Abstract
The basolateral amygdala (BLA) plays a key role in fear-related learning and memory, in the modulation of cognitive functions, and in the overall regulation of emotional behavior. Pathophysiological alterations involving hyperexcitability in this brain region underlie anxiety and other emotional disorders as well as some forms of epilepsy. GABAergic interneurons exert a tight inhibitory control over the BLA network; understanding the mechanisms that regulate their activity is necessary for understanding physiological and disordered BLA functions. The BLA receives dense cholinergic input from the basal forebrain, affecting both normal functions and dysfunctions of the amygdala, but the mechanisms involved in the cholinergic regulation of inhibitory activity in the BLA are unclear. Using whole cell recordings in rat amygdala slices, here we demonstrate that the α7-containing nicotinic acetylcholine receptors (α7-nAChRs) are present on somatic or somatodendritic regions of BLA interneurons. These receptors are active in the basal state enhancing GABAergic inhibition, and their further, exogenous activation produces a transient but dramatic increase of spontaneous inhibitory postsynaptic currents in principal BLA neurons. In the absence of AMPA/kainate receptor antagonists, activation of α7-nAChRs in the BLA network increases both GABAergic and glutamatergic spontaneous currents in BLA principal cells, but the inhibitory currents are enhanced significantly more than the excitatory currents, reducing overall excitability. The anxiolytic effects of nicotine as well as the role of the α7-nAChRs in seizure activity involving the amygdala and in mental illnesses, such as schizophrenia and Alzheimer's disease, may be better understood in light of the present findings.
Keywords: α7-nAChR, GABAA receptor, inhibition, basolateral amygdala
the amygdala is a group of nuclei in the temporal lobe that receives information from all sensory modalities, locally processes this information for its emotional significance, and plays a key role in the orchestration of the behavioral response (Pitkanen et al. 1995; Sah et al. 2003). The amygdala is particularly responsive to fear-evoking stimuli, and it appears to be the site where fear-related memories are consolidated and stored (Gale et al. 2004; LeDoux 2003, 2005; Muller et al. 1997; Pape and Pare 2010). Amygdalar dysfunction that involves hyperexcitability and hyperactivity is a key feature of anxiety disorders, including posttraumatic stress disorder (Davis et al. 1994; Etkin and Wager 2007; Shin et al. 2004). Furthermore, the amygdala is very prone to seizure generation, and, along with the hippocampus, it is the epileptic focus in certain types of epilepsy (Aroniadou-Anderjaska et al. 2008; Gloor 1992; Pitkanen et al. 1998). Therefore, knowledge of the mechanisms that regulate the excitability of the amygdala can have significant implications in understanding the pathophysiology of and treatment for anxiety and seizure disorders.
Neuronal excitability in the basolateral nucleus of the amygdala (BLA) is particularly relevant to both anxiety (Davis 1998; Davis et al. 1994; Etkin et al. 2004; Etkin and Wager 2007; LeDoux 2003) and seizure generation (Aroniadou-Anderjaska et al. 2008). GABAergic inhibition plays a primary role in the regulation of the excitability of neuronal networks (e.g., Lang and Pare 1998). Although GABAergic interneurons in the BLA make up only a small proportion (∼20%) of the total neuronal population (McDonald and Mascagni 2002; Sah et al. 2003; Spampanato et al. 2011), they tightly regulate principal cell excitability (Lang and Pare 1998; Spampanato et al. 2011; Woodruff and Sah 2007). A number of neuromodulatory systems participate in the regulation of GABAergic synaptic transmission in the BLA (Aroniadou-Anderjaska et al. 2007; Braga et al. 2003, 2004; Kishimoto et al. 2000; Ohshiro et al. 2011; Rainnie 1999). The cholinergic system is prominently present in the BLA (Ben-Ari et al. 1977; Muller et al. 2011; Power 2004), but its involvement in the regulation of GABAergic synaptic transmission is not well-understood.
The cholinergic projections to the BLA arise primarily from the nucleus basalis magnocellularis (Carlsen et al. 1985; Emson et al. 1979; Nagai et al. 1982; Woolf 1991), a collection of neurons in the substantia innominata of the basal forebrain. Afferents from these neurons synapse on both pyramidal cells and interneurons (Carlsen and Heimer 1986; Muller et al. 2011; Nitecka and Frotscher 1989), targeting nicotinic and/or muscarinic acetylcholine receptors, which are abundantly present in the BLA (Hill et al. 1993; Mash and Potter 1986; Muller et al. 2011; Segal et al. 1978; Swanson et al. 1987; van der Zee and Luiten 1999; Zhu et al. 2005).
Neuronal nicotinic acetylcholine receptors (nAChRs) are pentameric and are composed, in different subunit combinations, of α2–α10 and β2–β4 subunits (Alkondon and Albuquerque 2004; Dani 2001; Dani and Bertrand 2007; McGehee and Role 1995). The homomeric α7 and heteromeric α4β2 are the two major subtypes of nAChRs found in the mammalian brain (Albuquerque et al. 2009; Gotti et al. 2009). α7-nAChRs play an important role in the regulation of neuronal excitability in different brain regions either by presynaptically modulating neurotransmitter release (Barik and Wonnacott 2006; Dickinson et al. 2008; Livingstone et al. 2009; Quarta et al. 2009) or by their position on somatodendritic sites of interneurons and pyramidal cells, where they directly regulate neuronal activity (Alkondon and Albuquerque 2001; Alkondon et al. 1996, 1998, 2000; Arnaiz-Cot et al. 2008; Kalappa et al. 2010; Khiroug et al. 2003; Klein and Yakel 2006). In the BLA, α7-nAChRs are present on somatodendritic regions of glutamatergic neurons (Klein and Yakel 2006), and they are also involved in presynaptically facilitating glutamate release (Barazangi and Role 2001; Jiang and Role 2008). It is unclear whether α7-nAChRs are also present on somatodendritic sites of BLA interneurons. There is only one study in the literature addressing this question, where it is reported that in the BLA of neonatal [postnatal day 7 (P7)-10] rats, an increase in the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) by application of acetylcholine or nicotine was not reduced by the specific α7-nAChR antagonists α-bungarotoxin (α-BgTx) or methyllycaconitine, suggesting that α7-nAChRs do not play an important role in the regulation of GABAergic activity in the BLA (Zhu et al. 2005). The focus of the present study was to delineate the role of α7-nAChRs in the regulation of GABAergic activity in the BLA and determine the net effect of α7-nAChR activation on the excitability of the BLA network.
METHODS
Animals.
Experiments were performed using 25- to 40-day-old, male, Sprague-Dawley rats (Taconic Farms, Derwood, MD). Animals were housed in an environmentally controlled room [20–23°C, 44% humidity, 12:12-h light-dark cycle (350–400 lx), lights on at 6:00 AM] with food (2018 Teklad Global Diet, 18% protein rodent diet; Harlan Laboratories, Indianapolis, IN) and water available ad libitum. Cages were cleaned weekly and had no physical enrichment within the cage (Prager et al. 2011). All animal experiments were conducted following the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council) and were approved by the Institutional Animal Care and Use Committee.
Electrophysiological experiments.
Animals were anesthetized with isoflurane before decapitation. Coronal brain slices (400 μm thick) containing the amygdala (−2.64 to −3.36 from bregma) were cut using a vibratome (Leica VT1200 S; Leica Microsystems, Buffalo Grove, IL) in ice-cold cutting solution consisting of (in mM): 115 sucrose, 70 N-methyl-d-glucamine (NMDG), 1 KCl, 2 CaCl2, 4 MgCl2, 1.25 NaH2PO4, 30 NaHCO3, and 25 d-glucose. The slices were transferred to a holding chamber, at room temperature, in a bath solution containing (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 21 NaHCO3, 2 CaCl2, 1 MgCl2, and 11 d-glucose. Recording solution was the same as the holding bath solution. For field potential recordings, the bath/recording solution was same as above except for the concentration of MgCl2 (1.5 mM) and KCl (3 mM). All solutions were saturated with 95% O2-5% CO2 to achieve a pH near 7.4. For whole cell recordings, the slice chamber (0.7-ml capacity) had continuously flowing artificial cerebrospinal fluid (ACSF; ∼8 ml/min) at temperature 32∼33°C. The osmolarity of this external solution was adjusted to 325 mosM with d-glucose. Field potential recordings were obtained in an interface-type chamber, maintained at 32∼33°C, with a flow rate of the ACSF at 1.5 ml/min.
