Abstract
During attention to a painful cutaneous laser stimulus, event-related causality (ERC) has been detected in recordings from subdural electrodes implanted directly over cortical modules for the treatment of epilepsy. However, these studies afforded limited sampling of modules and did not examine interactions with a nonpainful stimulus as a control. We now sample scalp EEG to test the hypothesis that attention to the laser stimulus is associated with poststimulus ERC interactions that are different from those with attention to a nonpainful stimulus. Subjects attended to (counted) either a painful laser stimulus (laser attention task) or a nonpainful electrical cutaneous stimulus that produced distraction from the laser (laser distraction task). Both of these stimuli were presented in random order in a single train. The intensities of both stimuli were adjusted to produce similar baseline salience and sensations in the same cutaneous territory. The results demonstrated that EEG channels with poststimulus ERC interactions were consistently different during the laser stimulus versus the electric stimulus. Poststimulus ERC interactions for the laser attention task were different from the laser distraction task. Furthermore, scalp EEG frontal channels play a driver role while parietal temporal channels play a receiver role during both tasks, although this does not prove that these channels are connected. Sites at which large numbers of ERC interactions were found for both laser attention and distraction tasks (critical sites) were located at Cz, Pz, and C3. Stimulation leading to disruption of sites of these pain-related interactions may produce analgesia for acute pain.
Keywords: attention, human, pain, cortex, network, event-related causality
it has often been suggested that pain sensations are the result of activity and functional interactions in “pain networks” in the brain (Apkarian et al. 2005; Casey 2000; Lenz et al. 2010; Melzack 1990; Peyron et al. 1999; Price 2000). Our prior studies applied a painful cutaneous laser stimulus and measured functional interactions between local field potentials recorded directly from cortex through subdural electrodes implanted for seizure monitoring (Liu et al. 2011b, 2011c; Ohara et al. 2004a, 2004b, 2006, 2008). These studies demonstrated the interactions between human cortical modules, such as S1 (primary somatic sensory cortex), PS (parasylvian cortex including S2 and insular cortex), and MF (medial frontal cortex including cingulate and supplementary motor cortex).
These interactions change dynamically with tasks, such as attention to or distraction from a cutaneous laser stimulus (Liu et al. 2011b; Ohara et al. 2006). However, these recordings did not sample many cortical modules, which seem to be involved in “pain networks,” and did not compare interactions between painful and nonpainful (control) somatic stimuli (Apkarian et al. 2005; Lenz et al. 2010; Peyron et al. 2000). Therefore, the results did not demonstrate the extent of this “pain network,” or the degree to which it is specific to painful versus nonpainful somatic modalities.
Our prior studies also identified cortical sites (critical sites) that have strong widespread functional interactions both within their module and with other cortical modules. At critical sites, the number of interactions for sites overall was elevated both during attention to the painful laser stimulus and during distraction from the stimulus (Liu et al. 2011c). This result suggests that functional interactions at critical sites may exert powerful, task-independent causal influences throughout the “pain network.”
EEG and MEG recordings have previously been used to demonstrate non-phase-locked changes in power in response to painful stimuli (Babiloni et al. 2006; Backonja et al. 1991; Ferracuti et al. 1994; Iannetti et al. 2008; Mouraux et al. 2003; Ploner et al. 2006; Raij et al. 2004; Yamasaki et al. 1999). We have now tested the hypothesis that attention to the laser stimulus is associated with increased EEG functional interactions involving widespread cortical structures, which are different from the structures associated with attention to a nonpainful electrical stimulus. The widespread functional interactions were quantified by the event-related causality (ERC), an approach that has previously been used to study event-related interactions among brain areas (Boatman-Reich et al. 2010; Korzeniewska et al. 2003, 2008; Liu et al. 2011a, 2011b). As a corollary of this hypothesis, we tested whether the analysis of the EEG would show evidence of pain-related ERC interactions characteristic of critical sites. Subjects attended (counted) either the painful laser stimuli or the nonpainful electrical stimuli while both modalities of stimulation were presented in random order in a single train of stimuli. This protocol is similar to those that have been used in previous studies (Bushnell and Duncan 1989; Bushnell et al. 1985, 1999; Longe et al. 2001; Miron et al. 1989; Tremblay et al. 1993).
To minimize the effect of location of the stimulus on the results, both stimuli were presented in the same somatotopic area. To minimize differences in expectancy of the stimuli, both were presented with random order and random interstimulus intervals. To minimize differences in intrusiveness, the stimuli were applied at intensities that produced the same salience for both stimuli, as determined for each subject in a separate session prior to the experiment. These features were designed to examine the extent to which ERC interactions are specific to painful versus nonpainful stimuli.
METHODS
Participants
Sixteen healthy volunteers (10 men and 6 women; aged 22–57 yr) were recruited in the present study. Results derived from the present data set have not been published previously. The methods of this study are consistent with the Declaration of Helsinki. The Institutional Review Board of the Johns Hopkins University approved these studies, and all subjects signed an informed consent form for participation in this study.
Painful Laser and Nonpainful Electrical Stimulation
The experiment was conducted in a silent, dimly lit room with the room temperature set to 22–24°C during the experiment. Subjects sat in a comfortable chair and rested their arms on a table in front of them. Insert earphones (ER1-14A Eartips; Etymotic Research, Elk Grove Village, IL) delivered a constant white noise (60–80 dB) throughout the experiment (Click-Tone control module; Astro-Med, Grass Instrument Division).
Subjects were instructed to keep their eyes closed and sit quietly while painful laser and nonpainful electrical stimuli were delivered to their left arm. The laser stimuli were delivered with a thulium YAG laser (Neurotest; Wavelight, Starnberg, Germany) with a laser beam wavelength of 2 μm, a beam diameter of 6 mm, and a duration of 1 ms. The laser was applied to slightly different locations between stimuli to avoid fatigue or sensitization of nociceptors. The nonpainful electrical stimuli were single-pulse, constant-current square-wave pulses (1-ms duration, Grass S12 Isolated Biphasic Stimulator) delivered through skin electrodes (0.5-cm diameter) with a 1-cm interelectrode distance on the dorsum of the left wrist. The laser stimuli were all delivered on the dorsum of the left hand within the territory of the superficial radial nerve, while the nonpainful electrical shock produced sensations in the same cutaneous territory.
For the laser stimulus, pain intensity and unpleasantness were rated separately on numerical rating scales, which were anchored by 0 for the absence of pain and 10 for the maximum imaginable pain. For the nonpainful electrical stimulus, unpleasantness was rated on a numerical rating scale that was anchored by 0 for the absence of unpleasantness and 10 for the greatest imaginable unpleasantness. For both stimuli, salience was described as “the ability of the stimulus to capture attention” and was rated on a numerical rating scale for which 0 was the absence of salience and 10 was the most salient stimulus imaginable.
For each subject, a series of laser pulses were delivered at eight energy levels in increasing order as follows: 400, 480, 560, 640, 720, 800, 850, and 900 mJ. Prior to the experiment, the subjects were asked to rate the pain intensity for the given energy levels. For this study we selected the laser energy level that produced pain of ∼5–6 out of 10 on each subject's pain intensity rating. In addition, at each given energy level, subjects were asked to rate unpleasantness and salience. The average energy level was 730 ± 170 mJ, and corresponding pain intensity ranged from 4/10 to 6/10.
In a separate session prior to the experiment, all subjects were introduced to the electrical stimulus with a series of electric pulses at intensity levels from 5.5 to 18 mA. For each level, subjects were asked to rate the unpleasantness and salience for the stimulus. For each subject, the final intensity level for the electrical stimulus was selected so that the electrical stimulus produced a salience rating equal to that of the selected laser energy level (average current 12.8 ± 5.2 mA). For each subject these levels of electric and laser stimulation were applied during all blocks of stimuli so that at baseline painful and nonpainful stimuli were considered to be equally salient for all the stimulation blocks.
Experimental Design
Painful cutaneous laser and nonpainful electrical pulses were delivered in four blocks of 80 stimuli (40 of each stimulus type) in random order and with random interstimulus intervals of between 7 and 8 s. The randomization procedures in the experimental design were carried out in our studies by use of a standard random number generator (Oracle, Redwood Shores, CA).
