Abstract
A unique property of mitochondria in mammalian cells is their ability to physically interact and undergo dynamic events of fusion/fission that remodel their morphology and possibly their function. In cultured cells, metabolic perturbations similar to those incurred during exercise influence mitochondrial fusion and fission processes, but it is unknown whether exercise acutely alters mitochondrial morphology and/or membrane interactions in vivo. To study this question, we subjected mice to a 3-h voluntarily exercise intervention following their normal physical activity patterns, and quantified mitochondrial morphology and membrane interactions in the soleus using a quantitative electron microscopy approach. A single exercise bout effectively decreased blood glucose (P < 0.05) and intramyocellular lipid content (P < 0.01), indicating increased muscle metabolic demand. The number of mitochondria spanning Z-lines and proportion of electron-dense contact sites (EDCS) between adjacent mitochondrial membranes were increased immediately after exercise among both subsarcolemmal (+116%, P < 0.05) and intermyofibrillar mitochondria (+191%, P < 0.001), indicating increased physical interactions. Mitochondrial morphology, and abundance of the mitochondrial pro-fusion proteins Mfn2 and OPA1 were unchanged. Collectively, these results support the notion that mitochondrial membrane dynamics are actively remodelled in skeletal muscle, which may be regulated by contractile activity and the metabolic state. Future studies are required to understand the implications of mitochondrial dynamics in skeletal muscle physiology during exercise and inactivity.
Keywords: mitochondrial dynamics, skeletal muscle, exercise, fusion, metabolic state
in response to contraction, skeletal muscle fibers undergo significant metabolic and molecular remodelling contributing to the health benefits of exercise (14). These changes include but are not limited to translocation of glucose transporters (GLUT4) to the plasma membrane (32), allosteric activation of Ca2+-sensitive mitochondrial dehydogenases (36), demethylation of mitochondrial gene promoters in the nuclear genome (3), and phosphorylation of several transcription factors (25). Collectively, these acute (minutes to hours) changes enable adequate adaptation in the face of rising energy demands. Most of skeletal muscle energy requirements during submaximal exercise are met by mitochondria, specialized organelles that exhibit unique topology and morphology within myofibers (2, 29, 37, 43). An emerging aspect of mitochondrial biology is their ability to undergo dynamic morphological changes (6), which enable quality-control processes (51), preserve mitochondrial DNA integrity (9), and possibly modulate mitochondrial respiratory capacity, reactive oxygen species (ROS) production, and sensitivity to permeability transition (7, 40).
Mitochondrial morphology transitions in isolated cells occur in response to variations in cellular energy metabolism (34), whereby energetic deprivation (metabolic undersupply) acutely favours mitochondrial elongation (22, 46) and excess substrate supply (metabolic oversupply) lead to mitochondrial fragmentation (50, 53) (see Ref. 42 for a discussion). The redox state and oxidative stress can also modulate mitochondrial morphology (15, 47). Furthermore, we recently demonstrated the existence of membrane interactions among skeletal muscle mitochondria in vivo (43), and others have observed adjacent mitochondria exchanging matrix-located fluorescent molecules in myofibers (39) and cardiomyocytes (26), which constitute evidence of mitochondrial dynamics. However, whether an acute bout of exercise can remodel mitochondrial membrane interactions or acutely alter mitochondrial morphology is unknown.
To study this question, key technical and physiological factors were considered. First, examining skeletal muscle immediately following exhaustive exercise may cause mitochondria swelling and cristolysis (18, 21) rendering impracticable the study of subtle changes in mitochondrial morphology. Second, given that gene expression and hormonal mediators regulating cellular energy metabolism are under strong diurnal control (1, 17, 28), the timing of exercise interventions with the natural physical activity patterns of nocturnal laboratory animals appears essential to isolate the effect of physical activity. Third, psychological stress that may impinge upon animals during forced running or swimming paradigms could possibly have confounding deleterious effects on mitochondrial morphology and function (23, 35). Finally, a quantitative high-resolution approach is required to resolve mitochondrial membrane interactions and compare key aspects of mitochondrial morphology (43).
To meet these requirements, we established a nonstressful voluntary exercise paradigm by introducing a running wheel at the onset of the dark phase in the light-dark cycle, and examined mitochondrial morphology and membrane interactions immediately after exercise by using a novel electron microscopy approach combining views from two orthogonal planes (43). Furthermore, we applied our analyses to the two major mitochondrial populations in myofibers: subsarcolemmal (SS) and intermyofibrillar (IMF), which exhibit both functional (10, 31) and morphological (20, 43) differences. We complemented these measurements with assessment of key proteins known to mediate mitochondrial membrane interactions. Overall, our findings demonstrate that a single exercise bout acutely remodels mitochondrial membrane interactions, but not morphology, in mouse skeletal muscle.
METHODS
Animals and experimental design.
Eight-week-old female C57BL/6J mice were group housed with free access to food and water and maintained at constant temperature on a 12:12-h light-dark cycle. At 1000 on the day of experiments, a pair of mice derived from the same litter were randomly assigned to “sedentary” or “running” conditions, and transferred individually to new cages with normal access to food and water, layered with ∼3 cm of wood-chip bedding, and containing 1 cup of paper-shredded nestlet in a cage corner. For mice assigned to the running condition, the cage contained a 15-cm diameter plastic running wheel equipped with a magnetic rotation counter, whereas for the sedentary mice only the wheel support was introduced to control for the presence of a novel object. At 1100, the running wheel and support were removed from the cages, and mice were kept in a quiet room thereafter. Immediately before the onset of the dark phase of the light cycle (1855), the running wheel or wheel support were reintroduced, such that mice had undisrupted access to the wheel at 1900 when the lights were turned off. For the remaining 3 h, mice remained undisturbed in the dark. The experimental paradigm is illustrated in Fig. 1A.
