Abstract
Hyperpolarization-activated cyclic nucleotide-gated nonselective cation channels (HCN or h-channels) are important regulators of neuronal physiology contributing to passive membrane properties, such as resting membrane potential and input resistance (RN), and to intrinsic oscillatory activity and synaptic integration. The correct membrane targeting of h-channels is regulated in part by the auxiliary h-channel protein TRIP8b. The genetic deletion of TRIP8b results in a loss of functional h-channels, which affects the postsynaptic integrative properties of neurons. We investigated the impact of TRIP8b deletion on long-term potentiation (LTP) at the two major excitatory inputs to CA1 pyramidal neurons: Schaffer collateral (SC) and perforant path (PP). We found that SC LTP was not significantly different between neurons from wild-type and TRIP8b-knockout mice. There was, however, significantly more short-term potentiation in knockout neurons. We also found that the persistent increase in h-current (Ih) that normally occurs after LTP induction was absent in knockout neurons. The lack of Ih plasticity was not restricted to activity-dependent induction, because the depletion of intracellular calcium stores also failed to produce the expected increase in Ih. Interestingly, pairing of SC and PP inputs resulted in a form of LTP in knockout neurons that did not occur in wild-type neurons. These results suggest that the physiological impact of TRIP8b deletion is not restricted to the integrative properties of neurons but also includes both synaptic and intrinsic plasticity.
Keywords: hippocampus, Ih, intrinsic plasticity, Schaffer collateral, perforant path
ca1 pyramidal neurons of the hippocampus receive input from the entorhinal cortex (EC) via numerous excitatory inputs along their dendritic tree. These inputs from the EC to CA1 pyramidal neurons in the hippocampus are divided into two groups: direct input from layer III neurons via the perforant path (PP) onto the most distal dendrites in stratum lacunosum-moleculare and indirect input from layer II neurons via the Schaffer collateral (SC) pathway from CA3 neurons onto the dendrites in stratum radiatum (Amaral and Witter 1989; Steward 1976). Both of these input pathways are known to display long-term potentiation (LTP) in response to various patterns of activity (Bliss and Collingridge 1993; Colbert and Levy 1993; Lynch et al. 1983; Remondes and Schuman 2003). Furthermore, coactivation of these two pathways leads to several cellular phenomena including dendritic plateau potentials, widespread Ca2+ influx, and synaptic plasticity (Dudman et al. 2007; Takahashi and Magee 2009).
HCN or h-channels are highly enriched in the distal dendrites of CA1 pyramidal neurons, where they contribute to resting dendritic physiology and regulate excitability. The high density of h-channels in the dendrites allows h-current (Ih) to contribute significantly to the total membrane conductance and thereby exert strong influence over neuronal function in the subthreshold voltage range near rest. Subtle modifications in the physiology of h-channels can produce significant changes in synaptic integration and neuronal excitability (Magee 1998, 1999; Poolos et al. 2002). A persistent increase in Ih throughout the dendritic arbor occurs after induction of LTP in CA1 pyramidal neurons (Fan et al. 2005; Narayanan and Johnston 2007). The combined plasticity of Ih and synaptic strength results in preferential tuning of the dendrites to selected synaptic inputs. This form of homeostatic plasticity is believed to be critical for information storage and processing by neuronal networks (Turrigiano and Nelson 2000). Homeostatic changes in Ih are not restricted to synaptic plasticity mechanisms. In response to cellular stressors, such as depletion of intracellular calcium stores, there is also a persistent increase in h-channels (Narayanan et al. 2010). However, unlike after LTP induction, this increase in Ih is restricted to the perisomatic region. A loss of normal h-channel plasticity, with or without accompanying deficits in synaptic plasticity, could have profound effects on neuronal signal processing and output.
The density of h-channels increases with distance from the soma in CA1 pyramidal neurons (Lörincz et al. 2002; Magee 1998). This gradient of Ih is accomplished by the correct targeting of h-channels to the distal dendrites by the association of HCN subunits with the accessory subunit tetratricopeptide containing Rab8b-interacting protein (TRIP8b). TRIP8b binds to both HCN1 and HCN2, the primary subunits of h-channels in the hippocampus, and regulates both the trafficking and gating of h-channels (Lewis et al. 2009; Santoro et al. 2009, 2011; Zolles et al. 2009). TRIP8b binds to HCN1 at two locations: an -SNL motif in the C-terminal end, which allows for proper assembly and targeting of h-channels, and a location within the cyclic nucleotide binding domain where TRIP8b binding can inhibit channel activation (Santoro et al. 2011). TRIP8b binding to HCN2 impairs cAMP-dependent activation of h-channels (Zolles et al. 2009). The loss of dendritic Ih that occurs in a model of temporal lobe epilepsy is due to the reduced trafficking of h-channels to the dendrites, in part because of a reduced association between TRIP8b and h-channel subunits (Shin et al. 2008). Consistent with the trafficking role of TRIP8b, CA1 pyramidal neurons from mice in which the gene for TRIP8b was knocked out have little to no dendritic Ih and reduced h-channel dendritic surface expression despite only a modest reduction in total HCN1 and HCN2 protein expression (Huang et al. 2012; Lewis et al. 2011). Investigations into the role that h-channels play in neuronal function often utilize pore-forming HCN subunit-knockout mice, which lack expression of HCN protein. In contrast, the TRIP8b-knockout mouse, which still has HCN1 and HCN2 protein expression, is ideal for investigating the role that h-channel trafficking plays during activity-dependent modulation of neuronal function.
While the effect of TRIP8b deletion on hippocampal postsynaptic properties has been well described (Huang et al. 2012; Lewis et al. 2011; Santoro et al. 2011), there have been no investigations of the effect of TRIP8b deletion on synaptic and intrinsic plasticity. We therefore investigated the impact of TRIP8b deletion on LTP of SC and PP inputs to CA1 pyramidal neurons as well as two forms of intrinsic h-channel plasticity.
MATERIALS AND METHODS
Acute hippocampal slices.
Hippocampal slices (300 μm) were prepared from 8- to 10-wk-old male wild-type C57BL/6 or TRIP8b-knockout mice with standard techniques (Routh et al. 2009). Briefly, animals were anesthetized with a lethal dose of ketamine and xylazine. Once deeply anesthetized, animals were perfused intracardially with ice-cold modified ACSF containing (in mM) 210 sucrose, 2.5 KCl, 1.2 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7.0 MgCl2, and 7.0 dextrose bubbled with 95% O2-5% CO2. The brain was removed and bisected along the midline. To promote an orientation favoring dendritic projection in a plane parallel to the surface of the slice, an additional cut was made on the dorsal surface at a 30° angle lateral to the midline. The brain was mounted and sliced with a microtome (Vibratome, St. Louis, MO). Slices were placed in a holding chamber filled with ACSF containing (mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 2 MgCl2, and 12.5 dextrose warmed to 35°C for 20 min and then placed at room temperature for <6 h until needed for recording. All animal procedures were conducted in accordance with the approval of the University of Texas Institutional Animal Care and Use Committee.
