Abstract
Many diseases associated with sympathoexcitation also exhibit elevated reactive oxygen species (ROS). A recent animal study indicated that exogenous administration of the sympathetic neurotransmitter norepinephrine (NE) increased systemic ROS via circulating leukocytes. The mechanisms contributing to this effect of NE and whether these findings can be translated to humans is unknown. Thus we tested the hypothesis that NE increases superoxide production in human peripheral blood mononuclear cells (PBMCs) via NADPH oxidase. Primary human PBMCs were freshly isolated from healthy young men and placed in culture. After NE (50 pg/ml, 50 ng/ml, and 50 μg/ml concentrations) or control treatments, NADPH oxidase mRNA expression (gp91phox, p22phox, and p67phox) was assessed using real-time RT-PCR, and intracellular superoxide production was measured using dihydroethidium fluorescence. PBMCs were also treated with selective adrenergic agonists-antagonists to determine the receptor population involved. In addition, CD14+ monocyte-endothelial cell adhesion was determined using a fluorescent-based assay. NE significantly increased NADPH oxidase gene expression and intracellular superoxide production in a time-dependent manner (superoxide: 0.9 ± 0.2 fold, 6 h vs. 3.0 ± 0.3 fold, 36 h; NE, 50 μg/ml; P < 0.05). The sustained increase in NE-induced superoxide production was primarily mediated via α-adrenergic receptors, preferentially α2-receptors. The NADPH oxidase blocker diphenylene iodonium and protein kinase C inhibitor Staurosporine significantly attenuated NE-induced increases in superoxide production. Importantly, NE treatment increased CD14+ monocyte-endothelial cell adhesion. These findings indicate for the first time that NE increases superoxide production in freshly isolated primary human PBMCs via NADPH oxidase through α-adrenergic receptors, an effect facilitating monocyte adhesion to the endothelium.
Keywords: sympathetic nerve activity, reactive oxygen species, oxidative stress
chronically elevated sympathetic nerve activity is a hallmark characteristic of several cardiovascular diseases (14, 17, 29). Recently, many of these diseases have also been shown to exhibit elevated systemic oxidative stress (9, 19, 38). However, it is unclear whether elevated oxidative stress causes sympathoexcitation or vice versa.
Numerous studies performed in animals have convincingly shown that an increase in central superoxide levels induces activation of key brain areas, such as the rostral ventrolateral medulla, to cause sympathoexcitation (4, 33, 44, 53, 54). In contrast, to our knowledge, little consideration has been given to the potential ability of the sympathetic neurotransmitter norepinephrine (NE) to increase oxidative stress. In this regard, Schraml et al. have shown that, when rats were implanted subcutaneously with tablets releasing NE, oxidative stress in circulating leukocytes was significantly increased (40). These findings suggest a possible role for elevated plasma NE, as a consequence of sympathoexcitation, to increase systemic reactive oxygen species (ROS) formation in circulating immune cells. Indeed, peripheral blood mononuclear cells (PBMCs), particularly monocytes, have been shown to produce systemic ROS in conscious mice via an NADPH oxidase pathway (47). However, whether NE stimulates PBMCs and activates NADPH oxidase to increase ROS is unknown.
Thus, in the present study, a series of protocols were performed using freshly isolated primary human PBMCs in culture to test the hypothesis that NE increases superoxide production in human PBMCs. First, we examined the effect of NE treatment in varying concentrations on NADPH oxidase gene expression and intracellular superoxide production in PBMCs with and without NADPH oxidase blockade. Next, to determine the adrenergic receptor population and intracellular signaling events involved in mediating NE responses, studies were repeated with selective adrenergic agonists-antagonists and the protein kinase C inhibitor Staurosporine, respectively, with the latter being integral to α2-adrenergic receptor signaling in PBMCs (23). Last, in an attempt to begin to understand some of the functional implications of this work, we examined the influence of NE treatment on monocyte-endothelial cell adhesion.
METHODS
General Procedures
Blood draws were taken on multiple days from seven young healthy men (age, 30 ± 1 yr; body mass index, 26 ± 2 kg/m2). Subjects were nonsmokers and were recreationally active (<3 days/wk) but not training competitively. None of the subjects had a history or symptoms of cardiovascular, pulmonary, metabolic, or neurological disease, and none were taking medication. After receiving a detailed verbal and written explanation of the intended experimental protocol, each subject provided written, informed consent. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the University of Missouri Health Sciences Institutional Review Board. On experimental days, subjects arrived at the laboratory in the morning after an overnight fast and without alcohol and physical activity for 24 h. Subjects were positioned supine in a quiet, temperature-controlled room (22–23°C), and after a minimum of 10 min, 60 ml of blood were obtained from the antecubital vein and collected in sodium heparin tubes.
Isolation of PBMCs
Primary PBMCs were freshly isolated from whole blood with density gradient centrifugation using Histopaque (Sigma, St. Louis, MO) at 600 g for 30 min at room temperature followed by three washes in sterile Dulbecco's PBS (Invitrogen, Life Technologies) solution. Cells were counted using the trypan blue (Sigma) exclusion method (42, 43, 45) to quantify cell counts before and after protocols.
Cell Culture
Primary PBMCs were incubated at 37°C in enrichment medium (RPMI 1640; Invitrogen, Life Technologies) supplemented with 10% fetal bovine serum and antibiotics. Subsequently, the cells were then treated with NE at various concentrations over several time points (see Figs. 1 and 2, and detailed protocols below). Briefly, a stock solution of NE (50 mg/ml) was prepared in 0.5 M HCl, which was further diluted with PBS to make 50 μg/ml, 50 ng/ml, and 50 pg/ml concentrations. Of note, the range of NE in healthy and diseased populations is quite large, ranging from 100 pg/ml to 1 ng/ml (9, 12, 30, 34), and, therefore, falls within the NE concentration range of 50 pg/ml to 50 ng/ml in our study. The 50 ng/ml to 50 μg/ml concentration range can be considered as supraphysiological; however, this range is similar to those used in previous studies examining NE effects in cell culture (24, 28). The PBS solution (vehicle) devoid of NE was considered as control treatment for all conditions.
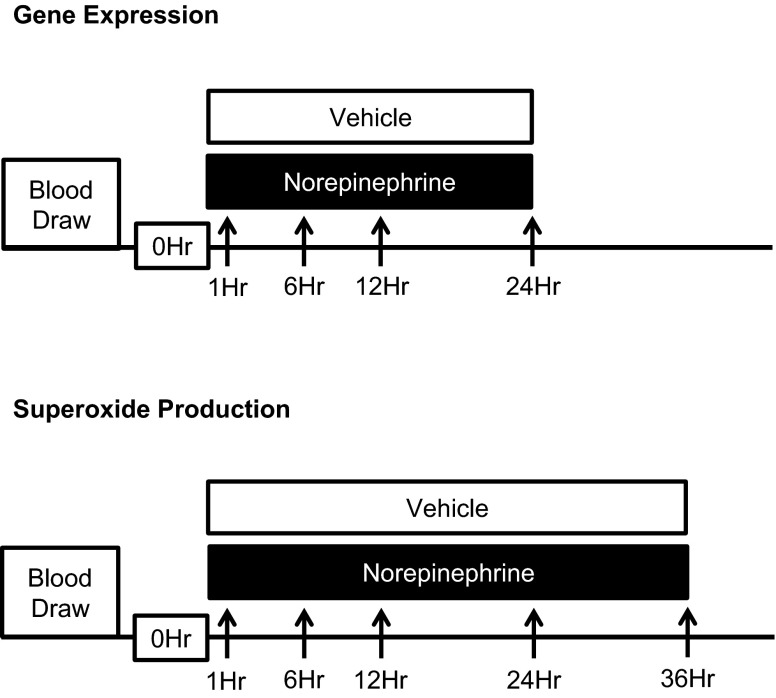
Fig. 1.