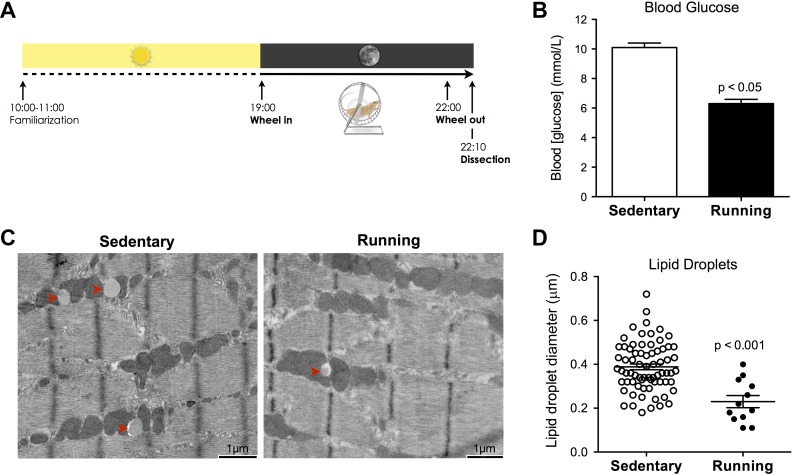
Fig. 1.
A single bout of exercise modulates skeletal muscle metabolic state. A: experimental paradigm showing the period (1 h) of familiarization and acclimatization early during the light phase, followed by a single 3-h period with free access to a running wheel during the dark phase of the light/dark cycle. B: blood glucose levels measured immediately after exercise (means ± SE). C: electron micrographs of the soleus depicting intramyocellular lipid droplets from sedentary and running exercised animals. D: quantification of lipid droplet size and number matched for surface area of muscle analyzed. Bars are means ± SE; n = 15–16 myofibers analyzed per group.
At 2200, lights were turned on, at which point mice stopped running. At 2210, mice in the running condition were killed by cervical dislocation (2215 for the sedentary mice). Blood glucose was measured immediately from trunk blood using a glucose monitor (AlphaTrak, Abbott). Within 1 to 1.5 min following decapitation, the soleus was dissected, cut into two equal halves, and fixed by immersion into fixative for transmission [transmission electron microscopy (TEM)] and scanning electron microscopy (SEM). Tissues remained in fixative overnight at room temperature, and >48 h at 4°C before processing for electron microscopy. Running distance was monitored by the number of wheel revolution over the 3-h period. Experiments were repeated three times, and a total of six mice were used. All animal procedures were approved by the University of Newcastle Animal Care and Ethics Committee (ACEC).
Transmission electron microscopy.
For TEM, half of the mouse soleus was immediately fixed in a 2% glutaraldehyde solution in 0.1 M cacodylate (TAAB Lab Equipment) buffer, pH 7.4 as described previously (43). Briefly, muscle samples were then postfixed and dehydrated, after which the half soleus was cut into smaller segments and embedded either in transverse (TS) or longitudinal (LS) orientation in 100% resin. Orientation and section quality was checked with 1-μm thick sections, and ultrathin sections of 70 nm were subsequently cut using a diamond knife on a Leica EM UC7 ultramicrotome. Sections were stretched with chloroform to eliminate compression and mounted on Pioloform filmed copper grids prior to staining with 2% aqueous uranyl acetate and lead citrate (Leica). Ultrathin sections were examined on a Phillips CM 100 Compustage (FEI) transmission electron microscope and digital micrographs were captured by an AMT CCD camera (Deben).
To analyze SS mitochondrial morphology and lipid droplets, muscle in the LS orientation was photographed at ×19,000 magnification. Mitochondria-rich regions were selected for analyses. Lipid droplets were quantified by measuring the diameter of all lipid droplets present in randomly selected micrographs in both groups. For IMF mitochondria, muscle in the TS orientation was imaged at ×7,900 magnification. To insure optimal transverse orientation at the subcellular level (relative to the Z-line), only muscle fibers presenting no more than two Z-lines separated by no less than 10 to 15 μm were selected for analysis (43). For each animal, five to six mitochondria-rich muscle fibers were analyzed in both TS and LS, for which at least two micrographs were captured, allowing the analysis of ∼40 IMF and 40 SS mitochondria per myofiber. A total of 505 (sedentary, SED) and 563 (running, RUN) SS, and 653 (SED) and 623 (RUN) IMF mitochondria were analyzed. Part of the data from the sedentary animals was previously reported in (43). For all morphological parameters, the coefficient of variation was significantly greater between different myofibers of a given animal than between animals.
