Abstract
A reversible photo-driven sensor for aluminum ions based on photochromic spiropyran was reported with rapid response time. The detection of aluminum was performed via the chelation of aluminum ions with the merocyanine form (MC) of photochromic spiropyran. 1H NMR studies confirmed the conversation from the MC form into the Al3+-MC form. Addition of aluminum ions to the spiropyran (SP) in a MeCN/H2O mixture results in obvious color changes with a loss in absorbance at 539 nm and an enhancement in absorbance at about 420 nm after irradiation at 365 nm. The metal chelation complex (Al3+-MC) can also be converted into the original SP form by irradiation with visible light. Aluminum ions can be detected down to 0.5 μM levels in a fast response of less than 5 seconds with no interference from other ionic species.
Keywords: photo-driven, photochromic spiropyran, sensor, aluminum ion
1. Introduction
Aluminum is the third most abundant element and widely used for industrial and domestic purposes. However, the toxicity of aluminum towards fish, algae, and plant roots in acidic media is well documented [1]. Toxic concentrations of Al are supposed to have ethological significance in primary degenerative dementia (Alzheimer's disease or senile dementia), in dialysis dementia, and in dialysis-related osteomalacia [2, 3].
A number of methods such as spectrometry (including graphite furnace atomic absorption spectrometry and inductively coupled plasma-atomic emission spectrometry) [4-6], chromatography [7-9], electrochemistry [10-12], spectrophotometry[13-15] and flow-injection [16-18] have been used to determine aluminum ions.
Though these methods provide accurate results they are not very convenient for the analysis of large numbers of environmental samples as they require sample pretreatment and sufficient infrastructure back-up. On the other hand, analytical procedures involving ion sensors are most appropriate for such determinations as they require no or minimum sample pretreatment and are fast, convenient and observable by the naked eye. However, these sensors suffer from the disadvantages of poor selectivity and significant interference from some cations (e.g. Hg2+, Cu2+, Ga3+, In3+, Pb2+, Mg2+, Fe2+and Fe3+), and also a very slow response time. A selective, sensitive and fast responding aluminum sensor with a long life time is required.
It is well-known that an important characteristic of a good sensor is that it performs reversibly, and the literature shows that a few sensors (e.g. Zn2+, Hg2+ and Cu2+) have been reported with good reversibility by addition of external metal ion chelators [19-21], then exhibit decreased stability and reproducibility after several reversible cycles. The development of optical methods to detect and monitor clinically and environmentally important species, such as metal ions, is an important area of contemporary sensor research [22, 23]. Therefore we proposed that an aluminum ion selective sensor can be reversibly photo-driven without any external influences.
Spiropyran molecules, one of promising families of photochromic compounds, can undergo reversible structural transformation in response to external inputs such as light, protons, and metal ions [24-29]. The spiropyran converts from closed form to open form (merocyanine) after ultraviolet light irradiation. The merocyanine form is thermally unstable and turns back to closed form in several minutes, however; the former displays a high tendency to coordinate with metal ions, and forms a thermally stable chelation complexes with metal ions, which lead to changes in the fluorescence or absorbance wavelength on metal ion binding. Several groups have reported that suitably substituted spiropyrans could be used to bind metal ions in the open merocyanine form [30-35]. Based on the reversible changes of the fluorescence or absorption spectra of spiropyrans responding to different external stimulations, the development of devices, such as sensors dependent on different wavelengths of light, would be inherently easier, and offer distinct advantages over those that employ other signaling mechanisms in terms of sensitivity and selectivity. As a result, photoreversibility of the photochromic spiropyran would be required to apply this strategy to metal ion sensors [36-37].
Towards this objective, we describe herein the extension of this spiropyran-based system to the design and synthesis of a photoreversible spiropyran “real-time” aluminum ion sensor with considerably great sensitivity (in the micromole range) and good selectivity.
2. Experimental Section
2.1. Reagents
3,3-Diimethyl-1-(2′-methylenepyridine)-5′-nitrospirobenzopyran was synthesized based on the established method [38] via the reaction of indoline derivatives and 5-nitrosalicylaldehyde.
Acetonitrile was of HPLC grade, and water was doubly distilled in quartz apparatus. The other commercially available chemicals used were of analytical grade. A standard aluminum ion solution was prepared using 0.002 mol/L of aluminum sulfate solution unless noted otherwise and stored in the dark. All metal ion solutions used in interference testing were prepared by dilution from their aqueous solutions of acetate or chloride salts to a concentration of 1.0×10−5 mol/L, and all anionic solutions used in interference testing were prepared by dilution from their aqueous solutions of sodium salts to a concentration of 1.0×10−5 mol/L.
2.2. Instrumentation
1H NMR spectra were measured on a Brucker AM 400 spectrometer. UV/Vis spectra were done on a Varian Cary 500 spectrophotometer with 1 cm quartz cell at 25°C. The photo-irradiation was carried on a CHF-XM 500 W high-pressure mercury lamp with suitable filters (365 nm, half width 30 nm, FAL type made in Germany) in a sealed 1 cm quartz cell. The distance between the lamp and the sample cell is 10 cm. Cyclic voltammetry measurements were performed using a platinum disk working electrode, a platinum wire counter electrode, an Ag/AgCl (in a saturated KCl solution) reference electrode and tetrabutylammonium hexafluorophosphate (0.1 M) as the electrolyte.