Experimental protocols for study 1. Arrows indicate time points for NADPH oxidase gene expression and superoxide production measurements. See methods for further details.
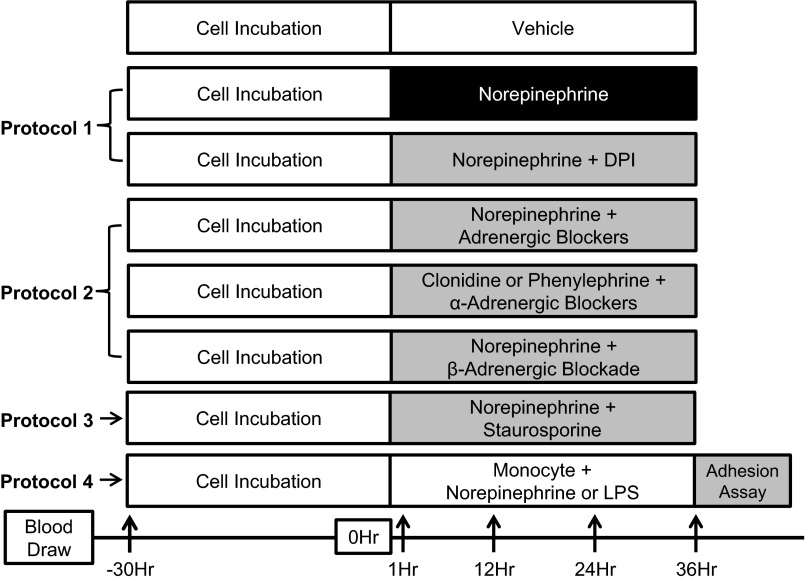
Fig. 2.
Experimental protocols for study 2. Arrows indicate time points for superoxide production measurements. All antagonists were given 60 min before norepinephrine (NE) or select agonist treatments. See methods for further details.
Assessment of NADPH Oxidase Gene Expression by Real-Time RT-PCR
To determine the effect of NE on NADPH oxidase gene expression in PBMCs, ∼3 × 106 cells per well were plated in 96-well plates. Total RNA was extracted using QIAmp RNA Blood Mini kit (Qiagen), according to manufacturer's instructions. Subsequently, total RNA was reverse transcribed using A5300 kit (Promega), and 5 ng of cDNA from each sample was then assayed for the determination of gene expression. mRNA levels of NADPH oxidase subunit genes (gp91phox, p22phox, and p67phox) were determined using gene-specific primers and Taqman chemistry (Taqman Gene Expression Assays, Life Technologies). The amplification program included the initial denaturation step at 95°C for 20 min, 40 cycles of denaturation at 95°C for 10 s, and annealing/extension at 60°C for 20 s. Fluorescence was measured at the end of each extension step. After amplification, melting curves were acquired and used to determine the specificity of PCR products. Real-time PCR was performed using A7900 Fast Real Time PCR System (Applied Biosystems). 18S rRNA was chosen as the internal control for these gene expression experiments because the cycle threshold (Ct) values for GAPDH were consistently high (>30 cycles), indicating very low expression and large variability. In contrast, 18S rRNA had much lower Ct values (∼17 cycles) and was quite stable with no significant differences between and within treatments as well as across time courses. For example, at 1 h, the Ct values for 18S rRNA during control and NE (pg/ml, ng/ml, and μg/ml) treatments ranged from 17.2 ± 0.3 to 17.5 ± 0.5 cycles. Likewise, at 24 h, the Ct values ranged from 16.5 ± 0.4 to 17.1 ± 0.6 cycles. In addition, primer amplification efficiencies were compared between 18S rRNA and all target genes for the validation of ΔCt calculation. A plot of the log cDNA dilution vs. ΔCt (target gene Ct − 18S rRNA Ct) (27) was made, and the slope of each relationship was close to zero, indicating that the efficiencies for target genes compared with 18S rRNA were similar.
Detection of Intracellular Superoxide Levels
Superoxide measurements were performed in duplicate according to previously published methods (21, 48). Briefly, ∼1.5 × 106 cells were incubated with 5 μM dihydroethidium (DHE; Life Technologies) and drug or control (PBS only) treatments in a final volume of 300 μl of serum-free PBS for 60 min at 37°C. Excess DHE was removed by centrifugation at 500 g for 5 min, and cells were resuspended in 300 μl of PBS. Superoxide fluorescence was measured on a fluorescent plate reader (Spectramax M2, Molecular Devices) using excitation and emission filters of 544 and 612 nm, respectively (21, 48). At the end of each time point, cells were recounted, and the cell number was confirmed to be similar compared with the 0-h time point. The overall intra-assay coefficient of variation for DHE fluorescence was determined to be 26 ± 3% in control samples (PBS only). In contrast, the effect size following NE treatments ranged from 89 to 288%, indicating that the NE-induced increase in superoxide production was significantly above the variability for the control signal. In addition, adequate sample size was achieved, as evidenced by power analysis (treatment × time) that was estimated to be above 0.8.
Experimental Protocols
Study 1.
to determine whether NE increases expression of NADPH oxidase subunits and superoxide production in primary human PBMCs.
Freshly isolated PBMCs were plated in 96-well plates treated with PBS (control) or NE (50 pg/ml, 50 ng/ml, and 50 μg/ml), and NADPH oxidase subunit gene expression and superoxide production were measured at various time points (Fig. 1).
Study 2.
PROTOCOL 1: TO DETERMINE WHETHER NE-INDUCED INCREASES IN SUPEROXIDE PRODUCTION IN PBMCS IS MEDIATED VIA NADPH OXIDASE.
Freshly isolated PBMCs were plated and incubated for 30 h in media. Following supplementation of media to maintain cell stability, cells were treated with PBS (control) and NE (50 pg/ml, 50 ng/ml, and 50 μg/ml) (Sigma). Intracellular superoxide production was measured at 1, 12, 24, and 36 h (protocol 1; Fig. 2). In addition, NADPH oxidase inhibitor diphenylene iodonium (DPI; 2 μM; Sigma) was added to the NE-treated cells, and superoxide production was measured at 36 h to determine the involvement of NADPH oxidase in mediating NE-induced superoxide production in PBMCs (protocol 1; Fig. 2).
PROTOCOL 2: TO DETERMINE WHETHER NE-INDUCED INCREASES IN SUPEROXIDE PRODUCTION IN PBMCS IS MEDIATED VIA ADRENERGIC RECEPTORS.
Cells were treated with a combination of adrenergic antagonists 60 min before NE (50 μg/ml) treatment and PBS (control) (protocol 2; Fig. 2). This was achieved by isolating each adrenergic receptor (α1, α2, β1, and β2) by blocking the other receptors. For example, α2-, β1-, and β2-adrenergic receptors were blocked to determine α1 contributions. Thus Doxazosine (10 μM), yohimbine (0.1 μM), metoprolol (0.1 μM), and ICI 118551 (0.1 μM) were used as selective antagonists to block α1-, α2-, β1-, and β2-adrenergic receptors, respectively. The nonselective β-adrenergic antagonist propranolol (1 μM) and the selective α1- and α2-adrenergic agonists phenylephrine (10 μM) and clonidine (100 μM), respectively, were also used to further delineate the involvement of α-adrenergic receptors in mediating the NE-induced increase in superoxide. Intracellular superoxide production was measured at 1, 12, 24, and 36 h (protocol 2; Fig. 2). All adrenergic receptor agonists and antagonists were obtained from Sigma.