For whole cell recordings, neurons were visualized under infrared light using Nomarski optics of an upright microscope (Axioskop 2; Zeiss, Thornwood, NY) through a ×40 water-immersion objective, equipped with a CCD-100 camera (Dage-MTI, Michigan City, IN). The patch electrodes had resistances of 3.5–4.5 MΩ when filled with the internal solution (in mM): 60 CsCH3SO3, 60 KCH3SO3, 10 KCl, 10 EGTA, 10 HEPES, 5 Mg-ATP, 0.3 Na3GTP (pH 7.2), 290 mosM. When sIPSCs and spontaneous excitatory postsynaptic currents (sEPSCs) were recorded simultaneously, the internal chloride concentration was 1 mM, and osmolarity was adjusted with potassium gluconate. We use KCl bridge electrode holders (ALA Scientific Instruments, Farmingdale, NY), which provide stable offset potentials and make the concentration of Cl− in the pipette solution irrelevant (the Ag+/AgCl wires are in constant contact with 2 M KCl). Tight-seal (>1 GΩ) whole cell recordings were obtained from the cell body of pyramidal-shaped neurons in the BLA region and from the cell body of interneurons, which were identified on the basis of their electrophysiological properties (Park et al. 2007; Sah et al. 2003). Access resistance (15–24 MΩ) was regularly monitored during recordings, and cells were rejected if the resistance changed by >15% during the experiment. Agonists of α7-nAChRs were applied either to the bath or by pressure injection. Pressure application was performed with the help of a push-pull experimental arrangement (Pidoplichko and Dani 2005) as used previously (Figueiredo et al. 2011; Williams et al. 2011). A motorizer (Newport, Fountain Valley, CA) was coupled with the approach/withdrawal (push-pull) actuator of a micromanipulator (Burleigh PCS-5000 series; EXFO Photonic Solution, Mississauga, Ontario, Canada). Motorizer movement and duration of application pulses were controlled with a Master-8 digital stimulator (AMPI, Jerusalem, Israel). The pipette was placed ∼50 μm away from the soma of the recorded neuron, and pressure was applied for 70–100 ms via a Picospritzer (General Valve Division, Parker Hannifin, Fairfield, NJ) set between 14 and 30 psi. Ionic currents and action potentials were amplified and filtered (1 kHz) using the Axopatch 200B amplifier (Axon Instruments, Foster City, CA) with a four-pole, low-pass Bessel filter, digitally sampled (up to 2 kHz) using the pClamp 10.2 software (Molecular Devices, Sunnyvale, CA), and further analyzed using the Mini Analysis Program (Synaptosoft, Fort Lee, NJ) and Origin (OriginLab, Northampton, MA). In some experiments, the charge transferred by postsynaptic currents was calculated using the Mini60 software by Synaptosoft.
Field potentials were evoked by stimulation of the external capsule at 0.05 Hz. Recording glass pipettes were filled with ACSF and had a resistance of ∼5 MΩ. Stimulation was applied with a bipolar concentric stimulating electrode made of tungsten (World Precision Instruments, Sarasota, FL). Signals were digitized using the pClamp 10.2 software, analyzed using Clampfit 10.2, and final presentation was prepared using Origin or GraphPad Prism (GraphPad Software, La Jolla, CA).
Drugs used were as follows: bicuculline methiodide, a GABAA receptor antagonist; atropine sulphate, a muscarinic AChR antagonist; dihydro-β-erythroidine (DHβE), an α4β2-nicotinic receptor antagonist; choline chloride and tricholine citrate, α7-agonists; α-BgTx, an α7-antagonist; and CNQX, an AMPA/kainate receptor antagonist (all purchased from Sigma-Aldrich, St. Louis, MO). We also used d-AP5, an N-methyl-d-aspartate receptor antagonist, SCH 50911, a GABAB receptor antagonist, α-conotoxin Au1B, an α3β4-nicotinic receptor antagonist, and LY 341495, a metabotropic glutamate group II/III receptor antagonist (all purchased from Tocris Bioscience, Ellisville, MO).
Statistical analysis.
Electrophysiological studies were analyzed using paired Student's t-tests. Results were considered statistically significant when P < 0.05. Data are presented as means ± standard error of the mean. Sample size “n” refers to the number of neurons for the whole cell experiments and the number of slices for the field potential recordings.
RESULTS
Functional α7-nAChRs are present on BLA interneurons.
Whole cell recordings were obtained from presumed interneurons in the BLA, which were identified on the basis of their small size compared with pyramidal/principal cells, their firing pattern in response to depolarizing current pulses in the current-clamp mode, and the absence of a current activated by hyperpolarizing voltage steps, in the voltage-clamp mode. Depolarizing current injections generated a high-frequency series of fast, nonaccommodating action potentials (Fig. 1B, right), which is typical of a significant subpopulation of BLA interneurons (Rainnie et al. 2006; Sah et al. 2003; Szinyei et al. 2000). Voltage-clamp recordings demonstrated linear change in leakage current and the absence of Ih (Fig. 1B, right), which is a cationic current activated by hyperpolarizing voltage steps that is commonly present in principal neurons of the BLA (Aroniadou-Anderjaska et al. 2012; Park et al. 2007; Womble and Moises 1993).
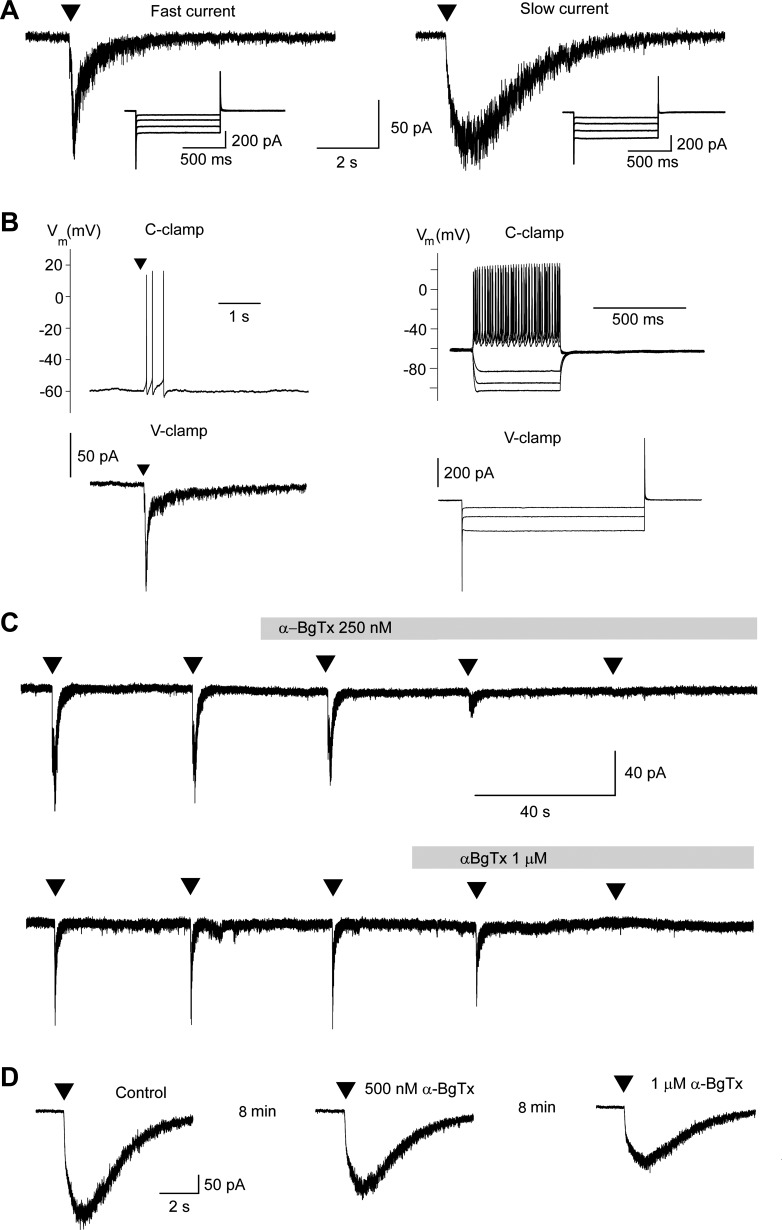
Fig. 1.
α7-Containing nicotinic acetylcholine receptors (α7-nAChRs) are present on basolateral amygdala (BLA) interneurons. Recordings were obtained from electrophysiologically identified interneurons in the BLA, in the presence of α-conotoxin Au1B (1 μM), dihydro-β-erythroidine (DHβE; 10 μM), atropine sulfate (0.5 μM), N-methyl-d-aspartate receptor antagonist d-AP5 (50 μM), AMPA/kainate receptor antagonist CNQX (20 μM), GABAB receptor antagonist SCH 50911 (10 μM), LY 3414953 (3 μM), and bicuculline (20 μM). A: examples of fast and slow currents evoked by pressure application of 10 mM choline chloride [arrowhead; 100 ms; 30 psi; holding potential (Vh) = −70 mV; internal Cl− concentration = 10 mM]. The insets show the absence of the current activated by hyperpolarization (Ih). B: in the current-clamp (C-clamp) mode, pressure application of 5 mM tricholine citrate (arrowhead) induced brief spiking [top left; the membrane potential (Vm) was held at −60 mV by passing low-amplitude depolarizing current]. The bottom left shows the inward current evoked in the same cell by tricholine citrate (5 mM; arrowhead) in the voltage-clamp (V-clamp) mode [Vh, −70 mV]. The right shows the fast, nonaccommodating spiking of the same neuron in response to depolarizing current injections and the absence of a “sag” in response to hyperpolarizing current injections (top) as well as the linear changes in leakage current (absence of Ih) during 1-s long, 10-mV hyperpolarizing steps, starting from the holding potential of −70 mV (bottom). C: the fast current activated by sequential (40-s interval) pressure application of 10 mM choline chloride (arrowheads; same settings as in A) was blocked by bath application of either 250 nM α-bungarotoxin (α-BgTx; top trace) or 1 μM α-BgTx (bottom trace). Top and bottom traces are from 2 different neurons. Gray bars over the recordings mark the duration of bath application of α-BgTx. D: example of the low sensitivity of choline-evoked, slow currents to α-BgTx. Arrowheads show the time point of pressure application of 10 mM choline chloride. α-BgTx was bath-applied initially at the concentration of 500 nM followed by 1 μM (duration of application, 8 min at each concentration; flow rate, 8 ml/min).
