Abstract
Despite saccades changing the image on the retina several times per second, we still perceive a stable visual world. A possible mechanism underlying this stability is that an internal retinotopic map is updated with each saccade, with the location of objects being compared before and after the saccade. Psychophysical experiments have shown that humans derive such location information from a corollary discharge (CD) accompanying saccades. Such a CD has been identified in the monkey brain in a circuit extending from superior colliculus to frontal cortex. There is a missing piece, however. Perceptual localization is established only in humans and the CD circuit only in monkeys. We therefore extended measurement of perceptual localization to the monkey by adapting the target displacement detection task developed in humans. During saccades to targets, the target disappeared and then reappeared, sometimes at a different location. The monkeys reported the displacement direction. Detections of displacement were similar in monkeys and humans, but enhanced detection of displacement from blanking the target at the end of the saccade was observed only in humans, not in monkeys. Saccade amplitude varied across trials, but the monkey's estimates of target location did not follow that variation, indicating that eye location depended on an internal CD rather than external visual information. We conclude that monkeys use a CD to determine their new eye location after each saccade, just as humans do.
Keywords: saccade, vision, stability, monkey
our stable visual perception, despite the shift of the retinal image with repeated saccadic eye movements, has been one of the most vexing problems in understanding the organization and function of our visual system. One view that has emerged is that the stability does not result from a higher-order map in the brain constructed in spatial coordinates but rather there is an updating of a map in retinotopic coordinates each time a saccade occurs (Duhamel et al. 1992). The source of this updating is thought to be a corollary discharge (CD) of the saccade (see review by Wurtz 2008). This CD is a copy of the motor command driving the saccadic eye movement, and this copy conveys information about the amplitude and direction of the upcoming saccade to relevant regions of the brain, including those relevant to spatial localization (Gottlieb et al. 1998; Hall and Colby 2011). In doing so, the CD updates information on the position of the eye just as the corresponding motor output changes the position of the eye.
A possible CD for providing the necessary updating of information has been identified in the old world monkey. A circuit extending from the superior colliculus (SC) through the medial dorsal nucleus of the thalamus (MD) to the frontal eye field (FEF) has been shown to have the characteristics expected of a CD (Sommer and Wurtz 2008). The neurons in MD, the relay of the circuit, discharge before saccades and could provide the vector information about the direction and amplitude of the impending saccade. Furthermore, inactivation of the relay does not alter the generation of the saccade. It does, however, produce deficits expected from a reduced CD, including a lack of the necessary target location information for the generation of sequential saccades (Sommer and Wurtz 2002, 2004a, 2004b, 2006). Interrupting the CD also leads to a reduction of the anticipatory updating of neuronal activity seen in FEF neurons (Sommer and Wurtz 2006) that precedes saccadic eye movements (Umeno and Goldberg 1997).
A critical aspect of the view that updating the visual map is a potential mechanism underlying visual stability is that for this updating the visual location of objects has to be compared before and after the saccade. Objects at or near a saccade target must be perceived to remain in the same relative location before and after the saccade if such a comparison is to be made and visual stability maintained. The key point is that the location of an object is with respect to the position of the eye. Such knowledge of eye position has recently been shown to be available to human observers. Displacement of targets during saccades can be detected, and the size and direction of the displacement with respect to eye position can be indicated by human observers (Deubel et al. 1996, 1998, 2002). In these target displacement detection tasks, subjects first look at a target spot on a screen in front of them (Fig. 1A), and when that spot jumps to another position, they make a saccade to follow it. During the saccade the target is turned off, and before the saccade ends it reappears either at the original target site or at a location shifted forward or backward from the original target site. Shifting the location of the target during the saccade avoids any visual motion cues from the displacement itself. Subjects then make a forced choice, using a manual response (turning a lever or pressing a button) to indicate in which direction they perceive the target to be displaced. In a variant of the task (Fig. 1B) the target reappears 250 ms after the initiation of the saccade, because previous work had shown that small steps in the target position often go unnoticed (saccadic suppression of displacement; Bridgeman et al. 1975), but this suppression is reduced when the target reappears at least 50 ms after the saccade ends (Deubel et al. 1996).
Fig. 1.
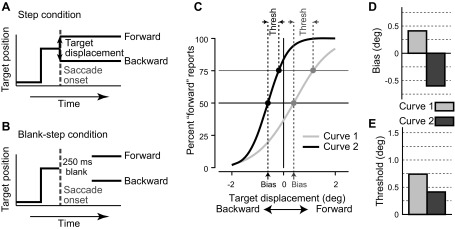
Target displacement detection task. In the task, the subject was required to make a saccade to a target, and the target was displaced during the saccade. A: in the Step condition, the target was displaced either forward or backward during the saccade and was present at saccade end. B: in the Blank-step, the target was removed for 250 ms beginning during the saccade and then reappeared after the saccade at the displaced position. In both conditions, the subject's task was to indicate the direction of the displacement. C: example psychometric curves. The frequency of forward responses (y-axis) is plotted as a function of target displacement (x-axis). The data were fitted to a cumulative Gaussian distribution (log likelihood) to determine the psychometric function. Shown are 2 sample psychometric functions with different parameters. D: perceptual bias, the step size at which the subject perceives the target displacement at chance level, was measured at the 50% points on the psychometric curves. The light gray bar is derived from the gray curve (curve 1) and the dark gray bar from the black curve (curve 2). E: perceptual threshold was taken as the difference in displacements between the 75% and 50% points on the psychometric curve. Thresholds are shown for both sample curves in C.
Over a series of trials with different target displacements, a psychometric curve is created that indicates the frequency of forward responses for each of the different target displacements (Fig. 1C). The perceptual null location (PNL) is the point where the forward and backward choices occur with equal frequency as derived from the psychometric curve. The PNL (“bias” in our plots) is thus the point at which the subject perceives that the target did not move and is taken as the best estimate of the perceived location of the presaccadic target. Judgments of target displacement necessarily depend on the CD, which provides the perceived location of the eye at the end of the saccade.
This location information in humans is a robust observation and has been studied by at least three laboratories (Collins et al. 2009; Deubel et al. 2002; Ostendorf et al. 2010). It is thought to depend on a CD accompanying each saccade, because the two other possible sources of visual location information, vision and proprioception, are not adequate under the test conditions of the experiment. The subject is usually in a dark room without other visual cues, so that vision is unlikely to contribute to localization. Proprioception is likely to be too slow to be of use (Wang et al. 2007). In net, this psychophysical technique for determining the perceived location of the target is a breakthrough in precisely measuring human perception of object location after the saccade. Understanding how we locate objects after the saccade may prove to be a major step in understanding how we derive perceptual stability from the repeatedly displaced retinal images produced by saccades.
