Abstract
In human cutaneous microvasculature, endothelium-derived hyperpolarizing factors (EDHFs) account for a large portion of vasodilation associated with local stimuli. Thus we sought to determine the role of EDHFs in active vasodilation (AVD) to passive heating in two protocols. Whole body heating was achieved using water-perfused suits (core temperature increase of 0.8–1.0°C), and skin blood flow was measured using laser-Doppler flowmetry. In the first protocol, four sites were perfused continuously via microdialysis with: 1) control; 2) tetraethylammonium (TEA) to block calcium-activated potassium (KCa) channels, and thus the actions of EDHFs; 3) N-nitro-l-arginine methyl ester (l-NAME) to inhibit nitric oxide synthase (NOS); and 4) TEA + l-NAME (n = 8). Data are presented as percent maximal cutaneous vascular conductance (CVC). TEA had no effect on AVD (CVC during heated plateau: control 57.4 ± 4.9% vs. TEA 63.2 ± 5.2%, P = 0.27), indicating EDHFs are not obligatory. l-NAME attenuated plateau CVC to 33.7 ± 5.4% (P < 0.01 vs. control); while TEA + l-NAME augmented plateau CVC compared with l-NAME alone (49.7 ± 5.3%, P = 0.02). From these data, it appears combined blockade of EDHFs and NOS necessitates dilation through other means, possibly through inward rectifier (KIR) and/or ATP-sensitive (KATP) potassium channels. To test this second hypothesis, we measured AVD at the following sites (n = 8): 1) control, 2) l-NAME, 3) l-NAME + TEA, and 4) l-NAME + TEA + barium chloride (BaCl2; KIR and KATP blocker). The addition of BaCl2 to l-NAME + TEA reduced plateau CVC to 32.7 ± 6.6% (P = 0.02 vs. l-NAME + TEA), which did not differ from the l-NAME site. These data combined demonstrate a complex interplay between vasodilatory pathways, with cross-talk between NO, KCa channels, and KIR and/or KATP channels.
Keywords: endothelium-derived hyperpolarizing factors, inward rectifier potassium channels, nitric oxide, thermoregulation, whole body heating
in addition to being subject to local signaling, the cutaneous vasculature is under both vasoconstrictor and vasodilator neural control. Small rises in core temperature (∼0.1–0.2°C) cause an increase in skin blood flow (approximately doubling baseline values) due to removal of tonic vasoconstrictor tone, mediated by sympathetic adrenergic nerves. Further increases in core temperature produce robust dilation due to activation of sympathetic vasodilator nerves, which release acetylcholine and a cotransmitter (16), possibly vasoactive intestinal polypeptide (VIP) (2, 17), pituitary adenylate cyclase activating peptide (PACAP) (17, 18), and/or substance P (48). Approximately 30–45% of the vasodilation to increased core temperature can be attributed to nitric oxide (NO) (15, 37, 45), for which neuronal NO synthase (nNOS) appears to be the predominant isoform responsible for its production (19, 20). However, prostanoids (24) and H1 histamine receptors (49) have been shown to play roles in active vasodilation, indicating that the endothelium is likely still involved. Additionally, heat-sensitive transient receptor potential vanilloid type 1 (TRPV-1) receptors located on the endothelium have been shown to contribute to the response (47).
Despite a large amount of research in this field, a portion of cutaneous active vasodilation remains unexplained. Endothelium-derived hyperpolarizing factors (EDHFs) are well known to play a prominent role in other vascular beds, and recently our lab has shown a robust role of EDHFs in the skin, in both the responses to local thermal hyperemia (5) and reactive hyperemia (22). EDHFs elicit vasodilation by stimulating calcium-activated potassium (KCa) channels on the endothelium and vascular smooth muscle. Potassium efflux through these channels then causes hyperpolarization, and thus relaxation of the smooth muscle. Possible EDHFs include the epoxyeicosatrienoic acids (EETs), the lipoxygensase derivatives 12-S-hydroxyeicosatetraenoic acid (12-S-HETE) and 11,12,15-trihydroxyeicosatrienoic acid (11,12,15-THETA), and H2O2 (11). Our lab has recently shown EETs to be involved in EDHF-mediated vasodilation in the skin (5). EDHF-mediated vasodilation can be blocked with the nonspecific KCa channel-inhibitor tetraethylammonium (TEA). In animal models, inhibition of KCa channels with TEA reduces VIP-induced relaxation (14, 21), lending to the possibility that the cotransmitters involved in active vasodilation cause vasodilation in part by stimulating the production of EDHFs.
Therefore, the purpose of our first experiment (protocol 1) was to examine whether KCa channels play a role in active vasodilation. We hypothesized that inhibition of KCa channels with TEA would cause an attenuated response to passive heating, and combined blockade of KCa channels and NO synthase (NOS) with N-nitro-l-arginine methyl ester (l-NAME) would reduce the response to a greater extent than NOS inhibition alone.