2.3. Photochromic experiments
The spectroscopic measurements were performed with a UV/Vis absorption spectrophotometer. The ultraviolet source of irradiation was a high-pressure mercury lamp obtained with a maximum at 365 nm. First, the spiropyran solution was irradiated with the Hg lamp for 10 min, the absorption spectra were recorded until the maximum absorbance decreased to that of the nonirradiated SP, and the variation of maximum absorbance was plotted against time. Then the SP was irradiated for subsequent times until no change in the photochromic properties of the SP was recorded. Again the plots of maximum absorbance with time were reported. The spectral region for the examined photochromic spiropyran was between 200 and 700 nm in order to follow the photo transformations between spiropyran and merocyanine.
3. Results and Discussion
3.1. Photochromic characteristics
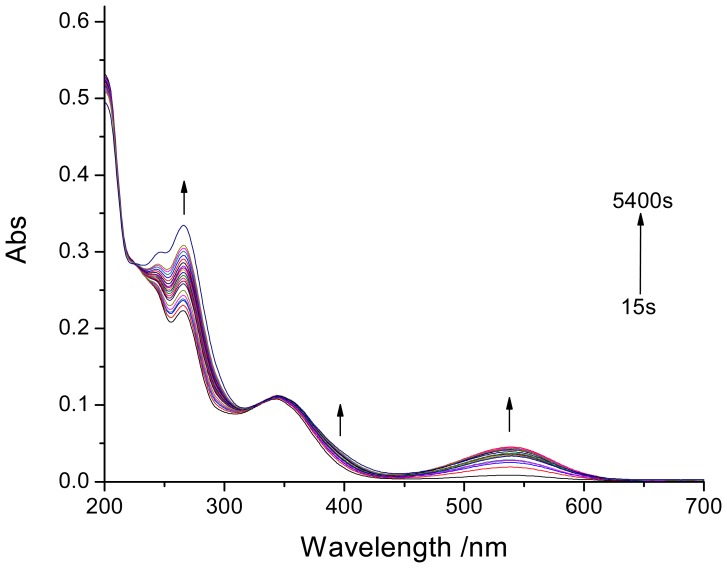
The photochromic spiropyran was excited by irradiation (365 nm light) for various time periods in order to follow the coloration. The result was the cleavage of the spiro carbon-oxygen bond, whereupon the molecule becomes a metastable amphoteric merocyanine ion, and the coloration of the spiropyran was due to the formation of this metastable ion. The latter may exist in different geometrical isomers, cis or trans, the cis isomer being unstable and transforming into the trans isomer. The spiropyran solutions were colorless before the irradiation and turned to red under irradiation at 365 nm, and then the solution color was changed to colorless upon fading. In Figure1 the absorption spectra of the spiropyran was depicted after the irradiation from 15 s to 1.5 h. The spectra of the spiropyran revealed that no absorption peak was observed in the range of 450-650 nm before irradiation. On the contrary, a well-formed peak at 539 nm was obtained with irradiation at 365 nm light. The peaks between 200 and 400 nm were attributed to the superposition of absorption bands of the spiropyran form and the merocyanine form. After 10 min of total irradiation time, the spiropyran solutions turned red and their photochromic properties were very stable.
Figure 1.
Absorbance spectra of spiropyran (1×10−5 mol/L, 298 K, MeCN/H2O = 6:4) upon irradiation at 365 nm light from 15 s to 5400 s.
Besides the changes caused in the absorption spectra of spiropyran by irradiation at different times, the decoloration rate of these films was also studied after irradiation with 365 nm light for 10 min. In order to follow the decoloration rate, the spectra were taken after each 3 s at 539 nm for the MC. As indicated, the decoloration rate was much quicker as shown by the maximum absorption intensity decrease. This can be clearly followed from the time dependence of the maximum absorption intensity. Considering the decoloration rate of the spiropyran as a first order reaction, then in the plot of the In(At-A∞) of the maximum absorbance against time a linear dependence must be observed. Indeed, a near linear dependence between In(At-A∞) and time (t) for spiropyran can be found by using equation [39].
This linearity in the dependence of the nature logarithmic of maximum absorption intensity against time shows the decoloration mechanism of spiropyran to be a first order reaction.
