Abstract
The study goal was to use membrane voltage changes during neurohypophysial action potential (AP) propagation as an index of nerve function to evaluate the role that circulating microparticles (MPs) play in causing central nervous system injury in response to decompression stress in a murine model. Mice studied 1 h following decompression from 790 kPa air pressure for 2 h exhibit a 45% broadening of the neurohypophysial AP. Broadening did not occur if mice were injected with the MP lytic agent polyethylene glycol telomere B immediately after decompression, were rendered thrombocytopenic, or were treated with an inhibitor of nitric oxide synthase-2 (iNOS) prior to decompression, or in knockout (KO) mice lacking myeloperoxidase or iNOS. If MPs were harvested from control (no decompression) mice and injected into naive mice, no AP broadening occurred, but AP broadening was observed with injections of equal numbers of MPs from either wild-type or iNOS KO mice subjected to decompression stress. Although not required for AP broadening, MPs from decompressed mice, but not control mice, exhibit NADPH oxidase activation. We conclude that inherent differences in MPs from decompressed mice, rather than elevated MPs numbers, mediate neurological injury and that a component of the perivascular response to MPs involves iNOS. Additional study is needed to determine the mechanism of AP broadening and also mechanisms for MP generation associated with exposure to elevated gas pressure.
Keywords: decompression sickness, nitric oxide synthase, NADPH oxidase, leukocytes, platelets
the goal of this investigation is to improve understanding of the mechanisms for neuropathology in decompression sickness (DCS). DCS can sometimes be a systemic pathophysiological process that occurs after tissues become supersaturated with gas and is a risk associated with deep-sea divers, high-altitude aviators, and astronauts. Neurological symptoms, although sometimes minor, are reported in the majority of SCUBA divers who suffer DCS (35). Inert gases inhaled while breathing are taken up by tissues in proportion to the ambient pressure, and when pressure is reduced, some of the gas released from tissues forms bubbles due to the presence of gas cavitation nuclei (7, 39, 40). The central place of bubbles as an inciting factor for DCS is widely accepted, but the pathophysiological responses that mediate tissue injury remain unclear.
Brain magnetic resonance imaging (MRI) studies of asymptomatic divers as well as symptomatic divers and high-altitude pilots suffering neurological DCS have shown abnormalities in cortical gray matter and subcortical white matter (6, 11, 15). White matter hyperintensity lesions on MRI were found in 54% of a series of pilots with neurological DCS, abnormalities thought to arise from microvascular inflammation and/or ischemia (15). Divers have been found to suffer multiple cerebral infarctions in the terminal and border zones of the brain arteries, but these abnormalities cannot all be explained by arterialized bubbles (17, 18). Hence, additional or alternative components to blood-borne gas bubbles may be involved. Some injuries may also arise from intraparenchymal (autochthonous) gas bubbles causing tissue distortion and/or destruction (8).
We hypothesized that circulating microparticles (MPs) play a central role in neurological DCS. Elevations of MPs occur in animals and humans after simulated or bona fide underwater diving (21, 31, 32, 36). MPs are defined as membrane lipid bilayer-enclosed vesicles with a diameter of 0.1–1.0 μm. Under some diving conditions there is a strong positive correlation between circulating MPs and ultrasound-detected bubbles (25, 32). In a murine model, MPs were shown to initiate an inflammatory process postdecompression due to whole-platelet or platelet MP interactions with neutrophils followed by neutrophil activation and tissue damage which was documented as neutrophil sequestration and an increased vascular leak (33, 34, 38).
For the present study we sought out a physiological index for nerve dysfunction. Given that data suggest intravascular and/or perivascular events are associated with decompression injuries, we hypothesized that neurohypophysial nerve terminal conductance, that is, membrane voltage changes during action potential (AP) propagation in the posterior pituitary, could be used to objectively evaluate neurological function following decompression stress. The neurohypophysis has an incomplete blood-brain barrier (10). Moreover, there is a close association between neurohypophysial nerve terminals and the perivascular space; only a thin glial sheath separates most neuronal processes and nerve terminals from brain capillary endothelium (12, 26). There is precedence for considering that perivascular neurological insults can be detected at the neurohypophysis. Morphological changes occur in the neurohypophysial microvasculature following anatomically distant focal cerebral ischemia, changes thought to occur due to bulk transport of vasoactive substances (9).
Recent decompression studies have highlighted a role for inflammatory/inducible or type 2 nitric oxide synthase (iNOS) because postdecompression neutrophil activation and vascular leak can be abrogated by injections of a specific iNOS inhibitor (1400 W), and there are fewer manifestations of decompression stress in iNOS knockout (KO) mice (34). However, the cause-effect relationships among MPs, bubbles, and decompression stress are complex. Phenomena related to iNOS may not explain all aspects of injuries because brains, but not omentum, leg skeletal muscle, or psoas, of iNOS KO mice still exhibit an elevated vascular leak (34). The goal of this study was to use neurohypophysial AP width as an index of neurological injury in the murine decompression model and assess the role of MPs.