Electrophysiology.
Slices were placed individually as needed into a submerged recording chamber continuously perfused with control extracellular saline (see below). Slices were viewed with a Zeiss Axioskop using infrared video microscopy and differential interference contrast (DIC) optics. For all recordings, the control ACSF solution contained (mM) 125 NaCl, 3 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, and 12.5 dextrose and was bubbled continuously with 95% O2-5% CO2 at 31–33°C. For all LTP experiments, a cut was made between area CA3 and area CA1 before the slice was placed in the recording chamber to prevent spontaneous epileptiform discharges, and GABAA- and GABAB-mediated inhibition were blocked by inclusion of 2 μM gabazine (Ascent Scientific) and 5 μM CGP55845 (Tocris), respectively.
Patch pipettes were pulled from borosilicate glass and had a resistance of 4–8 MΩ when filled with the internal recording solution containing (in mM) 120 potassium gluconate, 20 KCl, 10 HEPES, 4 NaCl, 4 MgATP, 0.3 Na-GTP, and 7 phosphocreatine (pH 7.3 with KOH). In some cases, Neurobiotin (0.1–0.2%) was included for morphological analysis. Whole cell recordings were made from the soma of CA1 pyramidal neurons with a Multiclamp 700A in current-clamp mode. Series resistance was compensated with bridge balance throughout the recording, and experiments in which the series resistance exceeded 30 MΩ were discarded. Excitatory postsynaptic potentials (EPSPs) were elicited with tungsten bipolar stimulating electrodes placed near (<20 μm) the main apical dendrite ∼130 μm from the soma in stratum radiatum and ∼250 μm from the soma in stratum lacunosum-moleculare.
Data acquisition and analysis.
Data were sampled at 10 kHz, filtered at 3 kHz, and digitized by an ITC-18 interface connected to a computer running AxoGraph X. Data analyses were performed with AxoGraph X. EPSPs were quantified by measuring the initial slope (linear fit over 1–2 ms). RN was determined by calculating the slope of the linear regression line through the plot of the steady-state voltage against the corresponding current injection from a family of 750-ms current steps. Rebound was determined as the slope of the linear regression fit to a plot of the posthyperpolarization rebound amplitude as a function of the steady-state hyperpolarization (Brager and Johnston 2007). Resonance frequency was calculated with a chirp stimulus (100 pA peak to peak), with its frequency linearly spanning 0–15 Hz in 15 s (Narayanan and Johnston 2007).
Statistical analyses.
All data are expressed as means ± SE. Statistical comparisons were made with one-way ANOVA followed by Tukey-Kramer multiple-comparison post hoc test or Student's t-test (paired or unpaired as appropriate) with Prism software (GraphPad). Linear fits and correlations were made with Prism. Data were considered statistically significant if P < 0.05.
RESULTS
Long-term potentiation of Schaffer collateral inputs.
Several studies have examined how the loss of TRIP8b alters the intrinsic and integrative properties of hippocampal and cortical neurons (Huang et al. 2012; Lewis et al. 2011; Piskorowski et al. 2011). Whether the loss of TRIP8b affects long-term synaptic plasticity is not known.
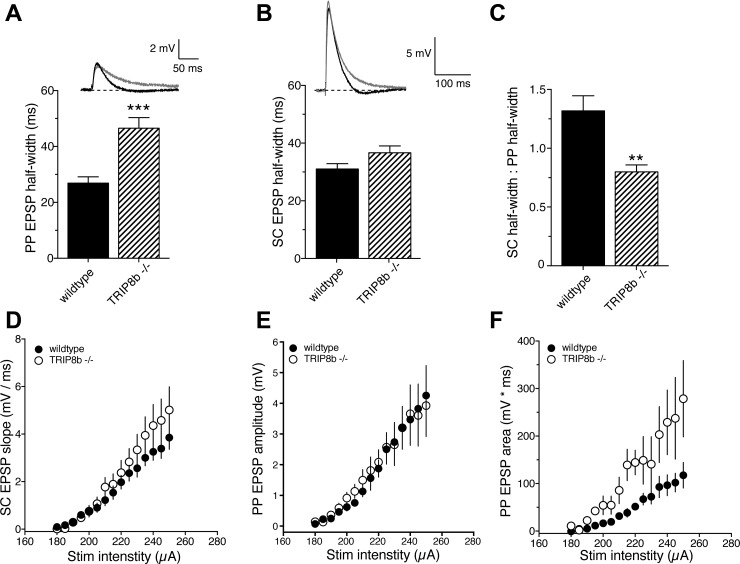
The presence of h-channels in the postsynaptic membrane greatly speeds the decay of single EPSPs (Magee 1998). In agreement with previous work on HCN1-knockout mice (Nolan et al. 2004; Piskorowski et al. 2011), we found that PP EPSPs in TRIP8b-knockout mice had a significantly greater half-width compared with wild-type mice (Fig. 1A). There was no significant effect on the half-width of SC EPSPs (Fig. 1B). Consistent with a role of TRIP8b in the targeting of h-channels to the distal dendrites (Piskorowski et al. 2011), the ratio of SC to PP EPSP half-width was significantly lower in TRIP8b-knockout neurons compared with wild-type neurons (Fig. 1C). To determine the effect of TRIP8b deletion on postsynaptic responsiveness to single presynaptic stimuli, we measured the postsynaptic response to single stimuli across a range of stimulus intensities. Deletion of TRIP8b did not affect the postsynaptic response to single stimuli for SC EPSPs (Fig. 1D). We found that although there was no difference in PP EPSP amplitude as a function of stimulus intensity (Fig. 1E), the area of PP EPSPs was greater for a given stimulus intensity in TRIP8b-knockout neurons (Fig. 1F). These data agree with previously published results from HCN1-knockout mice (Nolan et al. 2004; Piskorowski et al. 2011) and support a role for TRIP8b in targeting h-channels to the distal dendrites of CA1 pyramidal neurons.
Fig. 1.
TRIP8b deletion preferentially affects perforant path (PP) excitatory postsynaptic potentials (EPSPs). A: PP EPSP half-width was significantly greater in TRIP8b−/− neurons compared with wild-type neurons. B: Schaffer collateral (SC) EPSP half-width was not significantly different between wild-type and TRIP8b−/− neurons. C: the ratio of SC to PP EPSP half-width was significantly smaller in TRIP8b−/− neurons. D: relationship between SC EPSP slope and stimulus intensity for wild-type and TRIP8b−/− neurons. E and F: amplitude (E) and area (F) of PP EPSPs as a function of stimulus intensity. **P < 0.01 vs. wild type; ***P < 0.005 vs. wild type.