To further explore observations from protocol 1, we designed protocol 2 as a follow-up study to determine the involvement of inward rectifier (KIR) and/or ATP-sensitive (KATP) potassium channels in active vasodilation. KIR channels are found on both vascular endothelial (6, 42) and smooth muscle cells (34, 36) and are thought to be involved in resting membrane conductance and amplification of hyperpolarizing stimuli (30), suggesting they may be involved with cross-talk among the pathways controlling active vasodilation. KATP channels are responsive to a large number of other agents, including EETs (50), adenosine (8), prostacyclin (35, 39), and NO (28), also making it likely for them to be involved in cross-talk with KCa channels and/or NOS inhibition. Both KIR (13, 40) and KATP (4, 25), channels can be inhibited by extracellular barium ions, although this has only been shown to be true of KATP channels in nonvascular smooth muscle. In addition, the density of KATP channels is considerably lower than KIR channels in vascular smooth muscle (35), making it more likely that the effects of barium on the vasculature are the result of KIR channel inhibition. Accordingly, we administered barium chloride (BaCl2) to determine the role of KIR and/or KATP channels in active vasodilation.
MATERIALS AND METHODS
Subjects.
Sixteen subjects participated in the study (10 men, 6 women). Two subjects participated in more than one protocol. All subjects were young (18–30 yr of age), healthy, nonsmokers, did not have any history of cardiovascular disease, and were not taking any medications, with the exception of oral contraceptives. Subjects reported to the laboratory on the study day having refrained from all over-the-counter medications and heavy exercise for 24 h, and alcohol and caffeine for 12 h, and having fasted for at least 4 h. All female subjects were studied during menses, or during the placebo phase if taking oral contraceptives, to minimize the effects of the female sex hormones. Female subjects were also required to provide a negative pregnancy test prior to participation in the study day. All studies were conducted in a thermoneutral room (ambient temperature ∼20°C) with the subject resting in a supine position.
All subjects gave oral and written consent prior to participation in the study, as set forth by the Declaration of Helsinki. All experimental procedures were approved by the Institutional Review Board of the University of Oregon.
Subject instrumentation.
Subjects were instrumented with a five-lead electrocardiogram (CardioCap; Datex Ohmeda, Louisville, CO). Beat-by-beat blood pressure was measured on the middle finger of the nonexperimental arm with finger photoplethysmography (Nexfin; BMEye, Amsterdam, The Netherlands) and was verified via brachial auscultation (CardioCap) every 5 min. Subjects wore a water-perfused suit to control whole body skin temperature, which covered all skin surfaces except the experimental arm, face, hands, and feet, and a water-impermeable plastic garment over the suit to prevent heat evaporation. Oral temperature (Tor) was continuously monitored with a thermistor placed in the sublingual sulcus, which was held in place with tape on the cheek. While the thermistor was in place, subjects were instructed to breathe through their nose and not to talk or open their mouth. Mean skin temperature (Tsk) was calculated as the weighted average of six copper-constantan thermocouples, placed on the chest, abdomen, upper back, lower back, thigh, and calf (41).
Under aseptic conditions, four microdialysis fibers (30-kDa cutoff, 10-mm membrane; MD 2000; Bioanalytical Systems, West Lafayette, IN) were placed at least 5 cm apart in the ventral skin of the left forearm. Fibers were introduced using a 25-gauge needle, with entry and exit points ∼2.5 cm apart. Following needle insertion, fibers were threaded through the lumen of the needle. The needle was then removed, leaving just the fiber under the surface of the skin, with the semipermeable membrane (1 cm in length) of the fiber centered between the entry and exit points. A lactated Ringer's solution was infused through the fibers at a rate of 2 μl/min (CMA 102 Syringe Pump; CMA Microdialysis AB, Solna, Sweden) until the infusion of study drugs (see Pharmacological agents).
Laser-Doppler flowmetry (DRT-4 and MoorLab; Moor Instruments, Devon, UK) was used to measure red blood cell (RBC) flux, and thus attain an index of skin blood flow, at each microdialysis site.
Study protocol.
A period of 60–90 min was allowed following microdialysis fiber placement for the trauma associated with needle insertion to subside. Once skin blood flow had stabilized, a 5-min predrug baseline was recorded under thermoneutral conditions. Study drugs were then infused through the fibers at a rate of 2.0 μl/min. Drugs were infused for 60 min prior to the start of heating to achieve full efficacy of TEA (5) and were continued at the same rate throughout heating. Postdrug baseline was recorded as the last 5 min of the drug infusion period. During this time, water was circulated through the water-perfused suit at 33°C.
Passive heating was achieved by circulating water at 50°C through the water-perfused suit until Tor was increased by 0.8–1.0°C. Heating took approximately 45–60 min. A plateau in skin blood flow of at least 5 min was recorded at the highest temperature before ending heat stress.
Upon completion of heating, subjects were cooled down by reducing the water temperature to 28°C. At this time, maximal skin blood flux was attained by infusing 56 mM sodium nitroprusside (SNP; Nitropress, Ciba Pharmaceuticals, East Hanover, NJ).
Pharmacological agents.
We had two protocols in which we passively heated subjects to measure cutaneous active vasodilation. In each protocol, four microdialysis sites were established and continuously infused with the following drugs. All drugs were dissolved in a lactated Ringer's solution.