To determine whether α7-nAChRs are present on BLA interneurons, we pressure-applied tricholine citrate (5 mM, n = 7) or choline chloride (10 mM, n = 13) while recording from interneurons in the presence of α-conotoxin Au1B (1 μM), DHβE (10 μM), atropine sulfate (0.5 μM), d-AP5 (50 μM), CNQX (20 μM), SCH 50911 (10 μM), LY 3414953 (3 μM), and bicuculline (20 μM). The concentrations of the α7-nAChR agonists that we used have been shown in the hippocampus (Alkondon et al. 2007) or neocortical layer I interneurons (Christophe et al. 2002) to activate α7-nAChRs selectively; furthermore, the presence of the α4β2- and the α3β4-nicotinic receptor antagonists in the slice medium can increase confidence that tricholine citrate and choline chloride selectively activated α7-nAChRs. Out of 20 cells, 12 responded to the “puff application” of the α7-nAChR agonist with a fast-inactivating inward current of an amplitude 83.1 ± 8.4 pA (Fig. 1A, left trace). The remaining 8 cells responded with a slow current of an amplitude 236 ± 52.1 pA (Fig. 1A, right trace). The fast or slow kinetics of the evoked currents had no relation to whether tricholine citrate or choline chloride was applied. In the current-clamp mode, puff application of the α7-nAChR agonist elicited a brief train of action potentials (Fig. 1B, left, top trace). The fast-inactivating currents were blocked equally effectively by either 250 nM (n = 4; Fig. 1C, top trace) or 1 μM (n = 5; Fig. 1C, bottom trace) α-BgTx within 2–4 min of bath application of the toxin; the mean reduction was 94.5 ± 2.0% (n = 9). The slow currents were only partially reduced by 250 nM, 500 nM, or 1 μM α-BgTx. The blockade (36.5 ± 4.8%, n = 6) was slow (required at least 8 min of perfusion) and appeared to be more effective at the high concentration of the toxin (1 μM; Fig. 1D).
Glutamatergic excitation of interneurons following α7-nAChR activation.
The experiments described above indicate that α7-nAChRs are present on somatic or somatodendritic regions of BLA interneurons, and their activation depolarizes these cells. Since α7-nAChRs are also present on principal BLA neurons (Klein and Yakel 2006) as well as on glutamatergic terminals (Barazangi and Role 2001; Jiang and Role 2008), we investigated whether α7-nAChR activation in the BLA network, in addition to depolarizing interneurons directly, also depolarizes interneurons by increasing glutamatergic activity. Therefore, we conducted similar experiments as those shown in Fig. 1 except that CNQX was not included in the slice medium. Under these conditions, pressure application of 5 mM tricholine citrate induced long-lasting, high-frequency firing in the current-clamp mode and a transient inward current accompanied by sEPSCs in the voltage-clamp mode (n = 7; Fig. 2A). Similarly, when choline chloride (2.5 mM) was applied to the bath, it also induced repetitive, high-frequency firing of BLA interneurons (n = 4; Fig. 2B). Thus α7-nAChR activation in the BLA increases the activity of interneurons not only via direct depolarization, but also indirectly due to an enhancement of glutamatergic activity.
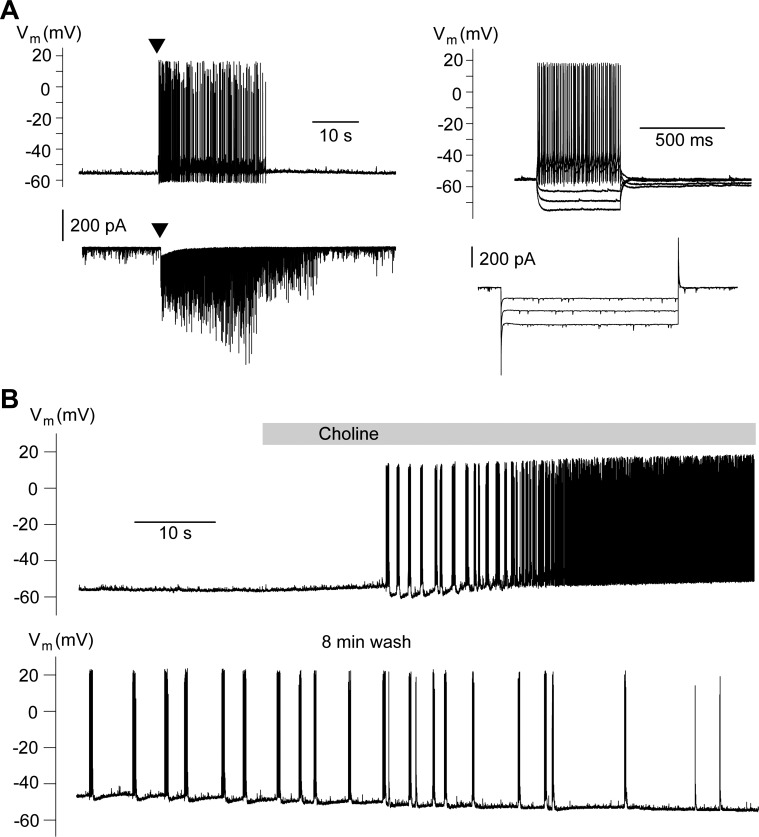
Fig. 2.
Indirect excitation of interneurons by activation of α7-nAChRs. Recordings are from electrophysiologically identified interneurons in the presence of α-conotoxin Au1B (1 μM), DHβE (10 μM), atropine sulfate (0.5 μM), d-AP5 (50 μM), SCH 50911 (10 μM), LY 3414953 (3 μM), and bicuculline (20 μM). A: pressure application of tricholine citrate (5 mM; arrowhead) induced high-frequency firing in the current-clamp mode (top left) and a transient inward current along with high-frequency spontaneous excitatory postsynaptic currents (sEPSCs) in the voltage-clamp mode (bottom left; Vh, −70 mV). The electrophysiological characteristics of this neuron are shown on the right. Responses to depolarizing or hyperpolarizing current injections (top right) and hyperpolarizing voltage steps (bottom right) are consistent with the properties of interneurons. B: in current-clamp mode, bath application of choline chloride (2.5 mM) induced high-frequency spiking.
Activation of α7-nAChRs enhances sIPSCs.
The neurons we recorded from in the experiments described above were presumed to be GABAergic interneurons. Therefore, the data presented would predict that activation of α7-nAChRs will enhance GABAergic inhibition in the BLA. To determine whether α7-nAChR activation increases GABAA receptor-mediated sIPSCs, we bath-applied choline chloride while recording from principal neurons (n = 12) in the presence of α-conotoxin Au1B (1 μM), DHβE (10 μM), atropine sulfate (0.5 μM), d-AP5 (50 μM), CNQX (20 μM), SCH 50911 (10 μM), and LY 3414953 (3 μM). Neurons were identified as principal cells based on their size and pyramidal-like shape as well as on their firing patterns in response to depolarizing current pulses and the presence of Ih (Aroniadou-Anderjaska et al. 2012; Park et al. 2007; Sah et al. 2003). Choline chloride (5 mM) was bath-applied at a flow rate of 8 ml/min. The effect was immediate and consisted of the appearance of a barrage of sIPSCs (Fig. 3). The frequency of sIPSCs was increased by choline from 22 ± 1 to 50 ± 3 Hz (n = 12; P < 0.001); the measurements of the sIPSC frequency in the presence of choline were made for a 20-s time window within a time period of 30 s after the initiation of the effect. The effect subsided within 40 s to 2 min despite the continual presence of choline, suggesting desensitization of the receptors. Reapplication of choline after only partial washout of the initially applied choline had virtually no effect, probably due to the lasting desensitization of the receptors while the exogenous choline was still present. However, after washing out choline for at least 8 min, reapplication of choline could evoke a similar effect, suggesting that desensitization was not lasting after removal of the 5 mM exogenous agonist. Higher concentrations of choline (we tried 10 and 20 mM) produced a similar increase in sIPSC amplitude and frequency, but longer washout times were required to be able to reproduce the effect on reapplication of choline.