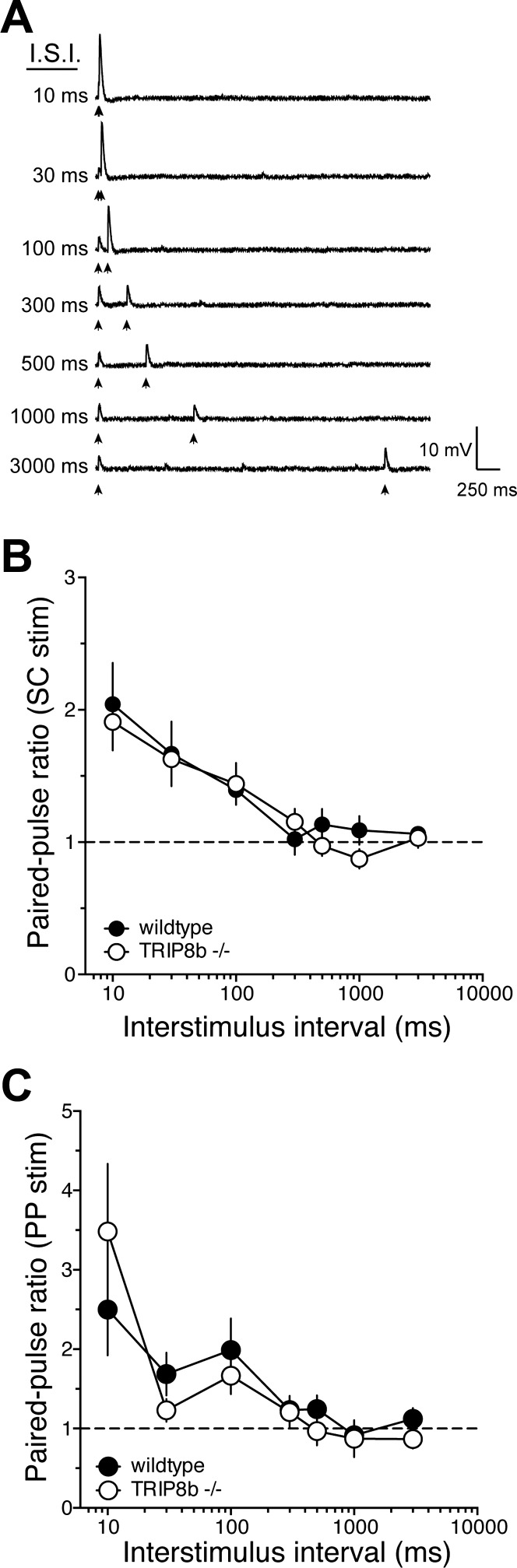
In addition to being localized in dendrites, h-channels can also be present in axons of cortical neurons (Bender et al. 2007; Huang et al. 2011; Notomi and Shigemoto 2004; Wilkars et al. 2012). To determine whether deletion of TRIP8b affected the release probability of neurotransmitter vesicles, we compared the paired-pulse ratio (PPR) at several interstimulus intervals (ISIs; 10–3,000 ms) between wild-type and TRIP8b-knockout mice (Fig. 2A). In agreement with recent results in EC (Huang et al. 2012), we found no significant difference in PPR at any ISI between wild-type and TRIP8b-knockout mice for either SC (Fig. 2B) or PP (Fig. 2C) EPSPs. These data suggest that deletion of TRIP8b does not affect vesicle release probability at SC or PP synapses.
Fig. 2.
TRIP8b deletion does not affect paired-pulse ratio at either SC or PP synapses. A: representative traces showing the effect of paired stimulation [interstimulus interval (ISI) 10–3,000 ms] used to determine paired-pulse ratio. B and C: paired-pulse ratio as a function of ISI for SC (B) and PP (C) EPSPs.
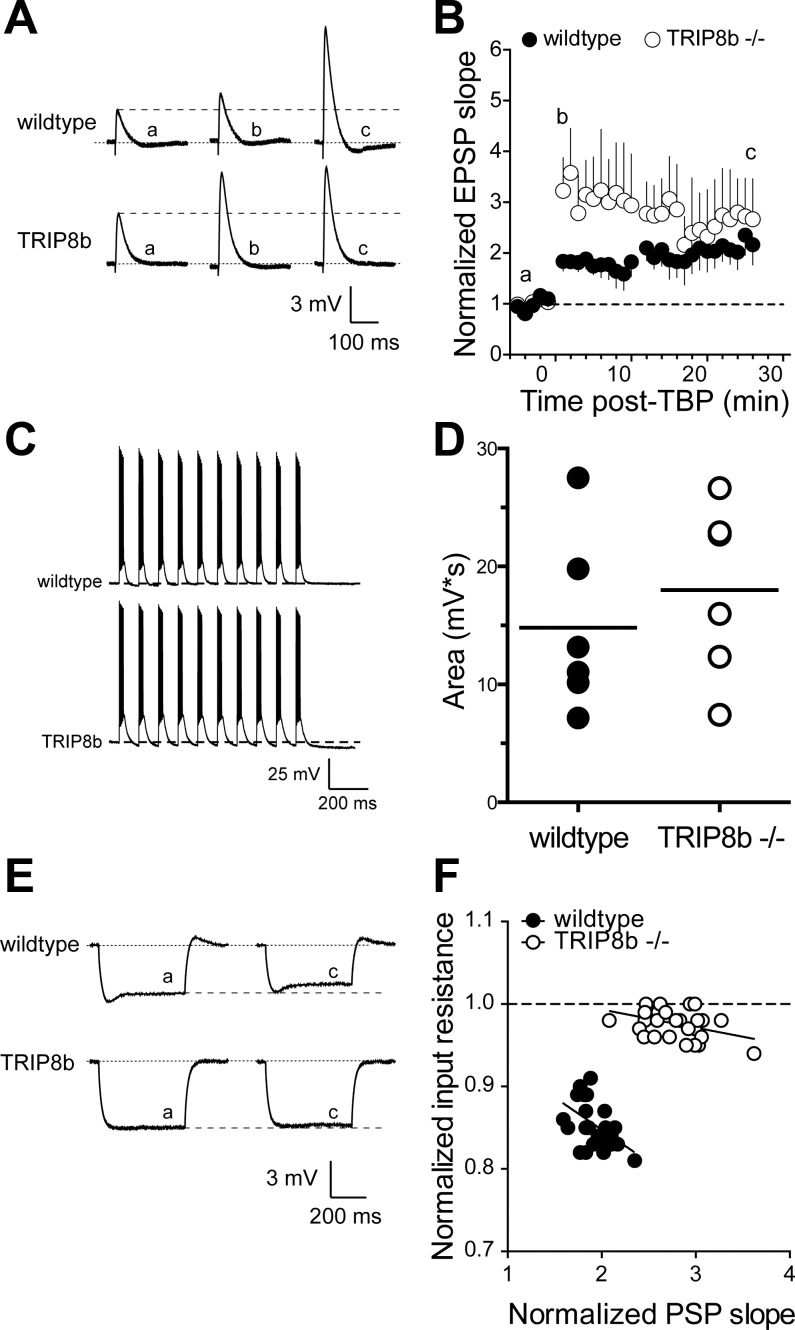
To examine the effect of TRIP8b loss on synaptic plasticity, we induced LTP using a theta burst pairing (TBP) protocol (Brager et al. 2012; Fan et al. 2005; Frick et al. 2004) in both wild-type and TRIP8b-knockout CA1 pyramidal neurons. The stimulus intensity was adjusted to produce an EPSP of 4–5 mV with initial slope of ∼1 mV/ms [wild type (wt): 189 ± 2 μA, knockout (ko): 192 ± 3 μA; P > 0.05]. SC EPSP slope was significantly increased 25 min after TBP in wild-type neurons (baseline: 1.2 ± 0.24 mV/ms, TBP: 2.4 ± 0.37 mV/ms; n = 6, P < 0.05; Fig. 3, A and B). In TRIP8b-knockout neurons there was a similar increase in SC EPSP slope 25 min after TBP (baseline: 1.1 ± 0.25 mV/ms, TBP: 3.1 ± 0.81 mV/ms; n = 7, P < 0.05). Although there was no significant difference in the magnitude of SC LTP 25 min after TBP between wild-type and TRIP8b-knockout neurons (Fig. 3B), there was significantly more short-term potentiation immediately following TBP in TRIP8b-knockout neurons (wt: 183 ± 19% of baseline, ko: 323 ± 62%; P < 0.05). Pharmacological block of h-channels or genetic deletion of HCN1 or TRIP8b results in greater postsynaptic summation (Huang et al. 2009; Lewis et al. 2011; Magee 1999; Poolos et al. 2002). Despite these known actions on EPSP summation, however, we found no significant difference in the depolarizing area produced by the TBP trains between wild-type and TRIP8b-knockout neurons (wt: 14.8 ± 3.1 mV·s, ko: 18 ± 3 mV·s; P > 0.05; Fig. 3, C and D).