The current limitation for understanding the neuronal mechanisms underlying stable visual perception is the development of tasks in the monkey that allow evaluation of the monkey's perception. The perceptual localization described above is measured in humans, but all the evidence for the possible CD circuit underlying it is from the monkey. Tasks that would allow direct comparison of perceptual behavior to the proposed CD neuronal mechanisms, including changes in behavior with the alteration of those mechanisms, are essential to end the disconnect between psychophysics and CD circuitry in the brain. Because identifying such a circuit in the human brain is beyond currently available techniques, the only possibility for relating perceptual stability to neuronal circuits is to study both perception and neuronal mechanisms in the monkey.
The goal of this report was to develop a psychophysical test of the monkey's visual perception at the time of the saccade. The first specific test is whether the human behavioral paradigm for assessing transsaccadic perceived location of an object can be used in monkeys. This would seem to be straightforward because the human transsaccadic test uses a forced choice response that should be equally objective whether the test is in humans or monkeys. In addition to applying the task to monkeys, it is essential to evaluate how consistent the human and monkey results are with each other in order to generalize between them. If developing this test in monkeys is successful, it opens the possibility of relating perceptual judgment to the neuronal activity argued to underlie it. Specifically, one possibility is that altering the CD in the monkey would alter changes in the monkey's perception of target location. The monkey provides an excellent animal model of the human visual system, and experiments such as these provide the opportunity to expand our ability to subject hypotheses about human visual stability to experimental test.
METHODS
The detection of transsaccadic changes in saccade target position has been used to assess the ability to maintain visual stability across saccades. We therefore first replicated the results obtained previously on such transsaccadic detection in humans by repeating these experiments (Collins et al. 2009; Deubel et al. 1998; Ostendorf et al. 2010). We then trained two monkeys on the same task with only minor variations in the procedures. Experimental protocols for the human subjects were approved by the Institutional Review Committee for human subjects, and informed consent was obtained from each participant. For the monkey subjects, all procedures were approved by the Institute Animal Care and Use Committee and complied with Public Health Service policy on the humane care and use of laboratory animals.
Basic Behavioral Tasks
In the target displacement detection task, the subject was required to make a saccade to a target, and the target was displaced during the saccade. In the Step condition (Fig. 1A), the target was displaced either forward or backward during the saccade and was present at saccade end. In the Blank-step condition (Fig. 1B), the target was also displaced during the saccade but only reappeared 250 ms later, after the saccade had ended. In both conditions, a fraction of the trials had no target displacement. The subject's task was to manually indicate the direction of the displacement by either turning a lever (monkey) or pressing one of two buttons (human). For the monkeys, a trial was rewarded if the manual response was correct and occurred within 1,500 ms of the saccade. The fixation point and the target spots were red spots produced by a laser back-projected on a tangent screen. A PC running real-time experimental control software (REX; Hays et al. 1982) controlled the position and timing of the lasers (onset and offset). Use of laser light permitted 1) precise timing of onset and offset with no possible interference from sweep refresh limitations and 2) the ability to carry out the experiments in the dark. Horizontal and vertical eye positions were recorded while the subject sat in a magnetic field (Robinson 1963) by using a scleral search coil on a contact lens in humans (Collewijn et al. 1975) with sampling at 1,000 Hz and with an implanted search coil with the same sampling rate in monkeys (Judge et al. 1980). Online saccade detection (when the eye left a 2° square virtual window around the fixation point for human subjects, a 1.5° square for the monkeys) was used for controlling the target displacement described above. For human subjects, the system was calibrated prior to data acquisition by having subjects fixate the known target locations used in the experiment.
Human Tests
We recorded the eye movements of five healthy human subjects (3 naive to the aim of the study) between the ages of 33 and 61 yr who had normal vision with correction. Subjects were seated in a dark room on a stationary chair in front of a tangent screen (183 cm by 138 cm in size) located 100 cm in front of the subject, and the head was fixed with a chin and forehead rest. Each trial began with the onset of a fixation target. The subject fixated this target for ∼1,200 ms, after which it was extinguished simultaneously with the appearance of a peripheral target 8° to the right or to the left. Manual responses indicating the perceived direction of displacement after the saccade to the target were recorded by the press of a button on either the right or the left to indicate whether the target stepped leftward or rightward during the saccade. The sign of the step (forward/backward) was randomly drawn with equal probability, and the maximum range of the target displacement was 4° (±2°). Throughout an experimental session trials were presented by a staircase method: with each correct manual response the absolute value of the target displacement decreased by 0.1°, and the absolute target displacement increased by 0.3° after an incorrect manual response. There were four concurrent staircases throughout the experimental session, one for each condition (Step and Blank-step) and saccade direction (right or left). With this method we quickly reached the subject's perceptual threshold and conducted the majority of the trials around this threshold displacement value. Subjects completed ∼500 trials for the session (∼125 trials for each condition and saccade direction). Subjects did not receive any training on the task prior to collection of the experimental data. Also in contrast to the monkey tests, there was no background light between trials to prevent dark adaptation and no feedback to indicate correct or incorrect responses. The human subjects did relatively few trials compared with the monkeys and found the background light to be disruptive, whereas the monkeys easily adapted to the light change over the thousands of trials they performed.
Monkey Tests
Two adult male monkeys (Macaca mulatta, monkeys Fln and Cap) weighing from 7 to 9 kg, were implanted with scleral search coils for measuring eye position and a post for immobilizing the head during experiments as described previously (Sommer and Wurtz 2000). Subjects sat facing a tangent screen 57 cm in front of them. Except for the laser spots themselves, the room was dark, and a background light was turned on between fixation trials to reduce dark adaptation and therefore any growing light sensitivity over the course of a series of trials.
Manual responses were made by moving a handle. Unlike the human subjects, trials were not run with a staircase procedure because there was a tendency for the monkey to cease performing when the task (reporting displacement size) became too difficult; monkeys simply skipped the difficult trials to reset the staircase. To counter this strategy, we ran the trials in blocks in which the condition (Step or Blank-step) was kept constant and the monkey had to accumulate enough correct trials (20) to switch to the next block (the other condition). In addition, the absolute target displacement was randomly drawn from a Gaussian distribution centered at 0° in steps that varied between 0.1° and 0.3°. In this way, the smaller (least detectable) displacements were sampled more than larger (more obvious) target displacements. With this method we were able to obtain many trials at target displacements close to and below perceptual threshold. As in the human subjects, the sign of the displacement (left or right) was randomly drawn with equal probability and the maximum range of the target displacement was 4° (±2°).
We tested monkeys on saccades to targets at different eccentricities (8°, 12°, and 16°), but within an experimental session the eccentricity was constant. We tested different eccentricities to have a full record of the behavior in the task that could be used to optimize subsequent experiments that concurrently examined the neuronal activity and perceptual report. The distribution of the target displacements (mean and standard deviation of the Gaussian distribution) was the same for each target eccentricity/session. Monkeys received significant training on the task before the collection of experimental data. Training was complete when the monkey could dissociate saccade direction and target displacement, as, for example, when the monkey reliably made correct “leftward” perceptual reports (>75%) for obvious leftward target displacements during rightward saccades. Monkeys completed ∼500–900 trials in a given session (∼125–225 trials for each condition and saccade direction) and 15 sessions overall (5 sessions at each target eccentricity).
