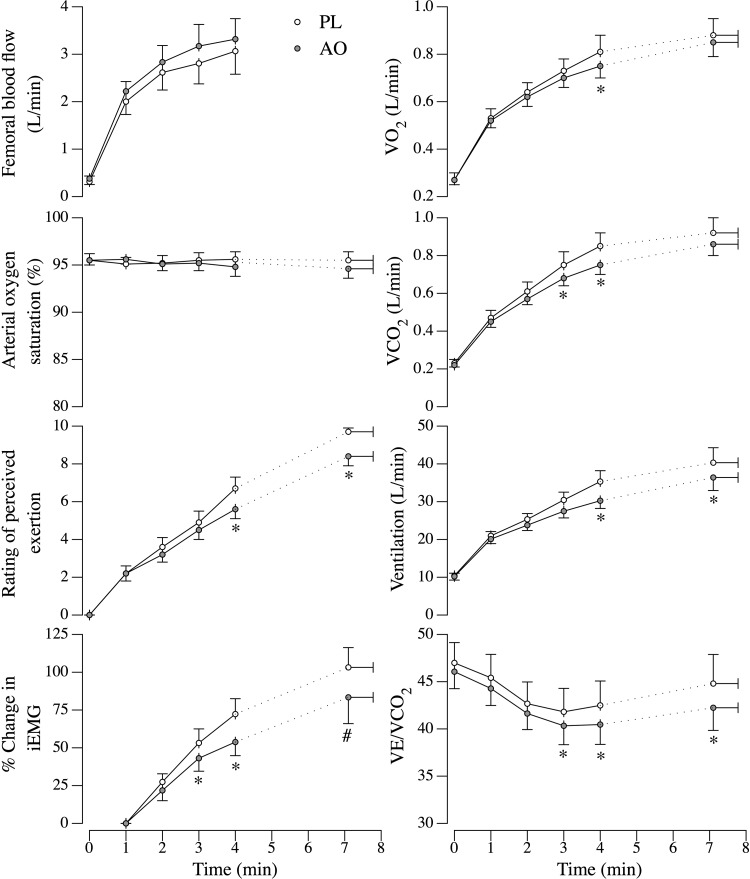
Acutely, MVC and Qtw,pot were unaffected by AO infusion (MVC: 374 ± 52 N to 374 ± 53 N, P > 0.05; Qtw,pot: 105 ± 13 N to 103 ± 13 N, P > 0.05, for pre- and postinfusion, respectively), and these values were not different from the pre- and post-PL infusion values. At baseline, before exercise, MAP was reduced following the AO infusion (Fig. 3). Despite the decrease in perfusion pressure, femoral artery blood flow was not different between conditions (P = 0.1), and thus femoral vascular conductance was significantly elevated in the AO condition (Fig. 3). Because of movement artifact, differences in MAP could not be evaluated throughout the isotime trials. The cardiorespiratory responses to the isotime trials are depicted in Fig. 4. As illustrated, oxygen consumption (Vo2) and carbon dioxide production (Vco2) were reduced during exercise following AO infusion at isotime minute 4, but reached similar levels at the end of exercise. In addition, ventilation rate (VE) and the ventilation relative to carbon dioxide production (VE/Vco2) ratio were reduced in the AO condition during exercise and at the end of the isotime trials. Arterial oxygen saturation and femoral artery blood flow were not different between conditions.
Fig. 3.
Resting mean arterial pressure and hemodynamic parameters following intravenous PL or AO administration. Values are presented as means ± SE. *Significantly different from the PL condition.
Fig. 4.
Physiological responses to constant workload isotime knee extensor exercise matched for intensity and duration following intravenous PL or AO administration. Group mean data (±SE) over the first 4 min of exercise, which were attained by all subjects. The final time point represents end-exercise values, which were not obtained for femoral blood flow due to loss of signal. VE/Vco2, ventilation relative to carbon dioxide production; Vco2, carbon dioxide production; Vo2, oxygen consumption; iEMG, integrated electromyogram from the vastus lateralis. *Significantly different from the PL condition. #P = 0.09.