PROTOCOL 3: TO DETERMINE WHETHER PROTEIN KINASE C (PKC) IS INVOLVED IN MEDIATING NE-INDUCED INCREASES IN SUPEROXIDE PRODUCTION IN PBMCS.
After the 30-h incubation in media, cells were treated with PBS (control), NE (50 μg/ml), or NE (50 μg/ml) + selective PKC inhibitor Staurosporine (10 nM; Calbiochem) up to 36 h, and intracellular superoxide production was measured at 1, 12, 24, and 36 h. The Staurosporine was added 60 min before NE treatment (protocol 3; Fig. 2). The 10 nM dose of Staurosporine was chosen for these studies since this dosage has been shown to block PKC-induced oxidative stress in a human monocyte cell line (22) without inducing apoptosis (51).
PROTOCOL 4: TO DETERMINE WHETHER NE-INDUCES CD14+ MONOCYTE-ENDOTHELIAL CELL ADHESION.
CD14+ monocytes were freshly isolated from primary PBMCs using immunomagnetic beads (Miltenyi Biotec) with positive selection approach followed by treatment with PBS (control), NE (50 μg/ml), and LPS (1 μg/ml) for 36 h (protocol 4; Fig. 2). LPS served as a positive control for the NE experiment, since it is a known pro-inflammatory agent that enhances monocyte-endothelial cell adhesion (10, 31). For this protocol, monocyte-endothelial cell adhesion was performed using a commercially available leukocyte cell adhesion assay kit (Cell Biolabs) (13, 26, 49). After the 36-h treatments, monocytes were washed to remove any excess NE or LPS from the media before co-incubating them with naive cultured human umbilical vein endothelial cells (HUVEC; American Type Culture Collection) at 37°C for 60 min in 96-well plates. The cellular mixture was washed with serum-free media, and the nonadherent cells were removed. The plate was read following cell lysis on a fluorescent plate reader using excitation and emission filters of 480 and 520 nm, respectively. This protocol was designed to determine the extent to which NE increased surface adhesion molecules on monocytes with the rationale that, after NE treatment, the monocytes would be more adherent to HUVEC compared with control. Although venous endothelial cells and arterial endothelial cells are not expected to be identical, we used naive HUVEC as a platform to test the adherence of the NE-activated monocytes, which is common for such cell adhesion-based assays (1, 7, 10, 41).
Data and Statistical Analysis
All fluorescence data are expressed as fold changes relative to the control (PBS only) condition at the 1-h time point, which is represented as onefold. However, the one exception is the monocyte-endothelial cell adhesion experiments where the data is expressed relative to the control (PBS only) condition at the 36-h time point. In addition, real-time RT-PCR data are presented as 2ΔCt mean ± SE values (ΔCt = 18S rRNA Ct − gene of interest Ct) (36) and are normalized to the 1-h time point (set at 1.0).
Statistical analyses were conducted using SigmaStat (Jandel Scientific Software, SPSS) software for Microsoft Windows. All results are presented as means ± SE. When the data were normally distributed, between- and within-treatment and time differences were analyzed using two-way, repeated-measures ANOVA (treatment × time), whereas when the data was not normally distributed, it was log transformed before ANOVA analyses. When a main effect was obtained, Fisher's least significant differences post hoc test was performed. All tests were two-sided, and a P value of <0.05 was considered statistically significant.
RESULTS
Study 1
NE increases NADPH oxidase subunit gene expression and superoxide production in human PBMCs.
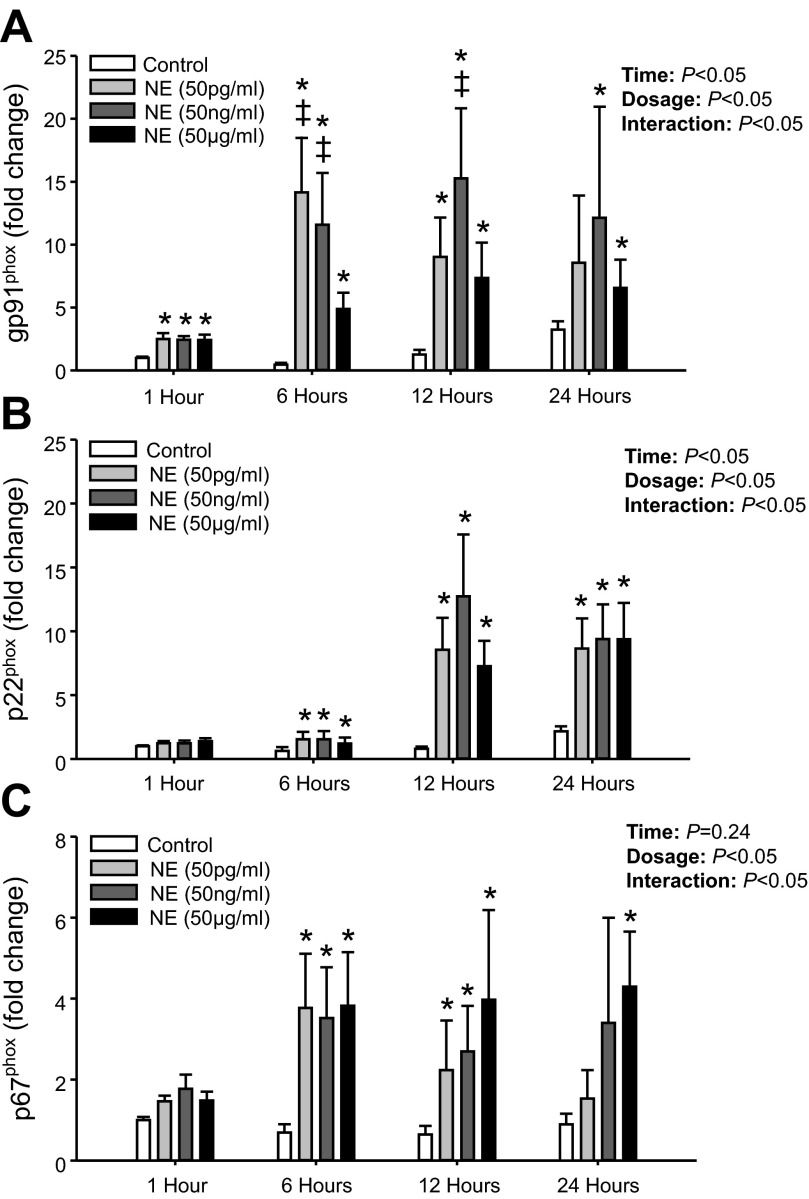
In initial experiments, NE at all concentrations studied (50 pg/ml, 50 ng/ml, and 50 μg/ml) significantly increased the gene expression for gp91phox in PBMCs at all time points compared with control, except for 50 pg/ml at 24 h (Fig. 3A). For p22phox, NE treatment significantly increased mRNA expression at all concentrations from 6 to 24 h compared with control (Fig. 3B). The NE-mediated increase in p22phox expression was delayed compared with gp91phox since no significant increase was observed at 1 h for any of the NE concentrations. Similarly, p67phox mRNA expression was not significantly increased at 1 h; however, NE significantly increased p67phox mRNA expression at all concentrations from 6 to 24 h (Fig. 3C) except for 50 pg/ml and 50 ng/ml at 24 h.
