Abstract
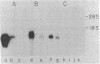
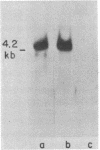
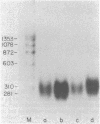
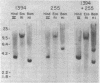
The expression of specific cellular genes in M1.19 rat hepatoma cells involves glucocorticoid regulation by mechanisms that are not well understood. To approach this problem we cloned cDNA prepared from dexamethasone-induced poly(A)-RNA and used a comparative colony hybridization method to identify recombinant clones containing hormone-regulated sequences. Two such cDNA clones, p1394 and p255, hybridize to a homogeneous RNA species of 900 nucleotides that is present in high abundance in 24-hr-induced cells but is undetectable in uninduced cells. This RNA can be seen as early as 1 hr after dexamethasone stimulation. Inhibition of protein synthesis with cycloheximide significantly reduces the accumulation of the RNA but does not abolish the induction response. In normal adult rat liver the RNA is abundant, and this RNA is induced by dexamethasone in adrenalectomized rats. Plasmids p1394 and p255 contain sequences that are homologous to the mRNA coding for the acute-phase reactant protein alpha 1-acid glycoprotein. Two other cDNA clones, p655 and p333, hybridize to a more heterogeneous RNA species 200-400 nucleotides in size with a lower induction response to dexamethasone. Southern blot analysis of M1.19 genomic DNA indicates that p1394 and p255 are complementary to a single DNA fragment, whereas p655 and p333 are complementary to repetitive sequences in the M1.19 genome. It appears that the genetic domain of glucocorticoid control in M1.19 rat hepatoma cells involves low copy number genes such as alpha 1-acid glycoprotein as well as repetitive sequence elements.
Full text
PDF

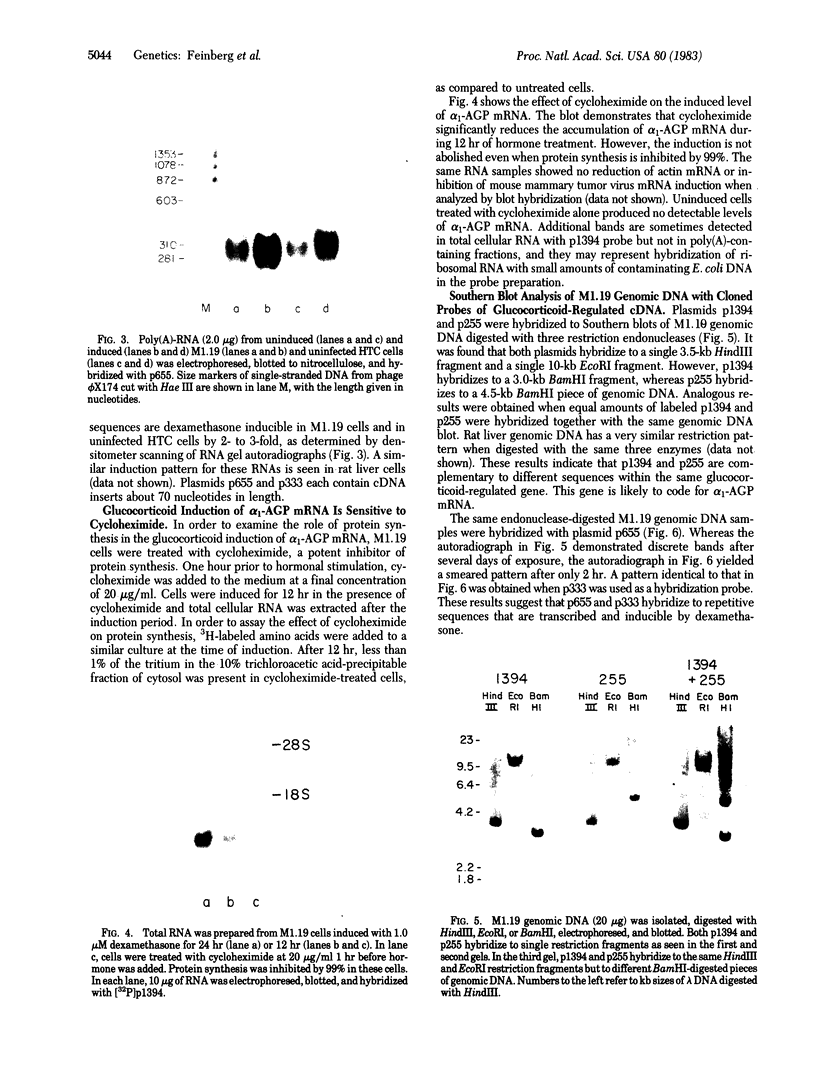


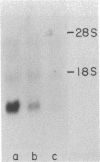
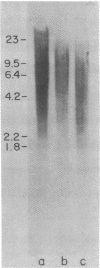
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberga A., Tran A., Baulieu E. E. Distribution of estradiol receptor and vitellogenin gene in chick liver chromatin fractions. Nucleic Acids Res. 1979 Dec 11;7(7):2031–2044. doi: 10.1093/nar/7.7.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André J., Raynaud A., Rochefort H. The extraction by micrococcal nuclease of glucocorticoid receptors and mouse mammary tumor virus DNA sequences is dissociated. Nucleic Acids Res. 1980 Aug 11;8(15):3393–3411. doi: 10.1093/nar/8.15.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrack E. R., Coffey D. S. The specific binding of estrogens and androgens to the nuclear matrix of sex hormone responsive tissues. J Biol Chem. 1980 Aug 10;255(15):7265–7275. [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Baumann H., Held W. A. Biosynthesis and hormone-regulated expression of secretory glycoproteins in rat liver and hepatoma cells. Effect of glucocorticoids and inflammation. J Biol Chem. 1981 Oct 10;256(19):10145–10155. [PubMed] [Google Scholar]
- Bennett M., Schmid K. Immunosuppression by human plasma alpha 1-acid glycoprotein: importance of the carbohydrate moiety. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6109–6113. doi: 10.1073/pnas.77.10.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu K. M., Mortensen R. F., Osmand A. P., Gewurz H. Interactions of alpha1-acid glycoprotein with the immune system. I. Purification and effects upon lymphocyte responsiveness. Immunology. 1977 Jun;32(6):997–1005. [PMC free article] [PubMed] [Google Scholar]
- Crook R. B., Louie M., Deuel T. F., Tomkins G. M. Regulation of glutamine synthetase by dexamethasone in hepatoma tissue culture cells. J Biol Chem. 1978 Sep 10;253(17):6125–6131. [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Leukocyte surface origin of human alpha1-acid glycoprotein (orosomucoid). J Exp Med. 1978 Aug 1;148(2):507–521. doi: 10.1084/jem.148.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Britten R. J., Davidson E. H. Significance of rare m RNA sequences in liver. Arch Biochem Biophys. 1977 Mar;179(2):584–599. doi: 10.1016/0003-9861(77)90147-3. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. The binding of estradiol-17 beta to the bovine endometrial nuclear membrane. J Biol Chem. 1974 Mar 10;249(5):1615–1626. [PubMed] [Google Scholar]