Psychometric Curves
We plotted the button or bar responses indicating the human's or monkey's perception of target displacement as a psychometric function (Fig. 1C). We plotted the percentage of forward responses (y-axis in Fig. 1C) for each size and direction of target displacement (x-axis in Fig. 1C). Forward responses are displacements in the direction of the saccade, so that for a rightward saccade a displacement to the right is a forward response. But the same rightward step is a backward target displacement for a leftward saccade. The data were then fit with a cumulative Gaussian distribution (log likelihood) as depicted in Fig. 1C by the two sample curves. From these curves we derived perceptual bias and perceptual threshold. The perceptual bias (Fig. 1D) was taken as the displacement from zero of the point on the psychometric curve where the forward and nonforward responses were equal at 50%. The bias quantified the postsaccadic judgment of the location of the presaccadic target. The perceptual threshold was determined as the difference in displacement size between the 75% and 50% points on the psychometric curve (see Fig. 1C). The perceptual threshold quantified the ability to perceive the target displacement (Fig. 1E). Larger thresholds (lower slopes) indicate more difficulty in perceiving the target displacement.
Statistical significance of any differences in the perceptual threshold and bias between experimental conditions was determined by a paired t-test. Statistical significance of multiple effects such as saccade direction (left vs. right), target eccentricity (8°, 12°, and 16°), and experimental condition (Step vs. Blank-step) on perceptual threshold and bias was determined by an ANOVA. Statistical significance of differences in the distribution of the saccade end points for the two experimental conditions was determined by a Kolmogorov-Smirnov test, and differences in the horizontal variance of the saccade end points was determined by Bartlett's test. For all tests the significance level was P < 0.05.
Saccade Measures
At the start of each trial the humans were required to fixate within an electronic window, a 2° square around the fixation point; the monkeys were required to be within a 1.5° square around the fixation point. Only trials in which the saccade ended within 3° of the original saccade target were analyzed. During the tasks, saccade initiation was taken as the time at which the eye position left the electronic fixation window. For off-line analysis, saccade initiation was identified as the time that both eye velocity and acceleration exceeded 100°/s and 5,000°/s2 for both humans and monkeys.
RESULTS
Perceptual Decisions
We first verified the results for humans to make certain that our methods produced results similar to those of previous investigators (Collins et al. 2009; Deubel et al. 1998; Ostendorf et al. 2010) and then compared the results in humans to those in monkeys performing the same task.
Humans.
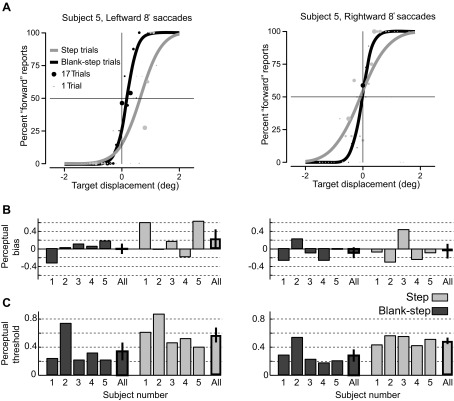
The psychometric curves for the target displacement detection task (leftward and rightward 8° saccades) for an example human subject (subject 5) are shown in Fig. 2A. The size of each data point represents the number of trials at that target displacement, direction, and condition (Step vs. Blank-step). For this subject there were asymmetries in the perceptual bias between saccade directions. The perceptual bias for both the Step condition (left: 0.63°, right: −0.09°) and the Blank-step condition (left: 0.17°, right: −0.02°) was positive for leftward saccades and slightly negative for rightward saccades. The negative biases on the right indicate PNLs in the direction opposite to the saccade. Additionally, the positive biases for leftward saccades indicate that PNLs were in the direction of the saccade. However, for both saccadic directions, the perceptual threshold for the Step condition (left: 0.40°, right: 0.51°) was higher than for the Blank-step condition (left: 0.21°, right: 0.20°), in keeping with previous observations (Deubel et al. 1998; Ostendorf et al. 2010). This means that detection of target displacement was more difficult for the subject to perceive in the Step condition than in the Blank-step condition.
Fig. 2.
Performance of human subjects on target displacement detection task. A: fitted psychometric curves for the target displacement detection task (leftward and rightward 8° saccades) for human subject 5. The size of each data point (filled circles) represents the number of trials at that target displacement, saccade direction, and experimental condition (gray, Step condition; black, Blank-step condition). B: perceptual bias for each human subject and the mean and SE of biases across subjects for both saccade directions. C: perceptual threshold for each human subject and the mean and SE across subjects for both saccade directions. Human performance was comparable to that in previous studies.
The perceptual bias and threshold for each subject and the average measure across subjects are summarized in Fig. 2, B and C, respectively. The measures for leftward and rightward saccades are displayed separately. Although there were asymmetries in the perceptual bias between the two saccade directions, neither saccade direction nor experimental condition had a significant effect on perceptual bias (2-way ANOVA, P = 0.13 for main effect of saccade direction, P = 0.27 for experimental condition). As in the example subject, the average perceptual threshold for the Step condition (left: 0.57 ± 0.09°, right: 0.49 ± 0.03°) was higher than for the Blank-step condition (left: 0.34 ± 0.11°, right: 0.28 ± 0.07°) for both saccade directions. The experimental condition did have a significant effect on perceptual threshold, but saccade direction did not (2-way ANOVA, P = 0.36 for main effect of saccade direction, P = 0.006 for experimental condition).
In summary, our results for human subjects were comparable to previous reports for human subjects (Collins et al. 2009; Deubel et al. 1998; Ostendorf et al. 2010). There was no significant difference in the perceptual bias between the Step and Blank-step conditions, or between leftward and rightward saccades. The lack of a significant difference in bias between the Step and Blank-step conditions suggests that saccadic suppression had little influence on the perceptual location of the saccade target. In contrast, the perceptual threshold in the Step condition was significantly higher than the threshold in the Blank-step task. These results replicate the finding of previous studies (Deubel et al. 1996) that target displacement is easier to detect in the Blank-step condition and that saccadic suppression does influence the perceptual awareness of visual changes during the saccade.
Monkeys.