3.2. Recognition mechanism
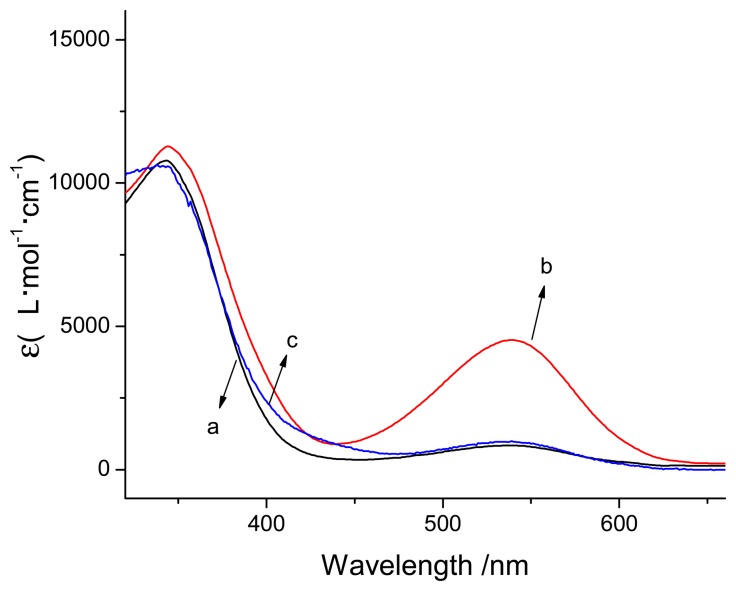
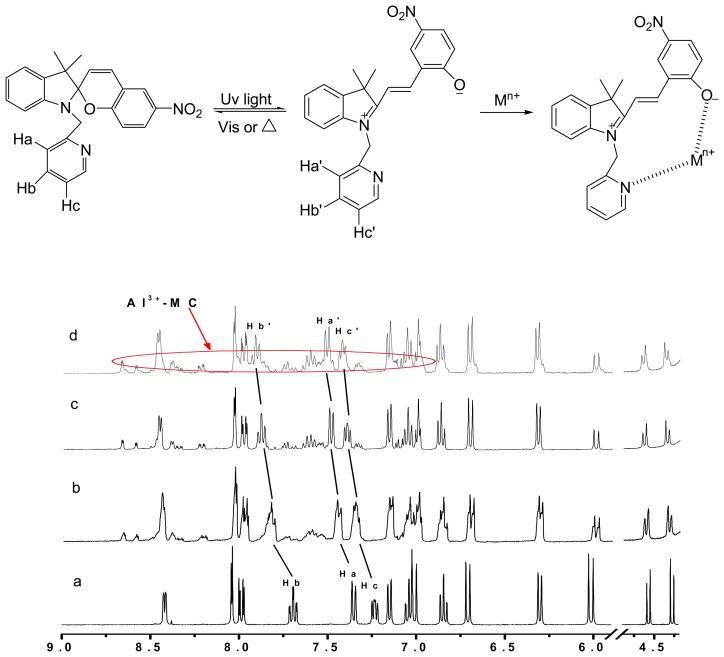
Figure 2 shows the visible absorption spectra of spiropyran upon irradiation at 365 nm light for 10 min before and after addition of aluminum ions. As a result, the absorption band in the visible region (539 nm) was enhanced, and attributed to the appearance of the MC after irradiation for 10 min. After addition of aluminum ions, this band disappeared immediately, and a new peak detected at about 420 nm, which was attributed to the metal-ion complexation with MC (Al3+-MC) [28]. The phenoxide and pyridine groups of the merocyanine fragment may be involved in the formation of the coordination bonds between the merocyanine and aluminum ion. 1H NMR (Figure 3) studies gave strong proof consistent with the above analysis. 1H NMR spectrum of spiropyran changes dramatically after the addition of aluminum ion upon irradiation at 365 nm light for 10 min. Moreover, the characteristic Ha, Hb and Hc of pyridine signals exhibited a remarkable downfield shift attributed to the interconversation from the MC form to aggregation forms by irradiation [24, 25].
Figure 2.
Absorbance spectra of spiropyran sensor (298 K, MeCN/H2O = 6:4, V/V) after irradiation for 10 min at 365 nm light with addition of Al3+ (c), without addition of Al3+ (b), and with addition of Al3+ after irradiation by visible light for 10 min (a)
Figure 3.
Partial 1H NMR spectra (400 MHz, CD3CN/D2O = 8:2, V/V) of spiropyran without Al3+ (a), and by irradiation at 365 nm for 10 min with adding Al3+of different concentration of 0.3 eq (b), 0.5 eq (c), and 1 eq (d).
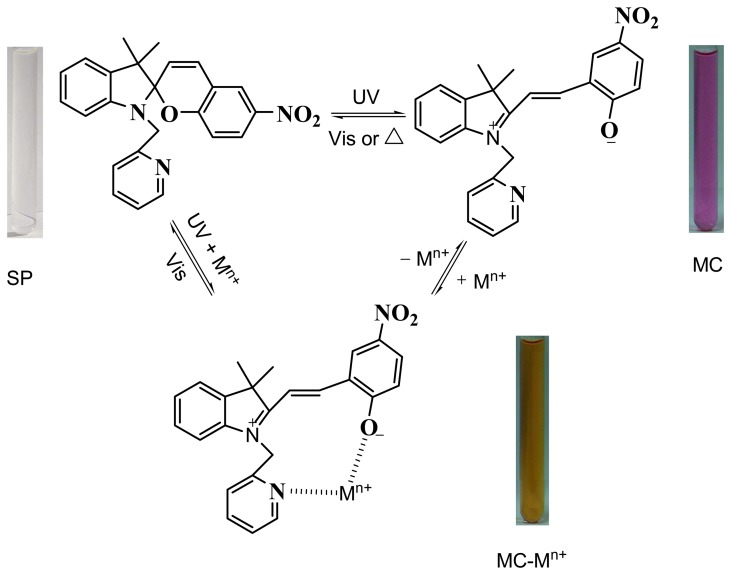
Furthermore, after irradiation for 10 min by visible light, a decrease occurred at about 420 nm (Figure 2), and the Al3+-MC released the aluminum ion and MC; the latter was simultaneously converted into the SP form. The reversible process (also shown in Scheme 1) provides a reversible detection of aluminum ion by photo controls.