In this study attention was directed, as subjects were instructed prior to each block to count either the number of laser stimuli (laser attention task) or the number of electrical stimuli (laser distraction task). The arrangement of tasks consisted of two blocks of “attend to laser” followed by two blocks of “attend to electric stimuli,” and vice versa. Therefore, there were a total of four blocks (blocks 1, 2, 3, and 4), and the first two blocks (blocks 1 and 2) were randomly assigned to either the laser attention task or the laser distraction task and counterbalanced across subjects. The task of counting stimuli was the same whether attention was directed to the laser or the electrical stimulus. Besides counting the number of attended stimuli, the experimental designs of directed attention to one of two stimulus modalities in a single train of stimuli have often been used in primate studies of the attentional modulation of painful stimuli (Bushnell et al. 1985, 1999; Longe et al. 2001; Miron et al. 1989; Tremblay et al. 1993). In studies of monkeys, attention was directed to a stimulus not by counting it but by a reward for identifying it.
Subjects were not told at the beginning of the first block of stimuli for the experiment that they would be asked to carry out ratings of the stimuli at the end of the block. Nevertheless, at the end of each block subjects were asked to report the number of attended stimuli in that block and to rate the pain intensity, pain unpleasantness, and salience of the laser stimulus. In addition, the subjects were asked to rate unpleasantness and salience for the electrical stimulus. The effect of priority upon these ratings is described in results.
Data Collection
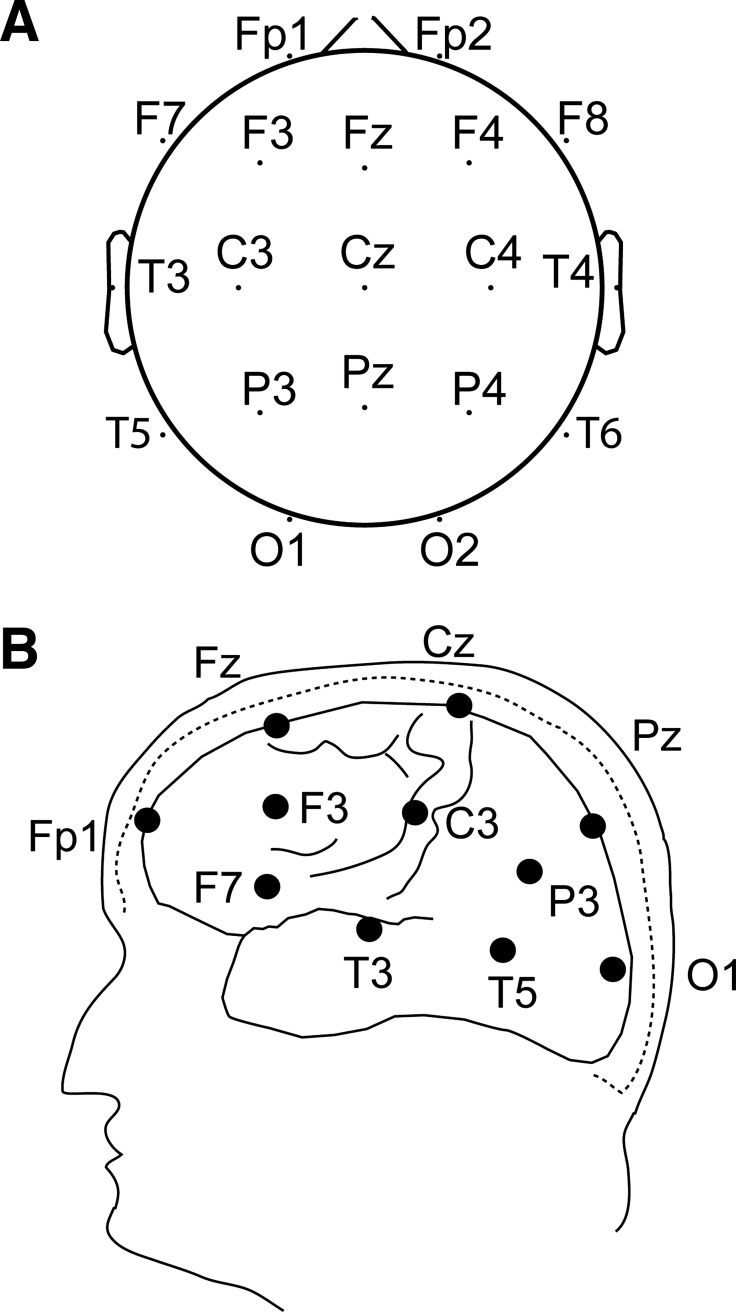
The EEG signals were recorded with 19 Ag-AgCl electrodes (Grass) placed on the scalp according to the International 10–20 System with a referential montage to a reference of linked earlobes, as shown in Fig. 1 (Fp1, Fp2, Fz, F3, F4, F7, F8, Cz, C3, C4, T3, T4, Pz, P3, P4, T5, T6, O1, O2) (Jasper 1958). The number of electrodes is relatively small, so we were not able to group electrodes into meaningful sets, e.g., there is only one central electrode on either side. Signals were amplified and digitized at the sampling rate of 500 Hz (SynAmps 5083, Neuroscan). The timings for the onset of the laser and electrical stimuli were acquired and embedded in the EEG recordings. All the EEG recordings were visually inspected, and trials with artifacts were removed before the analysis. The average event-related potential (ERP) waveforms were processed for each subject for both stimulus modalities and will be described in a subsequent report. EEG preprocessing was carried out with MATLAB (The MathWorks) and EEGLAB (Delorme and Makeig 2004).
Fig. 1.
Locations of EEG channels on the head. A: schematic of a view of the cranium from above by the standard arrangement for EEG channels (10-20 system). B: approximate location of electrodes on a lateral view of the head.
Event-Related Causality
Multivariate autoregressive (MVAR) modeling for causality analysis has become a primary technique for characterizing the ongoing activity of neuronal assemblies in the brain and their interactions in cortical networks, based on the theory of stochastic processes. Here we briefly describe ERC methods that have already been described in detail in previous reports (Boatman-Reich et al. 2010; Korzeniewska et al. 2003, 2008; Liu et al. 2011a, 2011b).
Phase-locked responses were first estimated by averaging the raw EEG signals from all trials separately by task (laser attention task vs. laser distraction task). These were then subtracted from the raw signals within each block. ERC analyses were performed on the resulting signals by MVAR modeling. This technique for subtracting phase-locked responses prior to ERC analysis has been commonly used in the literature (Boatman-Reich et al. 2010; Brovelli et al. 2004; Korzeniewska et al. 2008; Liu et al. 2011b, 2011d). It diminishes artifacts that can arise in the ERC analyses from phase-locked responses (Truccolo et al. 2002).
Directional interactions between brain areas were then estimated with the short-time direct directed transfer function (SdDTF). SdDTF is an MVAR modeling approach that is based on the concept of Granger causality (Granger 1969). In brief, for two observed time series X and Y, it is said that X is Granger causal of Y if the past knowledge of X significantly reduces the prediction error for Y. In this study, we refer to this as an electrode pair with a directed interaction, e.g., X > Y. The participation of any given electrode in network interactions can also be characterized by the total number of outgoing or incoming interactions with all other channels. The pattern of all directed interactions at any given time, as well as their evolution over time, can also be used to characterize the functional anatomy of large-scale cortical networks.
The SdDTF is an implementation of direct directed transfer function (dDTF) and is used for signals that are short in duration and have a large number of repetitions. In turn, the dDTF is a version of an earlier measure, the directed transfer function (DTF). The essential part of the ERC method is the statistical testing procedure that will reveal the significant changes in interchannel relationships after versus before the stimulus (i.e., event-related; Liu et al. 2011c).
SdDTF is superior to traditional spectral correlation approaches, such as coherence and partial direct coherence, because the latter methods do not provide information about the directionality of correlations (Korzeniewska et al. 2008; Liu et al. 2011b). The order for the MVAR model used for SdDTF estimates was determined by the Akaike information criteria (AIC) (Akaike 1974), which was an estimate for the number of coefficients chosen to optimize the MVAR analysis. As described above, for each task condition the ensemble average was subtracted from each single trial in order to meet the criteria for MVAR modeling.