In protocol 1 (n = 8, 5 men, 3 women), the four sites were 1) control (lactated Ringer's), 2) 50 mM TEA, 3) 20 mM l-NAME, and 4) 20 mM l-NAME + 50 mM TEA. TEA (Sigma-Aldrich, St. Louis, MO) was infused to block all subtypes of KCa channels. An infusion concentration of 50 mM was selected as it was shown to be the lowest concentration able to fully inhibit KCa channels in pilot work associated with other studies from our laboratory (22). We also performed pilot studies to ensure specificity of TEA at this concentration to KCa channels. In six subjects, 50 mM TEA had no effect on the dilation observed when pharmacologically stimulating the opening of KIR channels with potassium chloride, a response that was blocked by BaCl2. Although studies in isolated cell models have shown a concentration of 50 mM TEA to inhibit other types of potassium channels, equilibration of the pharmacological agents infused with the interstitium is unlikely to occur via microdialysis, particularly with infusion rates as high as 2.0 μl/min. Thus a lower concentration than 50 mM is reaching the blood vessels. Specific inhibitors of BKCa and SKCa channels, such as charybdotoxin and apamin, were not used as these drugs are toxic to humans (33). l-NAME (Tocris Bioscience; Minneapolis, MN) was infused through site 3 to inhibit NO synthase. A concentration of 20 mM was selected for l-NAME based on previous studies (5). NOS inhibition was used to separate the potential NO-dependent and NO-independent effects of TEA, especially as the full effects of EDHFs are commonly only observed in the presence of NOS inhibition (51). Site 4 served as a combination site, in which both 50 mM TEA and 20 mM l-NAME were infused.
In protocol 2 (n = 8, 6 men, 2 women), the four microdialysis sites were: 1) control (lactated Ringer's), 2) 20 mM l-NAME, 3) 20 mM l-NAME + 50 mM TEA, and 4) 20 mM l-NAME + 50 mM TEA + 100 μM BaCl2 to block KIR and KATP channels. Sites 1 and 2 were included in this protocol (despite having already been studied in protocol 1) to be compared with the fourth site, in which we simultaneously blocked NO, KCa channels, and KIR and KATP channels. BaCl2 was just added in the fourth site to investigate possible cross-talk between the NO and hyperpolarization pathways. A concentration of 100 μM BaCl2 was selected based on pilot studies as the concentration that gave the most effective inhibition of KIR-mediated vasodilation to KCl (KIR channel opener). This concentration was confirmed to completely block KCl-induced vasodilation up to concentrations of 10 mM KCl. Concentrations as low as 10 μM have been shown to completely block conductance through both KIR channels in isolated vascular smooth muscle cells (34); however, a higher concentration is required when delivered via microdialysis.
In two subjects in protocol 2 (1 man, 1 woman), we added a fifth site which was infused with 100 μM BaCl2 to differentiate whether KIR and/or KATP channels play a role in active vasodilation when all other vasodilatory pathways are intact, versus only in the presence of combined NOS and KCa channel inhibition.
Data analysis.
Data were digitized and stored on a computer at 20 Hz. Data were analyzed offline using signal-processing software (Windaq; Dataq Instruments, Akron, OH). Cutaneous vascular conductance (CVC) was calculated as RBC flux divided by arterial blood pressure. Blood pressure was attained from the Nexfin beat-by-beat measurements and corrected to mean arterial pressure (MAP) attained via brachial auscultation. All CVC data were normalized to 100% of maximal CVC, as attained through infusion of SNP.
To quantify the skin blood flow response to passive heating, CVC and Tsk were averaged over 5 min during the predrug baseline, postdrug baseline, and plateau at the end of heat stress, which ranged from a change of 0.8 to 1.0°C in ΔTor. CVC and Tsk were also averaged over 1-min periods following each 0.1°C increase in Tor. Threshold Tor for active vasodilation was determined by looking at the CVC tracing over time and identified as the Tor at which a sustained increase in CVC began after Tsk had increased to at least 38°C (18). Threshold was identified by an investigator blinded to the drug at each site and was confirmed by a second investigator.
Statistical analysis.
Differences in baseline, plateau at the end of heat stress, maximal flux, and Tor threshold for active vasodilation were compared across drug sites using one-way repeated measures analysis of variance (ANOVA). Pairwise interactions were analyzed using the Student-Newman-Keuls post hoc test. Drug effects on baseline (predrug baseline vs. postdrug baseline) and plateau CVC in the same drug site across protocols were compared with Student's paired t-test.
RESULTS
All subjects studied were young (23.9 ± 0.6 yr of age) and healthy (body mass index 23.8 ± 0.7 kg/m2; resting MAP 83.6 ± 0.7 mmHg). Infusion of the study drugs resulted in an attenuation of baseline CVC in the TEA, l-NAME + TEA, and l-NAME + TEA + BaCl2 sites, but not in the l-NAME site. These data are summarized in Table 1. There were no differences in maximal flux between sites (protocol 1: P = 0.25; protocol 2: P = 0.69).
Table 1.