Fig. 3.
Mean data from study 1 showing mRNA levels for gp91phox (A), p22phox (B), and p67phox (C) at 1, 6, 12, and 24 h following NE (50 pg/ml, 50 ng/ml, and 50 μg/ml) treatment. Data are expressed as fold change from control treatment (PBS only) at 1 h. *Significant difference vs. Control (P < 0.05; within time point). ‡Significant difference vs. NE (50 μg/ml) (P < 0.05; within time point).
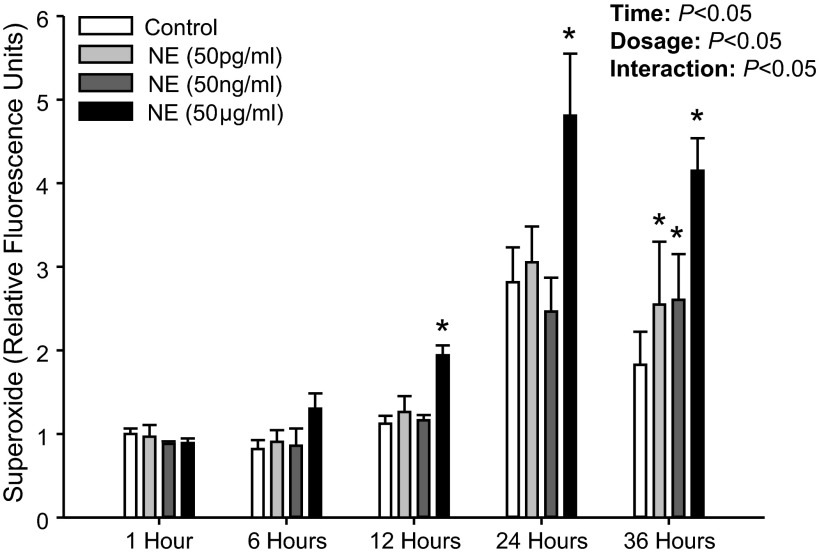
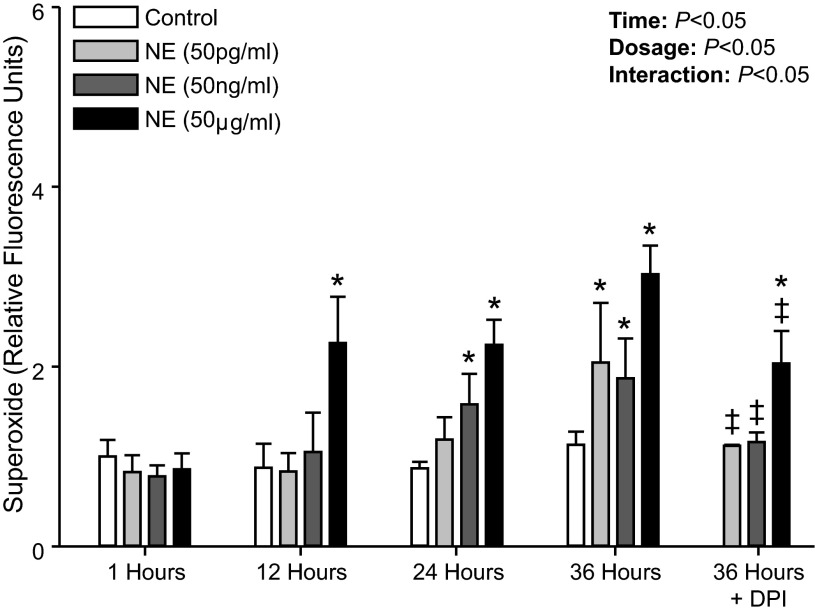
Figure 4 shows superoxide production following NE treatment from our initial experiments in which cells were immediately placed in media and treated with NE. Although NE significantly increased superoxide production with this protocol, there was also an unexpected increase in superoxide production in the control treatment (PBS only) at 24 and 36 h compared with 1 h (P < 0.05). Thus we modified our protocol to first incubate the PBMCs for 30 h and then, following media supplementation, NE treatments were performed (Fig. 2). This pre-incubation allowed for a maintained superoxide level during control treatments across all time points (P > 0.05; Fig. 5), and importantly, similar results were obtained in regard to NE treatment in that superoxide production was significantly elevated compared with control treatment (Fig. 5).
Fig. 4.
Mean data from study 1 showing intracellular superoxide production at 1, 6, 12, 24, and 36 h following NE (50 pg/ml, 50 ng/ml, and 50 μg/ml) treatment. Data are expressed as fold change from control treatment (PBS only) at 1 h. *Significant difference vs. Control (P < 0.05; within time point).
Fig. 5.
Mean data showing intracellular superoxide production at 1, 12, 24, and 36 h following NE (50 pg/ml, 50 ng/ml, and 50 μg/ml) treatment applied at 30 h. Also shown is mean data for superoxide production with the addition of DPI (NE + DPI) to block NADPH oxidase at 36 h. Data are expressed as fold change from control treatment (PBS only) at 1 h. *Significant difference vs. Control (P < 0.05; within time point). ‡Significant difference vs. NE at 36 h (P < 0.05).
Study 2
NE increases superoxide production in human PBMCs via NADPH oxidase.
NE at 50 μg/ml increased superoxide production at all time points (12 to 36 h; P < 0.05). In addition, NE at 50 ng/ml showed significant increases at 24 and 36 h, whereas 50 pg/ml raised superoxide levels at 36 h (P < 0.05; Fig. 5). The addition of DPI to inhibit NADPH oxidase significantly attenuated the superoxide production in the NE-treated cells at 36 h (Fig. 5). Of note, the numbers of viable PBMCs remained unchanged over the entire course of the experiment (150.4 ± 0.2 × 104 at incubation to 149.9 ± 0.3 × 104 at 66 h; P > 0.05). In addition, no significant difference was observed in the number of viable PBMCs between control and NE (50 μg/ml) treatments (P > 0.05) across time. Also, when the PBMC-derived superoxide production was compared between control and DPI-only treated cells, no significant difference was observed at any time point during the course of the experiment (e.g., 1.1 ± 0.1-fold control vs. 1.6 ± 0.3-fold DPI only at 36 h; P > 0.05), indicating that DPI also did not affect cell viability.
NE increases superoxide production in PBMCs via α-adrenergic receptors.