In the following subsections, we provide more detail for each of the above-mentioned approaches that contributed to the development of ERC in the present study.
Directed transfer function.
DTF is the first MVAR-based approach that was developed for analyzing the causal relationships for a multivariate system. DTF is related to the Granger causality; it measures the direction and strength of the causal interaction in both the time and frequency domains within a given system. In the case of two variables, the DTF is equivalent to the Granger causality and thus it may be interpreted in terms of Granger causality. The signals, which were acquired during the experimental trials, were treated as if they were produced by a common stochastic process and were used to estimate the MVAR model coefficients for that process. The MVAR model used for calculation of the ERC includes all 19 electrodes.
Direct directed transfer function.
DTF evaluates a linear combination of causal influences along all causal pathways either directly or indirectly by interaction with an unobserved site. Therefore, nonzero values of DTF between two channels do not necessarily imply that the causal influence between them is direct. The causal influence may be mediated by another channel, or by channels that are not even observed in the system.
The concept of partial correlation coefficient (partial coherence in frequency domain) is a well-known method that takes all other variables into account in a regression analysis. The partial coherence is introduced and incorporated in the computation of DTF to reveal the causal influence that is direct. Therefore, the DTF values are multiplied by the nondirectional partial coherence, and this step leads to the development of dDTF. As a result, the small or zero values of partial coherence and consequently the dDTF value indicate that interaction between a given pair of signals is mediated by other sites even if DTF has large values.
The original DTF and dDTF are not designed to analyze data with very short duration and thus cannot be used to study interactions that occur on short timescales. This particular limitation may be overcome when a large number of trials of a particular task are available. The short-time DTF (SDTF) is a modification of the original DTF, adapted for analyzing the causal interactions that occur in a short timescale, and has been applied to electrophysiological data.
Short-time direct directed transfer function.
Finally, the SdDTF was introduced by combining the dDTF and the SDTF. The SdDTF combines the advantages of dDTF and SDTF for estimating the directions, intensities, and spectral contents of direct causal influences between variables for recordings of short duration. Furthermore, a sliding window was applied when computing the SdDTF; this approach provided a smoothed estimator for SdDTF. In this study, the length of the sliding window and the length of the time window are chosen to be small enough to treat the data as stationary yet large enough to take into account the jitter of the recorded signal interactions across different trials.
Because the state of the brain is constantly and spontaneously changing over time, it is important to select the baseline data within the same subject and task condition for the ERC analysis. The same baseline approach has also been used in scalp EEG and MEG recordings for analyses of spectral power changes (Pfurtscheller and Aranibar 1977; Pfurtscheller and Lopes da Silva 1999).
The relationship between neuronal assemblies can be understood by frequency representations of their oscillatory activities. The SdDTF was developed to estimate the directions, intensities, and spectral contents of the causal interactions. Therefore, the results from ERC analysis provide both frequency and time information for significant causal influences found in time intervals of interest.
Poststimulus ERC interactions were identified if the value for the SdDTF was significantly higher than a preselected baseline interval (i.e., 1-s interval starting 2 s before the stimulus). For each subject, all 19 channels were included in the MVAR models and a sliding window approach was used to smooth estimates of ERC. The size of the sliding window was set to 0.1 s and was slid 0.04 s at consecutive windows. This sliding window provides smoother estimates for the SdDTF at a cost of decreased temporal resolution for the ERC analysis.
To determine the statistical significance of changes in SdDTF, and thus the ERC, a baseline statistical test was applied in this study, and the significance level was set to α = 0.05, with correction for multiple comparisons. For every paired electrode combination, this test compared the values for the SdDTF in the frequency-time domain between baseline and the interval of interest, using a semiparametric regression model (Crainiceanu et al. 2009; Korzeniewska et al. 2008; Liu et al. 2011b). A formal bivariate smoothing model for both frequency and time was also used here to reduce the effects of the inherent noise in the recorded signals.
As in prior publications, we scanned across the 0–50 Hz range in order to be able to measure frequencies of ERC including the low gamma band, which is often functionally significant (Boatman-Reich et al. 2010; Korzeniewska et al. 2008). In this study, significant causal influences were color coded by their magnitude as shown in time-frequency plots (see Fig. 2), across the 0–50 Hz band, which allows the possibility of detecting low gamma band ERC.
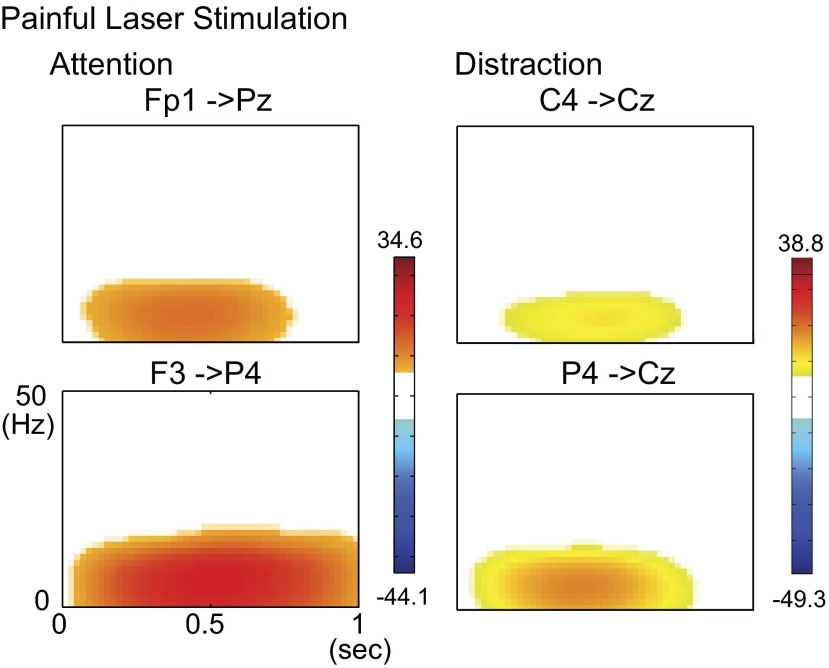
Fig. 2.
Examples of time-frequency plots of event-related causality (ERC) for different pairs of electrodes with the painful laser stimulus. Both displays are shown under the laser attention and laser distraction conditions with respect to the laser, as labeled. In the ERC time-frequency plots hot colors indicate significant increases in the raw ERC value. On the color scale, numbers indicate maximum and minimum values of ERC. All colored pixels indicate a significant change in ERC, and the maximum ERC indicates the highest strength of directed interaction for the pair of channels.
The computer programs for the above statistical tests were written in R language and have been previously tested and used in similar studies of multichannel human recordings (Liu et al. 2011c). All the programs for the ERC analysis were written in C language in-house, developed in the Linux environment, and run on a computer cluster, which was implemented as one distributed system.
Statistical Measures
This report is focused on the ERC, which is measured by the probability that the poststimulus period is different from the baseline period. There is no parametric value to be compared within individuals, only nonparametric tests of proportions of channels with significant ERC. The tests in which the numbers of subjects are multiplied by the numbers of electrodes are overall tests of proportions, such as differences in proportions between the attention tasks versus the distraction tasks overall. Individual tests of proportions among subjects are summarized by the ratios in Table 3. For any electrode, subjects are then taken as the independent variable and the presence of significant ERC for a subject as the dependent variable, which is examined statistically by tests of proportions.
Table 3.