To quantify mitochondrial interactions among SS and IMF mitochondria, micrographs of muscle photographed in the longitudinal orientation at 19,000 (SS) and 13,500 (IMF) were used. In the IMF compartment, a total of 750 (SED) and 636 (RUN) Z-lines possessing mitochondria on both sides were analyzed, either as pairs of discrete organelles, a continuous mitochondrion, or interacting organelles. The proportion of Z-lines spanned by a continuous mitochondrion or interacting mitochondria is reported as a percentage of the total. In addition, 437 (SED) and 391 (RUN) SS, and 667 (SED) and 489 (RUN) IMF physical contacts sites between adjacent mitochondria were evaluated for the presence of electron density contact sites (EDCS). The proportion of all mitochondrial contact sites between organelles that were electron dense is reported as a percentage of total contacts.
Scanning electron microscopy.
For SEM, the other half of the mouse soleus was immediately fixed in a 1% glutaraldehyde (TAAB Lab Equipment) and 0.5% paraformaldehyde (Sigma 158127) solution in a 0.060 M cacodylate (TAAB Lab Equipment) buffer, pH 7.4 as described previously (43). For processing, fixed samples were rinsed twice in 0.067 M cacodylate buffer and subsequently immersed in DMSO (Sigma D2650) for 5 to 10 min. Then, the fixed samples were snap frozen in liquid nitrogen-cooled isopentane (Sigma 277258) for 5 to 10 s and immediately fractured by applying lateral pressure within a liquid nitrogen-cooled cracking instrument. The resulting fractured specimens, ∼0.5 to 3 mm in size, were rinsed three times in 0.067 M cacodylate buffer and postfixed in osmium tetroxide (OsO4) 1% for 1 h. Samples were then transferred to OsO4 0.1% for 72–96 h to partially extract cytoplasmic components (37). Then, muscle samples were dehydrated in sequential steps of ethanol (25%, 50%, 75%, and 100% twice) and dried with CO2 in a Baltec Critical Point Dryer. Specimens were mounted on stubs covered with carbon disks, gold coated (15 nm) in Polaron SEM Coating Unit, and examined on a Stereoscan 240 Scanning Electron Microscope. Fracture sites were observed for exposed mitochondria and digital micrographs were captured at different magnifications with the Orion 6.60.6 software.
Confocal microscopy.
To image mitochondria by confocal microscopy, permeabilized myofibers were prepared as described previously (41). Briefly, a fresh mouse soleus was finely dissected on ice in relaxing buffer, permeabilized with saponin 0.05 mg/ml for 30 min, rinsed 3 times, and incubated for 20 min in Mitotracker Red ROX (Life Technologies M7512) 15 μM at 30°C. Stained muscle fibers were then sandwiched between a glass bottom 12-well plate (MatTek, P12G-1.5–14F) and circular coverslip. Images were immediately acquired using an inverted laser scanning confocal microscope (Zeiss LSM 710) with a PlanApo 63x/1.40 oil immersion objective. In this setting, most fibers lie in perfect longitudinal orientation when imaged (see Fig. 2D), although some overlapping fibers produced perpendicular segments enabling cross-sectional (transverse) imaging (see Fig. 2F). Three-dimensional reconstructions of Z-stacks were produced by the Zen software using default settings.
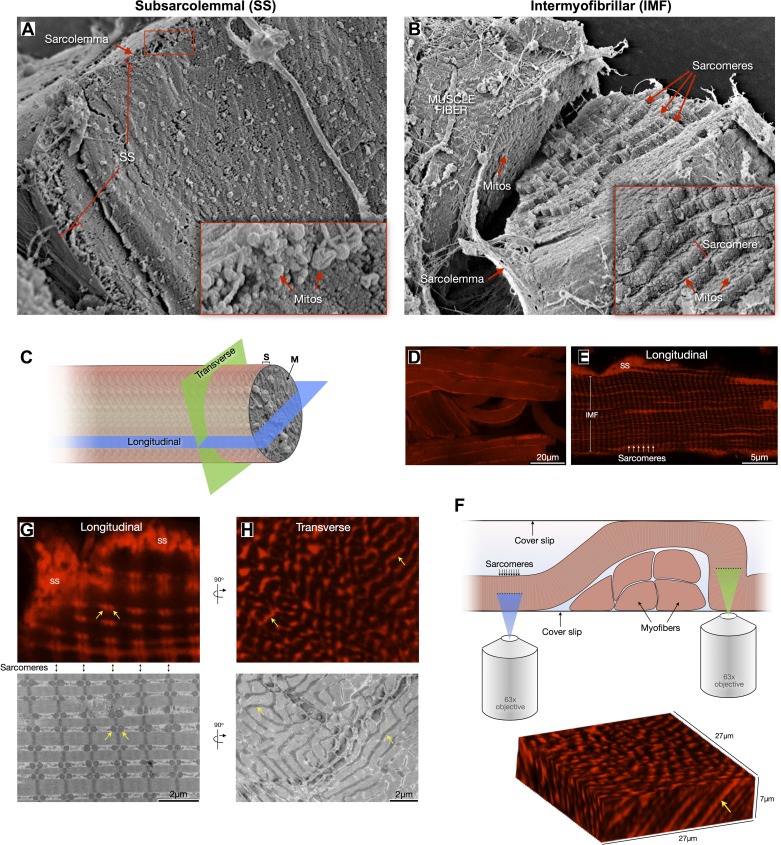
Fig. 2.