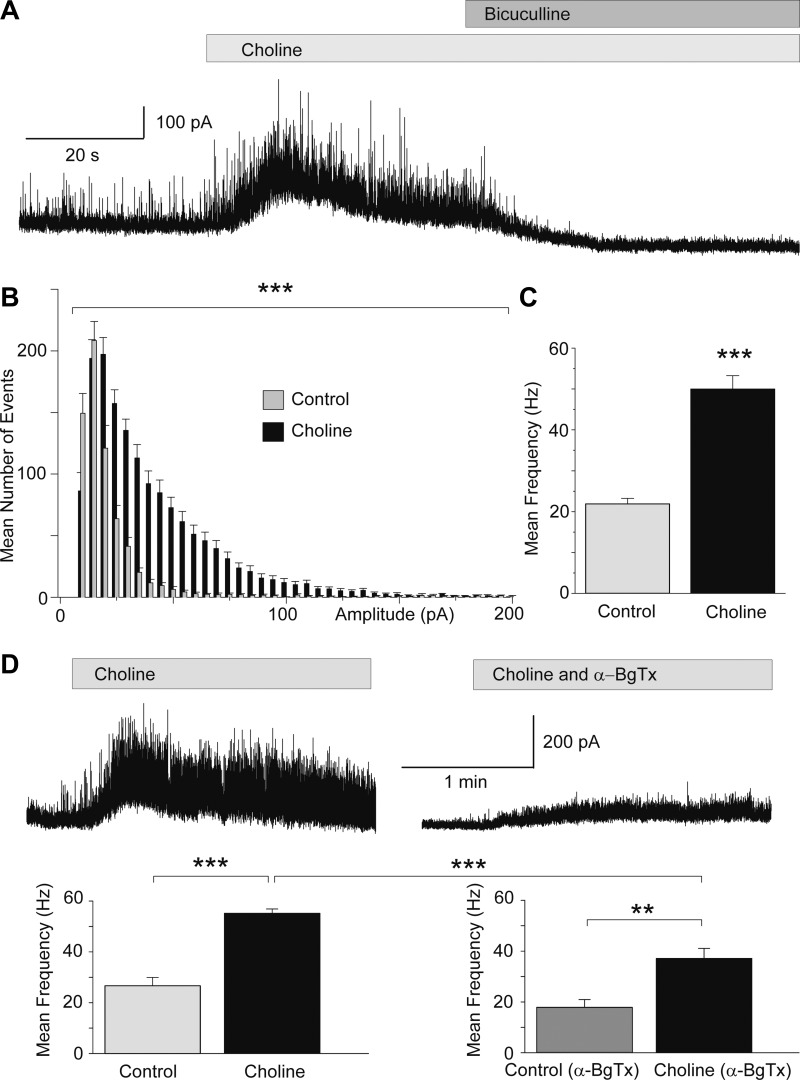
Fig. 3.
Activation of α7-nAChRs enhances spontaneous GABAA receptor-mediated inhibitory postsynaptic currents (sIPSCs). Recordings are from BLA principal cells in the presence of α-conotoxin Au1B (1 μM), DHβE (10 μM), atropine sulfate (0.5 μM), d-AP5 (50 μM), CNQX (20 μM), SCH 50911 (10 μM), and LY 3414953 (3 μM) at Vh +30 mV and internal chloride 10 mM. A: bath application of choline chloride (5 mM) induced a transient outward cationic current and increased the frequency and amplitude of sIPSCs. The sIPSCs were blocked by 20 μM bicuculline, indicating that they were mediated by GABAA receptors. B: amplitude-frequency histogram of the effect of choline on sIPSCs (n = 12; ***P < 0.001). C: group data (mean ± SE) of the change in the frequency of sIPSCs by bath application of choline chloride (n = 12; ***P < 0.001). D: pretreatment of the slices with α-BgTx reduced significantly the effects of choline. Traces show an example of the response of a principal neuron to bath application of choline chloride (5 mM) and the response of the same neuron when choline chloride was applied in the presence of α-BgTx (1 μM). The group data are shown in the bar graphs below (***P < 0.001, **P < 0.01; n = 9).
In some experiments, we washed out choline for 8 min and then applied α-BgTx (1 μM) for another 4 min. Subsequent application of 5 mM choline chloride (in the presence of α-BgTx) increased the frequency of sIPSCs from 18 ± 3 to 37 ± 4 Hz (n = 9; P < 0.01; Fig. 3D). Thus, even in the presence of α-BgTx, choline increased sIPSCs significantly, probably because of the activation of interneurons that respond to choline by generating a slow current with low sensitivity to α-BgTx (Fig. 1D). However, the increase in the frequency of sIPSCs in the presence of α-BgTx was significantly smaller than the increase in the absence of the α7-nAChR antagonist (P < 0.001; Fig. 3D), probably reflecting the blockade of the α-BgTx-sensitive α7-nAChRs on interneurons that generate fast-inactivating currents in response to choline (Fig. 1C).
The net effect of α7-nAChR activation in the BLA.
Since activation of α7-nAChRs in the BLA can increase both glutamatergic and GABAergic activity, an important question that arises is whether the overall, net effect of α7-nAChR activation is enhancement or suppression of excitatory activity in the BLA. To answer this question, we recorded both sIPSCs and sEPSCs simultaneously from principal neurons in the presence of α-conotoxin Au1B (1 μM), DHβE (10 μM), atropine sulfate (0.5 μM), d-AP5 (50 μM), SCH 50911 (10 μM), and LY 3414953 (3 μM) at a holding potential of −58 mV and internal chloride concentration of 1 mM. Bath application of choline chloride (5 mM) increased both sIPSCs and sEPSCs (Fig. 4). Out of nine cells, in three cells there was only a small increase in sEPSCs, whereas sIPSCs were dramatically increased (as in the example shown in Fig. 4A), in five cells both sEPSCs and sIPSCs were increased, but the increase in sIPSCs was clearly more prominent (as in the example shown in Fig. 4B), whereas one cell showed dramatic increase in both sIPSCs and sEPSCs. To quantify these effects, we calculated the total charge transferred. The charge, in picocoulombs, was calculated as the area delimited by the inhibitory or excitatory current and the baseline. Current areas were analyzed for a time period of 5 s. On average, the charge transferred by sIPSCs was increased from 1.3 ± 0.3 pC in control medium to 97 ± 24 pC in the presence of choline (n = 9; P < 0.01; Fig. 4C, left), whereas the charge transferred by sEPSCs was increased from 1.5 ± 0.3 to 20 ± 4 pC (n = 9; P < 0.01; Fig. 4C, right) during the initial phase of bath application of choline chloride (during a 5-s period after the initiation of the effect). The charge transferred by sIPSCs was significantly larger than the charge transferred by sEPSCs (n = 9; P < 0.01), suggesting that the net effect of α7-nAChR activation is inhibitory. As in the case where only sIPSCs were recorded (Fig. 3), the effects of choline subsided in less than 2 min despite the continual presence of the agonist. Lower concentrations of bath-applied choline chloride (1 mM, n = 2, and 0.5 mM, n = 3) had qualitatively similar effects (Fig. 4D).
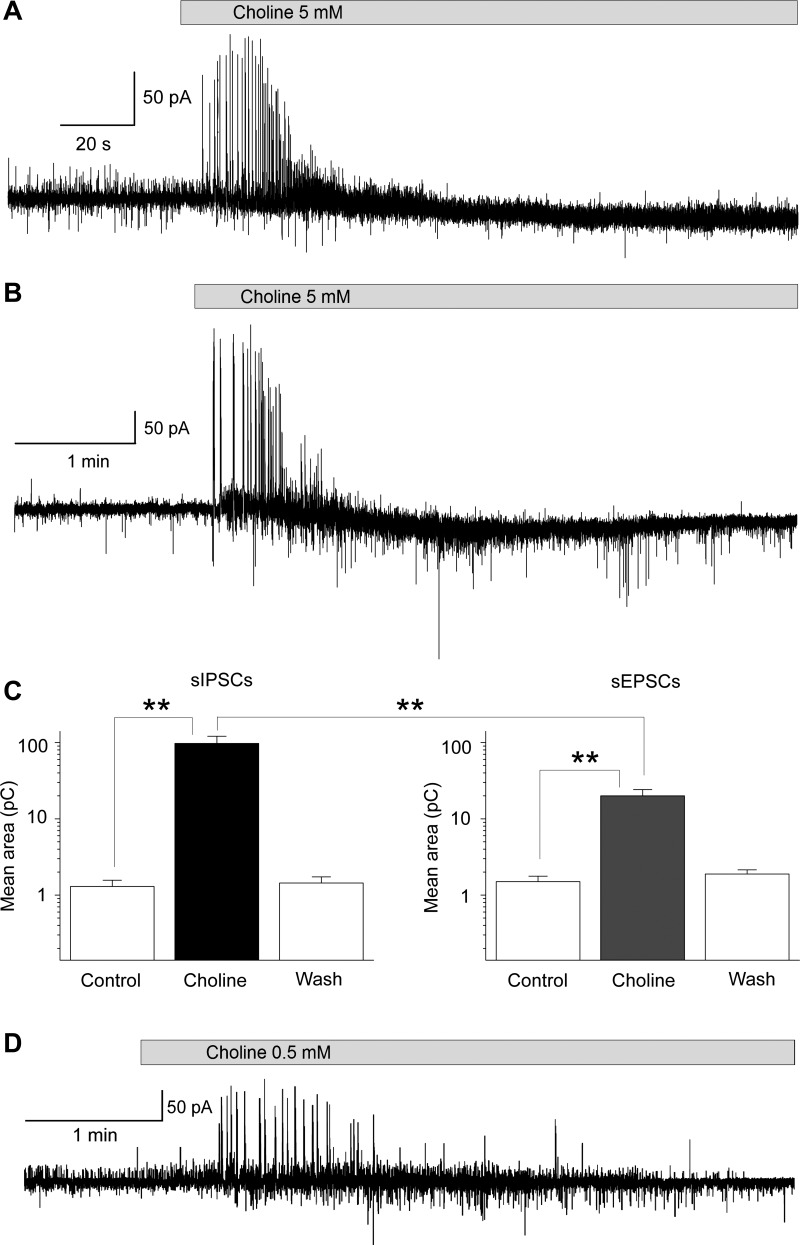
Fig. 4.