ERC results for individual channels by task and stimulus modality
| Laser Stimulus | Electric Stimulus | |
|---|---|---|
| Attention | ||
| Out | Fp1: 10/16 | Fp1: 9/16 |
| Pz: 10/16 | Fp2: 9/16 | |
| F3: 9/16 | Cz: 12/16 | |
| C3: 10/16 | C3: 10/16 | |
| Cz: 12/16 | ||
| In | O2: 9/16 | T6: 10/16 |
| Pz: 10/16 | Cz: 13/16 | |
| Cz: 13/16 | F4: 9/16 | |
| P3: 12/16 | ||
| T4: 10/16 | ||
| Total | Pz: 12/16 | Fp1: 10/16 |
| O2: 10/16 | Cz: 12/16 | |
| Cz: 10/16 | Fp2: 10/16 | |
| C3: 13/16 | C3: 13/16 | |
| Distraction | ||
| Out | C3: 12/16 | C4: 12/16 |
| Cz: 13/16 | T6: 9/16 | |
| T6: 10/16 | ||
| Pz: 12/16 | ||
| P4: 10/16 | ||
| In | Cz: 12/16 | F8: 10/16 |
| C4: 10/16 | O1: 19/16 | |
| O2: 9/16 | ||
| P4: 10/16 | ||
| C3: 13/16 | ||
| T3: 12/16 | ||
| Total | Cz: 12/16 | F4: 10/16 |
| C3: 12/16 | T6: 10/16 | |
| T6: 10/16 | C3: 12/16 | |
| P4: 12/16 | Fp1: 10/16 | |
| Pz: 13/16 | O1: 10/16 |
Channels with significantly larger numbers of event-related causality (ERC) pairs (1-way Wilcoxon, P = 0.01) are listed in increasing order. Ratios next to channel labels represent proportion of subjects who showed results consistent with the overall result.
In this study, the number of ERCs found for all 19 electrodes and 16 subjects under different combinations of task and modality were used to estimate the 99% confidence intervals or the median with one-sample Wilcoxon signed-rank test. This test is a nonparametric estimator of the confidence interval for the median of a population. Since it is a nonparametric test, it requires no assumption for the distribution of the measurement. The upper bounds for the 99% confidence intervals were used to determine whether observed numbers of ERC pairs in a channel were significantly greater than expected at random. All the confidence intervals were computed with Minitab (Minitab, State College, PA) (Hettmansperger and Sheather 1986).
RESULTS
Psychophysics Ratings
For all subjects, application of a painful laser evoked sharp or pinprick pain sensations and the electrical stimulus produced a nonpainful tingling sensation. For each subject, the final laser energy level was set to be around 5–6 on the pain intensity rating. The pain intensity was significantly greater (Table 1; 5.4 ± 2 vs. 1.9 ± 3; P < 0.01, paired t-test) when subjects attended to the laser (i.e., laser attention task) than when they attended to the electric stimulus (i.e., laser distraction task). The ability of the tasks to direct attention for the subjects was reflected in the low error rates for counting the total number of painful laser and nonpainful electric stimuli for attention and distraction tasks (0.12 ± 0.09 and 0.10 ± 0.12). In addition, the range for the error rate did not reveal outliers in performance for attention (maximum, minimum: 0.14, 0.05) and distraction (0.13, 0.0) tasks.
Table 1.
Psychophysical measures of pain and salience for laser and electric stimuli under laser attention and distraction tasks
| Laser: Attention vs. Distraction Task | Electric: Attention vs. Distraction Task | |
|---|---|---|
| Pain | 5.4 ± 2.0 vs. 1.9 ± 1.3* | n/a |
| Unpleasantness | 5.4 ± 2.3 vs. 2.0 ± 1.7† | 0.9 ± 0.8 vs. 1.9 ± 1.5† |
| Salience | 6.2 ± 2.6 vs. 2.0 ± 1.4† | 2.0 ± 1.7 vs. 4.8 ± 2.0† |
Values are means ± SE. Note that attention and distraction tasks always refer to the laser stimulus. n/a, Not applicable.
P < 0.05, †P < 0.001.
Prior to the study the current level for nonpainful electrical stimulus was selected to produce the same salience level as painful laser stimulus, and this level was kept unchanged in all blocks for an individual subject. Therefore, before the study the average salience level for both the laser and the electrical stimulus was 5.3 ± 1.9. The salience level under the laser attention task for the laser (Table 1, salience 6.2 ± 2.6) was similar to that for the electric stimulus under the laser distraction task (salience 4.8 ± 2.0). This was consistent with prior demonstrations that nonpainful somatic stimuli can be as salient as painful stimuli (Legrain et al. 2011; Mouraux and Iannetti 2009).
A two-way ANOVA for task (attention and distraction) and modality (painful laser stimulus and nonpainful electrical pulse) was performed for the ratings of unpleasantness and salience separately (Table 1). For the rating of unpleasantness, both factors (i.e., task and modality) revealed a main effect in the two-way ANOVA test (P = 0.0001). Significant interaction effects between task and modality were also found (P = 0.002). Post hoc comparisons using the two-sample t-test revealed that the unpleasantness ratings were significantly larger when the subject was asked to attend to the painful laser stimulus (5.4 ± 2.3) than to the nonpainful electric stimulus (1.9 ± 1.5, P < 0.001, 2-sample t-test), i.e., an analgesic effect of distraction.
For the salience ratings, the ANOVA results showed a main effect for task (P = 0.0001) but not for modality. Therefore, the salience ratings were greater in the attention task than the distraction task but were not significantly different between the laser and electric stimuli.
The subjects had not been told that they would rate the stimuli until the end of the first block. Therefore, we tested for an effect of priority on the ratings by comparing the ratings for the first versus the second block for the laser stimulus under the laser attention task and for the electric stimulus under the laser distraction task. These results are shown in Table 2 and demonstrate that differences in the ratings between these blocks were not significant (P > 0.05, t-tests).
Table 2.
Psychophysical ratings in attention and distraction tasks for both painful laser and nonpainful electric stimulus in first and second blocks
| Attention |
Distraction |
|||
|---|---|---|---|---|
| Block 1 | Block 2 | Block 1 | Block 2 | |
| Laser | ||||
| Pain intensity | 5.3 ± 0.32 | 5.5 ± 0.37 | 1.8 ± 0.9 | 2.1 ± 1.5 |
| Unpleasantness | 5.6 ± 0.53 | 5.2 ± 0.38 | 1.9 ± 1.5 | 2.1 ± 1.3 |
| Saliency | 6.1 ± 0.34 | 6.2 ± 0.61 | 1.9 ± 0.9 | 2.0 ± 1.5 |
| Electric | ||||
| Unpleasantness | 0.8 ± 0.5 | 1.0 ± 0.5 | 2.2 ± 1.3 | 1.7 ± 1.1 |
| Saliency | 1.9 ± 1.6 | 2.1 ± 1.3 | 4.7 ± 1.5 | 4.8 ± 1.8 |
Values are means ± SE.
ERC Analyses
ERC was calculated in the poststimulus interval for both tasks and stimulation modalities. Figure 2 shows ERC time-frequency plots for four separate pairs of electrodes under either the laser attention task or the laser distraction task. Figure 2, top left, shows outgoing ERC for Fp1 and incoming ERC for Pz. ERC was consistently found at frequencies of <20 Hz.
For the painful laser stimulus, the ERC analysis showed that the total number of ERC pairs was significantly greater in the attention task versus the distraction task [3,630/10,944 vs. 3,402/10,944 (denominator = 19 × 18 × 16 × 2); χ2, P < 0.05]. This significant difference was also found for nonpainful electric pulses (3,014/10,944 vs. 2,999/10,944; χ2, P < 0.05). For both tasks, the ERC results for individual channels are presented by task and stimulus modality in Table 3.
In Table 3, the results are given for the ERC analysis overall that indicates whether the numbers of ERC pairs (outgoing, incoming, or total) are more common than the median (see Statistical Measures). The table also includes the number of subjects who had significant ERC for the same electrode. The maximum number of subjects who could be expected to have significant ERC at any electrode at random was four, as determined by combinatorial analysis (binomial distribution with Bonferroni correction: 16 patients, probability of success 1/19). By this measure all ERC proportions were >50% and involved more individuals than expected at random.
Painful laser stimulus.
LASER ATTENTION TASK.
