Abstract
Physical inactivity promotes the development of cardiovascular diseases. However, few data exist examining the vascular consequences of short-term reductions in daily physical activity. Thus we tested the hypothesis that popliteal and brachial artery flow-mediated dilation (FMD) would be reduced and concentrations of endothelial microparticles (EMPs) would be elevated following reduced daily physical activity. To examine this, popliteal and brachial artery FMD and plasma levels of EMPs suggestive of apoptotic and activated endothelial cells (CD31+/CD42b− and CD62E+ EMPs, respectively) were measured at baseline and during days 1, 3, and 5 of reduced daily physical activity in 11 recreationally active men (25 ± 2 yr). Subjects were instructed to reduce daily physical activity by taking <5,000 steps/day and refraining from planned exercise. Popliteal artery FMD decreased with reduced activity (baseline: 4.7 ± 0.98%, reduced activity day 5: 1.72 ± 0.68%, P < 0.05), whereas brachial artery FMD was unchanged. In contrast, baseline (pre-FMD) popliteal artery diameter did not change, whereas brachial artery diameter decreased (baseline: 4.35 ± 0.12, reduced activity day 5: 4.12 ± 0.11 P < 0.05) following 5 days of reduced daily physical activity. CD31+/CD42b− EMPs were significantly elevated with reduced activity (baseline: 17.6 ± 9.4, reduced activity day 5: 104.1 ± 43.1 per μl plasma, P < 0.05), whereas CD62E+ EMPs were unaltered. Collectively, our results provide evidence for the early and robust deleterious impact of reduced daily activity on vascular function and highlight the vulnerability of the vasculature to a sedentary lifestyle.
Keywords: endothelial function, sedentary, atherosclerosis, physical inactivity
less than half (49.1%) of U.S. adults meet the recommended physical activity guidelines (1), resulting in a sedentary lifestyle, which accentuates the development of cardiovascular-related diseases. Epidemiological data clearly indicate that those individuals who are most inactive have the greatest risk for cardiovascular disease morbidity and mortality (8, 35). Likewise, Blair et al. (8) demonstrated that individuals with the lowest cardiorespiratory fitness have the highest risk of cardiovascular disease and all-cause mortality, further emphasizing the link between physical inactivity and cardiovascular disease risk. However, despite the known cardiovascular risk of being inactive, few studies have experimentally investigated the vascular consequences of reduced daily physical activity in humans. This becomes important because decreased endothelial function, as assessed by flow-mediated dilation (FMD), is associated with increased cardiovascular risk (56).
Previous human studies examining vascular function following physical inactivity are limited and have mainly used complete removal of physical activity via bed rest or limb immobilization (7, 9, 10, 15, 20, 36). Studies with bed rest have reported inward vascular remodeling, decreased reactive hyperemia, and decreased endothelium-dependent dilation (10, 15, 20). Limb immobilization also has been shown to cause vascular remodeling and impair endothelial function (7, 9, 19). Although these findings indicate that physical inactivity has detrimental effects on the vasculature, bed rest and immobilization are extreme models of inactivity that most individuals do not experience in their daily lives. In this regard, a recently described model for examining the effects of inactivity is to transition individuals from high to low levels of ambulatory activity by reducing daily step count (28). However, to date, no studies have examined the effects of this form of reduced daily physical activity on vascular endothelial function in humans.
In addition to the widely used measure of conduit artery FMD, endothelial microparticles (EMPs) are used as systemic markers of endothelial phenotype (25, 54). EMPs are small particles (≤1 μm) that are released from endothelial cells into circulation due to endothelial cell activation, apoptosis, or injury (12). Although EMPs are well established as valid markers of endothelial phenotype in the setting of chronic disease (22, 26), few studies have examined whether EMP levels change with alterations in physical activity (21, 23, 36). A recent study reported that circulating microparticles indicative of endothelial apoptosis were increased following extreme physical inactivity induced by 7 days of dry water immersion, a model of weightlessness (36). Whether more modest reductions in physical activity increase EMPs is not known.
The purpose of this study was to determine the early effects of a transition from high (>10,000 steps/day) to low daily physical activity (<5,000 steps/day) on endothelial function and phenotype in healthy, recreationally active men. These volumes of daily steps were chosen because 10,000 steps is generally considered a threshold of high daily activity, whereas 5,000 steps or below is close to the daily average step count of most sedentary US citizens (5). We hypothesized that 5 days of reduced daily physical activity would reduce FMD in the popliteal (leg) and brachial (arm) arteries. Furthermore, we anticipated that markers of endothelial apoptosis and activation (CD31+/CD42b− and CD62E+ EMPs, respectively) would be significantly elevated following 5 days of reduced daily physical activity.