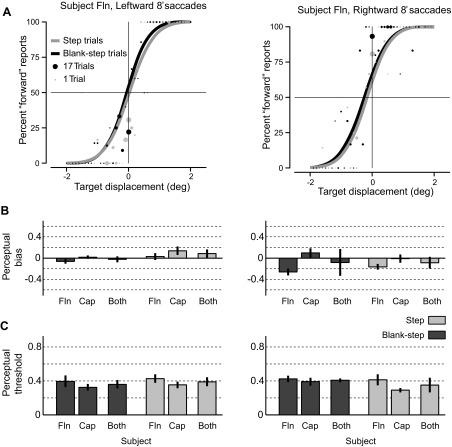
Figure 3A shows the psychometric curves for the target displacement detection task (leftward and rightward 8° saccades) for monkey Fln. Similar to the human subjects, there were asymmetries in the perceptual bias between the two saccade directions. The perceptual biases differed depending on the direction of the saccade but were similar for both tasks. Leftward biases (Step: 0.03°, Blank-step: −0.06°) were smaller in magnitude than rightward biases (Step: −0.17°, Blank-step: −0.26°). Perceptual thresholds, however, did not differ between leftward saccades (Step: 0.43°, Blank-step: 0.39°) and rightward saccades (Step: 0.41°, Blank-step: 0.42°) saccades, nor did they differ between tasks.
Fig. 3.
Performance of monkeys on target displacement detection task. A: psychometric curves for the target displacement detection task (leftward and rightward 8° saccades) for monkey Fln. As in Fig. 2, the size of each data point (filled circles) represents the number of trials at that target displacement, saccade direction, and experimental condition (gray, Step condition; black, Blank-step condition). B and C summarize the perceptual bias and threshold for both monkeys for leftward (left) and rightward (right) saccades. Vertical error bars represent SE across the 5 experimental sessions for each monkey. For the data from the 2 monkeys combined (Both) the error bar shows the sample variance. Results were similar to humans except that for monkeys perceptual threshold was not significantly different in Step and Blank-step conditions.
The perceptual bias and threshold for each monkey and the average for both monkeys for 8° saccades are summarized in Fig. 3, B and C, respectively. The average measures and standard error across sessions for leftward and rightward saccades for the Step and Blank-step conditions are summarized in Tables 1 and 2. Saccade direction did have a significant effect on perceptual bias, but the Step and Blank-step conditions did not (2-way ANOVA, P = 0.03 for main effect of saccade direction, P = 0.33 for experimental condition). Therefore, similar to human subjects, saccadic suppression of displacement had little influence on the perceptual location of the saccade target. Also, monkeys showed no effect of saccade direction on perceptual threshold (ANOVA, P = 0.89). The only difference we observed between humans and monkeys was in the effect on perceptual threshold of stimulus condition (Step vs. Blank-step). Recall that for humans perceptual threshold was greater for the Step condition. Monkeys, however, showed no difference in perceptual threshold between Step and Blank-step conditions (ANOVA, P = 0.70 for experimental condition).
Table 1.
Summary of the perceptual bias for the two monkeys
|
Monkey Fln |
Monkey Cap |
|||||||
|---|---|---|---|---|---|---|---|---|
| Leftward saccades |
Rightward saccades |
Leftward saccades |
Rightward saccades |
|||||
| Target Eccentricity | Step | Blank-step | Step | Blank-step | Step | Blank-step | Step | Blank-step |
| 8° | 0.03 ± 0.07° | −0.06 ± 0.04° | −0.17 ± 0.05° | −0.26 ± 0.06° | 0.14 ± 0.08° | 0.02 ± 0.03° | −0.01 ± 0.08° | 0.10 ± 0.09° |
| 12° | 0.03 ± 0.12° | −0.17 ± 0.04° | −0.46 ± 0.06° | −0.48 ± 0.14° | −0.13 ± 0.07° | −0.23 ± 0.8° | −0.20 ± 0.15° | −0.22 ± 0.13° |
| 16° | 0.28 ± 0.34° | −0.16 ± 0.12° | −0.81 ± 0.21° | −0.24 ± 0.21° | −0.15 ± 0.08° | −0.49 ± 0.10° | −0.30 ± 0.18° | −0.20 ± 0.12° |
Table 2.
Summary of the perceptual threshold for the two monkeys
|
Monkey Fln |
Monkey Cap |
|||||||
|---|---|---|---|---|---|---|---|---|
| Leftward saccades |
Rightward saccades |
Leftward saccades |
Rightward saccades |
|||||
| Target Eccentricity | Step | Blank-step | Step | Blank-step | Step | Blank-step | Step | Blank-step |
| 8° | 0.43 ± 0.05° | 0.39 ± 0.07° | 0.41 ± 0.06° | 0.42 ± 0.04° | 0.35 ± 0.04° | 0.32 ± 0.04° | 0.29 ± 0.03° | 0.39 ± 0.05° |
| 12° | 0.54 ± 0.06° | 0.35 ± 0.01° | 0.69 ± 0.11° | 0.57 ± 0.02° | 0.33 ± 0.03° | 0.45 ± 0.04° | 0.34 ± 0.05° | 0.42 ± 0.02° |
| 16° | 0.84 ± 0.08° | 0.72 ± 0.04° | 1.10 ± 0.23° | 0.92 ± 0.18° | 0.41 ± 0.05° | 0.51 ± 0.04° | 0.42 ± 0.07° | 0.57 ± 0.07° |
To summarize, the monkeys showed results similar to humans with the exception that there was little difference between the Blank-step and Step conditions for perceptual threshold for monkeys.
Saccadic Error and Perceptual Decisions
With repeated eye movements to the same target, saccades naturally vary from trial to trial. Because of the noisy nature of neuronal signals, variations are introduced at each stage of processing (as recognized by Ostendorf et al. 2010). To be of any use in perception, the CD should have a considerable degree of accuracy, and therefore must take into account the bulk of the saccadic noise. That is, the CD signal must be generated after most of the noise has been introduced into the system, and so should be derived near the end of the processing stream. With an accordingly accurate CD, perception should be immune to most of the saccadic noise.
Collins et al. (2009) and Ostendorf et al. (2010) observed that in humans the subject's judgment of displaced target location was independent of eye position at the end of the saccade, suggesting that their subjects were using their CD of the saccade for their perceptual judgments. Because this analysis directly addresses the role of CD in perceived eye location with respect to target location, we repeated the analysis for our observations.