Topological and morphological differences between subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria. A: scanning electron microscopy (SEM) imaging of a freeze-fractured soleus muscle fiber sectioned in the cross-sectional plane with its cytoplasm exposed. The SS region is outlined where mostly globular SS mitochondria are clustered. B: SEM of freeze-fractured muscle fiber cracked to expose the staircase-like sarcomere structure revealing the IMF mitochondrial reticulum between each sarcomeric plane. C: diagram representing the two main planes—longitudinal and transverse (i.e., cross-section)—used to quantify various aspects of mitochondrial morphology. D and E: confocal imaging of permeabilized muscle fibers labeled with Mitotracker Red showing SS and IMF mitochondria and sarcomeric organization. F: schematic of experimental setting used for optical sectioning of Mitotracker-labeled muscle fibers in the longitudinal (blue) and cross-sectional (green) planes. Below is a three-dimensional reconstruction of an oblique section across a myofiber, showing elongated organelles (arrow). G: the longitudinal view in both confocal and electron microscopy reveals the pairwise arrangement of IMF mitochondria across Z-lines at each sarcomeric plane (appearing as spherical organelles, arrows), whereas the transverse view reveals the actual tubular morphology of IMF mitochondria (H, arrows).
Morphological analyses and statistics.
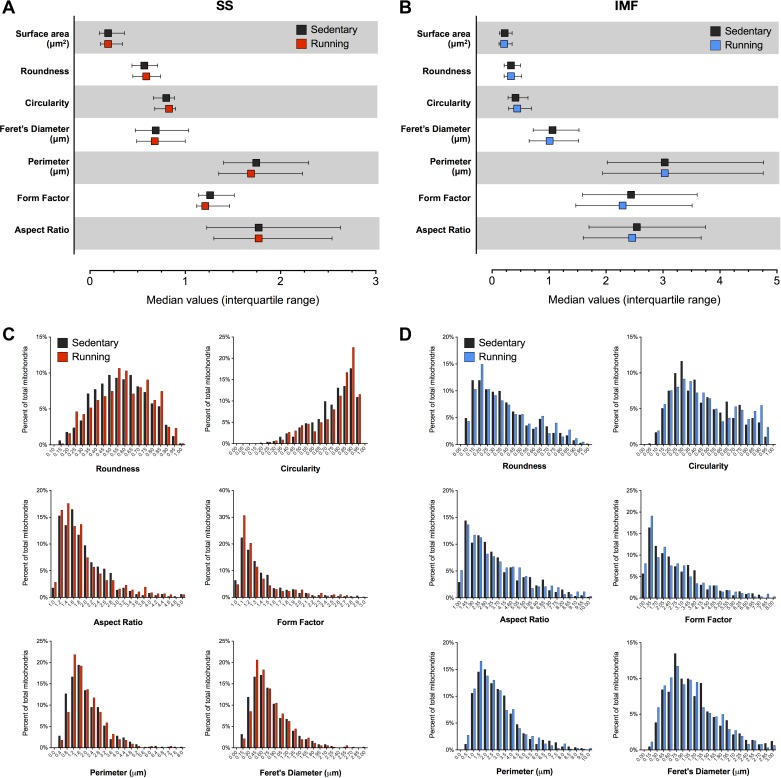
Mitochondrial shape descriptors and size measurements were obtained using Image J (version 1.42q, NIH, http://rsb.info.nih.gov/ij) by manually tracing only clearly discernible outlines of SS and IMF mitochondria on TEM micrographs, as described previously (43) and shown in Fig. 4D. Surface area (or mitochondrial size) is reported in μm2; perimeter in μm. Aspect ratio (AR) is computed as [(major axis)/(minor axis)] and reflects the “length to width ratio”; form factor (FF) [(perimeter2)/(4π·surface area)] reflects the complexity and branching aspect of mitochondria; circularity [4π·(surface area/perimeter2)] and roundness [4·(surface area)/(π·major axis2)] are two-dimensional indexes of sphericity with values of 1 indicating perfect spheroids; and Feret's diameter represents the longest distance (μm) between any two points within a given mitochondrion (30).
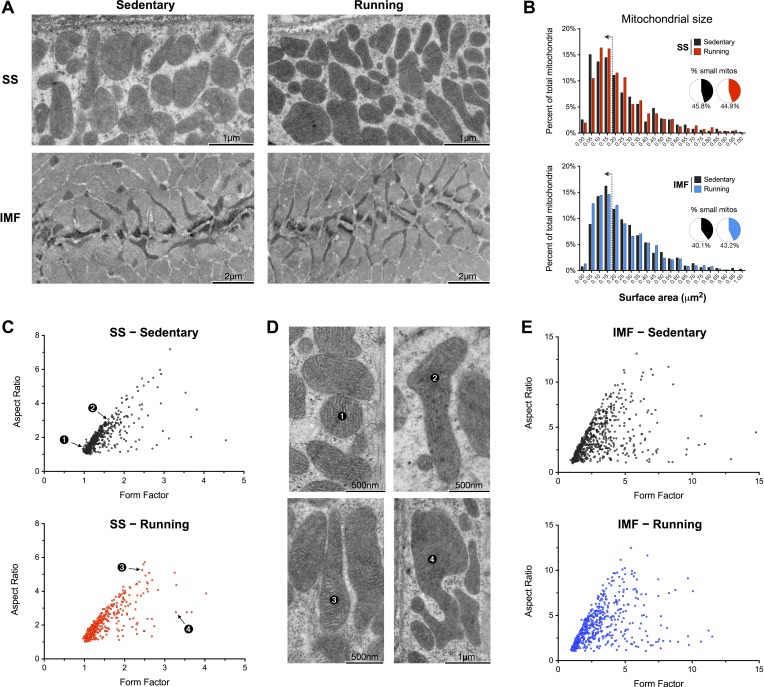
Fig. 4.