METHODS
Subjects
All protocols were approved by the University of Missouri Health Sciences Institutional Review board. Written informed consent was obtained from all subjects. Eleven apparently healthy (determined by a detailed health history questionnaire), recreationally active men (age: 25 ± 2 y, BMI: 24.8 ± 1.0 kg/m2, body fat: 19.3 ± 1.1%, V̇o2max: 51.8 ± 2.9 ml/kg/min) were recruited for the study. Recreationally active was defined as completing at least 90 min of primarily aerobic lower body exercise over ≥3 days per week and taking greater than 10,000 steps per day. Aerobic physical activity was self-reported, and pedometers were used to verify daily steps. Exclusion criteria included smoking, recent (<2 mo) change in body weight, or being involved in competitive endurance events.
Preintervention Testing
Body composition as assessed by DEXA (Hologic) and fitness as assessed by a graded maximal treadmill test to elicit maximal oxygen uptake (V̇o2max) were performed before starting the study intervention. Furthermore, subjects were provided standardized meals (57% carbohydrates, 28% fat, 15% protein) for three consecutive days before testing to assess average daily kilocalorie consumption. Average kilocalories consumed during this 3-day period were then prescribed to subjects during the study intervention. Furthermore, subjects also wore a pedometer to monitor daily steps and a physical activity monitor (Body Media) to estimate average daily metabolic equivalent of task (METs) and energy expended (kilojoules) above 3 METS during the 3-day monitoring period. Three days of physical activity monitoring has been shown to be representative of weekly physical activity (53). Kilojoules were then converted to kilocalories by the following equation: kilocalories = (kilojoules × 0.239).
Experimental Protocol
Three days before baseline testing, subjects wore a pedometer and physical activity monitor and were encouraged to follow their normal physical activity patterns. Two days before baseline testing, subjects began consuming the provided standardized meals. Twenty-four hours before baseline testing, subjects performed a 45-min supervised treadmill exercise session at 60% of heart rate reserve calculated from maximal heart rate obtained during the V̇o2max test. This supervised exercise bout was used to control for the amount and time of day when the subjects received their last bout of structured physical activity in an attempt to control for the exercise-induced hyperemia (i.e., increased blood flow and shear stress), which is a potent stimulus to the vasculature (32, 45). Subjects were asked to refrain from any additional planned exercise following this exercise bout.
All study visits were performed between 7:00 AM and 9:00 AM in a temperature-controlled room kept at 21–22°C. Upon arrival to the laboratory, height and weight were obtained via standard methods. Subjects were then placed in a supine position, and an intravenous catheter was placed. After a 15-min rest period, plasma samples were obtained for assessment of CD31+/CD42b− and CD62E+ EMPs. Following an additional 15 min of supine rest, brachial and popliteal artery FMD were assessed. FMD measures in the brachial and popliteal arteries were separated by a minimum of 15 min. Measures of EMPs and FMDs were made at baseline and repeated following days 1, 3, and 5 of reduced daily physical activity.
Intervention (Reduced Daily Physical Activity)
Immediately following baseline testing, subjects began 5 days of reduced daily physical activity (<5,000 steps/day). Subjects were instructed to avoid any planned exercise sessions and reduce daily physical activity. To achieve this goal and also verify it was met, pedometers were worn to monitor daily steps. In certain circumstances, some subjects were pushed in a wheel chair by study personnel to facilitate them achieving <5,000 steps/day.
Flow-Mediated Dilation Measurements
FMD of the brachial and popliteal arteries was assessed via high-resolution Doppler ultrasound (Logiq P5; GE Medical Systems, Milwaukee, WI) following present guidelines (48). Briefly, a 12-Mhz linear array transducer was used to scan the brachial and popliteal artery. Simultaneous velocity signals were obtained in duplex mode at pulsed frequency of 5 MHz and corrected with an insonation angle of 60° with the cursor set midvessel. Sample volume was adjusted to encompass the entire vessel lumen without extending beyond it. Once a satisfactory image was acquired, limbs were secured, and the transducer was stabilized using a custom-designed clamp. Transducer location was marked on the skin, and a photo was taken of each limb to ensure consistent placement throughout the study visits. Brachial and popliteal artery FMD across intervention days was measured by the same ultrasonographer, and all settings on the ultrasound were kept constant between intervention days. A cuff was positioned 3 cm distal to the antecubital fossa for brachial FMD and 5 cm distal to the fibular head for popliteal FMD. Baseline diameter and velocity were recorded for 2 min. The cuff was then inflated to 220 mmHg for 5 min. Diameter and velocity were recorded for 30 s before and for 3 min following cuff deflation. To ensure that any changes in FMD observed during reduced daily physical activity were not due to a time effect, popliteal FMD measurements were made before and following 5 days of maintained physical activity in three subjects. Popliteal artery FMD was not obtained in one subject due to an inability to obtain an adequate image. Brachial artery FMD was not obtained in one subject due to a computer-saving error.