Baseline data
| Predrug Infusion | Postdrug Infusion | P Value | n | |
|---|---|---|---|---|
| Control | 8.4 ± 1.3% | 7.5 ± 1.3% | 0.10 | 16 |
| TEA | 10.5 ± 1.8% | 4.7 ± 0.6% | <0.01 | 8 |
| l-NAME | 6.3 ± 0.8% | 6.2 ± 1.0% | 0.98 | 16 |
| l-NAME + TEA | 7.8 ± 0.6% | 4.1 ± 0.5% | <0.001 | 16 |
| l-NAME + TEA + BaCl2 | 7.8 ± 1.1% | 4.1 ± 0.5% | <0.001 | 8 |
Data are presented as means ± SE of % of maximal cutaneous vascular conductance (CVC). Baseline CVC pre and post 60 min of infusion with the study drugs are shown. Drugs include tetraethylammonium (TEA), N-nitro-l-arginine methyl ester (l-NAME), and barium chloride (BaCl2). CVC was significantly attenuated in the TEA, l-NAME + TEA, and l-NAME + TEA + BaCl2 sites.
Passive heating (both protocols) increased Tor from 36.42 ± 0.06°C to 37.37 ± 0.08°C. There were no significant differences between sites for Tor threshold at which active vasodilation was initiated when presented as absolute Tor or as ΔTor from baseline (Table 2), although Tor threshold trended toward being delayed in the l-NAME site (vs. control: protocol 1, P = 0.10; protocol 2, P = 0.15).
Table 2.
Tor threshold for active vasodilation
| Absolute Tor at Threshold, °C | ΔTor at Threshold, °C | |
|---|---|---|
| Protocol 1 | ||
| Control | 36.55 ± 0.07 | 0.20 ± 0.05 |
| TEA | 36.50 ± 0.08 | 0.14 ± 0.05 |
| l-NAME | 36.60 ± 0.07 | 0.25 ± 0.04 |
| l-NAME + TEA | 36.61 ± 0.09 | 0.27 ± 0.05 |
| Protocol 2 | ||
| Control | 36.57 ± 0.10 | 0.13 ± 0.03 |
| l-NAME | 36.71 ± 0.12 | 0.27 ± 0.06 |
| l -NAME + TEA | 36.59 ± 0.14 | 0.15 ± 0.05 |
| l-NAME + TEA + BaCl2 | 36.61 ± 0.10 | 0.17 ± 0.04 |
Data are presented as means ± SE in °C. Oral temperature (Tor) threshold at which active vasodilation was initiated in both protocols, presented as both absolute Tor and a change in Tor from normothermic baseline (ΔTor). Drugs include TEA, l-NAME, and barium chloride (BaCl2).
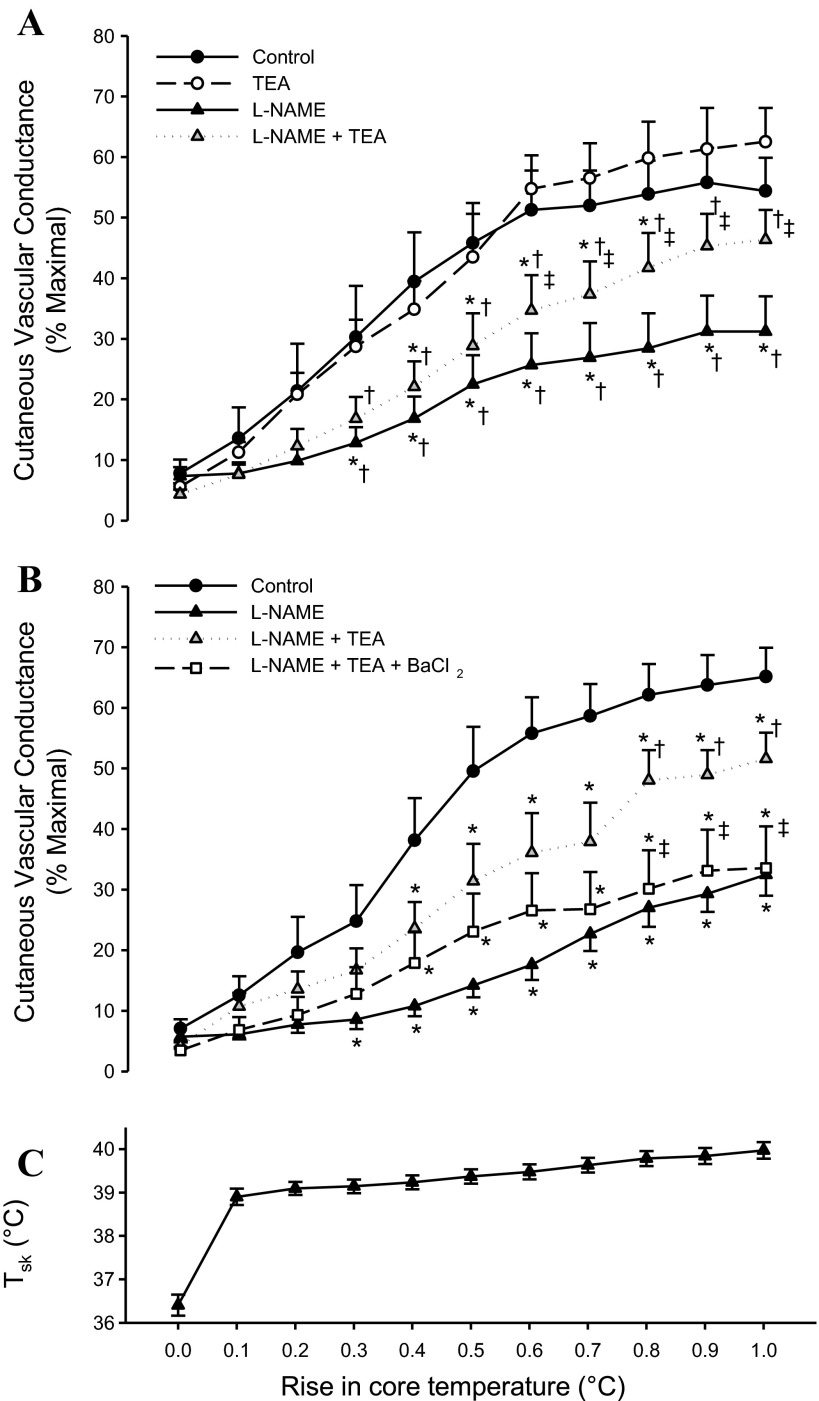
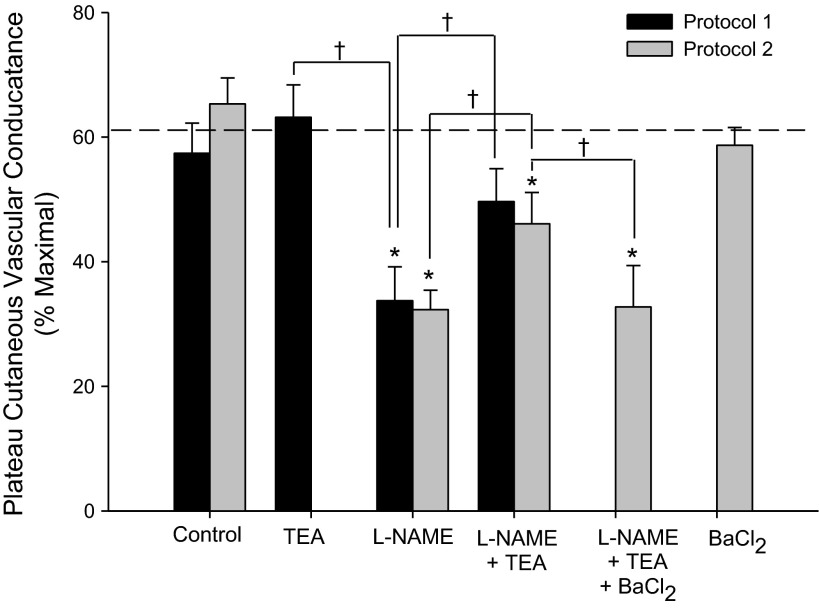