As described in methods, for all 19 channels a one-sample Wilcoxon test was used to construct the 99% confidence intervals. In the laser attention task, test results for painful laser stimulus showed that the Fp1, Pz, F3, C3, and Cz channels had significantly greater numbers of ERC interactions than the median for the outgoing direction (1-way Wilcoxon). By the same approach, the O2, Pz, Cz, P3, and T4 channels had greater numbers of ERC pairs in the incoming direction. Finally, the Pz, O2, Cz, and C3 channels had greater numbers of ERC pairs for the total of ERC directions (P < 0.01, 1-sample Wilcoxon test), which has a different distribution from incoming and outgoing directions.
During the laser attention task, Fp1 and F3 had a significantly larger number of outgoing (Fig. 3A, attention outgoing, hot colors) versus incoming ERC (Fig. 3A, attention incoming, cold colors) and thus played a driver role (Fp1: 124/288 vs. 75/288, F3: 116/288 vs. 81/288; χ2, P < 0.05). The denominator for these ratios was the number of possible ERC interactions in the direction of interest × the number of subjects (18 × 16 = 288). The temporal T4 and T6 channels were found to have significantly larger numbers of incoming versus outgoing ERC and thus played a receiver role during the laser attention task (T6: 100/288 vs. 72/288, T4: 105/288 vs. 77/288; χ2, P < 0.05). These results in the laser attention task are consistent with outgoing frontal ERC interactions and incoming parietal temporal ERC interactions.
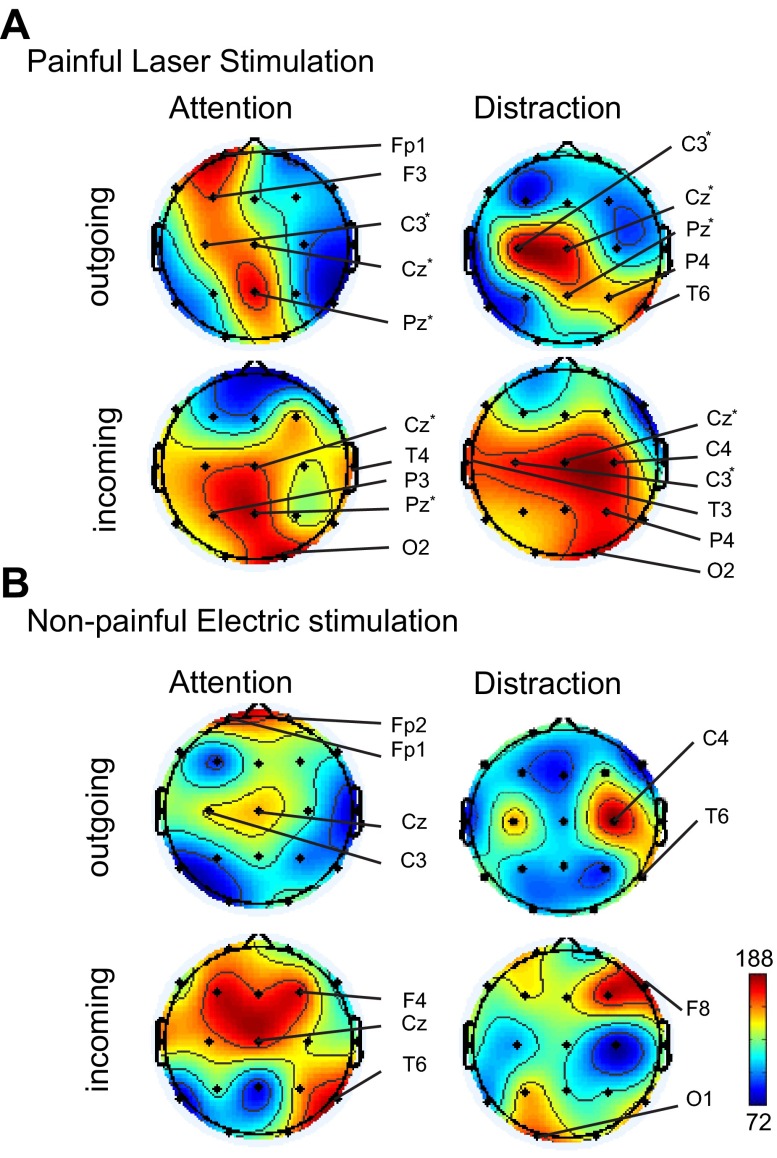
Fig. 3.
Schematics of the incoming or outgoing ERC for the laser (A) or the electric (B) modality by channel. These schematics show the numbers of ERC interactions involving each channel as summed across subjects and displayed by the direction of ERC (incoming and outgoing) and by task (laser attention or laser distraction). On the color scale, maximum and minimum values indicate the number of ERC interactions for each channel. Channel labels are plotted for the channels showing significantly greater numbers of ERC interactions than the median. Critical sites are indicated by an asterisk next to the label. Note that critical sites had larger numbers of total ERC pairs related to the laser stimulus during both the attention and distraction tasks. Therefore, not all critical sites had larger numbers of ERC pairs for all incoming and outgoing directions.
During the laser attention task, outgoing interactions were found for both laser and electric stimuli at channels FP1, C3, and Cz (Table 3). This proportion of channels was not significantly different from random selection of channels [P = 0.037, not significant (NS) with Bonferroni correction α = 0.0083], which suggests that different interactions subserve painful and nonpainful sensations. In this task, Cz was found to have incoming ERC interactions in response to both laser and electric stimuli, which was not different from random selection (P = 0.39). For the total interactions, C3 and Cz were found for both laser and electric stimuli modalities.
LASER DISTRACTION TASK.
In the distraction task, the test results for painful laser stimuli showed that the C3, Cz, T6, Pz, and P4 channels had greater numbers of ERC interactions than the median in the outgoing direction (P < 0.01, 1-sample Wilcoxon test). The Cz, C4, O2, P4, C3, and T3 channels were found to have significantly greater numbers of ERC pairs in the incoming direction. For the total of ERC directions (Table 3), the results showed that the Cz, C3, T6, P4, and Pz channels had greater numbers of ERC pairs.
F8 was found to have a significantly larger number of outgoing versus incoming ERC directions under the laser distraction task (F8: 65/288 vs. 87/288; χ2, P < 0.05) and thus played a driver role (Fig. 3A). T3 was found to have a significantly larger number of incoming ERC in the laser distraction task (T3: 97/288 vs. 72/288; χ2, P < 0.05) and thus played a receiver role (Fig. 3A). Therefore, for the laser stimulus both laser attention and distraction tasks were characterized by outgoing frontal ERC and incoming temporal parietal ERC, although these results do not prove that frontal channels are functionally connected for parietal temporal channels.
During the laser distraction task, only outgoing T6 ERC interactions were found for both the laser and electric stimuli, which is not significantly different from random selection of channels (P = 0.038, combinatorials NS with Bonferroni). No channels were found with incoming ERC interactions in response to both laser and electric stimuli, which was not significantly different from random selection of channels (P = 0.46). Therefore, for both tasks the interactions were not different for the painful versus nonpainful stimuli.
Nonpainful electric stimulus.
LASER ATTENTION TASK.
In the attention task, test results for nonpainful electric stimulus showed that the Fp1, Fp2, C3, and Cz channels (1-way Wilcoxon) had greater numbers of ERC pairs than the median in the outgoing ERC direction. The F4, Cz, and T6 channels were found to have greater numbers of ERC pairs in the incoming direction of ERC. For the total of directions, the results showed that the Fp1, Fp2, C3, and Cz channels had greater numbers of ERC interactions (P < 0.01, 1-sample Wilcoxon test).
For the nonpainful electrical stimulus, the Fp1 channel was found to have a larger number of outgoing versus incoming ERC (Fig. 3B) and thus played a driver role in the attention task (Fp1: 188/288 vs. 162/288; χ2, P < 0.05). In addition, the F3 channel was found to have a significantly larger number of incoming versus outgoing ERC pairs and thus played a receiver role in the attention task (F3: 170/288 vs. 143/288; χ2, P < 0.05). Therefore, frontal channels were both receivers and drivers for the electric stimulus under the laser attention task, while for the laser stimulus driver channels were frontal (FP1, F3) and the receiver channels were temporal parietal (T4, T6). Therefore, the pattern of frontal receiver channels and temporal parietal driver channels found for laser stimuli during the laser attention task was not found for the electrical stimulus.