Fig. 3.
SC EPSPs in TRIP8b-knockout mice show greater short-term potentiation but normal long-term potentiation after theta burst pairing (TBP). A: representative EPSPs in response to SC stimulation before, 1–2 min after, and 25 min after TBP. Note that a, b, c refer to the indicated time points in B. B: time course of EPSP potentiation after TBP. There was significantly more short-term potentiation, indicated by b, in TRIP8b-knockout neurons compared with control. C: representative traces from wild-type and TRIP8b-knockout neurons showing the voltage response during the TBP protocol. D: total depolarizing area under the voltage waveform during LTP induction for all TBP experiments. E: representative voltage traces indicating that input resistance decreased after TBP in wild-type but not TRIP8b-knockout neurons. Note that a and c refer to the indicated times in B. F: relationship between the change in input resistance and the change in EPSP slope. Data represent the time course of the change in input resistance and EPSP slope after TBP. Note the significantly reduced slope in TRIP8b-knockout neurons compared with wild-type neurons.
We previously demonstrated that the increase in EPSP slope following TBP is correlated with a corresponding decrease in RN in CA1 pyramidal neurons of both rats and mice (Brager et al. 2012; Fan et al. 2005). After TBP, there was a similar correlation between EPSP slope and RN in wild-type neurons (slope = −0.08 ± 0.02), which was greatly reduced in TRIP8b-knockout neurons (slope = −0.02 ± 0.01) (Fig. 3, E and F). The above results suggest that deletion of TRIP8b results in enhanced short-term potentiation but normal LTP at SC-CA1 synapses and that the concomitant decrease in RN following TBP present in wild-type neurons is absent in TRIP8b-knockout neurons.
Theta burst pairing-dependent h-channel plasticity.
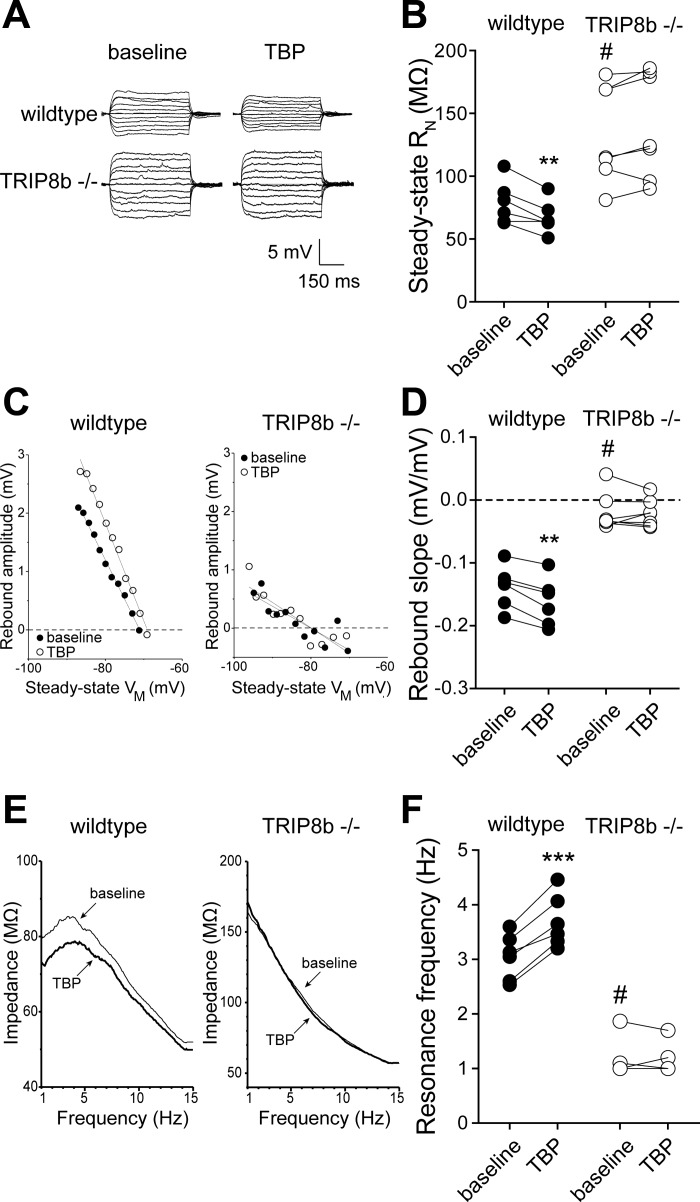
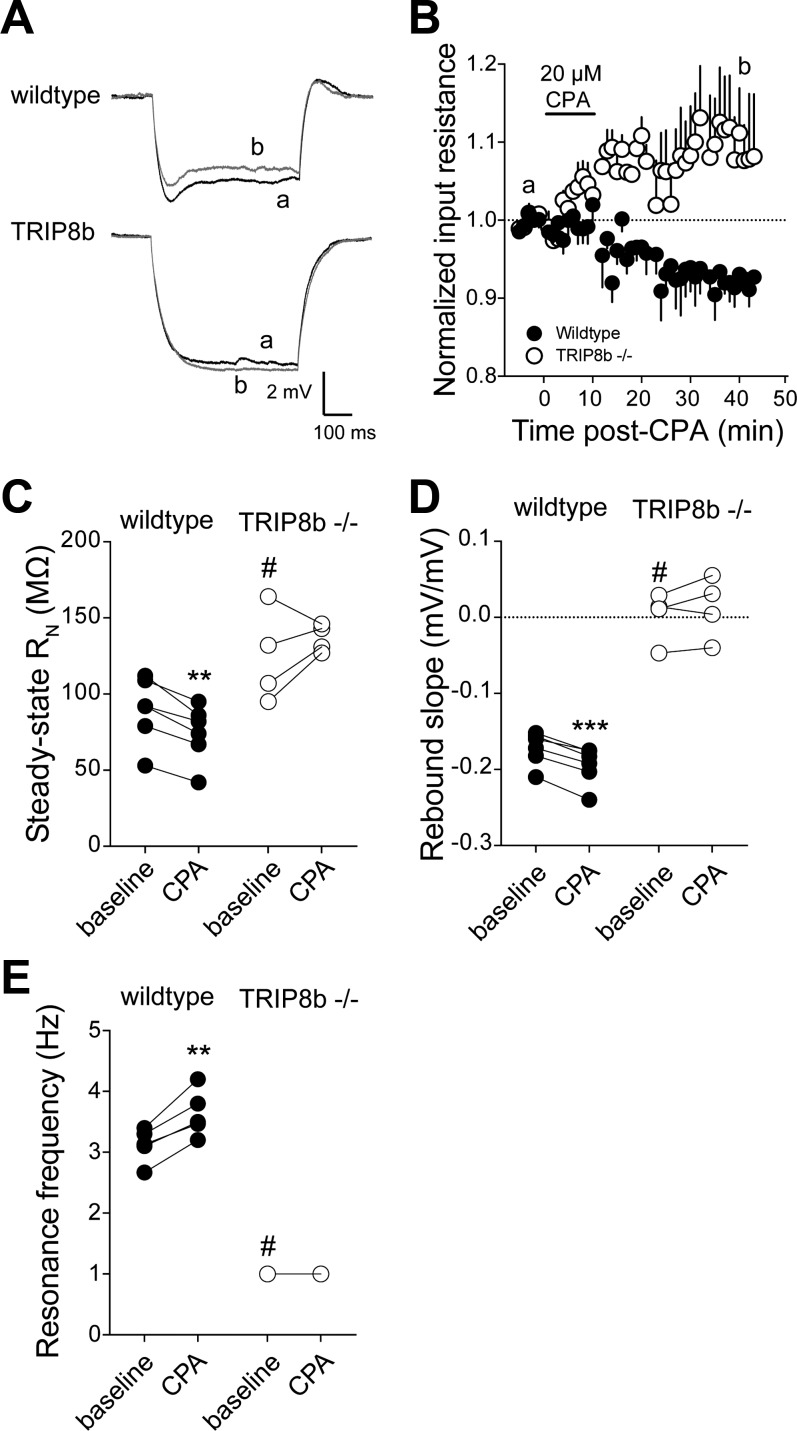
The decrease in RN during the development of LTP after TBP is due in large part to increases in Ih (Brager et al. 2012; Fan et al. 2005; Narayanan and Johnston 2007). We measured RN before and 30 min after TBP in both wild-type and TRIP8b-knockout neurons. Consistent with previous reports, we found that the baseline RN of TRIP8b-knockout neurons was significantly higher than wild-type neurons. RN significantly decreased 30 min after TBP