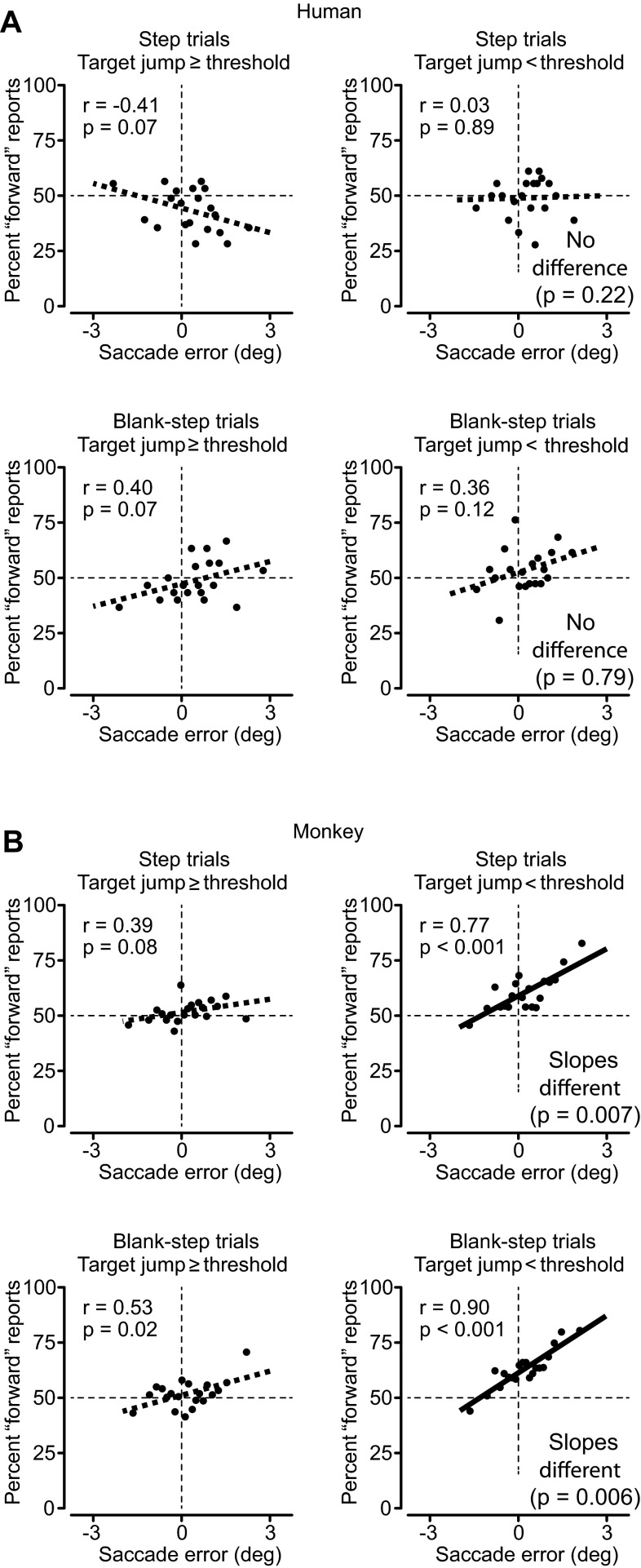
As in Collins et al. (2009) and Ostendorf et al. (2010), Fig. 4 shows the dependence of perceptual judgments on saccade variations for humans (Fig. 4A) and monkeys (Fig. 4B). In all graphs the x-axis shows saccadic error as the location of the original target relative to the end point of the saccade directed to that target. Positive errors indicate that the target was in front of the saccade end point (hypometric saccade), and negative values indicate that the target was behind the saccade (hypermetric saccade). Saccade errors were binned into 20 bins of equal size, and the perceptual judgments on the y-axis were calculated for each bin. We found that for our human subjects (Fig. 4A, left) there was little relation between saccade error at the end of the first saccade and the change in perceived target location for target steps above threshold (≥0.5°). This was true for both Step and Blank-step trials (Fig. 4A, left, top and bottom, respectively). Results for steps above threshold were similar for monkeys (Fig. 4B, left, top and bottom). For both humans and monkeys, perceptual judgments when target displacements were above threshold were not contaminated by saccade error, that is, by where the saccade landed.
Fig. 4.
Relation of saccade error and perceptual decision in humans (A) and monkeys (B). On each plot x-axis shows the saccade error (the difference between the original target location and the eye position after the saccade), with positive values indicating the target was in front of the saccade (hypometric saccade). Top: Step trials. Bottom: Blank-step trials. Saccade errors were divided into 20 sequential bins with equal numbers of trials in each. y-Axis plots the subjects' decisions—% forward responses (as on the psychometric curves). For each set of points, the correlation coefficient and its significance are noted at top left. The least-squares linear fit to the data is shown by the thick line through the data. This regression line is dashed if the slope was not significantly different from zero (P < 0.01, ANCOVA) and is solid otherwise. Left: graphs when the target stepped at least 0.5°. Right: steps < 0.5°. The 0.5° limen divided step sizes into those above and below threshold for both humans and monkeys. For both humans and monkeys, target steps ≥0.5° showed little relation of decision to saccade error. For monkeys, target steps <0.5° did show a significant linear relation between saccade error and step size. For monkeys, the slope of the linear regression was significantly different when displacements were below threshold (ANCOVA, P values shown at right), suggesting the use of retinal information on more difficult trials.
A different story emerges for displacements below perceptual threshold (Fig. 4, right). Humans (Fig. 4A) still exhibit no significant relationship between saccade error and judgment of smaller steps (<0.5°) for either task. However, monkeys (Fig. 4B) now exhibit a significant dependence of judgment on saccade error, a relationship significantly different from their judgments for displacements above threshold. Ostendorf et al. (2010) showed a similar dependence on saccade error for patient N.P., presumably caused by a stroke producing lesions observed in the CD pathway. Thus, just as the patient was presumably not using the CD for the perceptual judgment, neither was the monkey when the target step was so small as to prevent perception of the displacement.
In summary, we were able to observe the effect of saccade error on perceptual judgment in normal monkeys but only for small steps, which suggests that the saccade variations not included in the CD can contaminate perceptual judgment when they compete with the magnitude of the displacement to be detected. For a severely degraded CD, large steps also can be affected (as in Ostendorf et al. 2010). For an intact CD, the contamination of judgments by saccade variations occurs only for the smallest displacements, as in our monkeys.
Comparison of Saccade End Points Across Trial Types
The lack of a difference in perceptual threshold in the Blank-step and Step conditions for monkeys was initially puzzling (given the human results), and we investigated several factors that might be the source of this disparity. The first was related to the amplitude of the saccade to the original target position in the two variations of the task. The subject was required to compare the location of the target before and after the saccade. Thus one potential contributor to the differences in the perceptual measures between the Step and Blank-step conditions might be variations in saccade end point, particularly along the horizontal axis (the direction of the saccade). We therefore examined the saccade end points for both humans and monkeys. The variance in the direction of the saccade (along the horizontal axis) was similar for both humans and monkeys. Importantly, we found no significant difference in the horizontal end point variance for 8° saccades between the monkey and human subjects (Bartlett's test, P = 0.35). In addition, neither saccade direction (rightward vs. leftward) nor experimental condition (Step vs. Blank-step) had a significant effect on horizontal end point variance (Bartlett's test, P = 0.70 for experimental condition, P = 0.08 for saccade direction).
Across the sample of saccade records from humans and monkeys, we compared the distribution of saccade end points and the horizontal end point variance for the Step and Blank-step conditions. For each experimental session there were two comparisons: one for leftward saccades and one for rightward saccades. Comparisons were made over 15 sessions for the two monkeys (30 comparisons) and over the five human subjects (10 comparisons). There was rarely a significant difference in the saccade end point distribution between the two conditions (Kolmogorov-Smirnov test, P < 0.05, 2 of 30 for monkey Cap, 4 of 30 for monkey Fln, 1 of 10 for human subjects). These differences in the overall end point distributions were always due to upward deviations in the vertical direction in the Blank-step condition. Thus there was no indication of a systematic difference in saccade end points between the Step and Blank-step conditions, for either the humans or the monkeys.