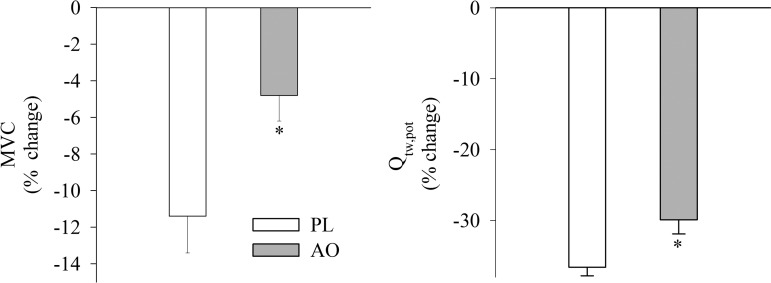
With respect to the development of peripheral fatigue during exercise, the percent increase in the integrated EMG signal (Fig. 4) was reduced during exercise in the AO condition and tended to be lower at end exercise (P = 0.09). Subjects' ratings of perceived exertion were also lower during exercise following AO infusion, as well as at the end of exercise. In line with these observations, the patients' dyspnea ratings were reduced at the end of the isotime trials in the AO condition (6.3 ± 1 vs. 4.8 ± 1, P > 0.05, for PL and AO, respectively). There were no changes in m-wave area (PL: 80 ± 9 mVms vs. 72 ± 10 mVms, P > 0.05; AO 85 ± 11 mVms vs. 82 ± 11 mVms, P > 0.05) or peak-to peak-amplitude (PL: 8.7 ± 0.8 mV vs. 8.1 ± 0.9 mV, P > 0.05; AO 9.3 ± 1.2 mV vs. 8.8 ± 1.2 mV, P > 0.05) in either condition from pre- to postexercise, and these values were not different between PL and AO trials. Voluntary activation was reduced following exercise to a similar extent in both conditions (AO: −3.1 ± 0.7% vs. PL: −3.5 ± 1.6%, P > 0.05). Additionally, the pre- to postexercise changes in MVC and Qtw,pot were reduced to a lesser extent in the AO condition, suggestive of less peripheral quadriceps fatigue (Fig. 5).
Fig. 5.
Changes from preexercise values in quadriceps muscle function following constant-load knee extensor exercise matched for intensity and duration preceded by either intravenous PL or AO administration. Data are presented as means ± SE. *Significantly different from the PL condition.
DISCUSSION
This study sought to evaluate the impact of intravenous AO on systemic antioxidant capacity and oxidative stress in patients with COPD and subsequently determine the effects of this intervention on skeletal muscle fatigue following exercise in this population. Before exercise, AO increased antioxidant capacity and reduced oxidative stress, and these changes in the pro- and antioxidant balance were accompanied by a reduction in MAP and an increase in femoral artery vascular conductance. Exercise after AO administration, matched for time with the PL trial, was associated with attenuated ventilatory and metabolic responses to the work and a slowed rate of fatigue development (rise in quadriceps iEMG). Thus the exercise bout ultimately resulted in less of a decrease in quadriceps MVC and evoked twitch force following exercise, revealing improved fatigue resistance during exercise. Collectively, these data demonstrate a beneficial effect of intravenous AO administration on systemic oxidative stress and skeletal muscle function in patients with COPD. Moreover, these data further implicate oxidative stress as a factor contributing to skeletal muscle dysfunction in COPD.
Oxidative stress and fatigue.
Previously, dynamic KE exercise has been documented to increase markers of oxidative damage in patients with COPD, but not in healthy control subjects (11, 12). In this prior study, within the patient group, the magnitude of increase in oxidative stress was negatively correlated with exercise time to exhaustion (12). Furthermore, when patients with COPD were pretreated with the pharmacological antioxidant N-acetylcysteine before performing KE exercise, markers of oxidative damage were reduced and exercise time to exhaustion was improved (24). Excessive elevations in free radicals, within muscle itself, have been suggested to impair function by decreasing the calcium sensitivity of the myofilaments and attenuating calcium reuptake by the sarcoplasmic reticulum, among other mechanisms (1). Collectively, these studies suggest that oxidative stress contributes to skeletal muscle dysfunction in patients with COPD, and decreasing the oxidant load has the potential to improve the intramuscular redox state and therefore skeletal muscle function in this population.
In the current study, intravenous AO decreased plasma markers of oxidative damage, improved the antioxidant status (Fig. 2), and attenuated exercise-induced fatigue (Fig. 5) in patients with COPD. Consequently, these data reveal that KE exercise performed by patients with COPD for the same duration and at the same intensity with AO infusion is associated with less peripheral quadriceps fatigue than without AO infusion. Specifically, the rate of increase in the integrated EMG signal from the vastus lateralis, an index of peripheral fatigue development during exercise, was attenuated (Fig. 4), and the magnitude of decrease in quadriceps MVC and Qtw,pot were diminished by ∼50% and ∼20%, respectively (Fig. 5). These data contrast with the lack of effect observed previously by our group following oral antioxidant administration in this population (32). However, the plasma concentration of ascorbate achieved in the current study, and consequently antioxidant capacity as assessed by FRAP, were elevated by approximately fivefold over the values obtained in the previous investigation (32), which may have enhanced the ability of the ascorbate to enter the muscle and exert beneficial effects on the myofilaments. It is therefore reasonable to hypothesize that the altered redox state following AO administration was translated into improved muscle function, potentially due to decreased intramuscular free radical accumulation and greater fatigue resistance during KE exercise. Thus these data suggest that oxidative stress contributes to skeletal muscle dysfunction in patients with COPD, and a reduction in oxidative stress lessens the magnitude of fatigue accumulated during high-intensity, small muscle mass exercise.