in wild-type (baseline 79 ± 7 MΩ, TBP 67 ± 5.3 MΩ; n = 6, P < 0.01) but not TRIP8b-knockout (baseline 134 ± 14.7 MΩ, TBP 140 ± 15.8 MΩ; n = 7, P > 0.05) neurons (Fig. 4, A and B). Deletion of TRIP8b results in a loss of somatic and dendritic Ih in CA1 pyramidal neurons (Lewis et al. 2011). In agreement with our previously published results, rebound slope (wt: −0.14 ± 0.01 mV/mV, ko: −0.02 ± 0.01 mV/mV; P < 0.005) and resonance frequency (wt: 3.1 ± 0.2 Hz, ko: 1.1 ± 0.1 Hz; P < 0.005) were significantly lower in TRIP8b-knockout compared with wild-type neurons. Consistent with an increase in Ih, rebound slope and resonance frequency were significantly increased after TBP in wild-type neurons (Fig. 4, C–F). In contrast, there was no significant change in either rebound or resonance frequency in TRIP8b-knockout neurons up to 30 min after TBP. These results suggest that the h-channel plasticity that normally accompanies TBP-LTP is absent in TRIP8b-knockout neurons.
Fig. 4.
TBP-dependent intrinsic plasticity is absent in neurons from TRIP8b-knockout mice. A: representative voltage traces used to measure input resistance before and after TBP in wild-type and TRIP8b-knockout neurons. B: summary graph showing a significant decrease in input resistance (RN) after TBP in wild-type but not TRIP8b-knockout neurons. Note that baseline input resistance in TRIP8b-knockout neurons was significantly higher than wild type. C: representative plots of rebound amplitude vs. steady-state voltage (VM) from wild-type and TRIP8b-knockout neurons used to calculate rebound slope. Note both the reduced slope and lack of change after TBP in TRIP8b-knockout neurons. D: summary graph showing a significant increase in rebound slope after TBP in wild-type but not TRIP8b-knockout neurons. Note that baseline rebound slope in TRIP8b-knockout neurons was significantly lower than wild type. E: representative impedance amplitude profiles for wild-type and TRIP8b-knockout neurons before and after TBP. Resonance frequency (fR) is the frequency at which the impedance is maximal. F: summary graph showing a significant increase in fR after TBP in wild-type but not TRIP8b-knockout neurons. Note that baseline fR in TRIP8b-knockout neurons was significantly lower than wild type. **P < 0.01, ***P < 0.005 vs. TBP; #P < 0.05 vs. wild type.
Intracellular store depletion-dependent h-channel plasticity.