Offline analyses of artery diameters and velocities were performed using a custom-designed edge-detection and wall-tracking software (Labview; National Instruments, Austin, TX) as previously described (17, 41, 46). FMD percentage change was calculated from three cardiac cycles averaged around the highest peak diameter using the following equation: FMD percentage change = (peak diameter − baseline diameter)/(baseline diameter * 100). Shear rate (s) was defined as 8·Vm/D (42). FMD percentage change was normalized to shear rate incremental area under the curve up to peak diameter (39, 40).
Plasma EMPs
Blood samples were collected from an antecubital vein into 8-ml vials containing acid citrate dextrose. The endothelial microparticle populations CD31+/CD42b− and CD62E+ were analyzed by flow cytometry as previously described by our group (25). Briefly, cell-free plasma samples were obtained and incubated with fluorochrome-labeled antibodies. Phycoerythrin-CD62E was used to determine levels of EMPs shed from activated endothelium (CD62E+). The combination of phycoerythrin-CD31 and fluorescein-isothiocyanate-CD42b was used to determine levels of EMPs released from apoptotic endothelial cells (CD31+/CD42b−). Samples were fixed in 2% paraformaldehyde and diluted with sterile PBS before flow cytometric analysis. Flow cytometry protocols were carried out on a Beckman Coulter CyAn ADP instrument in the University of Missouri Cell and Immunobiology Core Facility, as previously described (25). NIST Traceable polystyrene beads (Polysciences, Warrington, PA) were used as size calibrator beads to distinguish EMP events smaller than 1.0 μm. Unstained samples, samples stained with PE-conjugated mouse IgG isotype antibodies, and fluorescence minus one controls were used to discriminate true EMP events from noise. Our group has previously published within-subject reproducibility data for EMP measurements (25). CD31+/CD42b− and CD62E+ EMPs were not included for two subjects due to inadequate plasma sample volume for all time points.
Statistics
SigmaStat was used for analyses. Data were analyzed using a paired t-test and a one-way repeated measures ANOVA when appropriate. Friedman's Rank test was used when data were found to violate the assumption of homoscedasticity. Tukey's post hoc testing was performed in the event of a significant omnibus ANOVA. Significance was accepted at P < 0.05. All data are expressed as means ± SE.
RESULTS
Physical Activity Measurements
As shown in Table 1, steps per day, average daily METs, and Kcals expended per day above 3 METs decreased in the reduced daily physical activity phase compared with baseline, P < 0.05. Body weight was unchanged across the intervention (e.g., baseline: 78.8 ± 4.0 kg, day 5: 78.3 ± 4.1 kg, P > 0.05).
Table 1.
Physical activity-monitoring parameters
| Days | Steps | Average METs | Kcals >3 METs |
|---|---|---|---|
| BL | 12550 ± 743 | 1.98 ± 0.17 | 1214 ± 131 |
| RA1 | 3466 ± 381* | 1.60 ± 0.15* | 454 ± 82* |
| RA2 | 3303 ± 190* | 1.63 ± 0.19* | 464 ± 133* |
| RA3 | 4067 ± 197* | 1.53 ± 0.18* | 568 ± 195* |
| RA4 | 3841 ± 346* | 1.55 ± 0.12* | 320 ± 43* |
| RA5 | 3734 ± 227* | 1.61 ± 0.12* | 448 ± 99* |
Data are presented as means ± SE. Measurements are taken at baseline (BL) and days of reduced activity (RA1-5).
P < 0.05 vs. BL. MET, metabolic equivalent of task.
FMD and Conduit Artery Measurements
Popliteal artery.
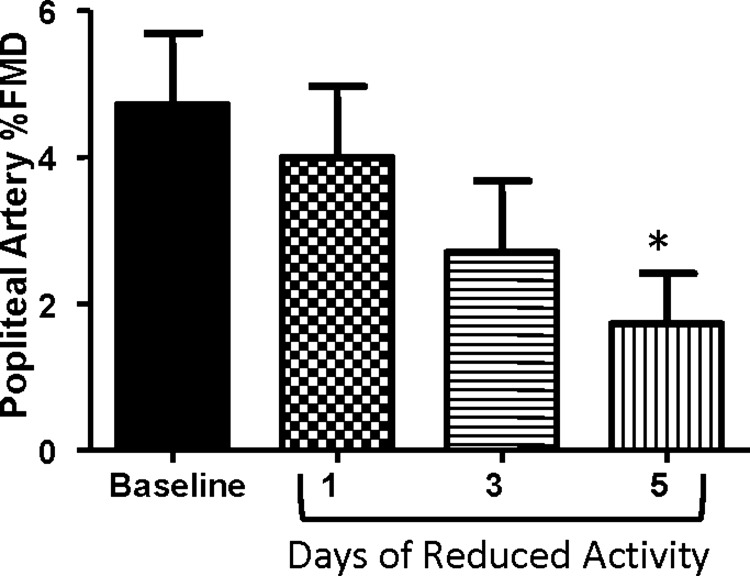
Percent FMD in the popliteal artery was reduced from baseline to day 5 of reduced daily physical activity (P < 0.05; Fig. 1). Likewise, popliteal artery FMD normalized to shear rate area under the curve was significantly decreased after 5 days of reduced daily physical activity (P < 0.05; Table 2). The inactivity-induced changes in popliteal artery FMD and steps per day from baseline to 5 days of reduced daily physical activity were correlated (r = 0.66, P = 0.05). No changes were found in baseline or peak diameter of the popliteal artery (Table 2). No alterations in shear rate area under the curve following forearm ischemia were observed in the popliteal artery (Table 2). In the subjects who maintained physical activity for 5 days, popliteal artery FMD was unchanged from baseline (baseline: 3.29 ± 0.66 and maintained activity day 5: 3.11 ± 0.51%; P > 0.05).