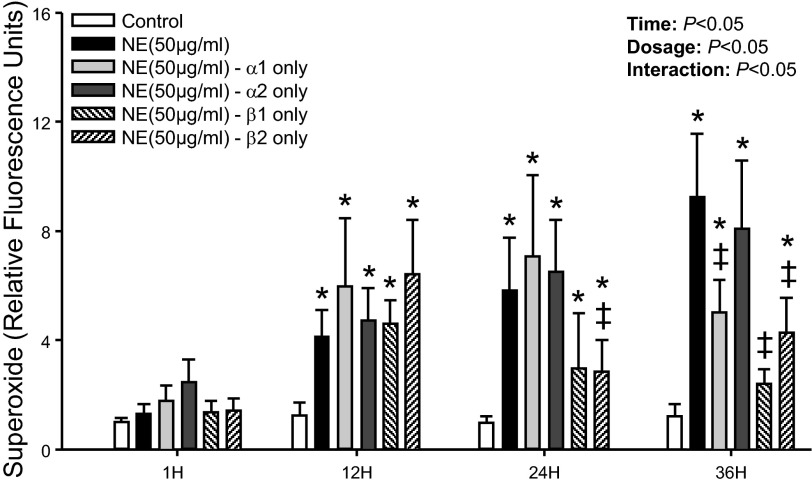
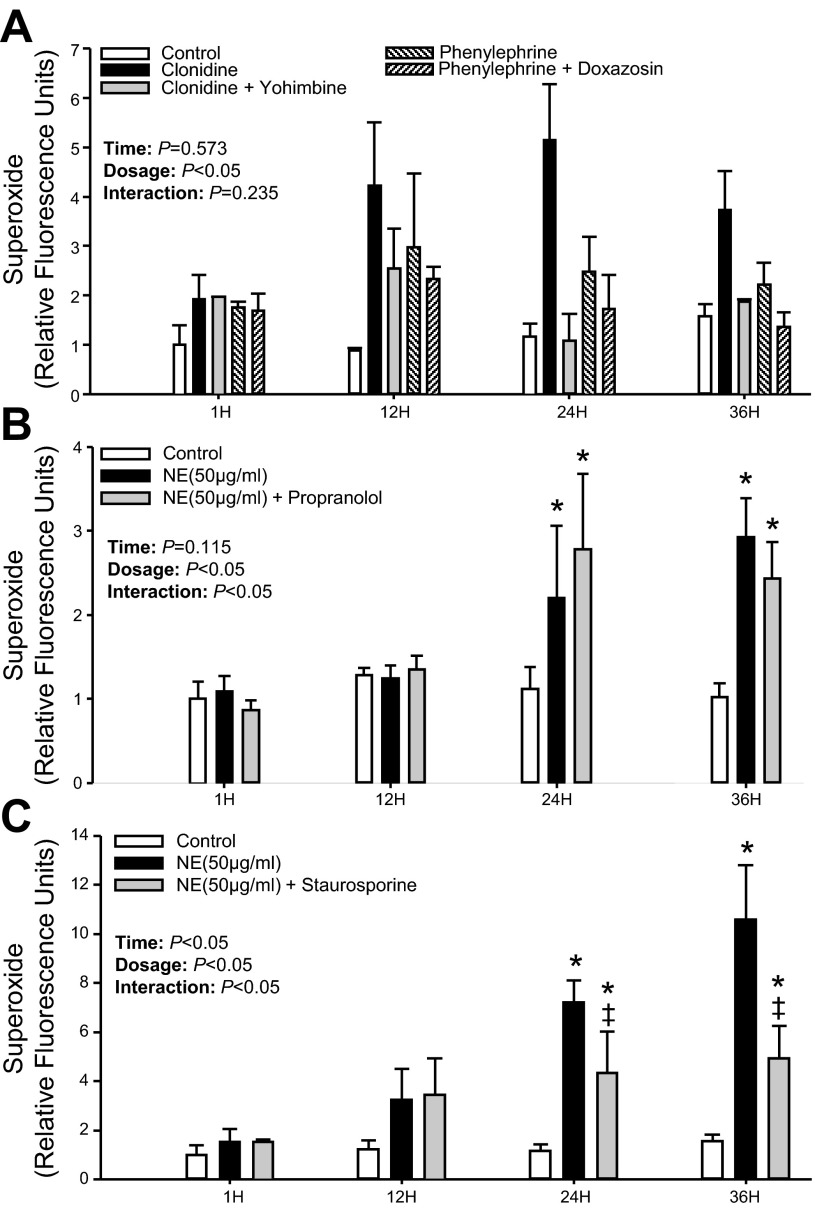
As expected, NE at 50 μg/ml increased superoxide production in PBMCs at 12, 24, and 36 h (P < 0.05; Fig. 6). When α2-adrenergic receptors were primarily available, intracellular superoxide production remained elevated at all time points. In contrast, although elevated at 12 and 24 h when α1-receptors were mainly available, superoxide production was blunted at 36 h (P < 0.05; Fig. 6). The NE-induced increase in superoxide production was unaffected at 12, 24, and 36 h following nonselective blockade of β-receptors to isolate α-adrenergic receptors (P > 0.05; Fig. 6). Furthermore, when β1- and β2-receptors were only available for NE binding, superoxide production was significantly blunted at 24 and 36 h (Fig. 6). Although treatment with the selective α1-adrenergic agonist phenylephrine showed only a trend for increased superoxide production, the α2-adrenergic agonist clonidine increased intracellular superoxide production at 24 and 36 h (P < 0.05; Fig. 7A) in a similar manner compared with NE (Fig. 6). This increase in superoxide production was significantly blunted by the selective α2-adrenergic antagonist yohimbine (P < 0.05; Fig. 7A), which was independent of time. In addition, when β1- and β2-receptors were blocked by propranolol, the increase in NE-induced superoxide production remained unchanged at 24 and 36 h (P > 0.05; Fig. 7B).
Fig. 6.
Mean data showing intracellular superoxide production at 1, 12, 24, and 36 h following NE (50 μg/ml) treatment applied at 30 h, whereas adrenergic receptor antagonists were applied 60 min before the NE treatment. Data are expressed as fold change from control treatment (PBS only) at 1 h. *Significant difference vs. Control (P < 0.05; within time point). ‡Significant difference vs. NE (P < 0.05; within time point).
Fig. 7.
Mean data showing intracellular superoxide production at 1, 12, 24, and 36 h following selective α-adrenergic receptor agonist-antagonist (A), NE (50 μg/ml) with and without propranolol (1 μM) (B), and Staurosporine (10 nM) (C) applied at 30 h while receptor antagonists were applied 60 min prior to the agonist treatment. Data are expressed as fold change from control treatment (PBS only) at 1 h. *P < 0.05 vs. Control (within time point). ‡P < 0.05 vs. NE (within time point).
NE increases superoxide production in PBMCs via PKC.
The NE-induced increase in superoxide production was significantly attenuated at 24 and 36 h by the selective PKC inhibitor Staurosporine (P < 0.05; Fig. 7C).
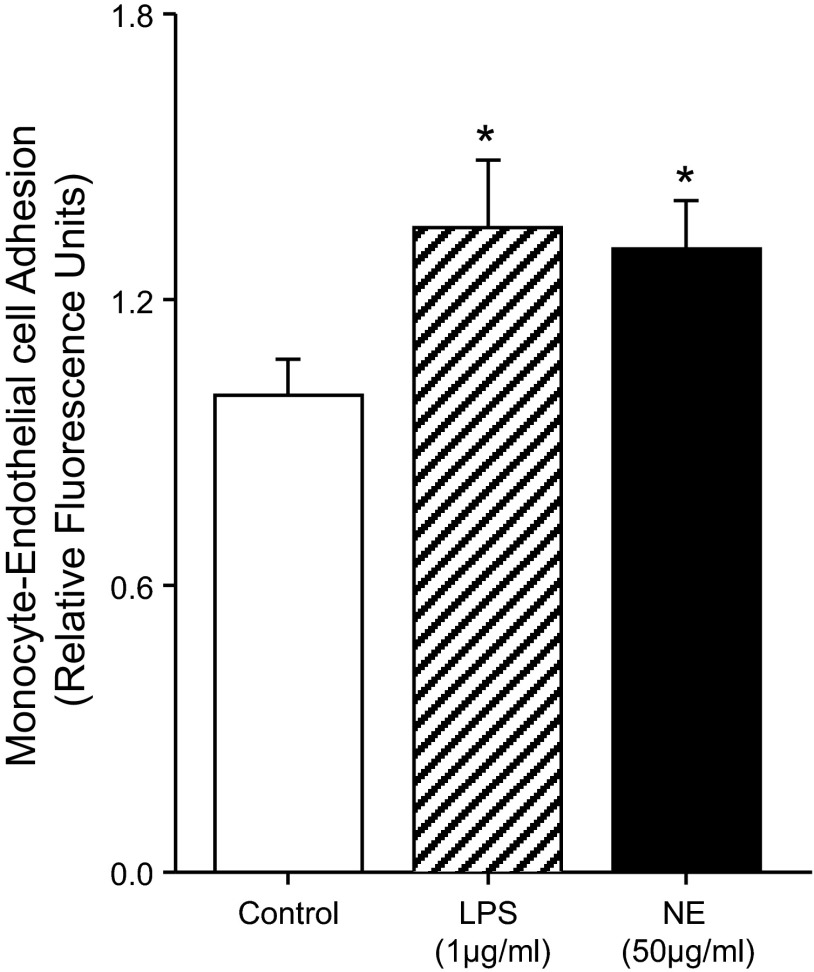
NE enhances monocyte-endothelial cell adhesion.