The above data indicate that the increase in Ih after TBP-LTP is absent in TRIP8b-knockout neurons. This form of h-channel plasticity occurs throughout the apical dendritic tree (Narayanan and Johnston 2007). The depletion of intracellular calcium stores by brief application of cyclopiazonic acid (CPA) also produces a persistent increase in Ih; however, this form of h-channel plasticity is restricted to the somatic region of CA1 pyramidal neurons (Narayanan et al. 2010). When TRIP8b is absent or the association of TRIP8b and h-channel subunits is reduced, there is a loss of distal dendritic targeting of h-channels (Lewis et al. 2011; Piskorowski et al. 2011; Shin et al. 2008). However, HCN1/2 mRNA levels are not different, and total HCN protein expression is only moderately reduced (Lewis et al. 2011). Immunofluorescence results suggest that there is little or no reduction in perisomatic HCN expression compared with the reduction in the distal dendrites (Lewis et al. 2011; Piskorowski et al. 2011). Because the increase in Ih following store depletion is restricted to the perisomatic region, we asked whether this form of h-channel plasticity is affected by deletion of TRIP8b. To address this question, we compared the changes in RN, rebound, and resonance frequency between wild-type and TRIP8b-knockout neurons after depletion of intracellular stores by CPA. Application of 20 μM CPA resulted in a time-dependent decrease in RN in wild-type but not TRIP8b-knockout neurons (Fig. 5, A and B). Consistent with previous results in rat (Narayanan et al. 2010), RN was significantly decreased 45 min after onset of CPA in wild-type neurons (baseline 90 ± 9 MΩ, post-CPA 74 ± 8 MΩ; n = 6, P < 0.01). In contrast, there was no significant change in RN in TRIP8b-knockout neurons after CPA application (baseline 125 ± 15 MΩ; post-CPA 137 ± 5 MΩ; n = 4, P > 0.05; Fig. 5C). Similar to TBP, both rebound slope (baseline −0.17 ± 0.009 mV/mV, post-CPA −0.21 ± 0.01 mV/mV; P < 0.005) and resonance frequency (baseline 3.1 ± 0.1 Hz, post-CPA, 3.6 ± 0.2 Hz; P < 0.01) were significantly increased after CPA application in wild-type neurons (Fig. 5, D and E). There was no significant change in either rebound slope or resonance frequency after CPA application in TRIP8b-knockout neurons. These results suggest that, just as TBP-dependent plasticity of Ih does not occur in TRIP8b-knockout neurons, store depletion-mediated increases in Ih are also absent in TRIP8b-knockout neurons.
Fig. 5.
Intracellular calcium store depletion-dependent intrinsic plasticity is absent in neurons from TRIP8b-knockout mice. A: representative voltage traces indicating that input resistance decreased after a 10-min application of 20 μM cyclopiazonic acid (CPA) in wild-type but not TRIP8b-knockout neurons. Note that a and b represent the indicated time points in B. B: summary graph showing the time course of the change in input resistance after CPA application. C: summary graph showing a significant decrease in input resistance after CPA application in wild-type but not TRIP8b-knockout neurons. D: summary graph showing a significant increase in rebound slope after CPA application in wild-type but not TRIP8b-knockout neurons. E: summary graph showing a significant increase in fR after CPA application in wild-type but not TRIP8b-knockout neurons. **P < 0.01, ***P < 0.005 vs. TBP; #P < 0.05 vs. wild type.
Long-term potentiation of perforant path inputs.
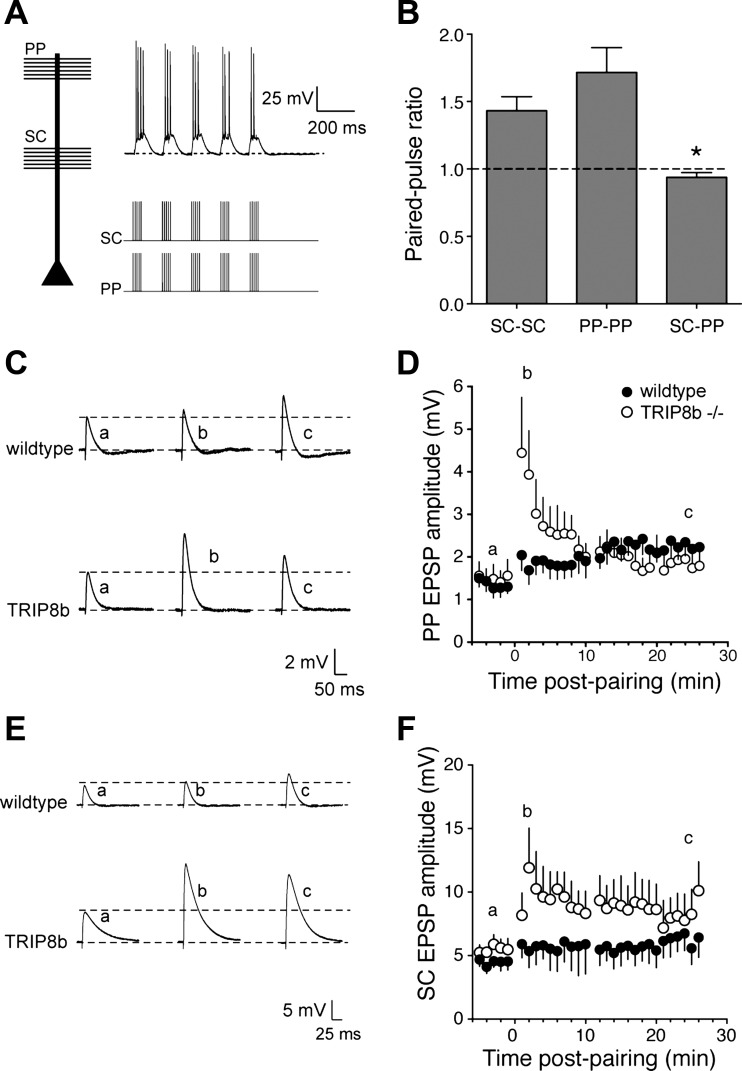
h-Channels are normally present at high density in the distal dendrites of CA1 pyramidal neurons, where they constrain synaptic input from the layer III EC neurons and limit dendritic excitability (Nolan et al. 2004; Tsay et al. 2007). Given that loss or reduction of TRIP8b expression results in a concomitant loss of dendritic Ih (Lewis et al. 2011; Santoro et al. 2011), we asked whether LTP of PP inputs was altered in TRIP8b-knockout neurons. The stimulus intensity was adjusted to produce SC EPSPs as described above (wt: 197 ± 5 μA, ko: 194 ± 3 μA; P > 0.05) and PP EPSPs of ∼1 mV (wt: 212 ± 6 μA, ko: 207 ± 4 μA; P > 0.05). PP LTP was accomplished by pairing stimulation of PP axons in stratum lacunosum-moleculare with SC inputs in stratum radiatum (Takahashi and Magee 2009) (Fig. 6A). The populations of axons activated by the two stimulating electrodes were considered nonoverlapping, as confirmed by paired-pulse analysis. Paired stimulation (100-ms ISI) of either SC or PP inputs resulted in paired-pulse facilitation (PPRSC: 1.4 ± 0.1, n = 10; PPRPP: 1.7 ± 0.2, n = 11). In contrast, when SC and PP were stimulated 100 ms apart there was no significant paired-pulse facilitation (Fig. 6B). Although the width of PP EPSPs was significantly greater in TRIP8b-knockout neurons (Fig. 1, A and F), there was no significant difference in either the depolarizing area (wt: 9.5 ± 2.3 mV·s, n = 8; ko: 10.2 ± 0.8 mV·s, n = 7) or number of spikes produced (wt: 14 ± 4 spikes, n = 8; ko: 16 ± 2 spikes, n = 7) during the induction protocol between wild-type and TRIP8b-knockout neurons.