METHODS
Materials.
Unless otherwise noted, chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Annexin binding buffer and fluorescein isothiocyanate (FITC)-conjugated anti-annexin V antibodies were purchased from BD Pharmingen (San Jose, CA). Anti-mouse CD66b and anti-mouse β actin were purchased from e-Biosciences (San Diego, CA). NADPH oxidase inhibitory peptide, Nox2ds, with the sequence NH3-CSTRVRRQL-CONH2 and a control scrambled amino acid peptide with sequence NH3-CLRVTRQSR-CONH2 were purchased from American Peptide (Sunnyvale, CA) (5).
Animals.
All aspects of this study were reviewed and approved by the institutional IACUC. Mice (Mus musculus) were purchased (Jackson Laboratories, Bar Harbor, ME), fed a standard rodent diet and water ad libitum, and housed in the University animal facility. Colonies of iNOS-KO and myeloperoxidase (MPO) KO mice, initially purchased from Jackson Laboratories, were also maintained in the vivarium. Mice were left to breathe room air (control) or subjected to 790 kPa (gauge pressure, 100 pounds/square inch) air pressure for 2 h following published procedures (33, 34, 38). Pressurization and decompression occurred at 200 kPa/min. Some mice were injected with 1400 W {N-[[3-(aminomethyl)phenyl]methyl]-ethanimidamide, dihydrochloride} at a dose of 25 mg/kg ip prior to exposure to pressure or in matched control mice, 3 h prior to being killed. Sterile polyethylene glycol (PEG) telomere B was infused intravenously at 0.7 μl of a 0.3% solution (wt/vol) per gram mouse. These injections were carried out immediately following decompression, and mice were killed 1 h later. Matching air-exposed control mice (no decompression) were also killed 1 h after injection.
Standard procedures for MPs acquisition and processing.
All reagents and solutions used for MPs studies were sterile and filtered (0.2-μm filter). Preparation of MPs from control or decompressed mice for reinjection into naive mice followed our published protocol (38). Briefly, heparinized blood was centrifuged for 5 min at 1,500 g and supernatant made (0.2 M EDTA) to diminish ex vivo MP aggregation. This supernatant was centrifuged at 15,000 g for 30 min to pellet the few remaining platelets and cell debris. Supernatant was parceled among centrifuge tubes at a ratio of 250 μl + 4 ml PBS and centrifuged at 100,000 g for 60 min (typically 3–4 tubes/experiment were used). Most fluid in the tubes was discarded and 250 μl remaining at the bottom used to resuspend the MPs pellet. After MPs were counted to match numbers among air-exposed control and decompressed mouse samples, naive mice were injected with MPs suspended in a total volume of 400 μl PBS via the tail vein. Under most circumstances this was performed ∼45 min after particle isolation. Where indicated, MPs from mice first subjected to 790 kPa air for 2 h were incubated with 10 μM Nox2ds, a peptide that specifically inhibits NADPH oxidase-2, or a scrambled amino acid sequence control peptide by adding the agent to heparinized blood and incubating for 15 min before proceeding with the standard isolation process described above (5).
Fluorescence probe studies.
Because of questions regarding the production of reactive species, MPs prepared in the presence of 10 μM Nox2ds or the control, scrambled peptide were suspended in 40 μl PBS that contained either 1.4 μM 4,5-diaminofluorescein (DAF), 0.5 μM 2,7-dihydrodichlorofluorescein (DCF), or 10 μM DCF-diacetate (DCF-DA), and fluorescence was monitored, similar to procedures described previously (34).
Neurohypophysial action potential.
Details of the neurohypophysis preparation and the associated apparatus have been reported previously (19, 27, 29). The neurointermediate lobe (comprising neurohypophysis and pars intermedia) was obtained from mice killed by exsanguination after anesthesia and pinned in a manner so that the infundibular stalk lies clasped between a pair of platinum-iridium electrodes. After the preparation had been bathed in oxygenated mouse Ringer's solution (in mM: 154 NaCl, 5.6 KCl, 1 MgCl2, 2.2 CaCl2, 10 glucose, 20 HEPES, adjusted to pH 7.4 with NaOH) containing 20 μM JPW 3031 (di-2-ANEPPDHQ), a fast-response potentiometric styryl dye (Molecular Probes/Life Technologies, Grand island, NY), for 15 min (23), balanced bipolar shocks (100–200 V) at a frequency of 15 Hz lasting 400 ms were delivered through a stimulus isolator, and the resulting changes in the extrinsic fluorescence of the stained tissue were recorded by a single large-area silicon photodiode (PV-444, Perkin-Elmer Optoelectronics, Vaudreuil, Canada), which is positioned in the image plane of an epifluorescence microscope (UEM, Carl Zeiss). The photocurrent is then converted into a voltage signal. The resulting optical record is a high-fidelity representation of the shape of the AP in the neurohypophysial nerve terminals (27). To quantify results across different experiments the mean AP width at the half-maximal height was calculated. From 4 to 12 replicate trials with each animal were carried out by quantitatively analyzing the first AP of each replicate.