Effects of α7-nAChR activation on simultaneously recorded sIPSCs and sEPSCs. Recordings were obtained from principal neurons in the presence of α-conotoxin Au1B (1 μM), DHβE (10 μM), atropine sulfate (0.5 μM), d-AP5 (50 μM), SCH 50911 (10 μM), and LY 3414953 (3 μM) at a holding potential of −58 mV and internal chloride concentration of 1 mM. A: bath application of choline chloride (5 mM) induced a transient increase in the frequency and amplitude of both sIPSCs (outward currents) and sEPSCs (inward currents), but the increase in sIPSCs was more pronounced. B: another example of the effects of choline chloride (5 mM) on sIPSCs and sEPSCs. The increase in sEPSCs is more apparent in this cell compared with the cell in A. C: group data (mean ± SE) showing the effects of 5 mM choline chloride on sIPSCs (left) and sEPSCs (right). Application of choline significantly increased the mean charge transferred by sIPSCs and sEPSCs (n = 9; **P < 0.01) measured during the 5-s period after the initiation of the effect. During this 5-s period, the charge transferred by sIPSCs was significantly larger than the charge transferred by sEPSCs (n = 9; **P < 0.01). D: lower concentrations of choline produced a similar effect; an example is shown where 500 μM choline chloride was bath-applied.
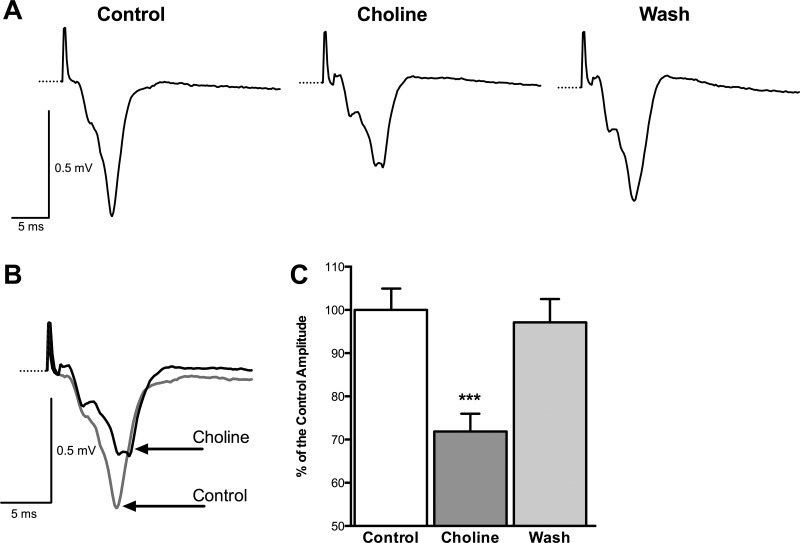
Since α7-nAChR activation enhances spontaneous GABAergic activity to a greater extent than glutamatergic activity, this effect should be reflected in the population responses. An enhanced “tonic” inhibition (sIPSCs) in the BLA network can be expected to reduce overall excitability. Field potentials in the BLA are reduced in amplitude by the GABAA agonist muscimol and are enhanced by bicuculline (E.M. Prager and V. Aroniadou-Anderjaska, unpublished observations), which implies significant presence of synchronized spiking activity (somatic and/or dendritic) in the generation of the field responses. Since choline enhances GABAergic inhibition, the amplitude of evoked population responses should be reduced in the presence of choline. To test this prediction, we bath-applied choline chloride while recording field potentials in the BLA, evoked by stimulation of the external capsule. In these experiments, slices were placed in an interface chamber, which has a much slower flow rate than in the whole cell recording experiments; the gradual exposure of the slice to low and slowly rising concentrations of choline chloride could desensitize the receptors, rendering the effect undetectable. Therefore, we used a high concentration of choline chloride (20 mM) to detect the effect. Choline chloride reversibly decreased the amplitude of the field response from 0.49 ± 0.02 to 0.34 ± 0.02 mV (n = 8; P < 0.001; Fig. 5). In percentages, choline reduced the evoked field potential to 71.87 ± 4.11% of the control amplitude (P < 0.001; Fig. 5C). These results are consistent with the view that activation of α7-nAChRs reduces the excitability of the BLA network.
Fig. 5.
Activation of α7-nAChRs reduces evoked field potentials in the BLA. A: an example of field potentials evoked in the BLA by stimulation of the external capsule before, during, and after washout of choline chloride (20 mM). Each trace is an average of 10 sweeps. B: same field potentials as in A, in control medium and in the presence of choline, superimposed for a clearer view of the effect of choline. C: group data from 8 slices showing the amplitude of the field responses as percentages of the control responses before, during, and after bath application of choline (n = 8; ***P < 0.001).
α7-nAChRs are active in the basal state contributing to background inhibition.
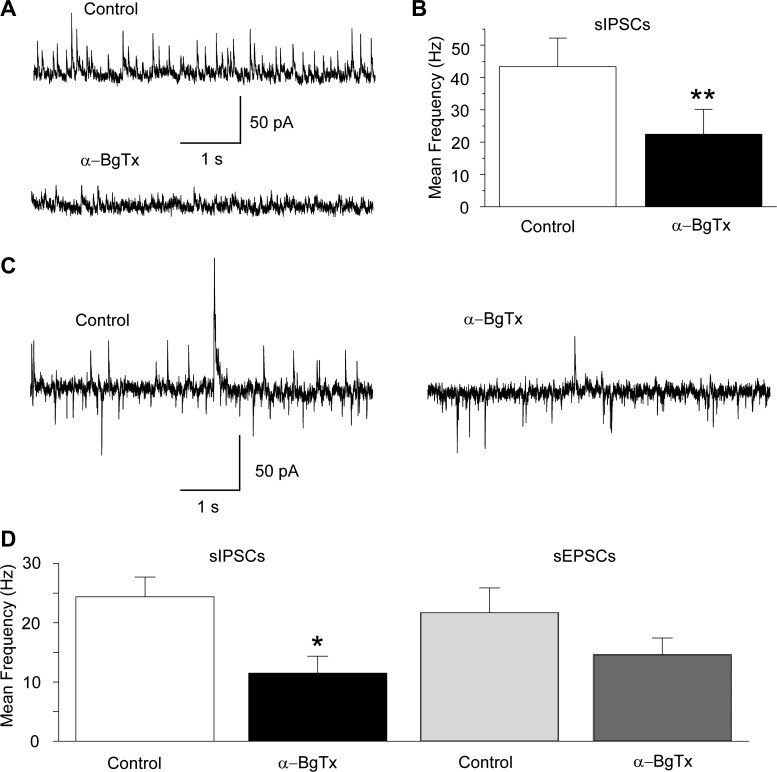
Considering that α7-nAChRs have low affinity for their endogenous agonists, acetylcholine and choline, and desensitize rapidly (Albuquerque et al. 2009), it is important to ask whether these receptors can be activated by ambient concentrations of acetylcholine or choline, thereby contributing to the background levels of inhibitory and/or excitatory activity. To answer this question, first we examined the effects of bath-applied α-BgTx (1 μM) on sIPSCs recorded from principal BLA neurons. The frequency of sIPSCs was reduced by bath application of α-BgTx from 43 ± 9 Hz in control medium to 22 ± 8 Hz in α-BgTx (n = 4; P < 0.01; Fig. 6, A and B). Next, we tested the effects of α-BgTx on simultaneously recorded sIPSCs and sEPSCs from principal neurons. α-BgTx (1 μM) significantly decreased the frequency of sIPSCs from 24 ± 3 to 12 ± 3 Hz (n = 4; P = 0.0316), whereas the frequency of sEPSCs was reduced from 22 ± 4 Hz in control medium to 15 ± 3 Hz in the presence of α-BgTx (n = 4; P = 0.141). These results suggest that basal activation of α7-nAChRs on interneurons (the postsynaptic α7-nAChRs demonstrated in the present study and/or presynaptic α7-nAChRs on GABAergic terminals) contributes significantly to spontaneous inhibitory activity.
Fig. 6.
Blockade of α7-nAChRs in the basal state decreases the frequency of sIPSCs. A: an example from a principal cell displaying high inhibitory synaptic activity in control medium and the effect of α-BgTx (1 μM) on sIPSCs (Vh = +30 mV); the reduction of the sIPSCs by α-BgTx was irreversible. The slice medium contains α-conotoxin Au1B (1 μM), DHβE (10 μM), atropine sulfate (0.5 μM), CNQX (20 μM), d-AP5 (50 μM), SCH 50911 (10 μM), and LY 3414953 (3 μM). B: group data showing the effect of α-BgTx on the frequency of sIPSCs (n = 4; **P < 0.01). C: an example from another principal cell where sIPSCs and sEPSCs were recorded simultaneously (Vh = −58 mV, internal chloride concentration is 1 mM). The reduction of the sIPSCs by α-BgTx (1 μM) was more pronounced than the reduction of sEPSCs. The slice medium contains α-conotoxin Au1B (1 μM), DHβE (10 μM), atropine sulfate (0.5 μM), d-AP5 (50 μM), SCH 50911 (10 μM), and LY 3414953 (3 μM). D: group data showing the effect of α-BgTx on the frequency of sIPSCs (n = 4; *P < 0.05) and sEPSCs (n = 4; P > 0.05).