Fig. 6.
Deletion of TRIP8b results in greater short-term potentiation of PP EPSPs and heterosynaptic potentiation of SC EPSPs. A: representative voltage traces showing the response to paired activation of PP and SC inputs onto wild-type and TRIP8b-knockout CA1 neurons. B: lack of paired-pulse facilitation during paired SC-PP stimulation (ISI = 100 ms), in contrast to SC-SC or PP-PP stimulation. C: representative EPSPs in response to PP stimulation before, 1–2 min after, and 25 min after TBP. Note that a, b, and c refer to the indicated time points in D. D: time course of PP EPSP potentiation after PP-SC pairing. There was significantly more short-term potentiation in TRIP8b-knockout neurons compared with control. E: representative EPSPs in response to SC stimulation before, 1–2 min after, and 25 min after TBP. Note that a, b, and c refer to the indicated time points in F. F: time course of SC EPSP potentiation after PP-SC pairing. Note that LTP was produced in the SC pathway in TRIP8b-knockout neurons but not in wild-type neurons. *P < 0.05.
In both wild-type and TRIP8b-knockout neurons, pairing of PP and SC inputs resulted in a small but significant increase in PP EPSP amplitude (wt: baseline 1.2 ± 0.3 mV, pairing 2.0 ± 0.3, n = 8, P < 0.05; ko: baseline 1.2 ± 0.3 mV, pairing 1.9 ± 0.3 mV, n = 7, P < 0.05). The amount of potentiation was not significantly different between wild-type and TRIP8b-knockout neurons (Fig. 6, B and C). Similar to TBP of SC-CA1 synapses (Fig. 3), there was significantly more short-term potentiation of PP EPSPs in TRIP8b-knockout neurons (wt: 152 ± 10% of baseline, ko: 300 ± 62% of baseline; P < 0.05). More interestingly, while PP-SC pairing produced no significant increase in SC EPSPs in wild-type neurons (baseline: 4.0 ± 0.5 mV; pairing: 6.2 ± 1.3 mV), SC EPSPs were significantly increased up to 25 min after pairing in TRIP8b-knockout neurons (baseline: 3.5 ± 0.6 mV, pairing: 9.8 ± 2.2 mV; P < 0.005) (Fig. 6, E and F). These results suggest that a form of heterosynaptic potentiation occurs in the absence of TRIP8b.
DISCUSSION
Auxillary subunits play a critical role in regulating the physiology of voltage-gated ion channels. The tetratricopeptide containing Rab8b-interacting protein (TRIP8b) is a scaffolding protein found in the distal apical dendrites of CA1 pyramidal neurons that regulates both the membrane expression and gating of h-channels (Lewis et al. 2009; Santoro et al. 2004, 2009; Zolles et al. 2009). Previous work using the TRIP8b-knockout mouse focused on changes in dendritic expression of h-channels (Lewis et al. 2011). In this report we investigated the impact the loss of TRIP8b had on LTP at both SC-CA1 and PP-CA1 synapses. There was no significant difference in presynaptic function, dendritic branching, or dendritic spines between TRIP8b-knockout and wild-type neurons. The amount of LTP was not affected by the absence of TRIP8b. However, there was significantly more short-term potentiation at both SC and PP inputs in TRIP8b-knockout neurons. We previously showed that LTP at SC synapses is accompanied by an increase in dendritic Ih (Brager et al. 2012; Fan et al. 2005; Narayanan and Johnston 2007). While the loss of TRIP8b does not affect the expression of LTP, h-channel-based intrinsic plasticity is distinctly absent in TRIP8b-knockout neurons. The lack of Ih plasticity was not limited to dendritic locations, as intracellular store-mediated h-channel plasticity, which is restricted to the soma and proximal dendrite, was also absent in TRIP8b-knockout mice. These results suggest that, in addition to normal membrane targeting of h-channels, TRIP8b may play a role in activity-dependent modulation of Ih, consistent with a proposed mechanism involving TRIP8b in recycling of intracellular h-channels to the neuronal membrane (Lewis et al. 2011).
The pairing of SC and PP inputs results in LTP of PP-CA1 synapses with no significant change at SC-CA1 synapses in wild-type neurons. Most interestingly, in TRIP8b-knockout neurons persistent potentiation of SC-CA1 EPSPs occurred concurrent with LTP at PP inputs. One factor that is known to control the synaptic location of LTP following SC-PP pairing is the timing difference between SC and PP stimulation. When SC and PP inputs are stimulated simultaneously, there is strong LTP of PP-CA1 synapses with little change at SC-CA1 synapses (Fig. 6; Dudman et al. 2007; Takahashi and Magee 2009). When the PP inputs precede the SC inputs by ∼20 ms, strong LTP is observed at SC-CA1 synapses with no change at PP-CA1 synapses (Dudman et al. 2007). As distal dendritic h-channels normally reduce temporal and spatial integration (Magee 1998, 1999) and constrain the synaptic depolarization in response to PP stimulation (Tsay et al. 2007), it is possible that the impaired distal dendritic targeting of h-channels in the absence of TRIP8b broadens the timing-dependent window of SC-CA1 and PP-CA1 LTP, resulting in LTP at both input locations.
h-Channels play a crucial role in setting the intrinsic membrane and integrative properties of cortical neurons. h-Channels contribute to the resting membrane potential, RN, and membrane time constant. The high density of h-channels in the dendrites limits the amount of depolarization produced by bursts of synaptic input by reducing the amount of temporal summation (Magee 1999). In addition, h-channels regulate the oscillatory dynamics of cortical neurons by tuning the neurons to be preferentially sensitive to inputs at a given frequency (Narayanan and Johnston 2007, 2008; Vaidya and Johnston 2012). Correct dendritic targeting of h-channels is accomplished in part by the auxiliary subunit TRIP8b (Lewis et al. 2009, 2011; Santoro et al. 2011). Deletion or knockdown of TRIP8b results in a loss of h-channel expression and reduced dendritic targeting (Lewis et al. 2011; Piskorowski et al. 2011). Changes in TRIP8b function or expression have been implicated in models of neurological disease. The loss of distal dendritic h-channels in a model of temporal lobe epilepsy is likely due to a reduction in TRIP8b binding to HCN1 (Shin et al. 2008), and deletion of TRIP8b results in an antidepressant phenotype (Lewis et al. 2011), most likely via its action on h-channels and Ih (Kim et al. 2012).