Vascular permeability and neutrophil sequestration analysis.
Anesthetized control and decompressed mice were exsanguinated and lysine-fixable tetramethylrhodamine-conjugated dextran (2 × 106 Da, Invitrogen, Carlsbad, CA) was injected exactly as described in a previous publication (33). Animals were then injected with colloidal silica to allow for endothelial enrichment of tissue homogenates following published techniques (33). To obtain isolated pituitary the entire dorsal and lateral portions of the skull were opened and the brains gently lifted to reveal the pituitary capsule, which was surgically opened to extract the entire pituitary gland. Pituitary gland was homogenized exactly as previously described for whole brains, and tissue supernatants were analyzed for perivascular rhodamine uptake normalized to a value obtained with a control mouse included in each experiment. Comparative analysis was done because of variability in molecular weight of the individual lots of dextran. The manufacturer warns that unlabeled dextran is polydispersed and becomes even more heterogeneous during modification and purification. Homogenates were also subjected to Western blotting to assess neutrophil sequestration by probing for CD66b using β actin to control for protein loading.
Statistical analysis.
We used Sigmastat software (Systat, Point Richmond, CA) for the statistical analysis. Data were analyzed by ANOVA followed by the Holm-Sidak test. Single comparisons were performed using Student's t-tests.
RESULTS
Neurohypophysial action potential broadening.
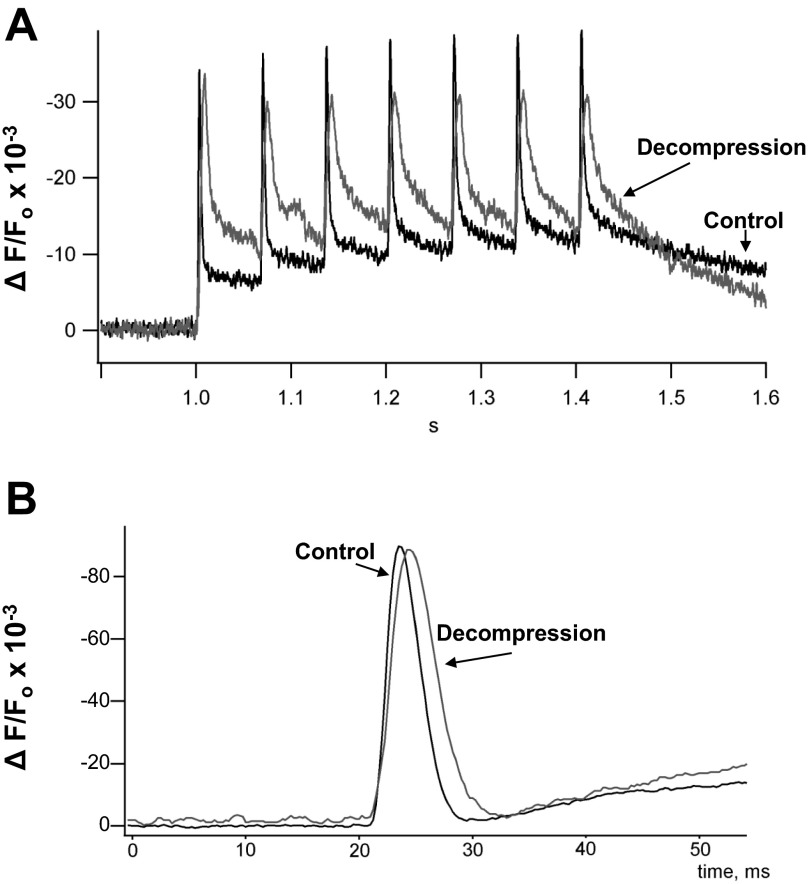
Figure 1A demonstrates the AP when a series of seven shocks was applied to neurohypophysial preparations from a control mouse and after a mouse was subjected to 790 kPa air pressure for 2 h and killed 1 h after decompression. To quantify results across different experiments the data are expressed as AP width at the half-maximal height using magnified images as shown in Fig. 1B. Mice studied 1 h after being subjected to decompression stress exhibited 45 ± 15% (SE, n = 9) broadening of the AP (Table 1). If mice were injected with PEG telomere B immediately after decompression and killed 1 h later, no significant AP broadening occurred. These studies were prompted because PEG will lyse intravascular MPs and abrogate the vascular insults that normally arise postdecompression (33). Data in Table 1 also demonstrate that no significant AP changes occurred in mice rendered thrombocytopenic prior to decompression or in MPO-KO mice. Similarly, if mice received an injection of the specific iNOS inhibitor 1400 W they too did not show AP broadening postdecompression. Finally, consistent with the data using 1400 W, AP broadening was not statistically significant in decompressed iNOS-KO mice.