Scheme 1.
Reversible photochromic reactions of spiropyran.
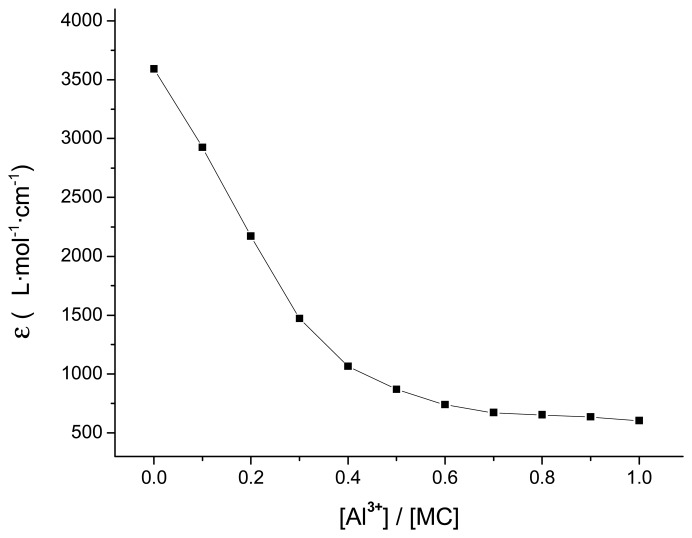
According to the spectra reported in Figure 3, addition of the spiropyran solution (1.0×10−5 mol/L) after irradiation for 10 min was carried out by the addition of microliter amounts of a concentrated standard solution of the aluminum ion in MeCN/H2O solution (2.0×10−3 mol/L) using a precalibrated micropipette, followed by absorption intensity reading at the λmax = 539 nm. Since the volume of titrant added during titration was negligible (at the most 0.01 mL) as compared with the initial volume of the ligand (2 mL), no volume correction was carried out. The absorption spectrum of MC showed a decrease of the band at 539 nm upon addition of increasing quantities of aluminum ions to MC, whereas the absorption intensity changes as a function of the [Al3+]/[MC] molar ratio (Figure 4). These changes could be attributed to the complexation between the MC and Al3+. From the inflection point in the absorbance/molar ratio plot at [Al3+]/[MC] values between 0.4 to 0.7, it can be inferred that 1:3 ([Al(MC)3]3+), 1:2 ([Al(MC)2]3+) and 1:1 ([Al(MC)]3+) complexes were formed in MeCN/H2O solution.
Figure 4.
Absorption intensity / mole ratio data of spiropyran (1×10−5 mol/L, 298 K, MeCN/H2O = 6:4, V/V) after irradiation for 10 min at 365 nm light with titration of Al3+ at different concentrations.
3.3. Optimization of experimental parameters
The performance of the spiropyran sensor was further assessed in partially non-aqueous media, i.e. acetonitrile–water mixtures. The influence of the rates between MeCN and H2O was studied. The spiropyran of concentration at 1.0×10−5 mol/L was irradiated for 10 min at 365 nm, the maximum absorption wavelength in the visible region was recorded, and the results showed that the maximum wavelength was hypsochromically shifted with decrease in non-aqueous content, and the absorption spectrum in pure water was very similar to which in the MeCN/H2O mixture solution at 6:4. Therefore, in order to improve the sensitivity, the spiropyran sensor can be used for further studies in mixtures containing only up to 60% MeCN content.
3.4. Response time
An important analytical feature of the spiropyran sensor is its response time for a sample solution of low Al3+ concentration. But chemsensors always have the problem of a long response time. In 2001 Mousavi reported a chemosensor based on an Al3+ - selective electrode method with a response time of 70 s [12], later lowered to 5 s by Gupta in 2007 [40]. Unexpectedly, the spiropyran sensor responded very fast. The spiropyran in MeCN/H2O mixture solution was irradiated for 10 min at 365 nm light, and when titrated with aluminum ion, the band at 539 nm disappeared, and a new weak band was detected very rapidly at about 420 nm within 5 s, remaining quite stable from 5 s to 30 min. As a consequence, the interconversion from MC to Al3+-MC was an immediate response, thus providing a new real-time method for aluminum detection.