In summary, we found that short-term synaptic potentiation was higher and that heterosynaptic plasticity was induced in CA1 pyramidal neurons from mice in which the gene for TRIP8b was deleted. These data suggest that, in addition to altering the integrative properties of cortical neurons, the loss of TRIP8b and subsequent mislocalization of h-channels has implications for the induction of both short- and long-term synaptic plasticity.
GRANTS
This work was supported by the Epilepsy Foundation of America (D. H. Brager) and National Institutes of Health Grants R01 NS-59934 (D. M. Chetkovich) and R01 MH-048432 (D. Johnston).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.H.B. conception and design of research; D.H.B. performed experiments; D.H.B. analyzed data; D.H.B. interpreted results of experiments; D.H.B. prepared Figs.; D.H.B. drafted manuscript; D.H.B., A.S.L., D.M.C., and D.J. edited and revised manuscript; D.H.B., A.S.L., D.M.C., and D.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank members of the Johnston Lab for input throughout this project.
Present address of A. S. Lewis: Dept. of Psychiatry, Yale University School of Medicine, New Haven, CT.
REFERENCES
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31: 571–591, 1989 [DOI] [PubMed] [Google Scholar]
- Bender RA, Kirschstein T, Kretz O, Brewster AL, Richichi C, Rüschenschmidt C, Shigemoto R, Beck H, Frotscher M, Baram TZ. Localization of HCN1 channels to presynaptic compartments: novel plasticity that may contribute to hippocampal maturation. J Neurosci 27: 4697–4706, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993 [DOI] [PubMed] [Google Scholar]
- Brager DH, Akhavan AR, Johnston D. Impaired dendritic expression and plasticity of h-channels in the fmr1(-/y) mouse model of fragile X syndrome. Cell Rep 1: 225–233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in Ih in hippocampal CA1 pyramidal neurons. J Neurosci 27: 13926–13937, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Levy WB. Long-term potentiation of perforant path synapses in hippocampal CA1 in vitro. Brain Res 606: 87–91, 1993 [DOI] [PubMed] [Google Scholar]
- Dudman JT, Tsay D, Siegelbaum SA. A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron 56: 866–879, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in Ih. Nat Neurosci 8: 1542–1551, 2005 [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci 7: 126–135, 2004 [DOI] [PubMed] [Google Scholar]
- Huang Z, Luján R, Kadurin I, Uebele VN, Renger JJ, Dolphin AC, Shah MM. Presynaptic HCN1 channels regulate Cav3.2 activity and neurotransmission at select cortical synapses. Nat Neurosci 14: 478–486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Luján R, Martinez-Hernandez J, Lewis AS, Chetkovich DM, Shah MM. TRIP8b-independent trafficking and plasticity of adult cortical presynaptic HCN1 channels. J Neurosci 32: 14835–14848, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Walker MC, Shah MM. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J Neurosci 29: 10979–10988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Chang PY, Johnston D. Enhancement of dorsal hippocampal activity by knockdown of HCN1 channels leads to anxiolytic- and antidepressant-like behaviors. Neuron 75: 503–516, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AS, Schwartz E, Chan CS, Noam Y, Shin M, Wadman WJ, Surmeier DJ, Baram TZ, Macdonald RL, Chetkovich DM. Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci 29: 6250–6265, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AS, Vaidya SP, Blaiss CA, Liu Z, Stoub TR, Brager DH, Chen X, Bender RA, Estep CM, Popov AB, Kang CE, Van Veldhoven PP, Bayliss DA, Nicholson DA, Powell CM, Johnston D, Chetkovich DM. Deletion of the hyperpolarization-activated cyclic nucleotide-gated channel auxiliary subunit TRIP8b impairs hippocampal Ih localization and function and promotes antidepressant behavior in mice. J Neurosci 31: 7424–7440, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörincz A, Notomi T, Tamás G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci 5: 1185–1193, 2002 [DOI] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 305: 719–721, 1983 [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci 2: 508–514, 1999 [DOI] [PubMed] [Google Scholar]
- Narayanan R, Dougherty KJ, Johnston D. Calcium store depletion induces persistent perisomatic increases in the functional density of h channels in hippocampal pyramidal neurons. Neuron 68: 921–935, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron 56: 1061–1075, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. The h channel mediates location dependence and plasticity of intrinsic phase response in rat hippocampal neurons. J Neurosci 28: 5846–5860, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsáki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 119: 719–732, 2004 [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1–4, in the rat brain. J Comp Neurol 471: 241–276, 2004 [DOI] [PubMed] [Google Scholar]
- Piskorowski R, Santoro B, Siegelbaum SA. TRIP8b splice forms act in concert to regulate the localization and expression of HCN1 channels in CA1 pyramidal neurons. Neuron 70: 495–509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci 5: 767–774, 2002 [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Molecular mechanisms contributing to long-lasting synaptic plasticity at the temporoammonic-CA1 synapse. Learn Mem 10: 247–252, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh BN, Johnston D, Harris K, Chitwood RA. Anatomical and electrophysiological comparison of CA1 pyramidal neurons of the rat and mouse. J Neurophysiol 102: 2288–2302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Hu L, Liu H, Saponaro A, Pian P, Piskorowski RA, Moroni A, Siegelbaum SA. TRIP8b regulates HCN1 channel trafficking and gating through two distinct C-terminal interaction sites. J Neurosci 31: 4074–4086, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Piskorowski RA, Pian P, Hu L, Liu H, Siegelbaum SA. TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain. Neuron 62: 802–813, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci 24: 10750–10762, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Brager D, Jaramillo TC, Johnston D, Chetkovich DM. Mislocalization of h channel subunits underlies h channelopathy in temporal lobe epilepsy. Neurobiol Dis 32: 26–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol 167: 285–314, 1976 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Magee JC. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron 62: 102–111, 2009 [DOI] [PubMed] [Google Scholar]
- Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron 56: 1076–1089, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol 10: 358–364, 2000 [DOI] [PubMed] [Google Scholar]
- Vaidya S, Johnston D. HCN channels contribute to the spatial synchrony of theta frequency synaptic inputs in CA1 pyramidal neurons (Abstract). Neuroscience Meeting Planner 2012: 435.15, 2012 [Google Scholar]
- Wilkars W, Liu Z, Lewis AS, Stoub TR, Ramos EM, Brandt N, Nicholson DA, Chetkovich DM, Bender RA. Regulation of axonal HCN1 trafficking in perforant path involves expression of specific TRIP8b isoforms. PLoS One 7: e32181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolles G, Wenzel D, Bildl W, Schulte U, Hofmann A, Müller CS, Thumfart JO, Vlachos A, Deller T, Pfeifer A, Fleischmann BK, Roeper J, Fakler B, Klöcker N. Association with the auxiliary subunit PEX5R/Trip8b controls responsiveness of HCN channels to cAMP and adrenergic stimulation. Neuron 62: 814–825, 2009 [DOI] [PubMed] [Google Scholar]