Fig. 1.
Popliteal artery endothelial function across the study intervention as assessed by flow-mediated dilation (FMD). *P < 0.05 vs. baseline.
Table 2.
Popliteal artery vascular measures at baseline and during the FMD
| Days | Baseline Diameter, mm | Peak Diameter, mm | Shear, AUC | Peak FMD:SRAUC Ratio, a.u. | Time to Peak, s |
|---|---|---|---|---|---|
| BL | 5.40 ± 0.14 | 5.66 ± 0.14 | 18108.84 ± 4192.08 | 0.58 ± 0.29 | 71.90 ± 15.34 |
| RA1 | 5.54 ± 0.14 | 5.76 ± 0.13 | 22213.98 ± 4196.80 | 0.29 ± 0.11 | 99.11 ± 20.90 |
| RA3 | 5.47 ± 0.14 | 5.61 ± 0.12 | 24722.05 ± 6451.97 | 0.17 ± 0.05 | 99.95 ± 22.23 |
| RA5 | 5.45 ± 0.13 | 5.54 ± 0.11 | 24888.69 ± 5049.87 | 0.11 ± 0.04* | 110.47 ± 14.68 |
Data are presented as means ± SE.
P < 0.05 from BL. FMD, flow-mediated dilation; AUC, area under the curve; SR, shear rate.
Brachial artery.
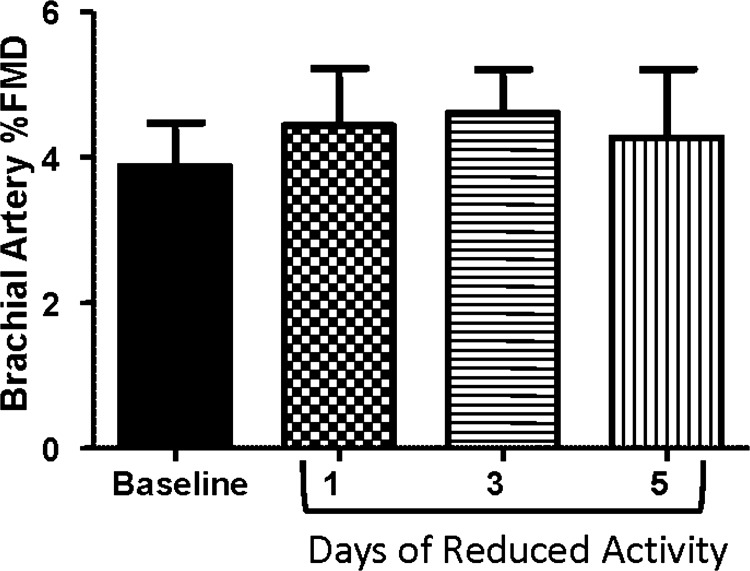
The brachial artery displayed no changes in percentage (Fig. 2) or normalized FMD (Table 3) throughout the intervention. However, baseline and peak diameter in the brachial artery were significantly decreased following 5 days of reduced daily physical activity (P < 0.05; Table 3). No alterations in shear rate area under the curve following forearm ischemia were observed in the brachial artery (Table 3).
Fig. 2.
Brachial artery endothelial function across the study intervention as assessed by FMD.
Table 3.
Brachial artery vascular measures at BL and during the FMD
| Days | Baseline Diameter, mm | Peak Diameter, mm | Shear, AUC | Peak FMD:SRAUC Ratio, a.u. | Time to Peak, s |
|---|---|---|---|---|---|
| BL | 4.35 ± 0.12 | 4.52 ± 0.13 | 38287.09 ± 8266.96 | 0.17 ± 0.05 | 59.74 ± 7.23 |
| RA1 | 4.32 ± 0.12† | 4.51 ± 0.12† | 28220.70 ± 2714.19 | 0.17 ± 0.04 | 48.95 ± 5.13 |
| RA3 | 4.17 ± 0.14* | 4.36 ± 0.14 | 33099.36 ± 4780.04 | 0.16 ± 0.03 | 55.25 ± 9.84 |
| RA5 | 4.12 ± 0.11* | 4.29 ± 0.13* | 36888.24 ± 6114.66 | 0.15 ± 0.05 | 61.33 ± 6.12 |
Data are presented as means ± SE.