Physiological responses to exercise.
Feedback from skeletal muscle group III and IV afferent fibers contribute to the cardiovascular and ventilatory response to dynamic exercise (2). In the current study, ascorbate infusion led to a reduction in VE and VE/Vco2 ratio (Fig. 4) as well as attenuated sensations of dyspnea at the same exercise time points in the PL condition. The attenuated increase in the integrated EMG signal during exercise in the AO condition suggests less peripheral fatigue development during exercise. This is largely determined by the accumulation of metabolic by-products such as hydrogen ions and inorganic phosphates (41), as well as reactive oxygen species, within the muscle (1). These exercise-induced metabolites, as well as oxidative stress, have also been documented to activate group III and IV afferent fibers (15, 28). Thus the attenuated ventilatory responses to the exercise bouts may have been the result of improved muscle function following antioxidant administration due to an improved intramuscular redox state. This reduced metabolic perturbation during exercise would, in turn, diminish the requisite increase in Vo2 and Vco2 during exercise in the AO condition (Fig. 4).
Alternatively, oxidative stress has been documented to directly stimulate group IV afferent fibers (15), and blocking afferent feedback with spinal anesthesia attenuated the ventilatory response to exercise in patients with COPD (17). Therefore, reducing oxidant-driven afferent activity with the AO infusion may have contributed to the reduced ventilatory response in the AO condition in the current study. Collectively, these data reveal that reducing oxidative stress by intravenous AO administration was associated with an overall attenuation in the ventilatory and metabolic responses to exercise. These, likely positive, changes may be attributed to reduced stimulation of lower limb afferent fibers potentially due to improved muscle function during exercise and decreased metabolite accumulation, or less direct stimulation of afferent fibers by oxidative stress.
Blood pressure and vascular conductance.
Research regarding the hypotensive effect of antioxidant administration is equivocal. However, in a small, tightly controlled experiment, our group has previously observed a tendency for acute oral antioxidant supplementation to reduce arterial blood pressure in normotensive, older individuals (44), whereas chronic antioxidant treatment has been associated with reduced blood pressure in young healthy males (33). Under basal conditions in the current study, as is not unusual in this population (35), the patients with COPD had an average “high-normal” MAP of ∼104 mmHg, and this tendency to exhibit elevated blood pressure is associated with increased cardiovascular disease risk (40). Intriguingly, intravenous AO administration resulted in an ∼10- to 15-mmHg reduction in MAP, such that the average resting blood pressure for the patients with COPD in the AO condition returned to a healthy, normal value (Fig. 3). Because COPD is an independent predictor of cardiovascular disease mortality, and cardiovascular disease is a leading cause of hospitalizations in patients with mild to moderate COPD (35), ameliorating cardiovascular disease risk factors is of utmost importance. Thus these data suggest that some form of antioxidant treatment may be important for cardiovascular disease risk management in patients with COPD.
Despite reduced arterial blood pressure with the AO, femoral artery blood flow at rest was unchanged (Fig. 3). Thus when femoral artery blood flow was normalized for the decrease in perfusion pressure, resting femoral vascular conductance was significantly elevated in the AO condition (Fig. 3). Interestingly, the magnitude of increase in femoral vascular conductance was similar to that demonstrated previously in healthy, older individuals following a similar intravenous AO infusion (20). AO has been documented to activate endothelial nitric oxide synthase (25), and coinfusion of AO and a nitric oxide synthase inhibitor negates the ability of AO to improve blood flow and vascular conductance (14). Thus, in the current study, intravenous infusion of AO may have improved nitric oxide bioavailability, perhaps by both reducing oxidative stress and promoting nitric oxide production by nitric oxide synthase, which resulted in a reduction in total peripheral resistance, leading to reduced MAP and improved femoral vascular conductance. The potential increase in nitric oxide may have also improved oxygen distribution in the working muscle and improved aerobic metabolism (37), increasing fatigue resistance during exercise. Collectively, these data support a favorable role of reducing oxidative stress on resting hemodynamic parameters in patients with COPD.