Fig. 1.
Neurohypophysial nerve terminal membrane voltage changes. A: representative tracings of voltage signals from the neurohypophysis of a control and decompressed animal in response to a train of 7 stimuli delivered at 15 Hz. Ordinate scale is (Δ fluorescence)/(fluorescence at time 0), and hence dimensionless. B: magnified image of single action potential (AP); note difference in abscissa scale vs. A, from a control mouse and a decompressed mouse. Ordinate again shows (Δ fluorescence)/(fluorescence at time 0); abscissas are either in s or ms.
Table 1.
Action potential width at half-maximum height for control mice and those killed 1 h after exposure to 790-kPa air pressure for 2 h
| Control | Decompression | |
|---|---|---|
| No manipulations | 1.00 ± 0.04 (20) | 1.45 ± 0.15 (9)* |
| + PEG | 0.92 ± 0.03 (3) | 1.00 ± 0.03 (9) |
| Thrombocytopenia | 1.05 ± 0.09 (3) | 1.13 ± 0.05 (3) |
| + 1400 W | 1.11 ± 0.02 (3) | 1.18 ± 0.04 (3) |
| MPO-KO | 1.14 ± 0.05 (6) | 1.20 ± 0.07 (3) |
| iNOS-KO | 1.02 ± 0.06 (3) | 1.18 ± 0.04 (3) |
Values were normalized to the mean wild-type mouse control action potential width (3.97 ± 0.14 ms) and are displayed as means ± SE (n = individual mice studied).Where shown wild-type mice were first rendered thrombocytopenic, injected with 25 mg/kg 1400 W immediately before pressure exposure or injected with PEG telomere B (PEG) after decompression and killed 1 h later. Knock-out (KO) mice used in some studies lacked myeloperoxidase (MPO) or iNOS.
P < 0.05.
Perivascular changes in neurohypophysis.
The question arose whether postdecompression neurohypophysial changes are similar to those observed in whole brain studies (33, 38). Neutrophil sequestration and vascular permeability were assayed in endothelium-enriched homogenates of pituitary glands obtained from control and decompressed mice. Western blots of the neutrophil-specific CD66b protein normalized to β actin was 1.66 ± 0.18 (n = 4, P < 0.05)-fold higher in tissue from decompressed mice vs. control mice. Vascular permeability to lysine-fixable 2 × 106 Da rhodamine-conjugated dextran did not differ significantly and was 1.11 ± 0.32 (n = 4, NS)-fold greater in decompressed vs. control mice. However, dextran deposition normalized to protein present in each sample for control pituitary was nearly fourfold higher than found in whole brain studies performed over the past several years [197.7 ± 65.1 (n = 3) vs. 50.2 ± 2.5 (n = 18) mg rhodamine/mg protein].
MP injections.
Manipulations administered to wild-type mice and the knockout mice used in experiments summarized in Table 1 have a limitation because they all decrease the numbers of circulating MPs generated postdecompression (33, 34, 38). Hence, it is unclear whether seeming protection from decompression stress occurs due to gross alterations in MP number, modification(s) within MPs themselves, and/or due to an agent-induced perivascular tissue change. Therefore, AP studies were conducted using harvested mouse MPs injected into naive mice so that equal numbers could be introduced. Results shown in Table 2 indicate that MPs from air-breathing control mice have no adverse effect on neurohypophysial AP whereas statistically significant broadening occurs when mice are injected with MPs from decompressed mice. Mice injected with MPs obtained from decompressed iNOS KO mice also exhibited AP broadening.
Table 2.
Action potential width at half-maximum height for injected mice relative to mean value for control mice
| Injection | No. of MPs Injected | Relative AP |
|---|---|---|
| PBS only | 0 | 1.01 ± 0.03 (6) |
| Control mouse MPs | 7.22 × 105 (± 19,123) | 0.95 ± 0.05 (3) |
| Decompressed WT MPs | 6.78 × 105 (± 29,025) | 1.24 ± 0.02 (3)* |
| Decompressed iNOS KO MPs | 8.59 × 105 (± 61,639) | 1.33 ± 0.11 (3)* |
| Decompressed WT MPs + Nox2ds | 7.04 × 105 (± 15,392) | 1.25 ± 0.02 (4)* |
| Decompressed WT MPs + sham Nox2ds | 7.26 × 105 (± 23,351) | 1.49 ± 0.03 (4)* |
Values are means ± SE; n = individual mice studied. The first column indicates source of microparticles (MPs) injected into naive mice. Mean numbers of MPs injected from different types of mice are shown in the second column (no significant difference among samples). Action potential (AP) width relative to the mean unmanipulated control mouse value (3.97 ± 0.14 ms) is shown in the third column. MPs were obtained from control wild-type (WT) mice not subjected to decompression stress, WT mice first subjected to 790 kPa air for 2 h, or iNOS KO mice first subjected to 790 kPa air pressure for 2 h. The last two rows show the effect of injecting MPs from WT mice first subjected to 790 kPa air pressure but incubated with 10 μM Nox2ds or a control scrambled sequence peptide during isolation.