P < 0.05 from BL;
P < 0.05 from RA5.
EMP Measurements
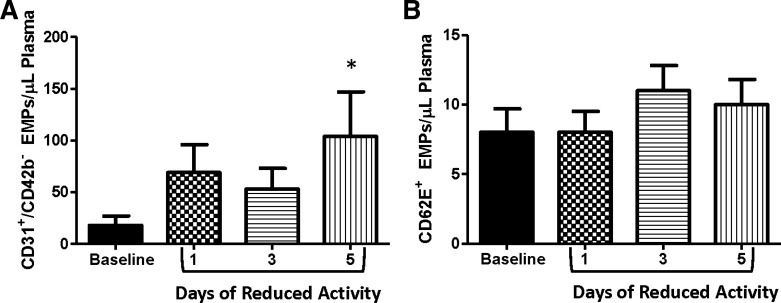
CD31+/CD42b− EMPs increased significantly from baseline to 5 days of reduced physical activity (P < 0.05; Fig. 3A); however, CD62E+ EMPs were not significantly altered by the 5-day intervention (Fig. 3B). The inactivity-induced changes in CD31+/CD42b− and popliteal artery FMD from baseline to 5 days of reduced daily physical activity were not significantly correlated (r = 0.33, P > 0.05).
Fig. 3.
CD31+/CD42b− (A) and CD62E+ (B) endothelial microparticles across the study intervention. *P < 0.05 vs. baseline. EMP, endothelial microparticles.
DISCUSSION
The major and novel findings of this study are that a transition from high to low levels of daily physical activity for only 5 days 1) impairs FMD in the popliteal artery but not brachial artery, 2) induces a decrease in diameter of the brachial artery but not popliteal artery, and 3) produces an increase in circulating levels of CD31+/CD42b− EMPs. This represents the first evidence in humans of deleterious vascular consequences of transitioning from high to low daily physical activity levels.
Herein, we used a recently described model of reduced daily physical activity (28, 34), which most sedentary individuals experience (52). Remarkably, after just 5 days of reducing activity in these young healthy men, we observed a marked reduction in endothelial function at the popliteal artery. To our knowledge, these are the first data demonstrating such detrimental vascular effects without “complete” removal of activity used in other experimental models of physical inactivity in humans. For example, Birk et al. (7) showed that 8 days of unilateral arm inactivity, via an arm sling, resulted in a significantly reduced postischemic blood flow suggestive of remodeling of the downstream resistance vasculature. In addition, 7 days of leg casting in healthy men resulted in decreased femoral artery distensibility (47). Findings from studies using bed rest also demonstrate structural impairments in the vasculature (10, 14). Another approach used to understand the impact of chronic inactivity was reported by Green et al. (18), in which arms of habitual tennis players were compared. Reactive hyperemia to limb ischemia was decreased in the nondominant arm compared with the dominant arm; however, no differences in blood flow were shown with any of the vasoactive drugs, suggesting that physical inactivity impairs peak vasodilator capacity without altering basal or stimulated nitric oxide release (18). Collectively, these human studies demonstrate the vulnerability of the vasculature to physical inactivity, lending insight into the contribution of a sedentary lifestyle to the vascular disease process.
Our finding that the detrimental effects of reduced daily physical activity on endothelial function were manifested in the popliteal but not the brachial artery is reinforced by recent rodent data by our group (38). Using a somewhat similar design to that employed in the present study, Padilla et al. (38) showed in rats that cessation of voluntary wheel running for 7 days produced increased expression of proatherogenic genes in the iliac but not the renal artery. Taken together, these findings suggest that a reduction in physical activity primarily affects the arteries perfusing the limbs exposed to the greatest reduction in activity. In this regard, a possible explanation for our observation of decreased popliteal but not brachial artery FMD relates to the fact that popliteal arteries, upon reduction of daily physical activity, may be subjected to a greater reduction in exercise hyperemia and thus shear stress, relative to the brachial arteries. Indeed, there is a growing body of evidence derived from both in vitro and in vivo studies indicating that removal of shear stress produces a detrimental signal to the endothelium (24, 29, 37). Interestingly, although brachial artery FMD was unaltered with 5 days of reduced activity, it is important to note that we did observe a decrease in brachial artery diameter suggestive of inward vascular remodeling (10, 14, 50) that was not found in the popliteal artery. Thus another plausible explanation for the preservation of brachial artery FMD may be due to the mathematical bias imposed when expressing FMD as percentage change in arterial diameter from baseline (3). Indeed, it is well documented that FMD percentage is negatively correlated to baseline diameter (45, 49). Of note, bed rest studies have also reported no change or increases in conduit artery FMD when decreases in baseline conduit artery diameter were observed (10, 14). Alternatively, it is also possible that the decreased diameter of the brachial artery in the present study is associated with a chronic increase in shear stress that in turn leads to a preservation of endothelial function (14, 51). However, more research is warranted to test this hypothesis.