The adhesion between NE-treated CD14+ monocytes and naive HUVEC was significantly greater compared with control conditions (P < 0.05; Fig. 8). In addition, the increase in the monocyte-HUVEC adhesion due to NE was similar to LPS (P > 0.05).
Fig. 8.
Mean data showing human monocyte-endothelial cell adhesion at 36 h following control (PBS-only), LPS (1 μg/ml), or NE (50 μg/ml) treatments. Data are expressed as fold change from control treatment. *P < 0.05 vs. Control.
DISCUSSION
The major novel finding of our study is that NE induced a sustained increase in superoxide production in freshly isolated primary human PBMCs via α-adrenergic receptors, preferentially α2-receptors. The observed NE-induced increase in intracellular superoxide production was mediated via NADPH oxidase enzyme complex and required PKC signaling. Several studies performed in animals have convincingly shown that increased superoxide levels in key brain areas, such as the rostral ventrolateral medulla, contribute to sympathoexcitation (4, 33, 44, 53, 54). Here, we provide the first evidence in humans that NE, the primary neurotransmitter for the sympathetic nervous system, can stimulate the production of superoxide in PBMCs that in turn may contribute to systemic oxidative stress. Furthermore, we demonstrate that stimulation of CD14+ primary human monocytes with NE increases adhesion with naive HUVEC. Overall, these findings indicate for the first time that NE increases superoxide production in freshly isolated primary human PBMCs via NADPH oxidase through α-adrenergic receptors, an effect facilitating monocyte adhesion to the endothelium.
PBMCs have recently been identified as one of the primary contributors to circulating ROS. For example, when circulating PBMCs, primarily monocytes, were selectively depleted in mice, systemic superoxide levels were significantly reduced (47). Restoration of these cells in the circulation reestablished the systemic oxidant status in these same animals, indicating that PBMCs were the major source of systemic oxidative stress (47). The findings from the present study demonstrated that NE can stimulate PBMC-derived superoxide production via NADPH oxidase, identifying a potential contributor to systemic oxidative stress. The importance of NE as a mediator of superoxide production in PBMCs is further substantiated by the work of Schraml et al., in which subcutaneously implanted NE tablets in conscious rats induced significant increases in oxidative stress in circulating leukocytes (40). Furthermore, Kang et al. showed in mice that splenic macrophages are activated via α2-adrenergic receptors and PKC (23). These findings support the results from the present study that NE acts preferentially via α2-adrenergic receptors and PKC in primary human PBMCs to increase intracellular superoxide production.
Various downstream pathways can be postulated to establish a connection between NE and superoxide production. Here, we focused on the NADPH oxidase enzyme complex, which is known to be present in PBMCs (19, 47). In this regard, we present the novel findings that NE increases the expression of key NADPH oxidase subunit genes with the associated increase in superoxide production in PBMCs. In addition, because PKC has been implicated as a major enzyme for NADPH oxidase subunit phosphorylation and activation, and is also integral to α2-adrenergic receptor signaling in PBMCs, we determined the extent to which PKC is involved in mediating the observed NE-mediated responses in PBMCs (3, 23, 52). Staurosporine significantly attenuated the NE-induced increases in superoxide production, indicating a requirement of PKC. Nevertheless, regardless of the intracellular signaling events involved, our finding that DPI largely prevented the NE-induced superoxide production indicates that NADPH oxidase was the primary molecular source of superoxide under the conditions of our study.
The time course for the increases in the NADPH oxidase mRNA expression induced by NE treatments were somewhat variable, with gp91phox (catalytic subunit) being significantly increased as early as 1 h (with all NE concentrations), followed by both p67phox (cytosolic subunit) and p22phox being increased at 6 h. Interestingly, NE at the highest concentration induced a smaller increase in mRNA expression of gp91phox than the lower concentrations. This lower mRNA expression might have resulted from negative feedback from the large increases in superoxide production with the higher concentration of NE (20); however, mRNA for the other subunits was elevated. For superoxide production, we observed the expected time delay required to translate the mRNA to protein and evoke a functional response. However, despite similar or greater gene expression between the low (50 pg/ml) and high (50 μg/ml) NE concentrations used, superoxide production was elevated much more rapidly with the highest NE concentration. One of the reasons for this discrepancy between mRNA expression and superoxide production may be due to posttranslational modification, as suggested by others (20). Thus, at any given time following the lower NE (pg/ml) treatment, not all NADPH oxidase subunits may be made active and available to form a functional enzyme complex to produce superoxide. However, at high NE (μg/ml) concentrations, the stimulus is robust enough to ensure posttranslational modification and activation of all NADPH oxidase subunit proteins to form a functional enzyme complex and increase superoxide production. It is also possible that a threshold may exist such that lower NE concentrations (pg/ml and ng/ml) may bind to the adrenergic receptors but may not be able to produce a strong enough response to activate the downstream pathways and allow the NADPH oxidase enzyme complex to be activated and produce an increase in superoxide. Nevertheless, it is important to note that, over time, the lower NE concentrations produced similar elevations in superoxide to the highest concentration (Fig. 5). Indeed, all three concentrations of NE significantly increased superoxide production.
In our studies, we chose to use PBMCs as our cellular model since it closely represents the physiological hematopoietic environment in circulation and the known cross talk between innate (monocytes) and adaptive (lymphocytes) immune cells (11, 35, 39). Thus, functionally, the net response observed from these heterogeneous cell populations is more representative of an intact system as opposed to individual populations of cells. Furthermore, it is important to note that these findings are from primary human cells and not from an immortalized cell line, providing a foundation for future patient studies to examine cellular mechanisms and also potentially to elucidate biomarkers for health and disease (8, 32). Interestingly, the observed variability in our gene expression data is similar to most physiological data obtained in humans (2). Although we would expect data derived from a cell line to have less variability, we believe that using freshly isolated primary cells (PBMCs) provided a stronger study design and potentially greater clinical relevance. Also, because these primary cells are a heterogeneous mixture of different cell types such as monocytes and T- and B-lymphocytes, a higher variability may also be anticipated. Although this approach allowed capturing of the potential cross talk present among these cells in vivo, the isolation of each of these immune cells and their individual responsiveness to NE certainly warrants future studies.