P < 0.05.
MPs incubated with Nox2ds.
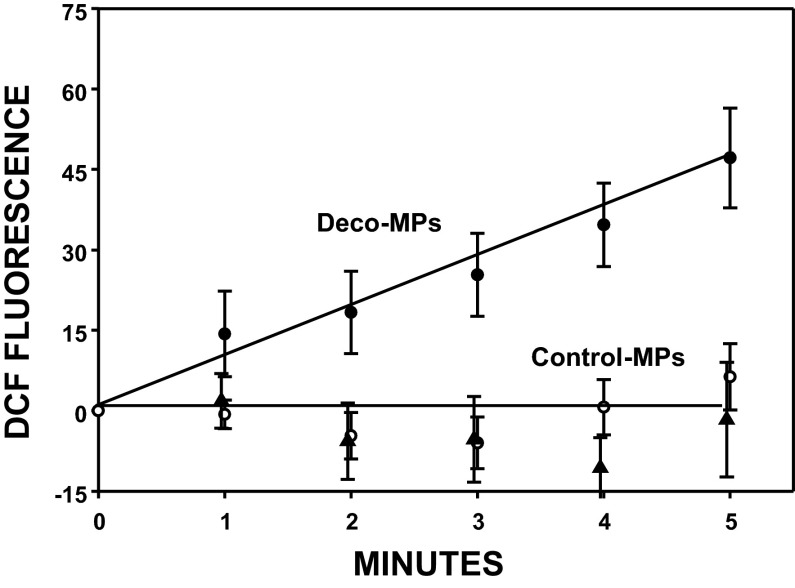
Observing AP broadening with MPs from decompressed iNOS KO mice caused us to consider that reactive species other than ·NO may be involved with the neurohypophysial insult. In addition to iNOS, others have reported that NADPH oxidase is present in some MPs (13, 14). Therefore, MPs were obtained from control (no decompression) or decompressed wild-type mice, split into two aliquots, and incubated with a specific NADPH oxidase inhibitor, Nox2ds, or a scrambled amino acid sequence control peptide. Fluorescence in the presence of membrane-impermeable 2,7-dihydrodichlorofluorescein (DCF) (Fig. 2) indicates that reactive species are liberated from MPs taken from decompressed mice incubated with sham, scrambled sequence peptide but not when incubated with Nox2ds. DCF fluorescence was also negligible with MPs from control mice. Interestingly, the rate of fluorescence increase was not significantly different when MPs were incubated with membrane-permeable DCF-DA. Expressed as fluorescence units per minute, the slope for MPs from control mice incubated with scrambled peptide was 5.4 ± 0.6 (SE, n = 6 for all studies), for control MPs plus Nox2ds was 5.5 ± 0.9, for MPs from decompressed mice incubated with scrambled peptide was 5.3 ± 0.6, and for decompressed MPs plus Nox2ds was 5.7 ± 0.9. Consistent with a previous study, no significant fluorescence signal was detected when MPs were incubated with 4,5-diaminofluorescein (DAF), an agent with greater sensitivity for reactive nitrogen vs. reactive oxygen species (data not shown) (34). When naive mice were injected with MPs obtained from decompressed mice, significant AP broadening was identified whether MPs had been incubated with the scrambled sequence control peptide or with Nox2ds (Table 2).
Fig. 2.
DCF fluorescence from 60,000 microparticles (MPs). Values are means ± SE (n = 6) for MPs obtained from decompressed mice that were incubated with 10 μM Nox2ds (closed triangles) or scrambled sequence peptide (Deco-MPs, closed circle), or control mice (no decompression stress) incubated with scrambled sequence peptide (Control-MPs, open circles). For sake of clarity, control MPs incubated with Nox2ds are not shown, but slope was insignificantly different from decompressed MPs + Nox2ds (slope = 1.7 ± 2.1 fluorescence units/min).
DISCUSSION
The goal of this investigation was to examine the role of MPs in causing central nervous system injury due to decompression stress. Decompression caused broadening of the neurohypophysial AP and MPs were implicated because AP broadening did not occur if mice were injected with PEG immediately following decompression. AP broadening also did not occur if mice were rendered thrombocytopenic and changes were not found in MPO KO mice. These observations support a role for circulating platelets and reactive species generated by neutrophil MPO in CNS decompression pathophysiology. They are consistent with previous studies evaluating systemic decompression injuries linked to MPs (33, 34, 38). Similarly, iNOS plays a role based on the inhibitory effect of 1400 W and lack of AP broadening in decompressed iNOS KO mice. A problem with interpreting the results, however, is that we have shown that iNOS activity is involved with MP formation and there are fewer circulating MPs generated in response to decompression in MPO-KO, iNOS-KO, and thrombocytopenic mice, and those treated with 1400 W (33, 34, 38).