The CD31+/CD42b− and CD62E+ EMP populations that we assessed are thought to be markers of endothelial apoptosis and activation, respectively (12). EMPs have been shown to correlate well with measures of FMD (16, 54), are associated with a number of cardiovascular disease risk factors (6, 13), and are elevated in a variety of patient populations (2, 43, 44, 55). In addition, we recently showed that an acute reduction in shear increases local concentrations of CD31+/CD42b− and CD62E+ EMPs in the human forearm (25). On the basis of this finding, we hypothesized that reduction of daily physical activity, and thus removal of the associated bouts of increased vascular shear stress, would result in an increase in EMPs. Consistent with this hypothesis, we found that 5 days of reduced activity induced an approximately fivefold increase in plasma levels of CD31+/CD42b− EMPs. Similarly, using a model of extreme physical inactivity and weightlessness, Navasiolava et al. (36) demonstrated increased concentrations of CD31+/CD41− EMPs following 7 days of dry water immersion. Interestingly, plasma concentrations of soluble CD62E protein were found to be unchanged following dry water immersion. One possible explanation for the lack of increase in CD62E+ EMPs in our study or soluble CD62E concentrations in plasma from Navasiolava et al. (31) is that this marker is expressed and released from endothelial cells when they are in an inflamed (i.e., “activated”) state. On the other hand, CD31+/CD42b− EMPs are thought to be markers of endothelial cell apoptosis; as such, substantial inflammation might not be required for their release (27). In this regard, Krogh-Madsen et al. (28) demonstrated no increase in circulating inflammatory markers IL-6, IL-15, TNF-α, adiponectin, and leptin following a 2-wk reduction in physical activity from >10,000 steps/day to <1,500. Given that this intervention duration and reduction in physical activity was greater than the one used in the present study, it is unlikely that a substantial rise in inflammatory markers occurred in the present study. However, this requires further investigation. Navasiolava et al. (36) also demonstrated that endothelium-dependent dilation in calf skin microvasculature was impaired following dry water immersion. We now advance these findings by demonstrating a reduction in endothelial function in the popliteal artery and decrease in diameter of the brachial artery using a model of physical inactivity, which most sedentary individuals experience.
Although previous in vitro studies have demonstrated that EMPs can impair the vascular endothelium (11, 33) and provide a potential mechanism for impaired vascular health in diseased states, the design of the present study does not allow us to make those comparisons or assumptions. The purpose of examining EMPs in the present study was to systemically assess endothelial function in addition to examining limb-specific endothelial function via FMD. The finding that increases in plasma CD31+/CD42b− EMPs were not correlated with reductions in FMD was surprising given that others have reported such relationships (16, 54). However, a recent study by Babbitt et al. (4) also demonstrated a lack of correlation between EMPs and FMD following a physical activity intervention in which both variables exhibited favorable changes (i.e., reduction in EMPs and increase in FMD). Thus it seems there is disconnect between EMP and FMD measures in response to changes in physical activity levels that requires future studies.
Perspectives
Despite the strong links between physical inactivity and cardiovascular disease risk from epidemiological data (30), there remains a relative paucity of experimental investigation into the effects of reduced daily physical activity on human vascular physiology. In this regard, our study was designed to emulate physical inactivity in “real-world” situations mimicking the degree of reduced daily physical activity, which most sedentary individuals experience. Specifically, our study volunteers were regularly active individuals meeting current guidelines of >10,000 steps/day in whom we enforced an acute period of reduced activity. As this reduction in activity brought them to the level previously reported for average sedentary adults in the U.S. (i.e., ∼5,000 steps/day) (52), we believe our findings have broad implications for understanding the effects of sedentary behavior on human vascular endothelial function and phenotype. Indeed, there was a clear relationship between reductions in popliteal artery FMD and the decrease in daily steps with reduced activity. Furthermore, it should be noted that, compared with alternative models of physical inactivity that have been more commonly used (e.g., bed rest, limb casting, etc.), the model of reducing daily steps by ∼50% might be regarded as a relatively modest inactivity “stimulus”. Thus it seems that the human vasculature is remarkably sensitive to alterations in activity status, as indicated by our observations of impaired popliteal artery FMD, decreased basal brachial artery diameter, and increased levels of EMPs with only 5 days of reduced daily physical activity. However, it should be noted that subjects in this study were only men; thus these findings should not be generalized to women.