- Kurtz D. T. Hormonal inducibility of rat alpha 2u globulin genes in transfected mouse cells. Nature. 1981 Jun 25;291(5817):629–631. doi: 10.1038/291629a0. [DOI] [PubMed] [Google Scholar]
- Levy B., Baxter J. D. Distribution of thyroid and glucocorticoid hormone receptors in transcriptionally active and inactive chromatin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1045–1051. doi: 10.1016/0006-291x(76)90301-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson P. S., Thompson E. B., Granner D. K. Regulation of hepatoma tissue culture cell tyrosine aminotransferase messenger ribonucleic acid by dexamethasone. Biochemistry. 1980 Apr 15;19(8):1705–1711. doi: 10.1021/bi00549a029. [DOI] [PubMed] [Google Scholar]
- Ordahl C. P., Kioussis D., Tilghman S. M., Ovitt C. E., Fornwald J. Molecular cloning of developmentally regulated, low-abundance mRNA sequences from embryonic muscle. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4519–4523. doi: 10.1073/pnas.77.8.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps D. S., Litwack G. An electrophoretic characterization of the glucocorticoid response of the Fu5-5 rat hepatoma cell line. Eur J Biochem. 1982 Aug;126(2):407–415. doi: 10.1111/j.1432-1033.1982.tb06795.x. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca G. A., Hamilton R. W., McLean J. W., Conn A., Kalinyak J. E., Taylor J. M. Rat alpha 1-acid glycoprotein mRNA. Cloning of double-stranded cDNA and kinetics of induction of mRNA levels following acute inflammation. J Biol Chem. 1981 Oct 25;256(20):10362–10368. [PubMed] [Google Scholar]
- Ricca G. A., Taylor J. M. Nucleotide sequence of rat alpha 1-acid glycoprotein messenger RNA. J Biol Chem. 1981 Nov 10;256(21):11199–11202. [PubMed] [Google Scholar]
- Ringold G. M., Shank P. R., Varmus H. E., Ring J., Yamamoto K. R. Integration and transcription of mouse mammary tumor virus DNA in rat hepatoma cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):665–669. doi: 10.1073/pnas.76.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G., Howlett G., Nagashima M., Millership A., Martin H., Urban J., Kotler L. The acute phase response of plasma protein synthesis during experimental inflammation. J Biol Chem. 1982 Sep 10;257(17):10271–10277. [PubMed] [Google Scholar]
- Schutz G., Killewich L., Chen G., Feigelson P. Control of the mRNA for hepatic tryptophan oxygenase during hormonal and substrate induction. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1017–1020. doi: 10.1073/pnas.72.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. W., Frankel F. R. Enrichment of estradiol-receptor complexes in a transcriptionally active fraction of chromatin from MCF-7 cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1291–1295. doi: 10.1073/pnas.77.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior M. B., Frankel F. R. Evidence for two kinds of chromatin binding sites for the estradiol-receptor complex. Cell. 1978 Aug;14(4):857–863. doi: 10.1016/0092-8674(78)90341-0. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Gutman G. A. Allelic forms of rat kappa chain genes: evidence for strong selection at the level of nucleotide sequence. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7064–7068. doi: 10.1073/pnas.78.11.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterman R. D., Lynch K. R., Nakhasi H. L., Dolan K. P., Hamilton J. W., Cohn D. V., Feigelson P. Cloning and sequence of several alpha 2u-globulin cDNAs. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3478–3482. doi: 10.1073/pnas.78.6.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]