Neutrophil sequestration assessed by Western blots of pituitary gland homogenates from decompressed mice is consistent with previously published whole brain studies (33). Contrary to prior studies, however, excessive vascular leakage was not identified in pituitary from decompressed mice. This may have occurred due to the unusually permeable neurovascular portal system in this tissue, as shown by others after injecting various tracer compounds (3, 37). We found substantially greater leakage of dextran in control mouse pituitary vs. whole brain in previously reported studies (33).
Injecting MPs from control (no decompression) mice did not cause AP broadening, unlike MPs from decompressed mice, thus showing that neurological injury is mediated by inherent differences within MPs obtained from decompressed mice rather than merely a postdecompression increase in MP number. In previous work, we found that MPs from decompressed mice are enlarged due to presence of a gas phase, and if they are subjected to hydrostatic recompression to diminish their size, MP injections did not cause neutrophil activation or an increased capillary leak (38). These and other studies have shown that iNOS is important for decompression pathophysiology in part because it generates a gas phase of nitrogen dioxide within some MPs that serves as a gas cavitation nucleus, thus allowing MP enlargement that we presume occurs due to inert gas uptake from supersaturated tissues (34).
The present study demonstrates that AP broadening is equal whether MPs from decompressed wild-type or iNOS KO mice are injected into naive mice; yet mice treated with 1400 W and iNOS KO mice do not show AP broadening. These observations lead us to conclude that postdecompression nerve dysfunction involves perivascular iNOS activation. There is, of course, ample precedence for this notion. Astrocyte and microglial iNOS are linked to CNS inflammation following a variety of insults, and iNOS synthesis is a component of traumatic and postischemic brain injury (4, 16, 22, 24).
Decompressed iNOS KO mice exhibit neutrophil activation and a small, but statistically significant leak from brain vessels (34). Naive mice injected with MPs from decompressed iNOS KO mice do not exhibit leakage from brain vessels although they show some neutrophil activation and also sustain an increased vascular leak in several tissues, although not to the same degree as caused by MPs obtained from wild-type decompressed mice (34). These findings indicate that iNOS is not critical for all elements of decompression pathophysiology. They also show that the mechanism for AP broadening is not the same as that causing the vascular leak because AP broadening does not occur in decompressed iNOS KO mice but does occur in naive mice injected with MPs from decompressed iNOS KO mice.
Finding AP broadening following injections of MPs from iNOS KO mice led us to hypothesize that reactive species other than ·NO may play a role in nerve injury. On the basis of DCF fluorescence observations, we conclude that reactive species are liberated from MPs obtained from decompressed mice but not control mice (Fig. 2). Inhibition of DCF fluorescence by Nox2ds strongly implicates NADPH oxidase as the reactive species generator, although it is surprising that all required cytosolic and membrane-associated enzyme complex components should be present. We reason that reactive species liberation from decompressed samples occurs because of membrane perturbations that activate the oxidase rather than a more simplistic view that membranes are damaged and thus “leaky” due to stretch associated with particle enlargement from the decompression process. Because fluorescence from membrane-permeable DCF-DA did not differ among MPs whether obtained from control or decompressed mice incubated with or without Nox2ds, we conclude that NADPH oxidase plays an insignificant role in production of reactive species contained within MPs. This novel finding will require further work to discern the mechanism(s), but the data demonstrate that DCF-detectable reactive species liberation from MPs are not responsible for AP broadening because nerve dysfunction still occurred when MPs from decompressed mice were incubated with Nox2ds prior to injection (Table 2). AP broadening in mice injected with MPs incubated with sham Nox2ds appears somewhat greater than when mice are injected MPs incubated with active Nox2ds, although the difference was not statistically significant. Because just 250 μl of solution containing these agents was injected, this amounts to only about 2 μM of peptide in a 20-g mouse [with plasma volume of ∼980 μl (2)]. The peptides had identical amino acids although different sequences, so selective neurotoxicity of the sham agent seems unlikely.
In conclusion, the present study advances our understanding of neurological DCS by directly implicating decompression-induced MPs as causing neurohypophysial AP broadening. Inflammatory conditions can lead to neurohypophysial dysfunction although pituitary injury is not known to occur with DCS (30). However, there is a growing body of literature showing that after global cerebral injuries neurohypophysial dysfunction may be subclinical or manifested in a delayed fashion (1, 20, 28). We view AP broadening postdecompression as a useful scientific index rather than a finding with diagnostic clinical relevance. In this regard, the mechanism for AP broadening will require additional study. Changes could occur due to the number of nerve terminals activated, changes in the action potential wave form, and/or alterations of intraterminal calcium elevation (e.g., release, buffering, influx, and/or extrusion). There also is need for improved understanding of mechanisms that generate MPs, whether it is bubbles that generate MPs, or vice versa, remains unclear. Studies in human divers have suggested that MP generation is influenced by differences in nitrogen and O2 partial pressures at depth or during the decompression (ascent) phase of the dive (32).