The comparison of ERC interactions that occur in both electric and laser stimuli is presented above under the painful laser stimulus for both tasks.
LASER DISTRACTION TASK.
The ERC results for nonpainful electric pulses in the laser distraction task showed that the C4 and T6 channels had greater numbers of ERC interactions than the median in the outgoing direction. The F8 and O1 channels had greater numbers of ERC pairs in the incoming direction of ERC. For the total of directions of ERC, the Fp1, F4, C3, T6, and O1 channels were found to have significantly greater numbers of ERC pairs (P < 0.01, 1-sample Wilcoxon test).
For the laser distraction task and the electric stimulus, the C3 and C4 channels were found to have significantly larger numbers of outgoing versus incoming ERC pairs (Fig. 3B) and thus played a driver role (C3: 166/288 vs. 142/288, C4: 174/288 vs. 150/288; χ2, P < 0.05). No channel was found to have a significantly larger number of incoming versus outgoing ERC pairs in the distraction task (χ2, P < 0.05). Therefore, for the laser distraction task central drivers were found for the electric stimulus, while for the laser stimulus and laser distraction task F8 was the driver channel and T3 was the receiver channel.
Painful laser stimulus vs. nonpainful stimulus.
For the laser attention task, the ERC analysis showed that the total number of ERC pairs was significantly greater for the painful laser stimulus than the nonpainful stimulus [3,630/10,944 vs. 3,014/10,944 (denominator = 19 × 18 × 16 × 2); χ2, P < 0.01]. For the laser distraction task, the total number of ERC pairs was significantly greater in the painful laser stimulus than the nonpainful stimulus (3,402/10,944 vs. 2,999/10,944; χ2, P < 0.01).
As described above, the overlap between electrodes with greater numbers of ERC pairs than the median for both the laser and the electrical stimulus was not greater than expected at random. These results address both the numbers of ERC pairs and the topographical arrangement of the response to the electrical stimulus. The results are that the overlap of electrical and laser ERC pairs for either task is not significant, which demonstrates that these tasks are subserved by different structures during both tasks.
Channels with reciprocal interactions.
Examination of Table 3 suggests that many channels with significant ERC interactions have both incoming and outgoing interactions. Channels with reciprocal interactions are shown in Table 4. More of these interactions involved the laser stimulus than the electric stimulus (5/6 vs. 1/6, P = 0.08).
Table 4.
Channels with significant ERC incoming and outgoing interactions by task
| Painful Laser | Nonpainful Electric | |
|---|---|---|
| Poststimulus: attention | Cz, Pz | Cz |
| Poststimulus: distraction | F7, O1 | F4, T6, O1 |
Critical sites.
Our prior studies of subdural recordings demonstrated the presence of critical sites that were associated with multiple significant ERC interactions in a laser attention-distraction protocol (Liu et al. 2011c). At critical sites, the numbers of ERC interactions during laser distraction and attention tasks were both larger than their median to a greater degree than expected at random. Therefore, to identify critical sites in the scalp data we looked for electrodes that were associated with larger numbers of ERC interactions than the median during both the laser attention and laser distraction tasks by the one-sample Wilcoxon signed-rank test (P < 0.01). This is a stringent test, since the chance of both conditions being met (P < 0.01 for each condition) at random for any channel is very small. The results showed that the critical sites for this data set were Cz, C3, Pz, and O2 channels (Table 3). The activity at these channels may reflect activity at critical sites in the brain as identified by subdural recordings (Liu et al. 2011c).
DISCUSSION
We have now tested the hypothesis that attention to the painful laser stimulus is associated with increased functional interactions involving widespread cortical modules, which are different from the modules associated with attention to a nonpainful electric stimulus. The results demonstrate ERC interactions that are much more widespread than those described in our subdural studies of S1, MF, and PS cortex (Liu et al. 2011b, 2011c). The results also demonstrate that ERCs of scalp EEG channels over the frontal lobe play a driver role while those over the parietal and temporal lobes play a receiver role for the laser stimulus during both the laser attention task and the laser distraction tasks. This pattern was not seen for ERC of the electrical stimulus in either task.
The channels with ERC in these results might be generated by particular cortical structures located within the brain. It is important to note that the precise structures involved in these ERC interactions cannot be inferred from the present results. Nevertheless, these results seem to be correlated with studies of subdural recordings that have demonstrated incoming ERC or a receiver role for PS (parietal and temporal) cortex during poststimulus intervals (Liu et al. 2011b, 2011c). The present results do not prove that frontal channels are functionally related to parietal and temporal channels. Nevertheless, the present results also seem to be related to fMRI studies, which found functional interconnections involving the fronto-parietal attention circuit during directed attention to pain (Ploner et al. 2011), perception of pain (Boly et al. 2007), and rating of pain (Kong et al. 2006) (also see Kong et al. 2010; Ploner et al. 2010).
Activations of these structures have been related to the salience of the stimulus, regardless of either the modality or the behavioral context of the stimulus (Downar et al. 2002, 2003). The present results do not reflect differences in salience because the baseline intensity of the stimuli was adjusted to make baseline salience equal between the painful and nonpainful somatic sensory stimuli. Differences in activations of these same cortical structures have been reported during attentional modulation of stimuli in different modalities (Corbetta and Shulman 2002; Gilbert and Sigman 2007). The present ERC interactions are likely to be associated with endogenous components of attention because of the directed attention task, and because the stimulus intensities, locations, and frequencies were constant for all blocks of the experiment within each subject.
Methodological Considerations
There are a number of limitations to ERC analysis. First, significant ERC roles might be detected between signals that are both active and correlated but not causal, as in the case of phase-locked activity. However, the coefficients of the MVAR model used in this analysis are calculated with correlation matrices, which include all observed channels. In this kind of analysis, correlation can influence the magnitude but not the direction of the ERC.
These ERC calculations cannot account for the effect of unobserved channels or indirect interactions, which are outside the 19 channels included in this analysis. The present ERC causal interactions did not identify particular causal pairs, only the numbers of incoming or outgoing interactions by electrode. Finally, ERC detects and quantifies linear relations, while some of the relationships that are present in the EEG may be nonlinear.
To mitigate the effect of phase-locked activity (e.g., ERPs) on our ERC analyses, we chose the most straightforward procedure that has been used in the literature to remove phase-locked activity prior to SdDTF estimations (Boatman-Reich et al. 2010; Brovelli et al. 2004; Korzeniewska et al. 2008; Liu et al. 2011b, 2011d). In particular, ERP subtraction is a required preprocessing step for multitrial MVAR model fitting (Ding et al. 2000). In addition, spurious causality can be produced when the subtraction is not done (Oya et al. 2007). Nevertheless, we cannot completely rule out the possibility that our results were affected by this procedure (Truccolo et al. 2002).
Salience is a property of painful and nonpainful somatic sensory stimuli and of stimuli of special sense, e.g., vision (Downar et al. 2002, 2003; Mouraux and Iannetti 2009). The response to the painful cutaneous laser may signal the salience of the stimulus, defined as “the ability of the stimulus to capture attention” (Mouraux and Iannetti 2009; see also Downar et al. 2002, 2003; Legrain et al. 2005; Lorenz and Garcia-Larrea 2003; Zaslansky et al. 1995). We controlled for the effect of baseline salience of stimuli within each subject by matching the salience of the painful laser stimulus to that of the nonpainful electrical stimulus through adjustments in the intensity of the stimuli. Salience of a stimulus was altered by task, so that salience was not matched under different tasks. In addition, both stimuli were applied in the same somatotopic location. This method was designed so that the presence or absence of pain was the main difference between the two stimuli, which allowed us to examine our hypotheses regarding ERC interactions between painful and nonpainful stimuli. The present results illustrate “the usefulness of laser-related activity... to explore the effect of a given experimental factor on the transmission and processing of nociceptive input” (Mouraux and Iannetti 2009).