In the present study, we did isolate CD14+ monocytes for our adhesion experiments and demonstrated for the first time that NE increases adhesion to endothelial cells. As such, aside from contributions to increases in systemic oxidative stress and potentially further elevations in sympathetic outflow, another deleterious outcome of NE stimulation of PBMCs may be an increase in monocyte adhesion to the endothelium. Indeed, we demonstrate that NE increases cellular adhesion between primary human monocytes and naive HUVEC. These findings are in line with studies performed in mouse models of atherosclerosis, where greater aortic lesion areas were observed in NE-treated animals (46). In addition, monocyte chemoattractant protein 1 is also increased in such animals, suggesting greater mononuclear cell infiltration into the lesions (46, 50). We now provide evidence that NE may be an important trigger of such events by making monocytes more adherent to endothelial cells. Because the monocyte-HUVEC co-incubation was performed for only 60 min, there was limited time for the expression of adhesion molecules such as ICAM or VCAM to increase on the surface of HUVEC. Thus the NE-mediated increase in monocyte-HUVEC adhesion is likely due to an increase in the surface expression of adhesion molecules on the monocytes. Importantly, treatment of monocytes with LPS, a known pro-inflammatory agent that enhances monocyte cell adhesion (10, 31), induced a similar increase in the monocyte-HUVEC adhesion as NE. Additionally, it is important to bear in mind that the pro-atherogenic effects of NE demonstrated by previous studies using in vivo mouse models have been largely attributed to increases in blood pressure (6, 46). The results from our in vitro experiments indicating that NE increases monocyte-endothelial cell adhesion suggest a novel means by which excessive NE creates a pro-atherogenic state, in addition to its well established pressor effects.
Experimental Considerations
Potential limitations in the design and interpretation of the present investigation should be considered. First, NADPH oxidase protein expression was not measured in conjunction with the mRNA measurements. This was attempted in initial experiments; however, the protein yield for each treatment along with each time point was too low to obtain a reliable measure of the various target proteins. Therefore, we measured the functional outcome of the NADPH oxidase protein (i.e., superoxide production), which was considered more informative than the estimation of the protein content alone. Second, although the specificity of DPI for inhibition of NADPH oxidase has been questioned, most pharmacological NADPH oxidase inhibitors available have approximately similar specificities toward this enzyme (15, 16). Likewise, although some reports have suggested that DPI may be toxic to cells, we do not believe this to be the primary contributor to the observed blunting of NE-mediated increase in superoxide production by DPI. Indeed, no significant difference was observed for PBMC-derived superoxide production between control and DPI-only treated cells at any time point. If DPI was toxic and killing cells, one would have expected lower superoxide production with DPI treatment compared with control. Nevertheless, we acknowledge that a more effective and specific approach for studying the role of NADPH oxidase would be the use of siRNA; thus future studies should employ this technique. Third, we have used Staurosporine to test the involvement of PKC in the NE-mediated superoxide production in human PBMCs. Although previous work suggests that Staurosporine induces apoptosis in human cells, this effect appears to occur at concentrations above 100 nM (51). In the present study, we have used a concentration of 10 nM where apoptosis does not appear to occur (51). Last, the stability of NE in culture for 36 h was not tested in our experiments. However, the increase in gene expression at 1 h and the sustained increase in superoxide with all concentrations suggest that NE remained stable. Likewise, trypan blue measures in our studies showed that the number of viable cells remained unchanged between control and the highest NE concentration (50 μg/ml) across time, indicating that NE did not exert any toxic effects due to its potential autoxidation on PBMCs. Based on these observations and other studies using longer NE treatment periods in culture (24), we believe that NE remained stable for the entire period of these experiments. Also, although we have not performed any pulse labeling studies, our protocol was designed to mimic the continual exposure of PBMCs to NE in vivo.
Perspectives and Significance
Numerous studies have reported that systemic oxidative stress is elevated in chronic disease conditions including heart failure, hypertension, and Type 2 diabetes mellitus (9, 19, 33, 37, 38). Likewise, NADPH oxidase activity has been reported to be increased in disease states, particularly hypertension (5, 15). Interestingly, many diseases with elevated oxidative stress also exhibit increased basal sympathetic nerve activity (14, 17, 29). Although the effects of angiotensin II in mediating elevations in oxidative stress via NADPH oxidase (16, 18, 54) as well as in promoting monocyte-endothelial cell adhesion are well known (1, 10), our results provide another perhaps under-appreciated novel mechanism by which oxidative stress and monocyte-endothelial cell adhesion can be enhanced in disease states. Furthermore, the findings that NE increases superoxide production via NADPH oxidase in human PBMCs suggest the existence of a positive feedback cycle that may contribute to the concomitant increase in oxidative stress and sympathetic nerve activity in disease states. Indeed, it is plausible that an increase in NE-mediated systemic oxidative stress would cause an increase in central sympathetic outflow that would, in turn, cause more NE release and further increase oxidative stress (4, 33, 44, 53, 54). Future studies are warranted to determine the potential contribution of this novel mechanism of NE-mediated superoxide production to the concomitant increase in sympathetic nerve activity and oxidative stress in many disease states. We have unpublished, preliminary data in heart failure patients demonstrating increased basal NADPH oxidase subunit protein expression and superoxide production in PBMCs compared with healthy controls. Although these data do not provide cause and effect, given the known high resting sympathetic nerve activity and elevated oxidative stress in heart failure (9, 25), they do extend the clinical significance of our findings and prompt the need for future studies.
In summary, these findings indicate for the first time that NE induced a sustained increase in superoxide production in freshly isolated primary human PBMCs via activation of NAPDH oxidase. This effect appeared to be preferentially mediated through α2-adrenergic receptors and required PKC activation. Perhaps more importantly, we show that NE treatment enhances monocyte-endothelial cell adhesion. Collectively, these data suggest NE can contribute to an increase in systemic oxidative stress through circulating PBMCs, which may have important implications for understanding the link between sympathetic overactivity and systemic oxidative stress in a number of cardiovascular diseases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-093167 (P. J. Fadel). J. Padilla was supported by a postdoctoral fellowship from the American Heart Association (AHA 11POST5080002), and N. T. Jenkins was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant T32-AR-048523.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.H.D., N.T.J., J.P., A.R.P., and P.J.F. conception and design of research; S.H.D. performed experiments; S.H.D. analyzed data; S.H.D., N.T.J., J.P., A.R.P., and P.J.F. interpreted results of experiments; S.H.D. prepared figures; S.H.D. drafted manuscript; S.H.D., N.T.J., J.P., A.R.P., and P.J.F. edited and revised manuscript; S.H.D., N.T.J., J.P., A.R.P., and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank LaNita Nichols for expert advice and assistance with the real-time RT-PCR analysis, and Charla Jay for assistance with subject recruitment and blood draws.