Evolution of Perceptual and Performance Measures During Training
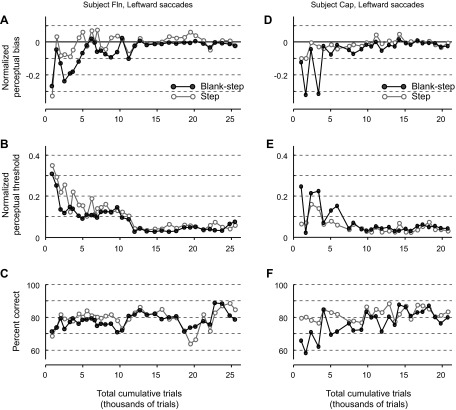
A second possible difference in Step and Blank-step conditions between humans and monkeys might be the duration of their experience with the task. We hypothesized that the monkeys' extensive performance of the task, with many thousands of trials, might lessen the difference between Step and Blank-step conditions. To investigate this further we examined the performance of the monkeys throughout the data acquisition period. We defined the start of this period as when the monkeys could dissociate saccade direction and target displacement, as, for example, when the monkey could make the correct perceptual report for a leftward target displacement for a rightward saccade. The perceptual bias, perceptual threshold, and percent correct trials for the two monkeys are shown in Fig. 5. For simplification, only the results for leftward saccades are presented since results for rightward saccades were not significantly different. We tested saccades to targets at various eccentricities throughout the training period and therefore normalized the measures of perceptual bias and threshold by the respective target eccentricity for a valid comparison.
Fig. 5.
Performance of monkeys during the course of training: perceptual bias (A and D), perceptual threshold (B and E), and % correct (C and F) as a function of total trials completed during the training period for each monkey. Each data point represents an experimental session. Target eccentricities varied between sessions (8°, 12°, and 16°). Thus perceptual bias and threshold are normalized by the target eccentricity of the respective experimental session.
As demonstrated in Fig. 5, there were obvious differences in both perceptual bias and threshold early in performance that settled over the course of the data acquisition period. For the lack of difference in the Step and Blank-step thresholds in the monkey, it would be revealing if the monkeys started out with a lower threshold for the Blank-step condition than the Step condition. In monkey Fln (Fig. 5B) the plot of the threshold indeed shows a somewhat lower threshold for the Blank-step than for the Step. For monkey Cap (Fig. 5E) the opposite is true, so no generalization on the direction of change over time can be made.
Even when these fluctuations in the perceptual measures decrease, there is still variability in the percentage of correct trials, signifying that there is still ongoing improvement in the overall task performance. Therefore the monkey is getting better at perceiving the displacement at the same time it is learning the task. In contrast, the humans are told what is expected and require very little training—perhaps a few trials. This makes it futile to choose an epoch of the monkey's behavior for comparison with the human's behavior because the human has no comparable training period. Thus, although a direct comparison of training between monkeys and humans is not possible and early differences in monkey performance are not revealing, we still feel that the amount of training monkeys receive likely contributes to the lack of a threshold difference between the Step and Blank-step conditions.
Perceptual Location Measures Across Target Eccentricities
To verify that our observations on the monkeys' localization performance was not dependent on the amplitude of the saccades, we extended the testing of the monkeys to three target eccentricities (8°, 12°, and 16°) at the end of the training period. Figure 6 displays the perceptual bias and threshold for both monkeys at the three different target eccentricities, and Tables 1 and 2 summarize the means and standard errors of the perceptual measures across the experimental sessions. For monkey Fln (Fig. 6, top), the difference between right and left saccade directions did have a significant effect on perceptual bias, but Step and Blank-step conditions and target eccentricity did not (3-way ANOVA, P < 0.001 for saccade direction, P = 0.53 for Step and Blank-step condition, and P = 0.31 for target eccentricity). However, saccade direction, Step and Blank-step condition, and target eccentricity all had a significant effect on perceptual threshold (3-way ANOVA, P = 0.03 for saccade direction, P = 0.005 for experimental condition, and P < 0.001 for target eccentricity). For monkey Cap (Fig. 6, bottom), target eccentricity did have a significant effect on perceptual bias, but Step and Blank-step condition and saccade direction did not (3-way ANOVA, P = 0.27 for experimental condition, P = 0.97 for saccade direction, and P < 0.001 for target eccentricity). For perceptual threshold, Step and Blank-step condition and target eccentricity had a significant effect, but saccade direction did not (3-way ANOVA, P = 0.0003 for experimental condition, P = 0.75 for saccade direction, and P < 0.001 for target eccentricity).
Fig. 6.
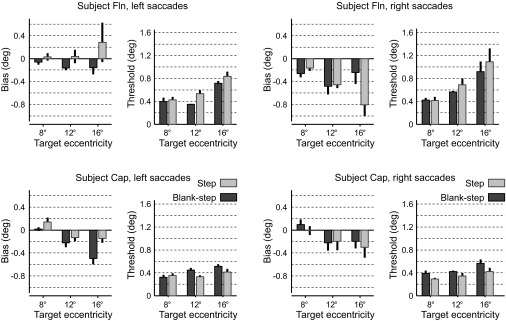
Perceptual measures for different target eccentricities for monkeys: summary of the perceptual bias and threshold for saccades to targets 8°, 12°, and 16° in eccentricity for leftward (left) and rightward (right) saccades. The results for both monkeys (monkey Fln, top; monkey Cap, bottom) are displayed (gray, Step condition; black, Blank-step condition). Vertical error bars represent SE.
To summarize, target eccentricity and experimental condition (Step/Blank-step) both had a significant effect on perceptual threshold across the 8°, 12°, and 16° saccades tested in both monkeys. Although the absolute magnitude of the perceptual bias tended to increase with movement size, this was not significant. Only saccade direction had a significant effect on perceptual bias.
DISCUSSION
Knowing how we determine the visual location of objects is a critical step in understanding how we maintain our perception of visual stability despite saccades. We have verified the effectiveness of a psychophysical paradigm used to determine perceptual location of targets in humans, and we have adapted the same task for use in monkeys. We find reproducible results in monkeys that are consistent with the target location results in humans. Monkeys and humans usually use a CD to determine target location after saccadic eye movements. The adaptation of this visual location task for monkeys now makes it possible to test perturbations of the CD circuit to frontal cortex to see whether this modifies monkeys' perception of visual location after saccades.