ERC of Painful vs. Nonpainful Stimuli
As predicted by our hypothesis, the channels with ERC interactions were consistently different between painful versus nonpainful stimuli for both laser attention and distraction tasks. These results were observed although the low resolution of scalp EEG recordings will lead to an overestimate of the number of cortical structures that play a driver or receiver role in response to both painful and nonpainful stimuli. These results are consistent with a study of MEG recordings that reported different causal interactions between primary somatic sensory cortex, secondary somatic sensory cortex, and motor cortex between painful versus nonpainful electrical stimulation (Ploner et al. 2009). A similar dichotomy of interactions for painful and nonpainful stimuli has also been demonstrated in the monkey somatic sensory thalamus (Apkarian et al. 2000).
The present results are consistent with imaging studies that have compared activations related to painful heat stimuli versus nonpainful stimuli, including vibrotactile (Coghill et al. 1994; Gelnar et al. 1999), moving tactile (Davis et al. 1998), and warm contact (Casey et al. 1996; Davis et al. 1998; Jones et al. 1991; Talbot et al. 1991) stimuli. Across all reports, there were similarities and differences in activations between painful and nonpainful stimuli. The most common similarities included pain-related and non-pain-related activation of cortical areas SI and SII; the most common differences included pain-related activation of the anterior insula or the insula.
In one of these studies, correlation between the extent of activation in different areas was taken as a measure of functional connectivity (Gelnar et al. 1999), although it will not reflect the direction of causal interactions (see Methodological Considerations). By this measure, more pairs of areas were correlated in response to either painful heat or vibration than were correlated for both painful heat and vibration stimuli. The present results, together with the referenced studies, support the hypothesis that different networks subserve painful and nonpainful somatic sensation, so that one modality could be disrupted by focal stimulation while the other is not disrupted.
Cortical Modules, Critical Sites, and Stimulation-Induced Analgesia
EEG has previously been used to assess the response to painful stimuli. For example, EEG recordings have been used to demonstrate changes in EEG power at particular frequency bands in response to painful stimuli. Immersion of the hand in painfully cold water can provoke a contralateral parietal decrease in power [event-related desynchronization (ERD)] in the alpha band (8–12 Hz) (Ferracuti et al. 1994). At longer latencies, bilateral frontal and posterior increases in power [event-related synchronization (ERS)] in the alpha band were observed in another study (Backonja et al. 1991). Alpha ERD over the vertex has been observed in scalp EEG in response to a painful cutaneous laser stimulus (Mouraux et al. 2003).
A prior study has examined causality based on analysis of EEG recordings during the response to painful stimuli (Weiss et al. 2008). This study identified significant causal interactions between midline and lateral “centroparietal” channels during the response to a painful cutaneous laser stimulus. These results may indicate causal interactions between the anterior cingulate, secondary somatosensory cortex, and insular cortexes.
A striking finding of this analysis is that channels with significant ERC are often involved in reciprocal incoming and outgoing functional interconnections for a given task (Table 4). The channels with reciprocal incoming and outgoing ERC might be due to jointly oscillating modules within the “pain network.” In general, oscillations in networks subserving a sensory modality may contribute to the representation of that sensation (Fetz and Shupe 2003; Sejnowski and Paulsen 2006).
As a corollary of our main hypothesis, we tested whether analysis of the EEG would show evidence of pain-related ERC interactions consistent with critical sites. The present studies of channels involved in critical sites and in reciprocal interactions commonly involve medial frontal, parietal, or central channels. The location of these electrodes may be consistent with the results of subdural recordings, which showed critical sites over the central sulcus and medial frontal cortex. In the present analysis, none of the critical sites or sites with reciprocal interactions was located over the frontal lobe. The location of such critical sites or sites with reciprocal activity may be used to determine sites for transcutaneous magnetic stimulation for the treatment of pain (Lefaucheur 2008; Liu et al. 2011b). This strategy is based on the assumption that disruption of a cortical network can interfere with pathological activity in a network by stimulation of a single module (Desmurget et al. 2009; Karnath et al. 2010; Sirigu et al. 2010) or a subcortical white matter pathway (De Lucia et al. 2007; Herbsman et al. 2009; Karnath et al. 2010).
GRANTS
This work was supported by grants to F. A. Lenz from both National Institutes of Health National Institute of Neurological Disorders and Stroke (NS-38493-11) and the Johns Hopkins Neurosurgery Pain Research Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.M. and C.-C.L. performed experiments; T.M., C.-C.L., and J.-H.C. analyzed data; T.M., C.-C.L., N.E.C., J.Z., and F.A.L. edited and revised manuscript; C.-C.L., N.E.C., and F.A.L. conception and design of research; C.-C.L., N.E.C., and F.A.L. interpreted results of experiments; C.-C.L., J.-H.C., and J.Z. prepared figures; C.-C.L. and F.A.L. drafted manuscript; C.-C.L. and F.A.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Anna Korzeniewska, who made suggestions on this manuscript.
REFERENCES
- Akaike H. New look at statistical-model identification. IEEE Trans Automat Contr AC19: 716–723, 1974 [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484, 2005 [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Shi T, Bruggemann J, Airapetian LR. Segregation of nociceptive and non-nociceptive networks in the squirrel monkey somatosensory thalamus. J Neurophysiol 84: 484–494, 2000 [DOI] [PubMed] [Google Scholar]
- Babiloni C, Brancucci A, Del Percio C, Capotosto P, Arendt-Nielsen L, Chen AC, Rossini PM. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. J Pain 7: 709–717, 2006 [DOI] [PubMed] [Google Scholar]
- Backonja M, Howland EW, Wang J, Smith J, Salinsky M, Cleeland CS. Tonic changes in alpha power during immersion of the hand in cold water. Electroencephalogr Clin Neurophysiol 79: 192–203, 1991 [DOI] [PubMed] [Google Scholar]
- Boatman-Reich D, Franaszczuk PJ, Korzeniewska A, Caffo B, Ritzl EK, Colwell S, Crone NE. Quantifying auditory event-related responses in multichannel human intracranial recordings. Front Comput Neurosci 4: 4, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 104: 12187–12192, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci USA 101: 9849–9854, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res 78: 415–418, 1989 [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Dubner R, Jones RL, Maixner W. Attentional influences on noxious and innocuous cutaneous heat detection in humans and monkeys. J Neurosci 5: 1103–1110, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci USA 96: 7705–7709, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KL. Concepts of pain mechanisms: the contribution of functional imaging of the human brain. Prog Brain Res 129: 277–287, 2000 [DOI] [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Morrow TJ, Koeppe RA. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol 76: 571–581, 1996 [DOI] [PubMed] [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci 14: 4095–4108, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215, 2002 [DOI] [PubMed] [Google Scholar]
- Crainiceanu CM, Staicu AM, Di CZ. Generalized multilevel functional regression. J Am Stat Assoc 104: 1550–1561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol 80: 1533–1546, 1998 [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004 [DOI] [PubMed] [Google Scholar]
- De Lucia M, Parker GJ, Embleton K, Newton JM, Walsh V. Diffusion tensor MRI-based estimation of the influence of brain tissue anisotropy on the effects of transcranial magnetic stimulation. Neuroimage 36: 1159–1170, 2007 [DOI] [PubMed] [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement intention after parietal cortex stimulation in humans. Science 324: 811–813, 2009 [DOI] [PubMed] [Google Scholar]
- Ding M, Bressler SL, Yang W, Liang H. Short-window spectral analysis of cortical event-related potentials by adaptive multivariate autoregressive modeling: data preprocessing, model validation, and variability assessment. Biol Cybern 83: 35–45, 2000 [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol 87: 615–620, 2002 [DOI] [PubMed] [Google Scholar]
- Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage 20: 1540–1551, 2003 [DOI] [PubMed] [Google Scholar]