DISCUSSION
This study demonstrated the presence of functional α7-nAChRs on somatic or somatodendritic regions of electrophysiologically identified interneurons in the BLA. Activation of α7-nAChRs by choline directly depolarized interneurons and elicited action potentials. In addition, α7-nAChR activation increased the frequency of sEPSCs recorded from interneurons, probably due to α7-nAChR-mediated depolarization of principal, glutamatergic neurons and/or presynaptic facilitation of glutamate release. The increase in interneuronal activity by α7-nAChR activation produced a dramatic increase in the frequency and amplitude of GABAA receptor-mediated sIPSCs recorded from BLA principal cells. Simultaneous recordings of sIPSCs and sEPSCs from principal neurons revealed that activation of α7-nAChRs by choline increases both types of currents, but the increase of the inhibitory currents is larger than the increase of the excitatory currents. This observation, along with the reduction of the population field response in the presence of choline, suggests that the net effect of α7-nAChR activation in the BLA network is suppression of excitability. This function of α7-nAChRs appears to be in effect even in the basal state, as blockade of these receptors decreased the frequency of sIPSCs.
The α7-subunit of the nicotinic receptors is expressed in the BLA (Zhu et al. 2005), and functional α7-nAChRs are present on somatodendritic regions of principal BLA neurons (Klein and Yakel 2006). However, it was unknown whether α7-nAChRs are also present on somatodendritic regions of BLA interneurons. This is the first study investigating the responses of BLA interneurons to the activation of α7-nAChRs by a specific agonist. We found that 60% of the recorded interneurons responded with a fast-inactivating current, which was blocked by α-BgTx; these characteristics are typical of the currents mediated by homomeric α7-nAChRs (Albuquerque et al. 2009; Khiroug et al. 2002). However, the remaining interneurons displayed a slow current, which was only partly sensitive to α-BgTx. Since this current was activated by choline in the presence of antagonists of all cholinergic receptors except for the α7-nAChRs, it was probably mediated by α7-nAChRs. The slow kinetics and the partial resistance to α-BgTx may suggest that the α7-nAChRs mediating this current are not homomeric. A different composition and stoichiometry of these α7-containing receptors may be responsible for the different kinetics and pharmacology of the currents they mediate. For example, the α7β2-subunit combination has different pharmacological properties and produces currents with slower kinetics compared with the homomeric α7-nAChRs (Khiroug et al. 2002). The presence of heteromeric α7-containing nicotinic receptors (α7β2-subunit combination) has been demonstrated in rat basal forebrain cholinergic neurons (Liu et al. 2009); it is possible, therefore, that such heteromeric α7-nAChRs exist also in other brain regions, including the BLA.
In regard to the role that α7-nAChRs play in the regulation of GABAergic activity in the rat BLA, there is only one previous study showing that α7-nAChR antagonists did not reduce the acetylcholine-induced increase in the frequency of sIPSCs recorded from BLA principal cells; therefore, it was suggested that α7-nAChRs may not participate in the regulation of inhibitory activity in the BLA (Zhu et al. 2005). One factor that may have contributed to the disparity between our results and those of Zhu et al. (2005) could be the age of the animals used, as the latter study was performed in neonatal rats (P7–10), and, at least in hippocampal interneurons, α7-nAChR-mediated currents increase with age (Alkondon et al. 2007). Another possibility, which the authors (Zhu et al. 2005) have considered, is that the increase in sIPSCs by acetylcholine [the agonist used in Zhu et al. (2005)] was not reduced by subsequent application of specific α7-nAChR antagonists because the α7-nAChRs were already desensitized; the increase in sIPSCs was sustained only by non-α7-nicotinic receptor subtypes that are slow to desensitize. This explanation for the divergent conclusions regarding the importance of α7-nAChRs in the regulation of GABAergic activity in the BLA is consistent with the present results where the effect of the specific α7-nAChR agonist, choline, on the sIPSCs did not last for more than 1–2 min, which probably reflects the fast desensitization of the α7-nAChRs.
What are the functional implications of the involvement of the α7-nAChRs in the regulation of GABAergic activity in the BLA? Like the α7-nAChRs on hippocampal interneurons (Alkondon et al. 1999), the α7-nAChRs in the BLA appear to be active in the basal state, preferentially increasing inhibitory activity. Therefore, even without significant activation of the cholinergic inputs to the amygdala above the basal level, these receptors may participate in the regulation of normal amygdala functions, primarily by limiting excitation. It should be considered, however, that since α7-nAChRs are present on both inhibitory and excitatory BLA neurons, their in vivo activation in the functioning BLA may promote either excitatory or inhibitory activity depending on a number of factors such as: 1) the concentration of acetylcholine at the vicinity of α7-nAChRs and the temporal pattern of its increase, which may differentially affect the desensitization of α7-nAChRs on principal cells vs. interneurons; 2) the role of presynaptic α7-nAChRs on glutamatergic terminals (Barazangi and Role 2001; Jiang and Role 2008); and 3) possible effects of other concomitantly acting neurotransmitters and neuromodulators that could potentially alter the neurons' response to α7-nAChR stimulation. Our results show only that inhibition is favored over excitation when α7-nAChRs are uniformly activated throughout the BLA network.
Relatively uniform activation of α7-nAChRs in the BLA can be expected during cigarette smoking, and our results suggest that these receptors may, in part, mediate the anxiolytic effects of nicotine. On an acute basis, nicotine is known to have anxiolytic (Bencan and Levin 2008; Cheeta et al. 2001; Cohen et al. 2009; Kassel and Unrod 2000; Szyndler et al. 2001) and antidepressant effects (Hawkins 1997; Vazquez-Palacios et al. 2004). The amygdala, and the BLA in particular, plays a central role in anxiety and depression (Davis 1998; Davis et al. 1994; Drevets 1999; Etkin et al. 2004; Hamilton et al. 2008; LeDoux 2003; Mitra et al. 2009). Our results suggest that the α7-nAChR-mediated increase in GABAergic activity in the BLA may be one of the mechanisms by which nicotine suppresses BLA excitability, thereby reducing anxiety and alleviating depression. This view receives support by the finding that systemic administration of an α7-nAChR agonist, in mice, has anxiolytic effects (Feuerbach et al. 2009). Because of the pronounced but only transient increase of inhibitory activity on activation of α7-nAChRs, followed by desensitization of the receptor, the anxiolytic effect of nicotine may be stronger when α7-nAChRs are stimulated in an intermittent fashion, as it probably occurs during cigarette smoking. Because of their low affinity for nicotine (Fenster et al. 1997), the α7-nAChRs may be transiently activated only during the peak levels of nicotine, whereas they recover from desensitization during the troughs.
Considering that the amygdala is a seizure-prone structure with an important role in certain forms of epilepsy (Aroniadou-Anderjaska et al. 2008), the question arises as to whether α7-nAChRs, by regulating both GABAergic and glutamatergic activity in the BLA, play a significant role in seizure generation and/or suppression. An involvement of the α7-nAChR in epilepsy is suggested by the association of juvenile myoclonic epilepsy with a mutation in the gene coding for the α7-subunit (Elmslie et al. 1997). In addition, systemic administration of an α7-nAChR agonist in mice showed anticonvulsant potential in the audiogenic seizure paradigm (Feuerbach et al. 2009), which is known to involve the amygdala (Feng and Faingold 2002; Hirsch et al. 1997). This finding along with the present data showing a preferential increase of inhibitory activity in the BLA by α7-nAChR activation suggest that in the amygdala these receptors may contribute to suppression of seizures.
The α7-nAChRs are known to be important in learning and memory (Boess et al. 2007; Hellier et al. 2012; Levin 2012; Robinson et al. 2011; Van Kampen et al. 2004), including memory involving the amygdala (Addy et al. 2003). In the BLA of Alzheimer's patients, there is accumulation of β-amyloid protein in GABAergic and glutamatergic neurons (Espana et al. 2010), which interacts and forms a protein complex with the α7-nAChRs (Dziewczapolski et al. 2009; Parri et al. 2011); the resulting inactivation of α7-nAChRs is considered to be important in the pathogenesis of Alzheimer's disease (Parri et al. 2011). Alzheimer's patients display impaired fear conditioning (Hamann et al. 2002; Hoefer et al. 2008), a form of fear-related memory, as well as increased fear and anxiety (Espana et al. 2010; Ferretti et al. 2001); these symptoms may be related to the inactivation of α7-nAChRs by β-amyloid protein, producing hyperexcitability in the BLA.
The involvement of α7-nAChRs in the regulation of GABAergic inhibition in the BLA may also have implications in the pathophysiology of schizophrenia. The expression and function of α7-nAChRs is reduced in schizophrenia (Adler et al. 1998; Guan et al. 1999; Martin et al. 2004; Olincy and Stevens 2007). The dysfunction of the amygdala in schizophrenic patients (Aleman and Kahn 2005; Benes 2010; Lawrie et al. 2003) and, in particular, the abnormally high activation of the amygdala during processing of stimuli that should not evoke fear (Hall et al. 2008) may imply dysregulation of inhibitory activity in the BLA, which could be, in part, due to abnormalities in α7-nAChR function.