Mitochondrial size and morphology in sedentary and running mice. A: representative TEM micrographs of SS and IMF mitochondria from the soleus of sedentary and running mice. B: quantification of mitochondrial size shown as frequency distributions, for which the bin center is indicated. Inset shows the proportion of small mitochondria (<0.175 μm2; left of the dotted line on histograms). C: form factor and aspect ratio distributions for individual, manually-traced SS mitochondria. Numbered data points represent individual mitochondria shown in D. E: data as in C for IMF mitochondria. n = 505–653 mitochondria analyzed per group.
Computed values were imported into Microsoft Excel and Prism 6 (GraphPad Software) for data analysis. Statistical significance was evaluated based on 95% confidence interval (C.I.) of the mean. To produce frequency distributions of morphological parameters, each mitochondrion was assigned to one of twenty bins of equal sizes and proportions were determined to produce frequency histograms.
Western blot analyses.
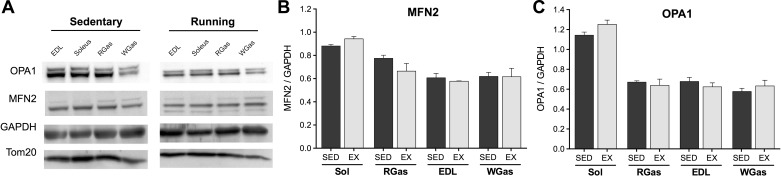
Thirty micrograms of muscle homogenates from the extensor digitorum longus (EDL), the soleus (Sol), the red gastrocnemius (RGas), and the white gastrocnemius (WGas) were prepared with Laemmeli buffer in reducing conditions. Protein abundance was analysed by Western blot using antibodies against mitofusin 2 (Mfn2) (Sigma), optic atrophy 1 (OPA1) (BD biosciences), translocase of outer mitochondrial membrane 20 kDa (TOM20) (Santa Cruz Biotech) and GAPDH (Abcam). Band intensities from immunoblots were quantified using ImageJ from three mice per condition, and normalized against GAPDH.
RESULTS
Exercise and the metabolic state.
During a 3-h period with free access to running wheel during the early part of the dark cycle (Fig. 1A), exercising mice ran intermittently for a total distance of 1893 ± 150 (mean ± SD) meters. To assess whether this single acute bout of exercise altered the metabolic state, blood glucose and intramuscular lipid stores were measured 10 min after the termination of exercise. Glycemia was 38% lower in exercising compared with sedentary mice (Fig. 1B). Intramyocellular lipid (IMCL) droplets in the soleus were less abundant and of reduced size in running mice (Fig. 1, C and D). Thus a single bout of voluntary exercise effectively increased metabolic demand within skeletal muscle, shifting the metabolic state towards undersupply.
Mitochondrial membrane interactions.
SS mitochondria are clustered beneath the plasma membrane whereas IMF mitochondria are layered in an interconnected reticulum near Z-lines between sarcomeres (Fig. 2, A and B). Examination of mitochondrial morphology in both longitudinal and cross-sectional (transverse) planes can be achieved by mounting two different muscle specimens in orthogonal orientation for EM (Fig. 2C), or by optical sectioning of muscle fibers in different orientation by confocal microscopy (Fig. 2, D–F). The typical pair of seemingly spherical mitochondria observed in longitudinal sections (Fig. 2G) are in fact tubular and branched organelles when visualized in cross section (Fig. 2H; see also Ref. 29).
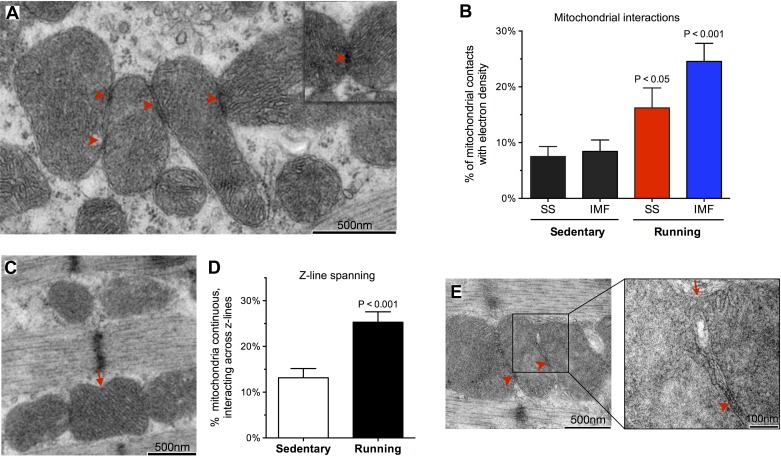
Mitochondrial dynamics involve the direct contact between outer mitochondrial membranes of adjacent organelles, followed by rapid (within seconds) sequential fusion of the outer and inner membranes (49). Electron-dense structures physically linking outer membranes are recognized as EDCS upon examination of skeletal muscle mitochondrial with electron microscopy (Fig. 3A) (2, 43). The abundance of EDCS increased by 1.2 fold (P < 0.05) in SS, and by 1.9 fold (P < 0.01) in IMF mitochondria after exercise, indicating increased mitochondrial interactions (Fig. 3B). In addition, the proportion of IMF mitochondria interacting or physically spanning the Z-line increased by 93% (Fig. 3, C and D). Muscles of exercising mice also contained rare events of membrane-bound bridges connecting the matrix space of tethered mitochondria (Fig. 3E), consistent with processes of mitochondrial dynamics.