- Ferracuti S, Seri S, Mattia D, Cruccu G. Quantitative EEG modifications during the cold water pressor test: hemispheric and hand differences. Int J Psychophysiol 17: 261–268, 1994 [DOI] [PubMed] [Google Scholar]
- Fetz EE, Shupe LE. Recurrent networks: neurophysiologic modeling. In: The Handbook of Brain Theory and Neural Networks, edited by Arbib MA. Cambridge, MA: MIT Press, 2003, p. 960–963 [Google Scholar]
- Gelnar PA, Krauss BR, Sheehe PR, Szeverenyi NM, Apkarian AV. A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. Neuroimage 10: 460–482, 1999 [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron 54: 677–696, 2007 [DOI] [PubMed] [Google Scholar]
- Granger CW. Investigating causal relations by econometric models and cross spectral methods. Econometrica 37: 424–438, 1969 [Google Scholar]
- Herbsman T, Forster L, Molnar C, Dougherty R, Christie D, Koola J, Ramsey D, Morgan PS, Bohning DE, George MS, Nahas Z. Motor threshold in transcranial magnetic stimulation: the impact of white matter fiber orientation and skull-to-cortex distance. Hum Brain Mapp 30: 2044–2055, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettmansperger TP, Sheather SJ. Confidence intervals based on interpolated order statistics. Stat Prob Lett 4: 75–79, 1986 [Google Scholar]
- Iannetti GD, Hughes NP, Lee MC, Mouraux A. Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol 100: 815–828, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol 10: 371–375, 1958 [PubMed] [Google Scholar]
- Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc R Soc Lond B Biol Sci 244: 39–44, 1991 [DOI] [PubMed] [Google Scholar]
- Karnath HO, Borchers S, Himmelbach M. Comment on “Movement intention after parietal cortex stimulation in humans.” Science 327: 1200, 2010 [DOI] [PubMed] [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. Pain 148: 257–267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp 27: 715–721, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewska A, Crainiceanu CM, Kus R, Franaszczuk PJ, Crone NE. Dynamics of event-related causality in brain electrical activity. Hum Brain Mapp 29: 1170–1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewska A, Manczak M, Kaminski M, Blinowska KJ, Kasicki S. Determination of information flow direction among brain structures by a modified directed transfer function (dDTF) method. J Neurosci Methods 125: 195–207, 2003 [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. TMS and pain. In: Handbook of Transcranial Stimulation, edited by Wassermann EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH. New York: Oxford Univ. Press, 2008, p. 717–736 [Google Scholar]
- Legrain V, Bruyer R, Guerit JM, Plaghki L. Involuntary orientation of attention to unattended deviant nociceptive stimuli is modulated by concomitant visual task difficulty. Evidence from laser evoked potentials. Clin Neurophysiol 116: 2165–2174, 2005 [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol 93: 111–124, 2011 [DOI] [PubMed] [Google Scholar]
- Lenz FA, Casey KL, Jones EG, Willis WD. The Human Pain System: Experimental and Clinical Perspectives. New York: Cambridge Univ. Press, 2010 [Google Scholar]
- Liu CC, Crone NE, Franaszczuk PJ, Cheng D, Schretlen DS, Lenz FA. Fear conditioning is associated with dynamic directed functional interactions between and within the human amygdala, hippocampus, and frontal lobe. Neuroscience 189: 359–369, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Ohara S, Franaszczuk PJ, Crone NE, Lenz FA. Attention to painful cutaneous laser stimuli evokes directed functional interactions between human sensory and modulatory pain-related cortical areas. Pain 152: 2781–2791, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Ohara S, Franaszczuk PJ, Lenz FA. Attention to painful cutaneous laser stimuli evokes directed functional connectivity between activity recorded directly from human pain-related cortical structures. Pain 152: 664–675, 2011c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Shi CQ, Franaszczuk PJ, Crone NE, Schretlen D, Ohara S, Lenz FA. Painful laser stimuli induce directed functional interactions within and between the human amygdala and hippocampus. Neuroscience 178: 208–217, 2011d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longe SE, Wise R, Bantick S, Lloyd D, Johansen-Berg H, McGlone F, Tracey I. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. Neuroreport 12: 2021–2025, 2001 [DOI] [PubMed] [Google Scholar]
- Lorenz J, Garcia-Larrea L. Contribution of attentional and cognitive factors to laser evoked brain potentials. Neurophysiol Clin 33: 293–301, 2003 [DOI] [PubMed] [Google Scholar]
- Melzack R. Phantom limbs and the concept of a neuromatrix. Trends Neurosci 13: 88–92, 1990 [DOI] [PubMed] [Google Scholar]
- Miron D, Duncan GH, Bushnell MC. Effects of attention on the intensity and unpleasantness of thermal pain. Pain 39: 345–352, 1989 [DOI] [PubMed] [Google Scholar]
- Mouraux A, Guerit JM, Plaghki L. Non-phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between Adelta- and C-fibre afferent volleys. Clin Neurophysiol 114: 710–722, 2003 [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol 101: 3258–3269, 2009 [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Kim JH, Lenz FA. Analysis of synchrony demonstrates that the presence of “pain networks” prior to a noxious stimulus can enable the perception of pain in response to that stimulus. Exp Brain Res 185: 353–358, 2008 [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates “pain networks” defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain 123: 244–253, 2006 [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Attention to a painful cutaneous laser stimulus modulates electrocorticographic event-related desynchronization in humans. Clin Neurophysiol 115: 1641–1652, 2004a [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Vogel H, Treede RD, Lenz FA. Attention to pain is processed at multiple cortical sites in man. Exp Brain Res 156: 513–517, 2004b [DOI] [PubMed] [Google Scholar]
- Oya H, Poon PW, Brugge JF, Reale RA, Kawasaki H, Volkov IO, Howard MA., 3rd Functional connections between auditory cortical fields in humans revealed by Granger causality analysis of intra-cranial evoked potentials to sounds: comparison of two methods. Biosystems 89: 198–207, 2007 [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain 122: 1765–1780, 1999 [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain: a review and meta-analysis (2000). Neurophysiol Clin 30: 263–288, 2000 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol 42: 817–826, 1977 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857, 1999 [DOI] [PubMed] [Google Scholar]
- Ploner M, Gross J, Timmermann L, Pollok B, Schnitzler A. Oscillatory activity reflects the excitability of the human somatosensory system. Neuroimage 32: 1231–1236, 2006 [DOI] [PubMed] [Google Scholar]
- Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci USA 107: 355–360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex 21: 719–726, 2011 [DOI] [PubMed] [Google Scholar]
- Ploner M, Schoffelen JM, Schnitzler A, Gross J. Functional integration within the human pain system as revealed by Granger causality. Hum Brain Mapp 30: 4025–4032, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 288: 1769–1772, 2000 [DOI] [PubMed] [Google Scholar]
- Raij TT, Forss N, Stancak A, Hari R. Modulation of motor-cortex oscillatory activity by painful Adelta- and C-fiber stimuli. Neuroimage 23: 569–573, 2004 [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ, Paulsen O. Network oscillations: emerging computational principles. J Neurosci 26: 1673–1676, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu A, Mottolese C, Desmurget M. Response to Comment on “Movement intention after parietal cortex stimulation in humans.” Science 327: 1200, 2010 [DOI] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science 251: 1355–1358, 1991 [DOI] [PubMed] [Google Scholar]
- Tremblay N, Bushnell MC, Duncan GH. Thalamic VPM nucleus in the behaving monkey. II. Response to air-puff stimulation during discrimination and attention tasks. J Neurophysiol 69: 753–763, 1993 [DOI] [PubMed] [Google Scholar]
- Truccolo WA, Ding M, Knuth KH, Nakamura R, Bressler SL. Trial-to-trial variability of cortical evoked responses: implications for the analysis of functional connectivity. Clin Neurophysiol 113: 206–226, 2002 [DOI] [PubMed] [Google Scholar]
- Weiss T, Hesse W, Ungureanu M, Hecht H, Leistritz L, Witte H, Miltner WH. How do brain areas communicate during the processing of noxious stimuli? An analysis of laser-evoked event-related potentials using the Granger causality index. J Neurophysiol 99: 2220–2231, 2008 [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Kakigi R, Watanabe S, Naka D. Effects of distraction on pain perception: magneto- and electro-encephalographic studies. Brain Res Cogn Brain Res 8: 73–76, 1999 [DOI] [PubMed] [Google Scholar]
- Zaslansky R, Sprecher E, Tenke CE, Hemli JA, Yarnitsky D. The P300 in pain evoked potentials. Pain 66: 39–49, 1995 [DOI] [PubMed] [Google Scholar]