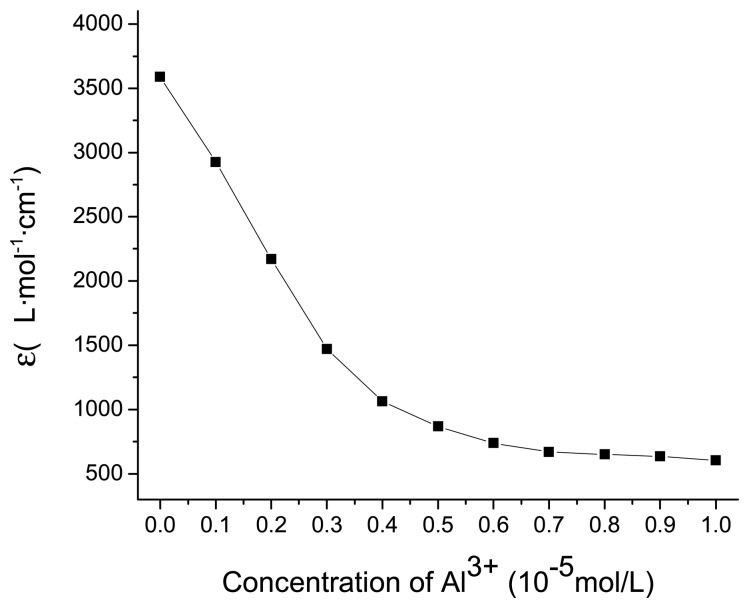
3.5. Detection limits
As mentioned above, the C-O bond of spiropyran at the spiro center can be cleaved upon irradiation and converted into merocyanine form, which chelated with aluminum ions, resulting in obvious color changes from red to pale yellow. Thus for spiropyran a highly sensitive colorimeter based on absorbance changed at 539 nm can be proposed as aluminum chemosensor. As shown in Figure 5, the chemosensor exhibited a good response for the aluminum ion with a linear working range from 0 to 4 × 10−6 mol/L. Thus micromolar concentrations of aluminum ion were sufficient to impose a detectable change on the absorption intensity, and the lowest detection limit was 5.0 × 10−7 mol/L.
Figure 5.
Absorbance changes at 539 nm for spiropyran (1.0 × 10−5 mol/L, 298 K MeCN/H2O = 6:4, V/V) with irradiation for 10 min at 365 nm light in the presence of different concentrations of Al3+.
3.6. Reversibility and stability
The long term stability was tested by setting the system in the dark, and the signal recorded at wavelength of 539 nm over a period of about 90 min. The results showed that no significant changes occurred during this time after the merocyanine chelated with aluminum ion. The spiropyran sensor preserved their performance characteristics unchanged for 90 min.
Furthermore, the reversibility of spiropyran sensor was tested by irradiation at a difference wavelength. As shown in Scheme 1, after irradiation for 10 min at 365 nm, the band at 539 nm was enhanced, then with addition of aluminum ions, a decrease of the absorption spectrum at 539 nm occurred, and the band at about 420 nm appeared simultaneously. Also after irradiation by visible light, a loss of the absorption spectrum at 420 nm occurred and came back to the initial spectrum before irradiation at 365 nm (Figure 2). As a result, the sensor gave good reproducibility of the reversible process. The merocyanine was in the thermally unstable open form, and converted into the original closed form within several minutes in the dark or by irradiation at visible light. With addition of aluminum ions, the metal-ion complexation with MC showed extraordinary thermally stability, resulting in a difference in the color fading to that of the open form of spiropyran. Thus aluminum ions can also control the merocyanine returning to the closed form by thermal fading.
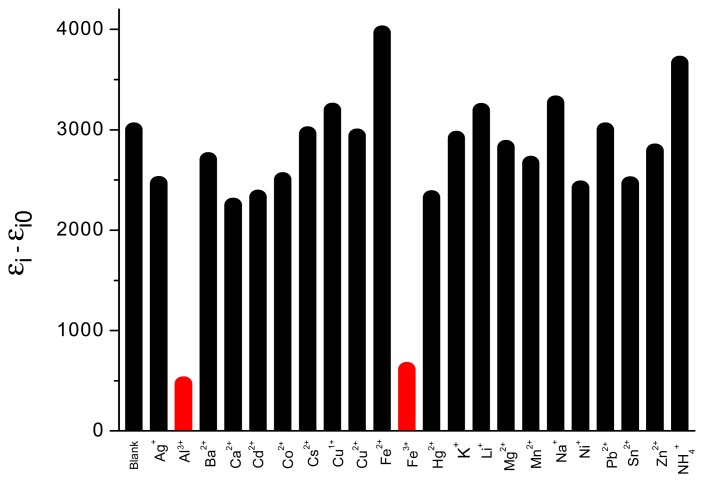
3.7. Selectivity
Selectivity is the sensor's most important characteristic as it determines the extent of its utility in real sample measurement. The colorimetric detection of Al3+ in a mixture of MeCN and H2O was generally complicated by the interference from some metal ions. Figure 6 illustrated the spectroscopic response of the spiropyran sensor following addition of a variety of metal ions in aqueous solution (including10-fold excess Li+, Na+, K+, Mg2+, Ca2+, Cu2+, Ba2+, Fe2+, Hg2+, Cd2+, Cr2+, Pb2+, Co2+, Ni2+, Cs+, Ag+, NH4+and 1-fold excess Sn2+), which indicated that the spiropyran chemosensor was essentially unaffected by the presence of these common ions except for the Fe3+ ion. Notably, the characteristic absorption peak at 539 nm for the MC form only disappeared in the presence of Al3+ and Fe3+, and was virtually insensitive to other cations. Differential pulse voltammetric experiment was performed in the MeCN/H2O mixture of the spiropyran with irradiation and addition of Al3+, Fe3+. Redox potentials of the spiropyran upon irradiation at 365 nm for 10 min and addition of Al3+ was found to be 1.16 V, and 1.07 V for Fe3+, respectively. The oxidation peak was shifted anodically by 90 mV. Therefore, the ability of the spiropyran sensor to electrochemically distinguish Al3+ from Fe3+ provided high efficiency and good selectivity.