In conclusion, we demonstrated that α7-nAChRs are expressed on somatic and/or dendritic regions of GABAergic interneurons in the BLA and that activation of these receptors increases GABAA receptor-mediated sIPSCs, even in the basal state. In addition, we showed that although activation of α7-nAChRs increases both sIPSCs and sEPSCs, the net effect is a preferential enhancement of inhibition. The presence of functional α7-nAChRs on GABAergic interneurons in the BLA may suggest a site for therapeutic treatments of diseases associated with amygdalar hyperactivity.
GRANTS
This work was supported by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurological Disorders and Stroke (Grant 5U01-NS-058162-07), and the Defense Threat Reduction Agency-Joint Science and Technology Office, Medical S&T Division (Grants CBM.NEURO.01.10.US.18 and CBM.NEURO.01.10.US.15).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
V.A.-A. and M.F.M.B. acquired funding for the research; V.I.P., E.M.P., V.A.-A., and M.F.M.B. conception and design of research; V.I.P. and E.M.P. performed experiments; V.I.P. and E.M.P. analyzed data; V.I.P., E.M.P., V.A.-A., and M.F.M.B. interpreted results of experiments; V.I.P. and E.M.P. prepared figures; E.M.P. drafted manuscript; V.A.-A. and M.F.M.B. edited and revised manuscript; V.I.P., E.M.P., V.A.-A., and M.F.M.B. approved final version of manuscript.
REFERENCES
- Addy NA, Nakijama A, Levin ED. Nicotinic mechanisms of memory: effects of acute local DHbetaE and MLA infusions in the basolateral amygdala. Brain Res Cogn Brain Res 16: 51–57, 2003 [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 24: 189–202, 1998 [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89: 73–120, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol 77: 283–298, 2005 [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor α7 and α4β2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol 86: 3043–3055, 2001 [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res 145: 109–120, 2004 [DOI] [PubMed] [Google Scholar]
- Alkondon M, Braga MF, Pereira EF, Maelicke A, Albuquerque EX. α7 Nicotinic acetylcholine receptors and modulation of gabaergic synaptic transmission in the hippocampus. Eur J Pharmacol 393: 59–67, 2000 [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. Age-dependent changes in the functional expression of two nicotinic receptor subtypes in CA1 stratum radiatum interneurons in the rat hippocampus. Biochem Pharmacol 74: 1134–1144, 2007 [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. α-Bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res 810: 257–263, 1998 [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. Mapping the location of functional nicotinic and gamma-aminobutyric acidA receptors on hippocampal neurons. J Pharmacol Exp Ther 279: 1491–1506, 1996 [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci 19: 2693–2705, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaiz-Cot JJ, Gonzalez JC, Sobrado M, Baldelli P, Carbone E, Gandia L, Garcia AG, Hernandez-Guijo JM. Allosteric modulation of alpha 7 nicotinic receptors selectively depolarizes hippocampal interneurons, enhancing spontaneous GABAergic transmission. Eur J Neurosci 27: 1097–1110, 2008 [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res 78: 102–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Pidoplichko VI, Figueiredo TH, Almeida-Suhett CP, Prager EM, Braga MF. Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors. Neuroscience 221: 157–169, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Qashu F, Braga MF. Mechanisms regulating GABAergic inhibitory transmission in the basolateral amygdala: implications for epilepsy and anxiety disorders. Amino Acids 32: 305–315, 2007 [DOI] [PubMed] [Google Scholar]
- Barazangi N, Role LW. Nicotine-induced enhancement of glutamatergic and GABAergic synaptic transmission in the mouse amygdala. J Neurophysiol 86: 463–474, 2001 [DOI] [PubMed] [Google Scholar]
- Barik J, Wonnacott S. Indirect modulation by alpha7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol 69: 618–628, 2006 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Zigmond RE, Shute CC, Lewis PR. Regional distribution of choline acetyltransferase and acetylcholinesterase within the amygdaloid complex and stria terminalis system. Brain Res 120: 435–444, 1977 [DOI] [PubMed] [Google Scholar]
- Bencan Z, Levin ED. The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiol Behav 95: 408–412, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology 35: 239–257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boess FG, De Vry J, Erb C, Flessner T, Hendrix M, Luithle J, Methfessel C, Riedl B, Schnizler K, van der Staay FJ, van Kampen M, Wiese WB, Koenig G. The novel α7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxamide improves working and recognition memory in rodents. J Pharmacol Exp Ther 321: 716–725, 2007 [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology 29: 45–58, 2004 [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci 23: 442–452, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. A correlated light and electron microscopic immunocytochemical study of cholinergic terminals and neurons in the rat amygdaloid body with special emphasis on the basolateral amygdaloid nucleus. J Comp Neurol 244: 121–136, 1986 [DOI] [PubMed] [Google Scholar]
- Carlsen J, Zaborszky L, Heimer L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. J Comp Neurol 234: 155–167, 1985 [DOI] [PubMed] [Google Scholar]
- Cheeta S, Irvine EE, Tucci S, Sandhu J, File SE. In adolescence, female rats are more sensitive to the anxiolytic effect of nicotine than are male rats. Neuropsychopharmacology 25: 601–607, 2001 [DOI] [PubMed] [Google Scholar]
- Christophe E, Roebuck A, Staiger JF, Lavery DJ, Charpak S, Audinat E. Two types of nicotinic receptors mediate an excitation of neocortical layer I interneurons. J Neurophysiol 88: 1318–1327, 2002 [DOI] [PubMed] [Google Scholar]
- Cohen A, Young RW, Velazquez MA, Groysman M, Noorbehesht K, Ben-Shahar OM, Ettenberg A. Anxiolytic effects of nicotine in a rodent test of approach-avoidance conflict. Psychopharmacology (Berl) 204: 541–549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry 49: 166–174, 2001 [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47: 699–729, 2007 [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry 44: 1239–1247, 1998 [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci 17: 208–214, 1994 [DOI] [PubMed] [Google Scholar]
- Dickinson JA, Kew JN, Wonnacott S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol 74: 348–359, 2008 [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann NY Acad Sci 877: 614–637, 1999 [DOI] [PubMed] [Google Scholar]
- Dziewczapolski G, Glogowski CM, Masliah E, Heinemann SF. Deletion of the alpha 7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer's disease. J Neurosci 29: 8805–8815, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmslie FV, Rees M, Williamson MP, Kerr M, Kjeldsen MJ, Pang KA, Sundqvist A, Friis ML, Chadwick D, Richens A, Covanis A, Santos M, Arzimanoglou A, Panayiotopoulos CP, Curtis D, Whitehouse WP, Gardiner RM. Genetic mapping of a major susceptibility locus for juvenile myoclonic epilepsy on chromosome 15q. Hum Mol Genet 6: 1329–1334, 1997 [DOI] [PubMed] [Google Scholar]
- Emson PC, Paxinos G, Le Gal La Salle G, Ben-Ari Y, Silver A. Choline acetyltransferase and acetylcholinesterase containing projections from the basal forebrain to the amygdaloid complex of the rat. Brain Res 165: 271–282, 1979 [DOI] [PubMed] [Google Scholar]
- Espana J, Gimenez-Llort L, Valero J, Minano A, Rabano A, Rodriguez-Alvarez J, LaFerla FM, Saura CA. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer's disease transgenic mice. Biol Psychiatry 67: 513–521, 2010 [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron 44: 1043–1055, 2004 [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164: 1476–1488, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HJ, Faingold CL. Repeated generalized audiogenic seizures induce plastic changes on acoustically evoked neuronal firing in the amygdala. Brain Res 932: 61–69, 2002 [DOI] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci 17: 5747–5759, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti L, McCurry SM, Logsdon R, Gibbons L, Teri L. Anxiety and Alzheimer's disease. J Geriatr Psychiatry Neurol 14: 52–58, 2001 [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Lingenhoehl K, Olpe HR, Vassout A, Gentsch C, Chaperon F, Nozulak J, Enz A, Bilbe G, McAllister K, Hoyer D. The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology 56: 254–263, 2009 [DOI] [PubMed] [Google Scholar]
- Figueiredo TH, Aroniadou-Anderjaska V, Qashu F, Apland JP, Pidoplichko V, Stevens D, Ferrara TM, Braga MF. Neuroprotective efficacy of caramiphen against soman and mechanisms of its action. Br J Pharmacol 164: 1495–1505, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci 24: 3810–3815, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P. Role of the amygdala in temporal lobe epilepsy. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction, edited by Aggleton JP. New York: Wiley-Liss, 1992, p. 505–538 [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol 78: 703–711, 2009 [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport 10: 1779–1782, 1999 [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, McKirdy JW, Romaniuk L, McGonigle D, McIntosh AM, Baig BJ, Gountouna VE, Job DE, Donaldson DI, Sprengelmeyer R, Young AW, Johnstone EC, Lawrie SM. Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry 64: 70–73, 2008 [DOI] [PubMed] [Google Scholar]
- Hamann S, Monarch ES, Goldstein FC. Impaired fear conditioning in Alzheimer's disease. Neuropsychologia 40: 1187–1195, 2002 [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry 13: 993–1000, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JW. Antidepressant effects of nicotine. J Clin Psychiatry 58: 324–325, 1997 [DOI] [PubMed] [Google Scholar]