Fig. 3.
Increased mitochondrial membrane interactions following exercise. A: representative TEM images of soleus SS mitochondria linked by electron-dense contact sites (EDCS). B: proportion of mitochondrial contacts exhibiting an enhanced electron density in SS and IMF mitochondria, from sedentary and running mice. C: pairwise (top) and continuous (bottom, arrow) mitochondria across a Z-line. D: proportion of continuous or interacting IMF mitochondria across Z-lines. E: neighboring mitochondria tethered by EDCS (arrowheads) and connected by a narrow (≈50 nm) tunnel of matrix space. n = 14–16 myofibers analyzed per group.
Mitochondrial morphology.
Acute voluntary exercise did not alter mitochondrial ultrastructure (Fig. 4A). The fusion of neighbouring mitochondria is expected to 1) increase mitochondrial size, and/or 2) affect mitochondrial shape descriptors such as length (aspect ratio) and branching complexity (form factor). Mitochondrial size and morphology were quantified by manually tracing mitochondria from electron micrographs in both the longitudinal (for SS) and transverse/cross-sectional (for IMF) orientations. Exercise running did not affect mitochondrial size (Fig. 4B). Likewise, neither mitochondrial aspect ratio nor form factor were altered after exercise (Fig. 4, C–E). Other morphological parameters including roundness, circularity, perimeter, and Feret's diameter did not differ between sedentary and running conditions (Fig. 5, A–D).
Fig. 5.
Mitochondrial morphology in sedentary and running soleus. Mitochondrial shape descriptors for SS (A) and IMF mitochondria (B). All nonsignificant vs. sedentary. C and D: frequency distributions for the same morphological parameters as in A and B.
Mitochondrial pro-fusion proteins.
To determine whether the increased membrane interactions in exercised muscles could be related to changes in the mitochondrial pro-fusion proteins, we analyzed the canonical fusion proteins Mfn2 and OPA1 (Fig. 6A). The abundance of the outer mitochondrial membrane fusion protein Mfn2 (Fig. 6B) and the inner mitochondrial membrane OPA1 (Fig. 6C) were similar between sedentary and exercised animals.
Fig. 6.
Mitochondrial fusion proteins in skeletal muscle of sedentary and exercised mice. A: Representative immunoblots for the mitochondrial pro-fusion proteins optic atrophy 1 (OPA1) and mitofusin 2 (Mfn2), the cytoplasmic marker GAPDH, and mitochondrial marker TOM20, in different muscles of the leg: extensor digitorum longus (EDL), the soleus (Sol), the red gastrocnemius (RGas), and the white gastrocnemius (WGas). Average protein abundance of Mfn2 (B) and OPA1 (C) normalized to GAPDH in different hind limb muscles. Data are means ± SE; n = 3 per group.
DISCUSSION
The ability of mitochondria to interact and fuse in skeletal muscle fibers is a relatively recently discovered property, and whether these processes are altered by exercise is unknown. To isolate the acute effect of exercise on mitochondrial dynamics, we applied a “nonstressful” voluntary running paradigm in mice, following their normal physical activity pattern, and quantified mitochondrial morphology on electron micrographs from the longitudinal and cross-sectional axes of skeletal muscle. Although neither mitochondrial size nor morphology were altered immediately after exercise in the soleus, mitochondrial membrane interactions were significantly increased, particularly in the IMF compartment where energy demand and mechanical stress might be greatest.
Based on in vitro studies indicating that energy deprivation can induce mitochondrial fusion and elongation (22, 46), our initial hypothesis was that the state of metabolic undersupply incurred during exercise—increased energy demand relative to supply—would provoke the elongation and enlargement of mitochondria. In addition, since oxidized glutathione (GSSG) can stimulate mitochondrial fusion (47), and skeletal muscle contraction can increase ROS production (44), this could further have stimulated mitochondrial fusion. However, excess ROS production triggered during exhaustive exercise (11) has been shown to trigger mitochondrial fragmentation in myotubes (15). Opposing forces promoting mitochondrial fusion on the one hand and fragmentation on the other may be simultaneously acting in the exercising skeletal muscle, possibly explaining the absence of overt morphological changes during or immediately after exercise.
Mitochondrial dynamics in skeletal muscle fibers may serve different potential functional roles contributing to normal mitochondrial function and skeletal muscle physiology (40). Physical tethers between mitochondrial membranes could 1) promote the exchange of molecules (ions, lipids, proteins) between adjacent organelles (8, 48), which may help to coordinate the functioning of otherwise physically distinct mitochondria; 2) protect healthy mitochondria from autophagy (22, 46) without necessarily engaging energy-consuming processes of complete outer mitochondrial membranes (OMM) and inner mitochondrial membranes (IMM) fusion; and 3) serve as prefusion events by tethering organelles' outer membranes to facilitate their rapprochement and possible fusion upon the appropriate microenvironment signals. These speculative functions deserve further experimental clarification.