GRANTS
Funding was provided by grants from the Office of Naval Research and the National Institutes of Health (NS-40966).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.Y., P.K., T.N.M., V.M.B., and S.R.T. performed experiments; M.Y., P.K., B.M.S., and S.R.T. analyzed data; M.Y. and S.R.T. drafted manuscript; M.Y., P.K., B.M.S., T.N.M., V.M.B., and S.R.T. edited and revised manuscript; M.Y., P.K., B.M.S., T.N.M., V.M.B., and S.R.T. approved final version of manuscript; P.K., B.M.S., and S.R.T. conception and design of research; S.R.T. interpreted results of experiments; S.R.T. prepared figures.
ACKNOWLEDGMENTS
Present address of M. Yang, V. M. Bhopale, and S. R. Thom: Dept. of Emergency Medicine, Univ. of Maryland, Baltimore, MD 21201.
REFERENCES
- 1. Agha A, Sherlock M, Phillips J, Tormey W, Thompson CJ. The natural history of posttraumatic neurohypophysial dysfunction. Eur J Endocrinol 152: 371–377, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Altman PL, Dittmer DS. Biology Databook. Washington, DC: FASEB, 1964, p. 264 [Google Scholar]
- 3. Broadwell RD. Entry of peroxidase into neurons of the central and peripheral nervous system from extracerebral and cerebral blood. J Comp Neurol 166: 257–283, 1976 [DOI] [PubMed] [Google Scholar]
- 4. Brown CM, Dela Cruz CD, Yang E, Wise PM. Inducible nitric oxide synthase and estradiol exhibit complementary neuroprotective roles after ischemic brain injury. Exp Neurol 210: 782–787, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Csanyi G, Cifuentes-Pagano E, Ghouleh IA, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ. Nox2 B-loop peptide, Noxds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erdem I, Yildiz S, Uzun G, Sonmez G, Senol MG, Mutuoglu M, Mutlu H, Oner B. Cerebral white matter lesions in asymptomatic military divers. Aviat Space Environ Med 80: 2–4, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Fox FE, Herzfeld KF. Gas bubbles with organic skins as cavitation nuclei. J Acoust Soc Am 26: 984–989, 1954 [Google Scholar]
- 8. Francis TJR, Pezeshkpour GH, Dutka AJ, Hallenbeck JM, Flynn ET. Is there a role for the autochthonous bubble in the pathogenesis of spinal cord decompression sickness? J Neuropath Exper Neurol 47: 475–487, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Frontczak-Baniewicz M. Focal ischemia in the cerebral cortex has an effect on the neurohypophysis. I. Ultrastructural changes in capillary vessels of the neurohypophysis after focal ischemia of the cerebral cortex. Neuroendocrinol Lett 22: 81–86, 2001 [PubMed] [Google Scholar]
- 10. Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin Exp Pharmacol Physiol 27: 422–427, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Gao GK, Wu D, Yang Y, Yu T, Xue JY, Wang X, Jiang YP. Cerebral magnetic resonance imaging of compressed air divers in diving accidents. Undersea Hyperb Med 36: 33–41, 2009 [PubMed] [Google Scholar]
- 12. Hatton GI, Perlmutter LS, Salm AK, Tweedle CD. Dynamic neuronal-glial interactions in hypothalamus and pituitary: implications for control of hormone synthesis and release. Peptides 5, Suppl 1: 121–138, 1984 [DOI] [PubMed] [Google Scholar]
- 13. Janiszewski M, Do Carmo AO, Pedro MA, Silva E, Knobel E, Laurnido FR. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: a novel vascular redox pathway. Crit Care Med 32: 818–825, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Jansen F, Yang X, Franklin BS, Hoelscher M, Schmitz T, Bedorf J, Nickenig G, Werner N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res 98: 94–106, 2013 [DOI] [PubMed] [Google Scholar]
- 15. Jersey SL, Baril RT, McCarty RD, Millhouse CM. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med 81: 64–68, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Jones NC, Prior MJW, Burden-Teh E, Marsden CA, Morris PG, Murphy S. Antagonism of the interleukin-1 receptor following traumatic brain injury in the mouse reduces the number of nitric oxide synthase-2 positive cells and improves anatomical and functional outcomes. Eur J Neurosci 22: 72–78, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Koch AE, Kirsch H, Reuter M, Warninghoff V, Rieckert H, Deuschl G. Prevalence of patent foramen ovale (PFO) and MRI-lesions in mild neurological decompression sickness. Undersea Hyperb Med 35: 197–205, 2008 [PubMed] [Google Scholar]