Figure 1, A (protocol 1) and B (protocol 2), depicts the rise in CVC across ΔTor from baseline. Data for the CVC plateau at the end of passive heating are summarized in Fig. 2. NOS inhibition with l-NAME attenuated the CVC response to passive heating, consistent with previous studies (15, 18, 37, 45, 47). KCa channel blockade with TEA tended to augment plateau CVC, although this effect was not significant (P = 0.27). A power analysis (power = 0.80, α = 0.05) revealed a sample size of n = 67 would be needed to show a significant difference between the control and TEA sites. Combined blockade of NOS and KCa channels (TEA + l-NAME) did not attenuate plateau CVC to as great an extent as l-NAME alone and, in protocol 1, was not significantly different from the control site (plateau CVC: P = 0.15). In protocol 2, the addition of BaCl2 to TEA + l-NAME attenuated plateau CVC compared with the TEA + l-NAME site back to the level of the l-NAME site. In the two subjects who had a fifth fiber infused with BaCl2 alone, there was no effect of BaCl2 compared with the control site. There were no differences in the control, l-NAME, nor l-NAME + TEA sites between protocols 1 and 2.
Fig. 1.
A: response of cutaneous vascular conductance (CVC) to passive heating relative to the rise in oral temperature (ΔTor) for protocol 1. Infusion of tetraethylammonium (TEA) had no effect on the CVC response compared with the control site, whereas infusion with N-nitro-l-arginine methyl ester (l-NAME) attenuated CVC. Combined infusion of TEA + l-NAME augmented the response compared with the l-NAME site. *P < 0.05 from the control site; †P < 0.05 from the TEA site; ‡P < 0.05 from the l-NAME site. B: response of CVC relative to ΔTor for protocol 2. The addition of barium chloride (BaCl2) to l-NAME + TEA attenuated CVC compared with the l-NAME + TEA, back to the level of the l-NAME alone site. *P < 0.05 from the control site; †P < 0.05 from the l-NAME site; ‡P < 0.05 from the l-NAME + TEA site. C: rise in mean skin temperature (Tsk) relative to changes in ΔTor, averaged across subjects in protocols 1 and 2. Data are presented as means ± SE of %CVCmax.
Fig. 2.
The plateau in CVC observed at the end of heat stress for protocols 1 and 2. Heat stress resulted in rise in core temperature ranging from 0.8 to 1.0°C. Drugs include tetraethylammonium (TEA), N-nitro-l-arginine methyl ester (l-NAME), and barium chloride (BaCl2). The dotted line represents the average plateau CVC for the control site between the two protocols. Data are presented as means ± SE of %CVCmax. *P < 0.05 from the control site within each protocol; †P < 0.05 between drug sites within each protocol.