Figure 6.
Absorbance changes at 539 nm for spiropyran (1.0 × 10−5 mol/L, 298 K, MeCN/H2O = 6:4, V/V) with irradiation for 10 min at 365 nm light (blank sample), and in the presence of different metal ions (1 eq). Note: εi0 was the absorption intensity at 539 nm with different ions without irradiation; εi was the absorption intensity at 539 nm with different ions with irradiation for 10 min at 365 nm.
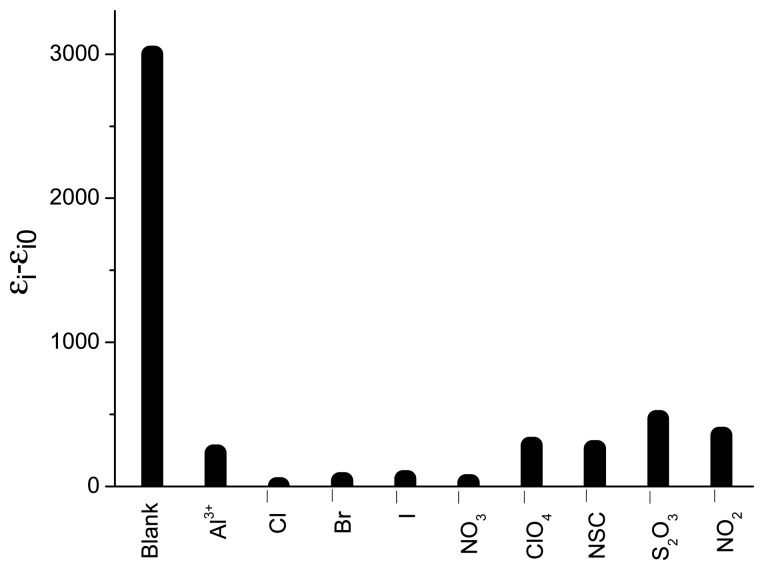
Also, the interference of foreign anions on the determination of Al3+ in MeCN/H2O was investigated experimentally, and the results (Figure 7) show that the presence of 10-fold excesses of Cl−, Br−, I−, NO2−, NO3−, SCN−, SO32− and S2O32− did not interfere in the determination of Al3+ at 1 ×10 −5 mol/L.
Figure 7.
Absorbance changes at 539 nm for spiropyran (1.0 × 10−5 mol/L, 298 K, MeCN/ H2O = 6:4, V/V) with irradiation for 10 min at 365 nm light in the absence of Al3+ (blank sample), and in the presence of Al3+(Al3+) or with other common anions (1 eq). Note: εi0 was the absorption intensity at 539 nm with different anions without irradiation in the presence of Al3+; εi was the absorption intensity at wavelength 539 nm with different anions with irradiation for 10 min at 365 nm in the presence of Al3+.
4. Conclusion
A highly sensitive and selective sensor for the aluminum ion based upon a photochromic spiropyran has been synthesized and studied. This spiropyran showed photochromic characteristics. After irradiation at 365 nm, the SP form transformed into the MC form, which chelated with Al3+, resulting in obvious changes in color. It showed a very fast response within 5 sec and could be detected by the naked eye. The metal ion chelated complex (Al3+-MC) can be converted into original SP form by irradiation at visible light. Thus, the spiropyran sensor provided a new, reversible real-time photodriven method for Al3+ detection, with the aluminum ion controling the fading process of spiropyran by thermal fading. The detection limit of this sensor is 5.0×10−7 mol/L. It fully meets the sensitivity standard by National Secondary Drinking Water Regulations (0.05 to 0.2 mg/L). Notably, the characteristic absorption peak changes for the spiropyran sensor can be observed only in the presence of Al3+, virtually insensitive to other cations or anions. The results clearly demonstrate that a highly selective detection system for an analyte can be developed with potential applications in a variety of settings requiring rapid and accurate analysis for the aluminum ion.
Supplementary data
The experimental details, synthetic, spectroscopic and electrochemical data are available in supplementary data.
Acknowledgments
This work was supported by NSFC/ China (50673025, 90401026), National Basic Research 973 Program (2006CB806200) and Scientific Committee of Shanghai.