REFERENCES
- 1.AbdAlla S, Lother H, Langer A, el Faramawy Y, Quitterer U. Factor XIIIA transglutaminase crosslinks AT1 receptor dimers of monocytes at the onset of atherosclerosis. Cell 119: 343–354, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson G, Taylor CE, Jones H. Inter-individual variability in the improvement of physiological risk factors for disease: gene polymorphisms or simply regression to the mean? J Physiol 588: 1023–1024; author reply 1025, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol 173: 5730–5738, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 287: H695–H703, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Campos RR, Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT. The role of oxidative stress in renovascular hypertension. Clin Exp Pharmacol Physiol 38: 144–152, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Capers Qt Alexander RW, Lou P, De Leon H, Wilcox JN, Ishizaka N, Howard AB, Taylor WR. Monocyte chemoattractant protein-1 expression in aortic tissues of hypertensive rats. Hypertension 30: 1397–1402, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Khismatullin DB. Synergistic effect of histamine and TNF-alpha on monocyte adhesion to vascular endothelial cells. Inflammation 36: 309–319, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Chon H, Verhaar MC, Koomans HA, Joles JA, Braam B. Role of circulating karyocytes in the initiation and progression of atherosclerosis. Hypertension 47: 803–810, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Deo SH, Fisher JP, Vianna LC, Kim A, Chockalingam A, Zimmerman MC, Zucker IH, Fadel PJ. Statin therapy lowers muscle sympathetic nerve activity and oxidative stress in patients with heart failure. Am J Physiol Heart Circ Physiol 303: H377–H385, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorffel Y, Franz S, Pruss A, Neumann G, Rohde W, Burmester GR, Scholze J. Preactivated monocytes from hypertensive patients as a factor for atherosclerosis? Atherosclerosis 157: 151–160, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Dragomir E, Simionescu M. Monocyte chemoattractant protein-1–a major contributor to the inflammatory process associated with diabetes. Arch Physiol Biochem 112: 239–244, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Esler MD, Thompson JM, Kaye DM, Turner AG, Jennings GL, Cox HS, Lambert GW, Seals DR. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation 91: 351–358, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol 32: 979–987, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci 148: 5–15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita M, Ando K, Nagae A, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension 50: 360–367, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol 288: H2271–H2279, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Grassi G. Sympathetic neural activity in hypertension and related diseases. Am J Hypertens 23: 1052–1060, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Sun M, Li D, Liu J, Guo H, Dong Y, Jiang L, Pan Q, Man Y, Wang S, Li J. Augmented NADPH oxidase activity and p22phox expression in monocytes underlie oxidative stress of patients with type 2 diabetes mellitus. Diabetes Res Clin Practice 91: 371–380, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Jayapal KP, Philp RJ, Kok YJ, Yap MG, Sherman DH, Griffin TJ, Hu WS. Uncovering genes with divergent mRNA-protein dynamics in Streptomyces coelicolor. PLos One 3: e2097, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins NT, Landers RQ, Prior SJ, Soni N, Spangenburg EE, Hagberg JM. Effects of acute and chronic endurance exercise on intracellular nitric oxide and superoxide in circulating CD34+ and CD34− cells. J Appl Physiol 111: 929–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin M, Kumar A, Kumar S. Ethanol-mediated regulation of cytochrome P450 2A6 expression in monocytes: role of oxidative stress-mediated PKC/MEK/Nrf2 pathway. PLos One 7: e35505, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang BY, Lee SW, Kim TS. Stimulation of interleukin-12 production in mouse macrophages via activation of p38 mitogen-activated protein kinase by alpha2-adrenoceptor agonists. Eur J Pharmacol 467: 223–231, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Lappin D, Whaley K. Adrenergic receptors on monocytes modulate complement component synthesis. Clin Exp Immunol 47: 606–612, 1982 [PMC free article] [PubMed] [Google Scholar]
- 25.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Liu G, Place AT, Chen Z, Brovkovych VM, Vogel SM, Muller WA, Skidgel RA, Malik AB, Minshall RD. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood 120: 1942–1952, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Lunemann JD, Buttgereit F, Tripmacher R, Baerwald CG, Burmester GR, Krause A. Effects of norepinephrine on oxygen consumption of quiescent and activated human peripheral blood mononuclear cells. Ann NY Acad Sci 966: 365–368, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Mazzeo RS, Rajkumar C, Jennings G, Esler M. Norepinephrine spillover at rest and during submaximal exercise in young and old subjects. J Appl Physiol 82: 1869–1874, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Min BK, Suk K, Lee WH. Stimulation of CD107 affects LPS-induced cytokine secretion and cellular adhesion through the ERK signaling pathway in the human macrophage-like cell line, THP-1. Cell Immunol 281: 122–128, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Nozawa N, Hibi K, Endo M, Sugano T, Ebina T, Kosuge M, Tsukahara K, Okuda J, Umemura S, Kimura K. Association between circulating monocytes and coronary plaque progression in patients with acute myocardial infarction. Circ J 74: 1384–1391, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep 8: 232–241, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Raskind MA, Peskind ER, Veith RC, Beard JC, Gumbrecht G, Halter JB. Increased plasma and cerebrospinal fluid norepinephrine in older men: differential suppression by clonidine. J Clin Endocrinol Metab 66: 438–443, 1988 [DOI] [PubMed] [Google Scholar]
- 35.Renier G, Mamputu JC, Desfaits AC, Serri O. Monocyte adhesion in diabetic angiopathy: effects of free-radical scavenging. J Diabetes Complications 17: 20–29, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Roberts MD, Childs TE, Brown JD, Davis JW, Booth FW. Early depression of Ankrd2 and Csrp3 mRNAs in the polyribosomal and whole tissue fractions in skeletal muscle with decreased voluntary running. J Appl Physiol 112: 1291–1299, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 34: 431–440, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Sawyer DB. Oxidative stress in heart failure: what are we missing? Am J Med Sci 342: 120–124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz G, Herr AS, Rothe G. T-lymphocytes and monocytes in atherogenesis. Herz 23: 168–177, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Schraml E, Quan P, Stelzer I, Fuchs R, Skalicky M, Viidik A, Schauenstein K. Norepinephrine treatment and aging lead to systemic and intracellular oxidative stress in rats. Exp Gerontol 42: 1072–1078, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Siekmann TR, Burgazli KM, Bobrich MA, Noll G, Erdogan A. The antiproliferative effect of pinostrobin on human umbilical vein endothelial cells (HUVEC). Eur Rev Med Pharmacol Sci 17: 668–672, 2013 [PubMed] [Google Scholar]
- 42.Strober W. Trypan blue exclusion test of cell viability. Curr Protocols Immunol Appendix 3B: Appendix 3B, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Tennant JR. Evaluation of the Trypan blue technique for determination of cell viability. Transplantation 2: 685–694, 1964 [DOI] [PubMed] [Google Scholar]
- 44.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol 139: 191–202, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg A, Zhang L, Brown D, Erice A, Polsky B, Hirsch MS, Owens S, Lamb K. Viability and functional activity of cryopreserved mononuclear cells. Clin Diagnostic Lab Immunol 7: 714–716, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation 103: 448–454, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124: 1370–1381, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation 9: 212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue J, Thippegowda PB, Hu G, Bachmaier K, Christman JW, Malik AB, Tiruppathi C. NF-kappaB regulates thrombin-induced ICAM-1 gene expression in cooperation with NFAT by binding to the intronic NF-kappaB site in the ICAM-1 gene. Physiol Genom 38: 42–53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi X, Xu L, Hiller S, Kim HS, Maeda N. Reduced alpha-lipoic acid synthase gene expression exacerbates atherosclerosis in diabetic apolipoprotein E-deficient mice. Atherosclerosis 223: 137–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang XD, Gillespie SK, Hersey P. Staurosporine induces apoptosis of melanoma by both caspase-dependent and -independent apoptotic pathways. Mol Cancer Therap 3: 187–197, 2004 [PubMed] [Google Scholar]
- 52.Zhao X, Xu B, Bhattacharjee A, Oldfield CM, Wientjes FB, Feldman GM, Cathcart MK. Protein kinase Cdelta regulates p67phox phosphorylation in human monocytes. J Leukoc Biol 77: 414–420, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol 84: 125–149, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002 [DOI] [PubMed] [Google Scholar]