In mammalian cells, mitochondrial morphology is remodelled by a group of GTPase proteins, including mitofusins (Mfn1, Mfn2) and OPA1, located respectively within the OMM and IMM (52). Mitochondrial fusion involves the rapprochement of OMMs from adjacent organelles, followed by sequential fusion of the OMM and IMM (33, 49). Mitochondrial membrane interactions are therefore a prerequisite for mitochondrial morphology transitions. The short exercise duration and absence of recovery period may explain the absence of difference in the abundance of Mfn2 and OPA1 with acute exercise. A study in humans also reported no change in the abundance of Mfn1 or Mfn2 following a single bout of exercise (38). In contrast, the transcriptional regulation of mitochondrial fusion and fission proteins appears more rapidly regulated and correlates with functional exercise capacity (19). One study in rats reported the transcriptional upregulation of fission protein Fis1 during intense exercise, with a concomitant downregulation of the fusion proteins Mfn1 and Mfn2 (12). In the recovery phase (0–2 h after exercise), this was followed by an early upregulation of Mfn1, and a delayed upregulation of Mfn2 at the mRNA level (12). Other studies in humans (5, 24) and rats (27) have also found increased transcript levels for mitochondrial fusion proteins in response to chronic muscle stimulation or endurance exercise. However, transcript levels for those genes are likely to bear more significant effects on the long-term adaptations than on immediate changes in mitochondrial morphology. In addition, transcript and protein levels of the key players in mitochondrial dynamics may not directly translate into altered dynamics since the pro-fusion activity of Mfn2 and OPA1 are regulated by posttranslational modifications and processing (13, 45, 47), respectively. Finally, it is also possible that mitochondrial tethers are mediated by proteins or membrane properties that do not involve the canonical mitochondrial fusion machinery.
SS mitochondria are located beneath the plasma membrane, whereas mitochondria in the IMF compartment are positioned between myofibrils in proximity to the T-tubules and sarcoplasmic reticulum where most of the ATP is hydrolyzed during and between contractions. Functionally, differences exist between SS and IMF mitochondria (10, 16, 31), and their basal morphologically also differ greatly (see Fig. 4 and Ref. 43). Exercise could therefore differentially affect mitochondrial membrane dynamics in the SS and IMF compartment. Our results indicate that although the proportion of EDCS was equivalent in sedentary conditions (about 7–8% of contacts are electron-dense), the increase with exercise was more robust in the IMF compartment. Another notable finding was that IMF mitochondria spanning the Z-lines were nearly twice as prevalent after acute exercise. Because mitochondria form reticular networks in the transverse plane (along sarcomeric planes) of myofibers, it is hypothesized that interconnecting these planes across sarcomeres would enable the transfer of metabolites, ions, or membrane potential, thus contributing to coordinate mitochondrial function along the myofiber.
In conclusion, we show that mitochondrial morphology is not affected immediately after exercise, but rather that EDCS tethers identified by electron microscopy are increased. This represents a novel immediate adaptation of skeletal muscle, especially among IMF mitochondria. Defining the impact of fluctuations in energy metabolism on mitochondrial dynamics, both during exercise and sedentary behaviour, has important implications for deciphering the mechanisms responsible for the health effects of physical in/activity (4, 42). Remaining questions include the molecular nature of EDCS, their exact role, and their long-term effects on skeletal muscle physiology. The exercise duration and intensity required for their formation and dissolution, and whether they eventually translate into membrane fusion, elongation, and enlargement of mitochondria remains to be explored.
GRANTS
M.P. was supported by a Canada Graduate Scholarship and a Michael Smith Foreign Study Supplement from the National Science and Engineering Research Council of Canada (NSERC), a Journal of Cell Science Travelling Fellowship, and holds a Canadian Institute of Health Research (CIHR) Postdoctoral Fellowship from the Institute of Neurosciences, Mental Health and Addiction as part of the Canadian Epigenetics, Environment and Health Research Consortium. Part of this work was performed in the Newcastle University Centre for Brain Ageing and Vitality supported by grants from the Biotechnology and Biological Sciences Research Council (BBRC), Engineering and Physical Sciences Research Council (EPSRC), Economic and Social Research Council (ESRC), Medical Research Council (MRC), the Wellcome Trust Centre for Mitochondrial Research and National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle upon Tyne Hospitals National Health Service Foundation Trust, and Newcastle University to D.M.T. Additional support came from NIH grant NS21328 and CA143351 and Simons Foundation grant 205844 awarded to D.C.W. Part of this work was supported by the Canadian Institutes of Health Research grant 86725 to B.J.G.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.P., S.E.G., and D.M.T. conception and design of research; M.P., M.J.M., K.W., and K.S.L. performed experiments; M.P. and B.J.G. analyzed data; M.P. and D.M.T. interpreted results of experiments; M.P. and B.J.G. prepared figures; M.P. drafted manuscript; M.P., B.J.G., M.J.M., and D.M.T. edited and revised manuscript; M.P., B.J.G., M.J.M., K.W., K.S.L., S.E.G., D.C.W., and D.M.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Tracey Davey (EM Research Services, Newcastle University) for expert technical assistance for the preparation and visualisation of samples, to Heidy McBride, Orian Shirihai and Yan Burelle for useful discussion of the data, and to Christopher Huggins for assistance with animal work.