References and Notes
- 1.Gensemer R.W., Playle R.C. The bioavailability and toxicity of aluminum in aquatic environments. Crit. Rev. Environ. Sci. Technol. 1999;29:315–450. [Google Scholar]
- 2.Rondeau V., Commenges D. In: Aluminum and Alzheimer's Disease: The Science that Describes the Link. Exley C., editor. Elsevier Press; London: 2001. pp. p. 59–73. [Google Scholar]
- 3.Flaten T.P. Aluminium as a risk factor in Alzheimer's disease, with emphasis on drinking water. Brain Res. Bull. 2001;55:187–196. doi: 10.1016/s0361-9230(01)00459-2. [DOI] [PubMed] [Google Scholar]
- 4.Lin T.W., Huang S.D. Direct and simultaneous determination of copper, chromium, aluminum, and manganese in urine with a multielement graphite furnace atomic absorption spectrometer. Anal. Chem. 2001;73:4319–4325. doi: 10.1021/ac010319h. [DOI] [PubMed] [Google Scholar]
- 5.Tangen G., Wickstrom T., Lierhagen S., Vogt R., Lund W. Fractionation and determination of aluminum and iron in soil water samples using SPE cartridges and ICP-AES. Environ. Sci. Technol. 2002;36:5421–5425. doi: 10.1021/es020077i. [DOI] [PubMed] [Google Scholar]
- 6.Bocca B., Alimonti A., Petrucci F., Violante N., Sancesario G., Forte G., Senofonte O. Quantification of trace elements by sector field inductively coupled plasma mass spectrometry in urine, serum, blood and cerebrospinal fluid of patients with Parkinson's disease. Spectrochimica Acta Part B. 2004;59:559–566. [Google Scholar]
- 7.Measures C.I., Edmond J.M. Shipboard determination of aluminum in seawater at the nanomolar level by electron capture detection gas chromatography. Anal. Chem. 1989;61:544–547. [Google Scholar]
- 8.Hara H., Kobayashi H., Maeda M., Ueno A., Kobayashi Y. Speciation of aluminum in rainwater using a fluoride ion-selective electrode and ion-exchange chromatography with fluorometric detection of the aluminum-lumogallion complex. Anal. Chem. 2001;73:5590–5595. doi: 10.1021/ac010428w. [DOI] [PubMed] [Google Scholar]
- 9.Lian H., Kang Y., Bi S., Arkin Y., Shao D., Li D., Chen Y., Dai L., Gan N., Tian L. Direct determination of trace aluminum with quercetin by reversed-phase high performance liquid chromatography. Talanta. 2004;62:43–50. doi: 10.1016/S0039-9140(03)00405-3. [DOI] [PubMed] [Google Scholar]
- 10.Yari A., Darvishi L., Shamsipur M. Al(III)-selective electrode based on newly synthesized xanthone derivative as neutral ionophore. Anal. Chim. Acta. 2006;555:329–335. [Google Scholar]
- 11.Di J., Bi S., Yang T., Zhang M. Voltammetric determination of aluminum(III) using a reagentless sensor fabricated by sol–gel process. Sens. Actuators, B. 2004;99:468–473. [Google Scholar]
- 12.Gupta V.K., Jain A.K., Maheshwari G. Aluminum(III) selective potentiometric sensor based on morin in poly(vinyl chloride) matrix. Talanta. 2007;72:1469–1473. doi: 10.1016/j.talanta.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 13.Carroll M.K., Bright F.V., Hieftje G.M. Fiber-optic time-resolved fluorescence sensor for the simultaneous determination of Al3+ and Ga3+ or In3+ Anal. Chem. 1989;61:1768–1772. [Google Scholar]
- 14.Zhang J., Xu H., Ren J.L. Fluorimetric determination of dissolved aluminium in natural waters after liquid–liquid extraction into n-hexanol. Anal. Chim. Acta. 2000;405:31–42. [Google Scholar]
- 15.Park C.I., Cha K.W. Spectrofluorimetric method for determination of aluminium with chromotropic acid and its application to water samples. Talanta. 2000;51:769–774. doi: 10.1016/s0039-9140(99)00351-3. [DOI] [PubMed] [Google Scholar]
- 16.Tóth I.V., Rangel A.O.S.S., Santos J. L. M., Lima J.L.F.C. Determination of aluminum(III) in crystallized fruit samples using a multicommutated flow system. J. Agric. Food Chem. 2004;52:2450–2454. doi: 10.1021/jf049812v. [DOI] [PubMed] [Google Scholar]
- 17.Albendín G., Míánuel-Vez M.P., Moreno C., García-Vargas M. Reverse flow-injection manifold for spectrofluorimetric determination of aluminum in drinking water. Talanta. 2003;60:425–431. doi: 10.1016/S0039-9140(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 18.Szunerits S., Walt D.R. Aluminum surface corrosion and the mechanism of inhibitors using pH and metal ion selective imaging fiber bundles. Anal. Chem. 2002;74:886–894. doi: 10.1021/ac0108257. [DOI] [PubMed] [Google Scholar]