- 18. Kohshi K, Katoh T, Abe H, Wong RM. Central nervous system involvement in patients with decompression illness. Sangyo Eiseigaku Zasshi 45: 97–104, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Kosterin P, Kim GH, Muschol M, Obaid AL, Salzberg BM. Changes in FAD and NADH fluorescence in neurosecretory terminals are triggered by calcium entry and by ADP production. J Membr Biol 208: 113–124, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Lieberman SA, Oberoi AL, Gilkison CR, Masel BE, Urban RJ. Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J Clin Endocrinol Metab 86: 2752–2756, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Madden LA, Chrismas BC, Mellor D, Vince RV, Midgley AW, McNaughton LR, Atkins SL, Laden G. Endothelial function and stress response after simulated dives to 18 msw breathing air or oxygen. Aviat Space Environ Med 81: 41–51, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Mayo L, Jacob-Hirsch J, Amariglio N, Rechavi G, Moutin MJ, Lund FE, Stein R. Dual role of CD38 in microglial activation and activation-induced cell death. J Immunol 181: 92–103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Obaid AL, Loew LM, Wuskell JP, Salzberg BM. Novel naphthylstyryl-pyridium potentiometric dyes offer advantages for neural network analysis. J Neurosci Methods 134: 179–190, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Oliva AA, Kang Y, Sanchez-Molano J, Furones C, Atkins CM. STAT3 signaling after traumatic brain injury. J Neurochem 120: 710–720, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Pontier JM, Gempp E, Ignatescu M. Blood platelet-derived microparticles release and bubble formation after an open-sea dive. Appl Physiol Nutr Metab 37: 1–5, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Reaves TA, Cumming R, Hayward JN. Light- and electron-microscopic characterization of electrophysiologically-identified, horseradish peroxidase-injected magnocellular neuroendocrine cells in goldfish preoptic nucleus. Neuroscience 7: 1545–1557, 1982 [DOI] [PubMed] [Google Scholar]
- 27. Salzberg BM, Obaid AL, Senseman DM, Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature 306: 36–40, 1983 [DOI] [PubMed] [Google Scholar]
- 28. Schmiegelow M, Lassen S, Poulson HS, Feldt-Rasmussen U, Schmiegelow K, Hertz H, Muller J. Growth hormone response to a growth hormone-releasing hormone stimulation test in a population-based study following cranial irradiation of childhood brain tumors. Horm Res 54: 53–59, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Senseman DM, Shimizu H, Horowitz IS, Salzberg BM. Multiple-site optical recording of membrane potential from a salivary gland. Interaction of synaptic and electronic excitation. J Gen Physiol 81: 887–908, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tashiro T, Sano T, Xu B, Wakatsuki S, Kagawa N, Nishioka H, Yamada S, Kovacs K. Spectrum of different types of hypophysitis: a clinicopathologic study of hypophysitis in 31 cases. Endocr Pathol 13: 183–195, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Thom SR, Milovanova TN, Bogush M, Bhopale VM, Yang M, Bushmann K, Pollock NW, Ljubkovic M, Denoble P, Dujic Z. Microparticle production, neutrophil activation and intravascular bubbles following open-water SCUBA diving. J Appl Physiol 112: 1268–1278, 2012 [DOI] [PubMed] [Google Scholar]
- 32. Thom SR, Milovanova TN, Bogush M, Yang M, Bhopale VM, Pollock NW, Ljubkovic M, Denoble P, Madden D, Lozo M, Dujic Z. Bubbles, microparticles and neutrophil activation: changes with exercise level and breathing gas during open-water SCUBA diving. J Appl Physiol 114: 1396–1405, 2013 [DOI] [PubMed] [Google Scholar]
- 33. Thom SR, Yang M, Bhopale VM, Huang S, Milovanova TN. Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J Appl Physiol 110: 340–351, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Thom SR, Yang M, Bhopale VM, Milovanova TN, Bogush M, Buerk DG. Intra-microparticle nitrogen dioxide is a bubble nucleation site leading to decompression-induced neutrophil activation and vascular injury. J Appl Physiol 114: 550–558, 2013 [DOI] [PubMed] [Google Scholar]
- 35. Vann RD, Butler FK, Mitchell SJ, Moon RE. Decompression illness. Lancet 377: 153–164, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Vince RV, McNaughton LR, Taylor L, Midgley AW, Laden G, Madden LA. Release of VCAM-1 associated endothelial microparticles following simulated SCUBA dives. Eur J Appl Physiol 105: 507–513, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Vorbrodt AW. Ultrastructural study on the interaction of native and cationized albumin-gold complexes with mouse brain microvascular endothelium. J Neurocytol 25: 645–657, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Yang M, Milovanova TN, Bogush M, Uzan G, Bhopale VM, Thom SR. Microparticle enlargement and altered surface proteins after air decompression are associated with inflammatory vascular injuries. J Appl Physiol 112: 204–211, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yount DE. On the elastic properties of the interfaces that stabilize gas cavitation nuclei. J Colloid Interface Sci 193: 50–59, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Yount DE, Kunkle TD, D'Arrigo JS. Stabilization of gas cavitation nuclei by surface active compounds. Aviat Space Environ Med 48: 185–189, 1977 [PubMed] [Google Scholar]