DISCUSSION
The present study was designed to determine whether endothelium-derived hyperpolarization plays a role in the response of skin blood flow to passive heating. Contrary to our hypotheses, blockade of KCa channels with TEA had no effect on active vasodilation, and furthermore, blockade of KCa channels in combination with NOS inhibition (with l-NAME) augmented active vasodilation compared with NOS inhibition alone. Secondarily, we tested the follow-up hypothesis that this augmentation of active vasodilation was the result of activation of KIR and/or KATP channels, which was confirmed, as blockade of KIR and KATP channels (with BaCl2) in combination with KCa channel and NOS inhibition returned active vasodilation back to the level of NOS inhibition alone. This is the first time KCa channels have been studied in cutaneous active vasodilation, and the first time KIR channels have been studied in cutaneous tissue.
Protocol 1: role of EDHFs in active vasodilation.
EDHFs make up a class of vasodilators that elicit vasodilation via hyperpolarization of the vascular smooth muscle. Hyperpolarization is achieved via stimulation of KCa channels on the endothelium and smooth muscle. Common EDHFs include EETs and the lipoxygenase derivatives 12-S-HETE and 11,12,15-THETA (11), among others. EDHFs, especially EETs, play an important role in vasodilation in other vascular beds and are known to contribute to vasodilation in the skin (5, 7).
In the present study, blockade of KCa channels with TEA had no measurable effect on the cutaneous response to passive heating, suggesting no role of EDHFs in active vasodilation when other vasodilatory pathways are intact. In the context of the current theory of cotransmission, this means that, normally, the neurotransmitters released in response to increases in core temperature do not cause vasodilation through stimulating the production of EDHFs. The likely cotransmitters, VIP (2, 17) and PACAP (17, 18), have been shown to act, in part, via EDHFs in animal vessels (14, 21); however, it appears that they do not in cutaneous active vasodilation. Vasodilation induced by substance P, another possible cotransmitter (48), is not affected by KCa channel inhibition, as shown in porcine coronary arteries (31). To the best of our knowledge, no studies have explored links between EDHFs and these neurotransmitters in humans. Our results also raise the question of what other downstream vasodilatory agents are involved. Combined inhibition of NOS and prostanoids abolishes approximately two-thirds of active vasodilation, (24); but the remainder is still unknown. Furthermore, inhibition of NOS only blocks ∼50% of vasodilation to exogenous VIP (44); thus VIP must have other effects in the skin. Perhaps the remaining dilation is the result of VIP and/or other cotransmitter(s) acting directly on smooth muscle. While this may be true of VIP, it is likely not the case with PACAP as a recent study demonstrated that, while blockade of PACAP receptors during whole body heating reduced CVC compared with the control site, combined NOS and PACAP receptor inhibition did not attenuate CVC beyond NOS inhibition alone (18), indicating PACAP works solely in series with NOS.
Despite no role of EDHFs under normal conditions, when TEA was infused in combination with l-NAME, active vasodilation was augmented compared with the NOS inhibition-only site, and to an extent such that CVC was not statistically different from that in the control site. These results were opposite of what was hypothesized but still support the conclusion that KCa channels do not directly contribute to active vasodilation. There are two possible explanations for this observation. First, TEA may be acting on potassium channels in the sympathetic cholinergic neurons. Although KCa channels have never been shown to exist in cutaneous sympathetic neurons, they are present in other types of neurons (12, 27). TEA may also inhibit voltage-gated potassium (Kv) channels. A concentration of 50 mM TEA (although likely lower than this once in the interstitium) is high enough to inhibit some types of Kv channels (23). Inhibition of neuronal KCa and/or Kv channels would cause depolarization and increased neurotransmitter release, thus augmenting active vasodilation. However, if this were occurring, we would expect to have also seen an augmentation of active vasodilation in the TEA-only site, which was not observed.
A more likely explanation is that blockade of KCa channels in addition to NOS inhibition augments active vasodilation by necessitating vasodilation via other pathways. EDHFs have often been implicated as a ‘back-up’: mechanism when other routes of vasodilation are impaired, as the effects of EDHFs are commonly not observed until NOS is blocked (1, 26, 38). Furthermore, cross-talk is known to exist between EDHFs and other vasodilatory pathways (3, 43). Two candidates for vasodilatory pathways that may be upregulated during active vasodilation when NOS and KCa channels are simultaneously blocked are KIR and KATP channels.
Protocol 2: cross-talk between KCa and KIR channels.
Protocol 2 was designed to test the hypothesis that when NOS and KCa channels are blocked, KIR and/or KATP channels are activated, thus allowing dilation to still occur, as shown in protocol 1. Our results from protocol 2 confirm this hypothesis as the augmentation of active vasodilation observed with combined blockade of NOS and KCa channels compared with NOS block alone was removed by the addition of BaCl2. Furthermore, BaCl2 alone had no effect on dilation, indicating that the involvement of KIR and/or KATP channels is triggered by combined blockade of NOS and KCa channels.