- 19.Joshi B. P., Cho W.M., Kim J., Yoon J., Lee K.H. Design, synthesis, and evaluation of peptidyl fluorescent probe for Zn2+in aqueous solution. Bioorg. Med. Chem. Lett. 2007 doi: 10.1016/j.bmcl.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Ha-Thi M.H., Penhoat M., Michelet V., Leray I. Highly selective and sensitive phosphane sulfide derivative for the detection of Hg2+ in an organoaqueous medium. Org. Lett. 2007;9:1133–1136. doi: 10.1021/ol070118l. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y., Orbulescu J., Ji X., Andreopoulos F.M., Pham S.M., Leblanc R.M. Development of fluorescent film sensors for the detection of divalent copper. J. Am. Chem. Soc. 2003;125:2680–2686. doi: 10.1021/ja0293610. [DOI] [PubMed] [Google Scholar]
- 22.Collins G.E., Choi L.S., Ewing K.J., Michelet V., Bowen C.M., Winkler J.D. Photoinduced switching of metal complexation by quinolinospiropyranindolines in polar solvents. Chem. Commun. 1999:321–322. [Google Scholar]
- 23.Winkler J.D., Bowen C.M., Michelet V. Photodynamic fluorescent metal ion sensors with parts per billion sensitivity. J. Am. Chem. Soc. 1998;120:3237–3242. [Google Scholar]
- 24.Berkovic G., Krongauz V., Weiss V. Spiropyrans and spirooxazines for memories and switches. Chem. Rev. 2000;100:1741–1753. doi: 10.1021/cr9800715. [DOI] [PubMed] [Google Scholar]
- 25.Kawata S., Kawata Y. Three-dimensional optical data storage using photochromic materials. Chem. Rev. 2000;100:1777–1788. doi: 10.1021/cr980073p. [DOI] [PubMed] [Google Scholar]
- 26.Shipway A. N., Willner I. Electronically transduced molecular mechanical and information functions on surfaces. Acc. Chem. Res. 2001;34:421–432. doi: 10.1021/ar000180h. [DOI] [PubMed] [Google Scholar]
- 27.Raymo F.M., Giordani S. Signal processing at the molecular level. J. Am. Chem. Soc. 2001;123:4651–4652. doi: 10.1021/ja005699n. [DOI] [PubMed] [Google Scholar]
- 28.Wojtyk J.T.C., Kazmaier P.M., Buncel E. Modulation of the spiropyran-merocyanine reversion via metal-ion selective complexation: trapping of the “transient” cis-merocyanine. Chem. Mater. 2001;13:2547–2551. [Google Scholar]
- 29.Raymo F. M. Digital processing and communication with molecular switches. Adv. Mater. 2002;14:401–414. [Google Scholar]
- 30.Inouye M., Akamatsu K., Nakzumi H. New crown spirobenzopyrans as light- and ion-responsive dual-mode signal transducers. J. Am. Chem. Soc. 1997;119:9160–9165. [Google Scholar]
- 31.Wojtyk J.T.C., Kazmaier P.M., Buncel E. Effects of metal ion complexation on the spiropyran–merocyanine interconversion: development of a thermally stable photo-switch. Chem. Commun. 1998:1703–1704. [Google Scholar]
- 32.Andréasson J., Straight S. D., Kodis G., Park C. D., Hambourger M., Gervaldo M., Albinsson B., Moore T. A., Moore A. L., Gust D. All-photonic molecular half-adder. J. Am. Chem. Soc. 2006;128(50):16259–16265. doi: 10.1021/ja0654579. [DOI] [PubMed] [Google Scholar]
- 33.Guo X., Zhou Y., Zhang D., Yin B., Liu Z., Liu C., Lu Z., Huang Y., Zhu D. 7-Trifluoromethylquinoline-functionalized luminescent photochromic spiropyran with the stable merocyanine species both in solution and in the solid state. J. Org. Chem. 2004;69:8924–8931. doi: 10.1021/jo0487799. [DOI] [PubMed] [Google Scholar]
- 34.Guo X., Zhang D., Zhu D. Logic control of the fluorescence of a new dyad, spiropyran-perylene diimide-spiropyran, with light, ferric ion, and proton: construction of a new three- input “AND” logic gate. Adv. Mater. 2004;16:125–130. [Google Scholar]
- 35.Roxburgh C. J., Sammes P.G. Synthesis of some new substituted photochromic N, N'-bis (spiro[1-benzopyran-2, 2′-indolyl]) diazacrown systems with substituent control over ion chelation. Eur. J. Org. Chem. 2006:1050–1056. [Google Scholar]
- 36.Shao N., Zhang Y., Cheung S., Yang R., Chan W., Mo T., Li K., Liu F. Copper ion-selective fluorescent sensor based on the inner filter effect using a spiropyran derivative. Anal. Chem. 2005;77:7294–7303. doi: 10.1021/ac051010r. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura M., Fujioka T., Sakamoto H., Kimura K. High stability constants for multivalent metal ion complexes of crown ether derivatives incorporating two spirobenzopyran moieties. New J. Chem. 2002;26:554–559. [Google Scholar]
- 38.Yuan W., Sun L., Tang H., Wen Y., Jiang G., Huang W., Jiang L., Song Y., Tian H., Zhu D. A novel thermally stable spironaphthoxazine and its application in rewritable high density optical data storage. Adv. Mater. 2005;17:156–160. [Google Scholar]
- 39.Dürr H. Perspectives in Photochromism: A novel system based on the 1, 5-electrocyclization of heteroanalogous pentadienyl anions. Angew. Chem., Int. Ed. Engl. 1989;28:413–431. [Google Scholar]
- 40.Mousavi M. F., Arvand-Barmchi M., Zanjanchi M. A. Al(III)–selective electrode based on furil as neutral carrier. Electroanalysis. 2001;13:1125–1128. [Google Scholar]