In summary, our data indicate that the effects of reduced daily physical activity on conduit artery function are limb specific, reducing endothelial function in the lower limbs and decreasing arterial diameter in the upper limbs. There also seems to be a significant systemic effect of reduced physical activity on the vascular endothelium, as reflected by an approximately fivefold increase in plasma levels of CD31+/CD42b− EMPs. Collectively, our results provide evidence for the early and robust deleterious impact of reduced daily activity on vascular function and highlight the vulnerability of the vasculature to a sedentary lifestyle.
GRANTS
This work was supported by National Institutes of Health grants R01-HL093167 (P. Fadel). J. Thyfault was partially supported by NIH R01DK088940, N. Jenkins was supported by NIH T32-AR048523, J. Padilla was supported by an American Heart Association postdoctoral fellowship (AHA 11POST5080002), and L. Boyle was supported by an AHA predoctoral fellowship (12PRE12080242) and by an ACSM Foundation Research Grant from the American College of Sports Medicine Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.J.B., J.P.T., and P.J.F. conception and design of research; L.J.B. and D.P.C. performed experiments; L.J.B., D.P.C., and N.T.J. analyzed data; L.J.B., D.P.C., N.T.J., J.P., H.J.L., J.P.T., and P.J.F. interpreted results of experiments; L.J.B. prepared figures; L.J.B. drafted manuscript; L.J.B., D.P.C., N.T.J., J.P., H.J.L., J.P.T., and P.J.F. edited and revised manuscript; L.J.B., D.P.C., N.T.J., J.P., H.J.L., J.P.T., and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Charla Jay, R.N., for assistance and the participants for time and cooperation.
REFERENCES
- 1.Centers for Disease Control and Prevention Facts About Physical Activity. Online. http://www.cdc.gov/physicalactivity/data/facts.html [Google Scholar]
- 2.Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ, Mendez A, Ferreira A, de Marchena E, Ahn YS. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol 98: 70–74, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson G, Batterham AM, Thijssen DH, Green DJ. A new approach to improve the specificity of flow-mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens 31: 287–291, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Babbitt DM, Diaz KM, Feairheller DL, Sturgeon KM, Perkins AM, Veerabhadrappa P, Williamson ST, Kretzschmar J, Ling C, Lee H, Grimm H, Thakkar SR, Crabbe DL, Kashem MA, Brown MD. Endothelial activation microparticles and inflammation status improve with exercise training in African Americans. Int J Hypertens 2013: 538017, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassett DR, Jr, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in US adults. Med Sci Sports Exerc 42: 1819–1825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernal-Mizrachi L, Jy W, Fierro C, Macdonough R, Velazques HA, Purow J, Jimenez JJ, Horstman LL, Ferreira A, de Marchena E, Ahn YS. Endothelial microparticles correlate with high-risk angiographic lesions in acute coronary syndromes. Int J Cardiol 97: 439–446, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Birk GK, Dawson EA, Timothy Cable N, Green DJ, Thijssen DH. Effect of unilateral forearm inactivity on endothelium-dependent vasodilator function in humans. Eur J Appl Physiol 113: 933–940, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 276: 205–210, 1996 [PubMed] [Google Scholar]
- 9.Bleeker MW, De Groot PC, Poelkens F, Rongen GA, Smits P, Hopman MT. Vascular adaptation to 4 wk of deconditioning by unilateral lower limb suspension. Am J Physiol Heart Circ Physiol 288: H1747–H1755, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bleeker MW, De Groot PC, Rongen GA, Rittweger J, Felsenberg D, Smits P, Hopman MT. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study. J Appl Physiol 99: 1293–1300, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol 286: H1910–H1915, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res 335: 143–151, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Constans J, Conri C. Circulating markers of endothelial function in cardiovascular disease. Clin Chim Acta 368: 33–47, 2006 [DOI] [PubMed] [Google Scholar]
- 14.de Groot PC, Bleeker MW, Hopman MT. Magnitude and time course of arterial vascular adaptations to inactivity in humans. Exerc Sport Sci Rev 34: 65–71, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Demiot C, Dignat-George F, Fortrat JO, Sabatier F, Gharib C, Larina I, Gauquelin-Koch G, Hughson R, Custaud MA. WISE 2005: chronic bed rest impairs microcirculatory endothelium in women. Am J Physiol Heart Circ Physiol 293: H3159–H3164, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Esposito K, Ciotola M, Schisano B, Gualdiero R, Sardelli L, Misso L, Giannetti G, Giugliano D. Endothelial microparticles correlate with endothelial dysfunction in obese women. J Clin Endocrinol Metab 91: 3676–3679, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green DJ, Fowler DT, O'Driscoll JG, Blanksby BA, Taylor RR. Endothelium-derived nitric oxide activity in forearm vessels of tennis players. J Appl Physiol 81: 943–948, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Green DJ, O'Driscoll JG, Blanksby BA, Taylor RR. Effect of casting on forearm resistance vessels in young men. Med Sci Sports Exerc 29: 1325–1331, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, Gokce N, Ruderman NB, Keaney JF, Jr, Vita JA. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol 27: 2650–2656, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison M, Murphy RP, O'Connor PL, O'Gorman DJ, McCaffrey N, Cummins PM, Moyna NM. The endothelial microparticle response to a high fat meal is not attenuated by prior exercise. Eur J Appl Physiol 106: 555–562, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Horstman LL, Jy W, Jimenez JJ, Ahn YS. Endothelial microparticles as markers of endothelial dysfunction. Front Biosci 9: 1118–1135, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Jenkins NT, Landers RQ, Thakkar SR, Fan X, Brown MD, Prior SJ, Spangenburg EE, Hagberg JM. Prior endurance exercise prevents postprandial lipaemia-induced increases in reactive oxygen species in circulating CD31+ cells. J Physiol 589: 5539–5553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins NT, Martin JS, Laughlin MH, Padilla J. Exercise-induced signals for vascular endothelial adaptations: implications for cardiovascular disease. Curr Cardiovasc Risk Rep 6: 331–346, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins NT, Padilla J, Boyle LJ, Credeur DP, Laughlin MH, Fadel PJ. Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension 61: 615–621, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Bidot CJ, Ahn YS. Endothelial microparticles (EMP) as vascular disease markers. Adv Clin Chem 39: 131–157, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res 109: 175–180, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Krogh-Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, van Hall G, Booth FW, Pedersen BK. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol 108: 1034–1040, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 104: 588–600, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lees SJ, Booth FW. Sedentary death syndrome. Can J Appl Physiol 29: 447–460; discussion 444–446, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology 77: 543–549, 1992 [PMC free article] [PubMed] [Google Scholar]
- 32.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035–2042, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Mezentsev A, Merks RM, O'Riordan E, Chen J, Mendelev N, Goligorsky MS, Brodsky SV. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol 289: H1106–H1114, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc 44: 225–231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet 265: 1111–1120, 1953 [DOI] [PubMed] [Google Scholar]
- 36.Navasiolava NM, Dignat-George F, Sabatier F, Larina IM, Demiot C, Fortrat JO, Gauquelin-Koch G, Kozlovskaya IB, Custaud MA. Enforced physical inactivity increases endothelial microparticle levels in healthy volunteers. Am J Physiol Heart Circ Physiol 299: H248–H256, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Newcomer SC, Thijssen DH, Green DJ. Effects of exercise on endothelium and endothelium/smooth muscle cross talk: role of exercise-induced hemodynamics. J Appl Physiol 111: 311–320, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Padilla J, Jenkins NT, Roberts MD, Arce-Esquivel AA, Martin JS, Laughlin MH, Booth FW. Differential changes in vascular mRNA levels between rat iliac and renal arteries produced by cessation of voluntary running. Exp Physiol 98: 337–347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Adjusting flow-mediated dilation for shear stress stimulus allows demonstration of endothelial dysfunction in a population with moderate cardiovascular risk. J Vasc Res 46: 592–600, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol 297: H1103–H1108, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker BA, Trehearn TL, Meendering JR. Pick your Poiseuille: normalizing the shear stimulus in studies of flow-mediated dilation. J Appl Physiol 107: 1357–1359, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Pirro M, Schillaci G, Paltriccia R, Bagaglia F, Menecali C, Mannarino MR, Capanni M, Velardi A, Mannarino E. Increased ratio of CD31+/CD42- microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arterioscler Thromb Vasc Biol 26: 2530–2535, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 41: 211–217, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol 110: 389–397, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugawara J, Hayashi K, Kaneko F, Yamada H, Kizuka T, Tanaka H. Reductions in basal limb blood flow and lumen diameter after short-term leg casting. Med Sci Sports Exerc 36: 1689–1694, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol 295: H1927–H1934, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thijssen DH, Green DJ, Hopman MT. Blood vessel remodeling and physical inactivity in humans. J Appl Physiol 111: 1836–1845, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Thijssen DH, Maiorana AJ, O'Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol 108: 845–875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 34: 1–8, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Tudor-Locke C, Burkett L, Reis JP, Ainsworth BE, Macera CA, Wilson DK. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med 40: 293–298, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Tushuizen ME, Nieuwland R, Rustemeijer C, Hensgens BE, Sturk A, Heine RJ, Diamant M. Elevated endothelial microparticles following consecutive meals are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care 30: 728–730, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 26: 112–116, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]