These data suggest cross-talk between NO, KCa channels, and KIR and/or KATP channels. Currently, there is little known about the potential mechanisms responsible for cross-talk between KCa and KIR channels, mostly owing to the fact that KIR channels are still relatively poorly understood. EDHFs activate KCa channels by eliciting calcium sparks from the sarcoplasmic reticulum (10); however, calcium has been shown to inhibit both KIR (36) and KATP channels (32). KIR channels have been shown to be activated by protein kinases (46). It is possible the change in calcium conductance within the cell, combined with other changes that occur during passive heating, leads to a protein kinase signaling cascade which activates KIR channels. KATP channels can be opened by EETs (50), which may occur to a greater extent when KCa channels are also blocked. Additionally, hyperpolarizing pathways may have been triggered due to changes in membrane potential. TEA significantly reduced baseline CVC, indicating depolarization at rest. Although depolarization likely would not directly open KIR or KATP channels during whole body heating (35), it may have played some role in their involvement.
Limitations: pharmacological agents.
The present study and all studies conducted in intact humans in vivo are limited in two ways. First, we are limited to using only pharmacological agents that can safely be administered in humans, and thus we are unable to more specifically pharmaco-dissect the mechanisms at play. While more specific blockers exist than the ones used in the present study, they are unsuitable for use in humans. Second, we are faced with the challenge of not being able to determine exactly which pathways are being affected by our interventions. Many pharmacological agents can have multiple effects, which may or may not be dependent on the dose of the drug. The technique of microdialysis, while providing an excellent means of examining molecular pathways relatively noninvasively, presents a greater challenge to dosing as it is not possible to determine the exact concentration of the drug once it reaches the interstitium.
The present study used TEA to examine the role of EDHFs in active vasodilation. TEA has been reported to inhibit other types of potassium channels (other than KCa) at concentrations lower than what was used in the present study (9, 29). Although the concentration of the drug once it reached the blood vessels would have been <50 mM, our results should be interpreted considering the possibility that TEA may have inhibited other potassium channels; however, this does not change the interpretation that KCa channels do not contribute to active vasodilation. At a minimum, it appears unlikely that TEA was also inhibiting barium-sensitive pathways.
We used BaCl2 to inhibit both KIR and KATP channels. A more specific inhibitor of KIR channels is not available for use in humans, and we did not specifically test the involvement of KATP channels in this study. The commonly used KATP-channel inhibitor, glibenclamide, also has other effects, namely on prostaglandin-induced vasodilation, the Na+-K+-ATPase pump, and other potassium channels, although it does not affect KCa or KIR channels (35), and so would not allow us to conclude a role of only KATP channels in active vasodilation.
Last, it is important to recognize that the responses observed when pharmacologically manipulating a system may not reflect the true physiology of the intact system. For example, our results suggest cross-talk between KCa channels and KIR and/or KATP channels to be the cause of the augmentation of active vasodilation when administering l-NAME + TEA relative to the l-NAME only site. But perhaps drug interactions between l-NAME and TEA, or even l-NAME and BaCl2, are (at least partially) responsible for our results. Perhaps TEA prevents l-NAME from effectively inhibiting NOS, thus allowing some NO-dependent dilation to still occur. There is no way of testing this in vivo, but the reader should be aware of the limitations of our techniques.
Conclusions and perspectives: endothelium-derived hyperpolarization and the cutaneous microcirculation.
To summarize, the present study has demonstrated that EDHFs and KCa channels do not contribute to cutaneous active vasodilation under normal conditions; however, combined inhibition of KCa channels and NOS initiates cross-talk between these pathways and other vasodilatory pathways, namely KIR and/or KATP channels. Previously, EDHFs have been shown to significantly contribute to the cutaneous responses to reactive hyperemia (7, 22) and local thermal hyperemia (5). Taken together with the results of the present study, while EDHFs may represent a predominant means of vasodilation in response to local stimuli, their role when vasodilation is mediated by sympathetic cholinergic nerves is clearly very different.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-081671.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.E.B., N.F., and C.T.M. conception and design of research; V.E.B. and N.F. performed experiments; V.E.B. analyzed data; V.E.B. and C.T.M. interpreted results of experiments; V.E.B. prepared figures; V.E.B. drafted manuscript; V.E.B., N.F., and C.T.M. edited and revised manuscript; V.E.B., N.F., and C.T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We graciously thank the subjects for participation.