REFERENCES
- 1.Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci U S A 107: 19090–19095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakeeva LE, Chentsov Yu S, Skulachev VP. Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta 501: 349–369, 1978 [DOI] [PubMed] [Google Scholar]
- 3.Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O'Gorman DJ, Zierath JR. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 15: 405–411, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Booth FW, Laye MJ, Lees SJ, Rector RS, Thyfault JP. Reduced physical activity and risk of chronic disease: The biology behind the consequences. Eur J Appl Physiol 102: 381–390, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 567: 349–358, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan DC. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu Rev Genet 46: 265–287, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Chan DC. Physiological functions of mitochondrial fusion. Ann N Y Acad Sci 1201: 21–25, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130: 548–562, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cogswell AM, Stevens RJ, Hood DA. Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol Cell Physiol 264: C383–C389, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107: 1198–1205, 1982 [DOI] [PubMed] [Google Scholar]
- 12.Ding H, Jiang N, Liu H, Liu X, Liu D, Zhao F, Wen L, Liu S, Ji LL, Zhang Y. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta 1800: 250–256, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, Chomyn A, Bauer MF, Attardi G, Larsson NG, Neupert W, Reichert AS. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem 281: 37972–37979, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Fan X, Hussien R, Brooks GA. H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic Biol Med 49: 1646–1654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira R, Vitorino R, Alves RM, Appell HJ, Powers SK, Duarte JA, Amado F. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics 10: 3142–3154, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Froy O. The circadian clock and metabolism. Clin Sci (Lond) 120: 65–72, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Gale JB. Mitochondrial swelling associated with exercise and method of fixation. Med Sci Sports 6: 182–187, 1974 [PubMed] [Google Scholar]
- 19.Garnier A, Fortin D, Zoll J, N'Guessan B, Mettauer B, Lampert E, Veksler V, Ventura-Clapier R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle FASEB J 19: 43–52, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Gauthier GF, Padykula HA. Cytological studies of fiber types in skeletal muscle. A comparative study of the mammalian diaphragm J Cell Biol 28: 333–354, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gollnick PD, King DW. Effect of exercise and training on mitochondria of rat skeletal muscle. Am J Physiol 216: 1502–1509, 1969 [DOI] [PubMed] [Google Scholar]
- 22.Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13: 589–598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Y, Chai Y, Ding JH, Sun XL, Hu G. Chronic mild stress damages mitochondrial ultrastructure and function in mouse brain. Neurosci Lett 488: 76–80, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, Finucane F, Liesa M, Chiellini C, Naon D, Zorzano A, Nolan JJ. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1{alpha}/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care 33: 645–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol 209: 2265–2275, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Sun L, Ji S, Zhao T, Zhang W, Xu J, Zhang J, Wang Y, Wang X, Franzini-Armstrong C, Zheng M, Cheng H. Kissing and nanotunneling mediate intermitochondrial communication in the heart. Proc Natl Acad Sci U S A 110: 2846–2851, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iqbal S, Ostojic O, Singh K, Joseph AM, Hood DA. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151: 2117–2127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkwood SP, Munn EA, Brooks GA. Mitochondrial reticulum in limb skeletal muscle. Am J Physiol Cell Physiol 251: C395–C402, 1986 [DOI] [PubMed] [Google Scholar]
- 30.Koopman WJ, Visch HJ, Smeitink JA, Willems PH. Simultaneous quantitative measurement and automated analysis of mitochondrial morphology, mass, potential, and motility in living human skin fibroblasts. Cytometry A 69: 1–12, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Krieger DA, Tate CA, McMillin-Wood J, Booth FW. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol 48: 23–28, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Lauritzen HP, Galbo H, Toyoda T, Goodyear LJ. Kinetics of contraction-induced GLUT4 translocation in skeletal muscle fibers from living mice. Diabetes 59: 2134–2144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legros F, Lombes A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell 13: 4343–4354, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17: 491–506, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology 24: 420–429, 2001 [DOI] [PubMed] [Google Scholar]
- 36.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70: 391–425, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec 248: 214–223, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham AH, Michael J, Chan DC. Mouse lines with photo-activatable mitochondria (PhAM) to study mitochondrial dynamics. Genesis 50: 833–843, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picard M, Shirihai OS, Gentil BJ, Burelle Y. Mitochondrial morphology transitions and functions: Implications for retrograde signaling? Am J Physiol Regul Integr Comp Physiol 304: R393–R406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MT, Romestaing C, Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods PLoS One 6: e18317, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picard M, Turnbull DM. Linking the metabolic state and mitochondrial DNA in chronic disease, health and aging. Diabetes 62: 672–678, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picard M, White K, Turnbull DM. Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: a quantitative three-dimensional electron microscopy study. J Appl Physiol 114: 161–171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quiros PM, Ramsay AJ, Sala D, Fernandez-Vizarra E, Rodriguez F, Peinado JR, Fernandez-Garcia MS, Vega JA, Enriquez JA, Zorzano A, Lopez-Otin C. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice EMBO J 31: 2117–2133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation Proc Natl Acad Sci U S A 108: 10190–10195, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shutt T, Geoffrion M, Milne R, McBride HM. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skulachev VP. Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci 26: 23–29, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell 20: 3525–3532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trudeau K, Molina AJ, Guo W, Roy S. High glucose disrupts mitochondrial morphology in retinal endothelial cells: Implications for diabetic retinopathy. Am J Pathol 177: 447–455, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11: 872–884, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103: 2653–2658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]