Comparison of Human and Monkey Psychometric Performance
Our conclusion that the target displacement detection task for monkeys provides useful information about the monkey's perception rests on the points of similarity of the human and monkey results. First, the psychometric curves in Fig. 2 for humans and Fig. 3 for monkeys are similar; as the forward and backward target steps became smaller, the correct choice frequency consistently declined. Humans and monkeys both show small differences in perceptual bias (the location of the PNL). The perceptual bias is a few tenths of a degree for humans and is about the same for the monkeys. The perceptual threshold is ∼0.2–0.8° for humans and close to 0.4° for monkeys, but the more consistent threshold for monkeys may be the result of more extensive training. Second, the nature of the monkeys' behavior while learning the task also gave confidence that the monkeys were making the same decision as humans. All that was required was the movement of a bar in the direction of the target displacement at the end of the saccade. In the course of their training, this was the major roadblock for the monkeys because they tended to perseverate in making a bar turn in the same direction as the saccade before learning to indicate the direction of the target step. Third, the horizontal spreads in the saccadic end points to the target were comparable in humans and monkeys, although the vertical end point spread for monkeys was somewhat greater. One cannot be certain that monkeys do the task in the same way as humans (or that humans all do it the same way), but the nature of the task coupled with the consistency of results over a series of experimental days provides support for the assumption that they do.
There were some minor differences in the tasks for the humans and monkeys starting with the lever turn for the monkeys and the button press for the humans, but this technical difference seemed to be negligible. In addition, the human experiments used a staircase procedure that we did not use for the monkeys. We found that monkeys using the staircase tended to stop performing consistently as the target step became smaller, and that they tended to accumulate errors until the staircase made the task easier. By eliminating the staircase and instead using displacements randomly chosen from a Gaussian distribution of step sizes with a width that focused on smaller displacements, we concentrated the trials where we needed them without providing the monkey the option of waiting out a series of difficult trials. Both methods of choosing target displacements yielded sufficient data to generate a subject's psychometric function. Finally, we believe the most critical methodological difference between our tests on humans and monkeys was that monkeys received a reward after each correct trial, and so received immediate feedback as to whether their judgments were correct or not. This may have had repercussion in the results, as discussed below.
The major difference between the performance of humans and monkeys was that the monkeys' perceptual threshold did not differ between the Step condition and the Blank-step condition. Our results for humans did show a higher perceptual threshold for the Step compared with the Blank-step condition, consistent with the previous human experiments (Deubel et al. 1996, 1998). In humans, perception of small target steps normally detectable during fixation is suppressed during the Step task. During saccades, displacements only become evident with larger steps (Bridgeman et al. 1975), and when a blank appears before the target step after the end of the saccade, smaller steps then become detectable (see discussion in Deubel et al. 2002). This saccadic suppression of displacement, like most saccadic suppressions, does not eliminate perception of the event but rather raises the threshold for the perception (Volkmann 1986).
Consequently, differences in threshold (or the lack thereof) between Step and Blank-step trials in monkeys relate directly to our perception of visual stability across saccades. Deubel et al. (2002) proposed that we assume that the visual world is stable across saccades. During Step trials, the target is present at the end of the saccade, so the smallest variations are ignored and the subject concludes that the target did not move. For larger target displacements on Step trials the detected change is assumed to have occurred in the visual world, and the displacement is reported. During Blank-step trials, a blank is inserted at the end of the saccade, so even for smaller steps there is a large difference in the target at the end of the saccade (i.e., the target is not there). This change is therefore assumed to be in the real world, and when the target reappears after 250 ms, even small displacements are reported. The effect of the visual stability assumption on perceptual judgments postulated by Deubel et al. (2002) explains the lower threshold in the Blank-step than the Step condition in humans. Monkeys, however, do not show a difference in threshold between Step and Blank-step trials.
Obviously there are several possible explanations for the difference between humans and monkeys, and we present three here. One possibility (as set forth in results) is that the lack of a difference between the Step and Blank-step conditions in the monkeys results from overtraining of the monkeys (Fig. 5). It is possible that with thousands of practice trials the monkeys learned to detect small displacements, that is, they learned to overcome saccadic suppression of displacement. For monkey Fln (Fig. 5B) there was some indication in early trials of a lower threshold for the Blank-step trials, but that was not the case for monkey Cap (Fig. 5E). It would be interesting to see whether the difference in threshold between Step and Blank-step trials in humans diminishes with substantial practice. Human subjects might simply become better at detecting changes in the Step task, showing that practice is a factor.
Another possibility involves the monkeys receiving feedback (rewards) during the task. If monkeys do assume a stable visual world, they may begin by assuming that small displacements are not in the visual world. During training they learn that the smallest displacements are in fact real displacements, and they quickly overcome the assumption that small displacements are not in the real world. Humans have not been given the opportunity to question this assumption, as they receive no feedback during the task as to whether their judgments of displacement are correct. We believe that the influence of feedback during training in monkeys is the most likely reason for the lack of a threshold difference between Step and Blank-step trials.
A final alternative is that monkeys do not assume a stable visual world between saccades, and therefore have no reason to assume that small displacements are to be ignored. In this case, monkeys might be expected to solve the problem of visual stability somewhat differently from humans. We think that of the alternatives this is the least likely, because of the many other striking similarities between the responses of humans and monkeys.
Regardless of why this difference between humans and monkeys occurs, the net point is that psychometric curves for monkeys' perceptual decisions during transsaccadic target location are similar to those of humans. The logic behind the experiment is that at the end of the saccade to the original location of the target, the location of the eye is determined by a CD. The subject then uses the difference in eye location as designated by the CD and the retinal location of the visible target after it reappears to determine the target displacement. The monkeys perform essentially the same as humans, with the exception noted above, and we conclude that the monkey uses the CD to determine eye position after saccades, as does the human.
Relation of Perceptual Decisions to Saccadic Errors in Humans and Monkeys
One of the striking points from Collins et al. (2009) and Ostendorf et al. (2010) was that humans' perceptual judgments of displaced target location were independent of eye position at the end of the saccade. We found the same for monkeys, but only for those target displacements that were clearly above the monkey's threshold for seeing the displacements (Fig. 4, left).
This suggests that in both cases the subjects were using their CD of the saccade for their perceptual judgments rather than the end point of the saccade itself. Under specific circumstances, however, dependence of perceptual judgment on the saccade end point can be observed. Ostendorf et al. (2010) saw such dependence with a presumably degraded CD for patient N.P. We saw it in normal monkeys (with supposedly normal CD) but only for small target displacements below the monkey's threshold for detection of displacement (Fig. 4B, right).
Our interpretation is that dependence of perceptual judgment on saccade error becomes evident only when the uncertainty of the judgment rivals the accuracy of the CD signal. If the CD is perfect, the subject never needs to guess, because there is no uncertainty. But when the imprecision in the CD rivals the magnitude of the displacement to be detected, the subject must guess. For naive human subjects, the guesses appear random (Fig. 4A, right). Given enough time (thousands of trials for our monkeys and months for Ostendorf's patient N.P.), guessing strategies can develop. For an undependable CD, the retinal position of the final target location, which is determined by where the saccade ends, becomes an efficient estimator of target displacement. If one's guesses rely on retinal position, the dependence emerges, as in the monkey's behavior with small displacements (Fig. 4B, right).