Perspectives and Significance
This study documents the ability of an intravenous AO infusion to improve antioxidant capacity and decrease oxidative stress in patients with COPD. These changes in redox balance were associated with a reduction in resting blood pressure and elevated femoral vascular conductance. In addition, dynamic KE exercise performed for the same duration and at the same intensity as the placebo condition, was associated with an attenuated rate of development of peripheral quadriceps fatigue, improved metabolic and ventilatory responses, and less of a reduction in quadriceps force production assessed after exercise. These data further implicate oxidative stress in the systemic, pathophysiological consequences of COPD and suggest a beneficial role for reducing oxidative stress in this population. Therefore, targeting oxidative stress with some form of antioxidant therapy in a clinical setting may represent an important therapeutic avenue for patients with COPD.
GRANTS
This study was funded by the National Heart, Lung, and Blood Institute Grant PO1 HL-09830.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.R., M.A., and R.S.R. conception and design of research; M.J.R., R.S.G., H.J.G., and M.A. performed experiments; M.J.R., V.R., and J.Z. analyzed data; M.J.R., R.S.G., M.A., and R.S.R. interpreted results of experiments; M.J.R. prepared figures; M.J.R., M.A., and R.S.R. drafted manuscript; M.J.R., R.S.G., H.J.G., M.A., and R.S.R. edited and revised manuscript; M.J.R., R.S.G., H.J.G., V.R., J.Z., M.A., and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol 104: 861–870, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239: 70–76, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics, 1998 [Google Scholar]
- 8.Bradley DW, Emery G, Maynard JE. Vitamin C in plasma: a comparative study of the vitamin stabilized with trichloroacetic acid or metaphosphoric acid and the effects of storage at −70 degrees, −20 degrees, 4 degrees, and 25 degrees on the stabilized vitamin. Clin Chim Acta 44: 47–52, 1973 [DOI] [PubMed] [Google Scholar]
- 9.Bronstad E, Rognmo O, Tjonna AE, Dedichen HH, Kirkeby-Garstad I, Haberg AK, Bjork Ingul C, Wisloff U, Steinshamn S. High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur Respir J 40: 1130–1136, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 23: 932–946, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Couillard A, Koechlin C, Cristol JP, Varray A, Prefaut C. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J 20: 1123–1129, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Couillard A, Maltais F, Saey D, Debigare R, Michaud A, Koechlin C, LeBlanc P, Prefaut C. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167: 1664–1669, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Couillard A, Prefaut C. From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J 26: 703–719, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y. Reactive oxygen species activate the group IV muscle afferents in resting and exercising muscle in rats. Pflügers Arch 459: 143–150, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gagnon P, Bussieres JS, Ribeiro F, Gagnon SL, Saey D, Gagne N, Provencher S, Maltais F. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186: 606–615, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94: 6–9, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Hornig B, Arakawa N, Kohler C, Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation 97: 363–368, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol 103: 1715–1721, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Jeffery Mador M, Kufel TJ, Pineda L. Quadriceps fatigue after cycle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161: 447–453, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Killian KJ. Limitation to muscular activity in chronic obstructive pulmonary disease. Eur Respir J 24: 6–7, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koechlin C, Couillard A, Simar D, Cristol JP, Bellet H, Hayot M, Prefaut C. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med 169: 1022–1027, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Ladurner A, Schmitt CA, Schachner D, Atanasov AG, Werner ER, Dirsch VM, Heiss EH. Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic Biol Med 52: 2082–2090, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lepers R, Maffiuletti NA, Rochette L, Brugniaux J, Millet GY. Neuromuscular fatigue during a long-duration cycling exercise. J Appl Physiol 92: 1487–1493, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy MN, Mizuno M, Mitchell JH, Smith SA. Cardiovascular regulation by skeletal muscle reflexes in health and disease. Am J Physiol Heart Circ Physiol 301: H1191–H1204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve 19: 549–555, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Remels AH, Gosker HR, Langen RC, Schols AM. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol 114: 1253–1262, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol 292: H1516–H1522, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Rossman MJ, Groot HJ, Reese V, Zhao J, Amann M, Richardson RS. Oxidative stress and COPD: the effect of oral antioxidants on skeletal muscle fatigue. Med Sci Sports Exerc 45: 1235–1243, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schutte AE, Huisman HW, Oosthuizen W, van Rooyen JM, Jerling JC. Cardiovascular effects of oral supplementation of vitamin C, E and folic acid in young healthy males. Int J Vitam Nutr Res 74: 285–293, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A, Gosker HR, Schols AM, Moxham J, Polkey MI, Wouters EF. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 36: 81–88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sin DD. Is COPD really a cardiovascular disease? Chest 136: 329–330, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 97: 2222–2229, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci USA 98: 355–360, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 97: 22–28, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 345: 1291–1297, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Westerblad H, Allen DG. Cellular mechanisms of skeletal muscle fatigue. Adv Exp Med Biol 538: 563–571, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW., Jr Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem 184: 193–199, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O'Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 116: 433–441, 2009 [DOI] [PubMed] [Google Scholar]