- Hellier JL, Arevalo NL, Smith L, Xiong KN, Restrepo D. α7-Nicotinic acetylcholine receptor: role in early odor learning preference in mice. PLoS One 7: e35251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Jr, Zoli M, Bourgeois JP, Changeux JP. Immunocytochemical localization of a neuronal nicotinic receptor: the beta 2-subunit. J Neurosci 13: 1551–1568, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Danober L, Simler S, Pereira de Vasconcelos A, Maton B, Nehlig A, Marescaux C, Vergnes M. The amygdala is critical for seizure propagation from brainstem to forebrain. Neuroscience 77: 975–984, 1997 [DOI] [PubMed] [Google Scholar]
- Hoefer M, Allison SC, Schauer GF, Neuhaus JM, Hall J, Dang JN, Weiner MW, Miller BL, Rosen HJ. Fear conditioning in frontotemporal lobar degeneration and Alzheimer's disease. Brain 131: 1646–1657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Role LW. Facilitation of cortico-amygdala synapses by nicotine: activity-dependent modulation of glutamatergic transmission. J Neurophysiol 99: 1988–1999, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalappa BI, Gusev AG, Uteshev VV. Activation of functional alpha7-containing nAChRs in hippocampal CA1 pyramidal neurons by physiological levels of choline in the presence of PNU-120596. PLoS One 5: e13964, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Unrod M. Smoking, anxiety, and attention: support for the role of nicotine in attentionally mediated anxiolysis. J Abnorm Psychol 109: 161–166, 2000 [DOI] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Klein RC, Fayuk D, Yakel JL. Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci 23: 9024–9031, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, Yakel JL. Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol 540: 425–434, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Koyama S, Akaike N. Presynaptic modulation of synaptic gamma-aminobutyric acid transmission by tandospirone in rat basolateral amygdala. Eur J Pharmacol 407: 257–265, 2000 [DOI] [PubMed] [Google Scholar]
- Klein RC, Yakel JL. Functional somato-dendritic alpha7-containing nicotinic acetylcholine receptors in the rat basolateral amygdala complex. J Physiol 576: 865–872, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Pare D. Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience 83: 877–889, 1998 [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Job DE, Johnstone EC. Structural and functional abnormalities of the amygdala in schizophrenia. Ann NY Acad Sci 985: 445–460, 2003 [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727–738, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol 46: 209–235, 1995 [DOI] [PubMed] [Google Scholar]
- Levin ED. α7-Nicotinic receptors and cognition. Curr Drug Targets 13: 602–606, 2012 [DOI] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, Hu G, Chang Y, Lukas RJ, Wu J. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci 29: 918–929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone PD, Srinivasan J, Kew JN, Dawson LA, Gotti C, Moretti M, Shoaib M, Wonnacott S. α7 and non-α7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur J Neurosci 29: 539–550, 2009 [DOI] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 174: 54–64, 2004 [DOI] [PubMed] [Google Scholar]
- Mash DC, Potter LT. Autoradiographic localization of M1 and M2 muscarine receptors in the rat brain. Neuroscience 19: 551–564, 1986 [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res 943: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Psychol 57: 521–546, 1995 [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390: 607–611, 1997 [DOI] [PubMed] [Google Scholar]
- Mitra R, Ferguson D, Sapolsky RM. SK2 potassium channel overexpression in basolateral amygdala reduces anxiety, stress-induced corticosterone secretion and dendritic arborization. Mol Psychiatry 14: 847–855, 827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci 111: 683–691, 1997 [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Cholinergic innervation of pyramidal cells and parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Comp Neurol 519: 790–805, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Kimura H, Maeda T, McGeer PL, Peng F, McGeer EG. Cholinergic projections from the basal forebrain of rat to the amygdala. J Neurosci 2: 513–520, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitecka L, Frotscher M. Organization and synaptic interconnections of GABAergic and cholinergic elements in the rat amygdaloid nuclei: single- and double-immunolabeling studies. J Comp Neurol 279: 470–488, 1989 [DOI] [PubMed] [Google Scholar]
- Ohshiro H, Kubota S, Murakoshi T. Dopaminergic modulation of oscillatory network inhibition in the rat basolateral amygdala depends on initial activity state. Neuropharmacology 61: 857–866, 2011 [DOI] [PubMed] [Google Scholar]
- Olincy A, Stevens KE. Treating schizophrenia symptoms with an alpha7 nicotinic agonist, from mice to men. Biochem Pharmacol 74: 1192–1201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Lee S, Kang SJ, Choi S, Shin KS. Hyperpolarization-activated currents control the excitability of principal neurons in the basolateral amygdala. Biochem Biophys Res Commun 361: 718–724, 2007 [DOI] [PubMed] [Google Scholar]
- Parri HR, Hernandez CM, Dineley KT. Research update: alpha7 nicotinic acetylcholine receptor mechanisms in Alzheimer's disease. Biochem Pharmacol 82: 931–942, 2011 [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Dani JA. Applying small quantities of multiple compounds to defined locations of in vitro brain slices. J Neurosci Methods 142: 55–66, 2005 [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, Amaral DG. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol 356: 288–310, 1995 [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Tuunanen J, Kalviainen R, Partanen K, Salmenpera T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res 32: 233–253, 1998 [DOI] [PubMed] [Google Scholar]
- Power AE. Muscarinic cholinergic contribution to memory consolidation: with attention to involvement of the basolateral amygdala. Curr Med Chem 11: 987–996, 2004 [DOI] [PubMed] [Google Scholar]
- Prager EM, Bergstrom HC, Grunberg NE, Johnson LR. The importance of reporting housing and husbandry in rat research. Front Behav Neurosci 5: 38, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Naylor CG, Barik J, Fernandes C, Wonnacott S, Stolerman IP. Drug discrimination and neurochemical studies in alpha7 null mutant mice: tests for the role of nicotinic alpha7 receptors in dopamine release. Psychopharmacology (Berl) 203: 399–410, 2009 [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol 82: 69–85, 1999 [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ. Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. J Comp Neurol 498: 142–161, 2006 [DOI] [PubMed] [Google Scholar]
- Robinson L, Platt B, Riedel G. Involvement of the cholinergic system in conditioning and perceptual memory. Behav Brain Res 221: 443–465, 2011 [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390: 604–607, 1997 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev 83: 803–834, 2003 [DOI] [PubMed] [Google Scholar]
- Segal M, Dudai Y, Amsterdam A. Distribution of an alpha-bungarotoxin-binding cholinergic nicotinic receptor in rat brain. Brain Res 148: 105–119, 1978 [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 61: 168–176, 2004 [DOI] [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology 60: 765–773, 2011 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM, Whiting PJ, Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci 7: 3334–3342, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinyei C, Heinbockel T, Montagne J, Pape HC. Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. J Neurosci 20: 8909–8915, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyndler J, Sienkiewicz-Jarosz H, Maciejak P, Siemiatkowski M, Rokicki D, Czlonkowska AI, Plaznik A. The anxiolytic-like effect of nicotine undergoes rapid tolerance in a model of contextual fear conditioning in rats. Pharmacol Biochem Behav 69: 511–518, 2001 [DOI] [PubMed] [Google Scholar]
- Tang J, Wotjak CT, Wagner S, Williams G, Schachner M, Dityatev A. Potentiated amygdaloid auditory-evoked potentials and freezing behavior after fear conditioning in mice. Brain Res 919: 232–241, 2001 [DOI] [PubMed] [Google Scholar]
- van der Zee EA, Luiten PG. Muscarinic acetylcholine receptors in the hippocampus, neocortex and amygdala: a review of immunocytochemical localization in relation to learning and memory. Prog Neurobiol 58: 409–471, 1999 [DOI] [PubMed] [Google Scholar]
- Van Kampen M, Selbach K, Schneider R, Schiegel E, Boess F, Schreiber R. AR-R 17779 improves social recognition in rats by activation of nicotinic alpha7 receptors. Psychopharmacology (Berl) 172: 375–383, 2004 [DOI] [PubMed] [Google Scholar]
- Vazquez-Palacios G, Bonilla-Jaime H, Velazquez-Moctezuma J. Antidepressant-like effects of the acute and chronic administration of nicotine in the rat forced swimming test and its interaction with fluoxetine [correction of flouxetine]. Pharmacol Biochem Behav 78: 165–169, 2004 [DOI] [PubMed] [Google Scholar]
- Williams LR, Aroniadou-Anderjaska V, Qashu F, Finne H, Pidoplichko V, Bannon DI, Braga MF. RDX binds to the GABA(A) receptor-convulsant site and blocks GABAA receptor-mediated currents in the amygdala: a mechanism for RDX-induced seizures. Environ Health Perspect 119: 357–363, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble MD, Moises HC. Hyperpolarization-activated currents in neurons of the rat basolateral amygdala. J Neurophysiol 70: 2056–2065, 1993 [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. J Neurophysiol 98: 2956–2961, 2007 [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37: 475–524, 1991 [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Stewart RR, McIntosh JM, Weight FF. Activation of nicotinic acetylcholine receptors increases the frequency of spontaneous GABAergic IPSCs in rat basolateral amygdala neurons. J Neurophysiol 94: 3081–3091, 2005 [DOI] [PubMed] [Google Scholar]