What CD Characteristics Do Bias and Threshold Measure?
We measured differences in two characteristics of the psychometric functions: the crossing at the 50% correct judgment point, which we refer to as the perceptual bias, and the difference between 50% and 75% detection of forward responses, which we refer to as the perceptual threshold. Because we trained the monkeys on this task in order to determine the contribution of the CD to perception, it is interesting to consider what aspects of the CD we might expect to be related to these two perceptual measures.
Experiments conducted by Sommer and Wurtz (2002, 2004a, 2004b) have delineated a pathway that transmits the CD signal from the SC through the MD of the thalamus to the FEF. They found that the pathway provided information about eye location for rapid sequential saccades. Specifically, MD inactivation by the GABA agonist muscimol affected performance on a double-step task (Hallett and Lightstone 1976) during which a subject makes two rapid sequential movements to two target locations without visual feedback. Under normal conditions, a subject compensates for errors that occur on the first saccade by making a CD-based adjustment in the second saccade. However, blocking the pathway at MD significantly reduced the accuracy of the second saccade; most second saccades were incorrectly oriented as if the initial saccade fell short of the first target, even when this was not the case.
In these previous experiments, the CD that was manipulated provided eye location information for the guidance of the second saccade. The same eye location information could logically be used as an internal marker for perception of visual object location after saccades. The transsaccadic localization test we use requires just such localization information. Inactivation of the CD circuit as it passes through MD ought to produce a weaker CD and therefore an altered perceptual report of the location of a target after the saccade, that is, a change in the perceptual bias.
The underlying basis of the perceptual threshold is much more speculative, but one possible explanation relating to shifting receptive fields (RFs) should be mentioned. In addition to influencing information about the location of the target, a reduction in the CD has previously been shown to reduce the anticipatory remapping activity in FEF (Sommer and Wurtz 2006). This remapping or shifting RF activity refers to an increased sensitivity of an FEF neuron to stimuli falling on the location that the RF of the neuron will occupy after the saccade (Sommer and Wurtz 2006; Umeno and Goldberg 1997). Such anticipatory activity before the saccade might be used as an internal signal that impending changes in RF stimulation are the result of a saccade. But exactly what the shift contributes remains unknown. One possibility, raised previously (for a discussion see Wurtz 2008), is that the future field activity before the saccade and the RF activity after the saccade (the same location in the visual field) might be what is being compared in any match of objects before and after the saccade, as proposed by Deubel et al. (2002). Our perceptual threshold measurement might be indicating how easy or hard the match of the target location is before and after the saccade. If the shifting RFs did convey information about objects in the future RF, an insufficient CD and the consequent error in the future field might result in a more difficult discrimination between the targets in the pre- and postsaccade periods. This might be reflected by a change in the perceptual threshold.
A caution on this point of associating threshold changes to shifting RFs is provided by recent observations by Crapse and Sommer (2012), who found that the visual responses of FEF neurons distinguished between a match and the lack of a match between pre- and postsaccadic stimuli. The CD to FEF provides information for such a match, as it does for the shifting RFs in FEF (Sommer and Wurtz 2006). But one surprising aspect of these experiments was that the difference in neuronal responses was observed even in FEF neurons that did not have shifting RFs. Without a shifting RF, there can be no mismatch between the RF and the shifting RF. Therefore shifting RFs could not account for the neuronal differences observed by Crapse and Sommer. A more parsimonious explanation is that the CD contributed to neuronal responses directly rather than through shifting RFs. The net effect, however, would still be that changes in the CD would affect perceptual thresholds, just as they affect the perceived location of stimuli after saccades.
GRANTS
This work was supported by the National Eye Institute Intramural Research Program at the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.M.J., J.C., and R.H.W. conception and design of research; W.M.J. and E.J.F. performed experiments; W.M.J. and J.C. analyzed data; W.M.J., J.C., E.J.F., and R.H.W. interpreted results of experiments; W.M.J. and J.C. prepared figures; W.M.J., J.C., and R.H.W. drafted manuscript; W.M.J., J.C., E.J.F., and R.H.W. edited and revised manuscript; W.M.J., J.C., E.J.F., and R.H.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Thérèse Collins for emphasizing the importance of the saccade error analysis and two reviewers whose insightful criticisms improved the paper. We are grateful to Altah Nichols and Tom Ruffner for machine shop support.
REFERENCES
- 1.Bridgeman B, Hendry D, Stark L. Failure to detect displacement of the visual world during saccadic eye movements. Vision Res 15: 719–722, 1975 [DOI] [PubMed] [Google Scholar]
- 2.Collewijn H, van der Mark F, Jansen TC. Precise recording of human eye movements. Vision Res 15: 447–450, 1975 [DOI] [PubMed] [Google Scholar]
- 3.Collins T, Rolfs M, Deubel H, Cavanagh P. Post-saccadic location judgments reveal remapping of saccade targets to non-foveal locations. J Vis 9: 29.1–29.9, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Crapse TB, Sommer MA. Frontal eye field neurons assess visual stability across saccades. J Neurosci 32: 2835–2845, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deubel H, Bridgeman B, Schneider WX. Immediate post-saccadic information mediates space constancy. Vision Res 38: 3147–3159, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Deubel H, Schneider WX, Bridgeman B. Postsaccadic target blanking prevents saccadic suppression of image displacement. Vision Res 36: 985–996, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Deubel H, Schneider WX, Bridgeman B. Transsaccadic memory of position and form. Prog Brain Res 140: 165–180, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science 255: 90–92, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature 391: 481–484, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Hall NJ, Colby CL. Remapping for visual stability. Philos Trans R Soc Lond B Biol Sci 366: 528–539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallett PE, Lightstone AD. Saccadic eye movements to flashed targets. Vision Res 16: 107–114, 1976 [DOI] [PubMed] [Google Scholar]
- 12.Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc 2: 1–10, 1982 [Google Scholar]
- 13.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- 14.Ostendorf F, Liebermann D, Ploner CJ. Human thalamus contributes to perceptual stability across eye movements. Proc Natl Acad Sci USA 107: 1229–1234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145, 1963 [DOI] [PubMed] [Google Scholar]
- 16.Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol 83: 1979–2001, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science 296: 1480–1482, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol 91: 1381–1402, 2004a [DOI] [PubMed] [Google Scholar]
- 19.Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. J Neurophysiol 91: 1403–1423, 2004b [DOI] [PubMed] [Google Scholar]
- 20.Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature 444: 374–377, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci 31: 317–338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I. Predictive visual responses. J Neurophysiol 78: 1373–1383, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Volkmann FC. Human visual suppression. Vision Res 26: 1401–1416, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat Neurosci 10: 640–646, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Wurtz RH. Neuronal mechanisms of visual stability. Vision Res 48: 2070–2089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]