REFERENCES
- 1.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation 94: 3341–3347, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Bennett LAT, Johnson JM, Stephens DP, Saad AR, Kellogg DL. Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol 552: 223–232, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 368: 850–853, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Bonev AD, Nelson MT. ATP-sensitive potassium channels in smooth muscle cells from guinea pig urinary bladder. Am J Physiol Cell Physiol 264: C1190–C1200, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colden-Stanfield M, Schilling WP, Ritchie AK, Eskin SG, Navarro LT, Kunze DL. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res 61: 632–640, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Cracowski JL, Gaillard-Bigot F, Cracowski C, Sors C, Roustit M, Millet C. Involvement of cytochrome epoxygenase metabolites in cutaneous postocclusive hyperemia in humans. J Appl Physiol 114: 245–251, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol 471: 767–786, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies NW, Spruce AE, Standen NB, Stanfield PR. Multiple blocking mechanisms of ATP-sensitive potassium channels of frog skeletal muscle by tetraethylammonium ions. J Physiol 413: 31–48, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earley S. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Félétou M, Vanhoutte PM. EDHF: an update. Clin Sci 117: 139–155, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Gruol DL. Single channel analysis of voltage-sensitive K+ channels in cultured Purkinje neurons. Biophys J 45: 53–55, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagiwara S, Miyazaki S, Moody W, Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol 279: 167–185, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki J, Kobayashi S, Miyagi Y, Nishimura J, Fujishima M, Kanaide H. The mechanisms of the relaxation induced by vasoactive intestinal peptide in the porcine coronary artery. Br J Pharmacol 121: 977–985, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellogg DL, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Kellogg DL, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Kellogg DL, Zhao JL, Wu Y, Johnson JM. VIP/PACAP receptor mediation of cutaneous active vasodilation during heat stress in humans. J Appl Physiol 109: 95–100, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellogg DL, Zhao JL, Wu Y, Johnson JM. Nitric oxide and receptors for VIP and PACAP in cutaneous active vasodilation during heat stress in humans. J Appl Physiol 113: 1512–1518, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Kellogg DL, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellogg DL, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol 107: 1438–1444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishi M, Takeuchi T, Suthamnatpong N, Ishii T, Nishio H, Hata F, Takewaki T. VIP- and PACAP-mediated nonadrenergic, noncholinergic inhibition in longitudinal muscle of rat distal colon: involvement of activation of charybdotoxin- and apamin-sensitive K+ channels. Br J Pharmacol 119: 623–630, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 585: 295–303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathie A, Wooltorton JR, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol 30: 13–24, 1998 [DOI] [PubMed] [Google Scholar]
- 24.McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006 [DOI] [PubMed] [Google Scholar]
- 25.McHugh D, Beech DJ. Inhibition of delayed rectifier K+-current by levcromakalim in single intestinal smooth muscle cells: effects of cations and dependence on K+-flux. Br J Pharmacol 114: 391–399, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michel FS, Man GS, Man RYK, Vanhoutte PM. Hypertension and the absence of EDHF-mediated responses favour endothelium-dependent contractions in renal arteries of the rat. Br J Pharmacol 155: 217–226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller YL, Yool AJ. Increased calcium-dependent K+ channel activity contributes to the maturation of cellular firing patterns in developing cerebellar Purkinje neurons. Brain Res Dev Brain Res 108: 193–203, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol 486: 47–58, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Cell Physiol 259: C3–C18, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Ohnishi Y, Hirano K, Nishimura J, Furue M, Kanaide H. Inhibitory effects of brefeldin A, a membrane transport blocker, on the bradykinin-induced hyperpolarization-mediated relaxation in the porcine coronary artery. Br J Pharmacol 134: 168–178, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orie NN, Thomas AM, Perrino BA, Tinker A, Clapp LH. Ca2+/calcineurin regulation of cloned vascular KATP channels: crosstalk with the protein kinase A pathway. Br J Pharmacol 157: 554–564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickkers P, Hughes AD, Russel FG, Thien T, Smits P. Thiazide-induced vasodilation in humans is mediated by potassium channel activation. Hypertension 32: 1071–1076, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Quayle JM, McCarron JG, Brayden JE, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol Cell Physiol 265: C1363–C1370, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 77: 1165–1232, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Robertson BE, Bonev AD, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat coronary arteries: block by Mg2+, Ca2+, and Ba2+. Am J Physiol Heart Circ Physiol 271: H696–H705, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Ku DD, Man RYK, Vanhoutte PM. Augmented endothelium-derived hyperpolarizing factor-mediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. J Pharmacol Exp Ther 318: 276–281, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Siegel G, Carl A, Adler A, Stock G. Effect of the prostacyclin analogue iloprost on K+ permeability in the smooth muscle cells of the canine carotid artery. Eicosanoids 2: 213–222, 1989 [PubMed] [Google Scholar]
- 40.Standen NB, Stanfield PR. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol 280: 169–191, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor WF, Johnson JM, Donal O, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 57: 191–196, 1984 [DOI] [PubMed] [Google Scholar]
- 42.von Beckerath N, Dittrich M, Klieber HG, Daut J. Inwardly rectifying K+ channels in freshly dissociated coronary endothelial cells from guinea-pig heart. J Physiol 491: 357–365, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Circ Res 79: 1024–1030, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol 97: 1291–1298, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilatation in humans. J Physiol 548: 963–969, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wischmeyer E, Döring F, Karschin A. Acute suppression of inwardly rectifying Kir2.1 channels by direct tyrosine kinase phosphorylation. J Biol Chem 273: 34063–34068, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Wong BJ, Fieger SM. Transient receptor potential vanilloid type 1 channels contribute to reflex cutaneous vasodilation in humans. J Appl Physiol 112: 2037–2042, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilatation in humans. J Physiol 577: 1043–1051, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye D, Zhou W, Lee HC. Activation of rat mesenteric arterial KATP channels by 11,12-epoxyeicosatrienoic acid. Am J Physiol Heart Circ Physiol 288: H358–H364, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Zhou MS, Raij L. Cross-talk between nitric oxide and endothelium-derived hyperpolarizing factor: synergistic interaction? J Hypertens 21: 1449–1451, 2003 [DOI] [PubMed] [Google Scholar]