Abstract
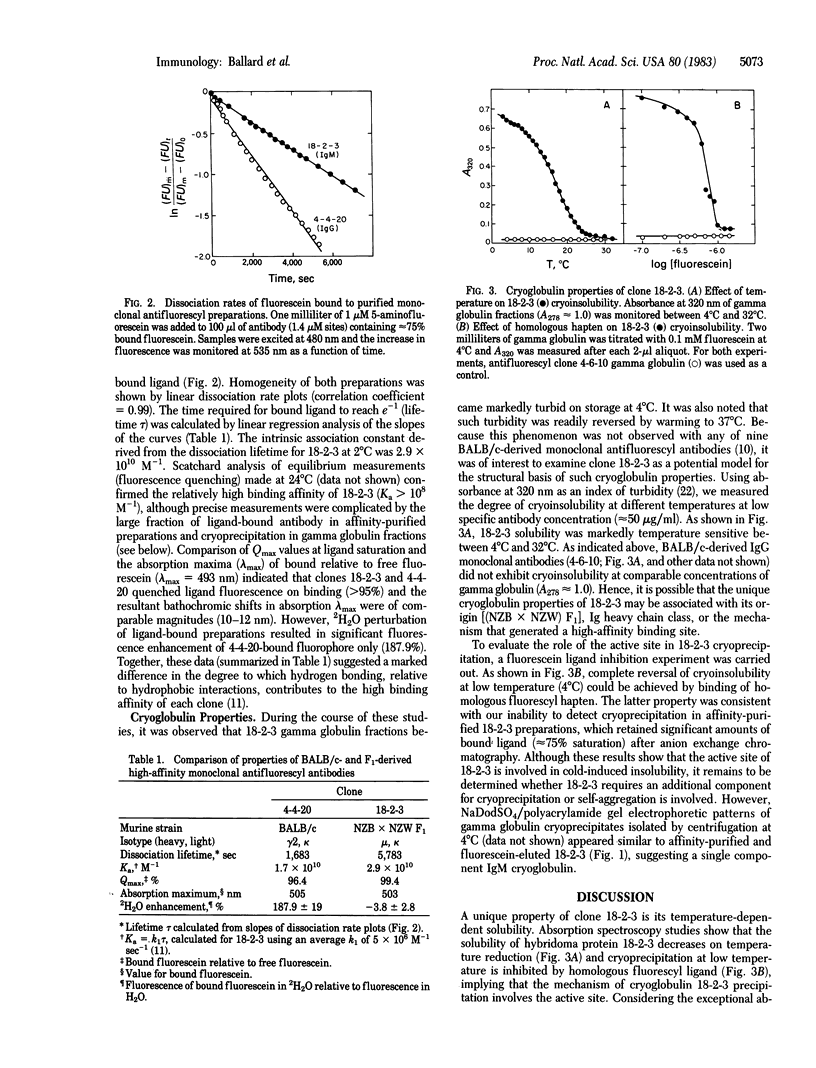
A monoclonal IgM antibody (18-2-3) derived from cell fusion of (NZB X NZW) F1 splenocytes following secondary immunization with fluorescein-conjugated keyhole limpet hemocyanin was shown to exhibit high intrinsic binding affinity and cryoinsolubility. Affinity-purified preparations were determined to be IgM by immunochemical, electrophoretic, and chromatographic analyses. An intrinsic association constant (Ka) of 2.9 X 10(10) M-1 (at 2 degrees C) was measured by first-order dissociation-rate analysis. Antibody solubility at low concentration (approximately equal to 50 micrograms/ml) was shown, by absorption spectroscopy, to be temperature dependent between 4 degrees C and 32 degrees C. Insolubility at low temperature (4 degrees C) was reversible in the presence of homologous fluorescyl hapten, indicative of active site involvement in the mechanism of cryoglobulin-18-2-3 complex formation. Characteristics of clone 18-2-3 are discussed in terms of (i) its potential use as a model for examining the mechanism of cryoprecipitation and (ii) the proposed relationship between affinity maturation and the IgM to IgG class switch.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Chang S. P., Brown M., Rittenberg M. B. Immunologic memory to phosphorylcholine III. IgM includes a fine specificity population distinct from TEPC 15. J Immunol. 1982 Oct;129(4):1559–1562. [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Davie J. M., Osterland C. K., Miller E. J., Krause R. M. Immune cryoglobulins in rabbit streptococcal antiserum. J Immunol. 1968 Apr;100(4):814–820. [PubMed] [Google Scholar]
- Decastel M., Bourrillon R., Frénoy J. P. Cryoinsolubility of peanut agglutinin. Effect of saccharides and neutral salts. J Biol Chem. 1981 Sep 10;256(17):9003–9008. [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Erickson B. W., Gerber-Jenson B., Wang A. C., Litman G. W. Molecular basis for the temperature-dependent insolubility of cryoglobulins--XI. Sequence comparison of the heavy-chain variable regions of the human cryoimmunoglobulins McE and Hil by metric analysis. Mol Immunol. 1982 Mar;19(3):357–365. doi: 10.1016/0161-5890(82)90201-2. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Hijmans W., Radema H., van Es L., Feltkamp T. E., van Loghem J. J., Schaap O. L. Cryoglobulins in New Zealand Black mice. Clin Exp Immunol. 1969 Feb;4(2):227–239. [PMC free article] [PubMed] [Google Scholar]
- Howie J. B., Helyer B. J. The immunology and pathology of NZB mice. Adv Immunol. 1968;9:215–266. doi: 10.1016/s0065-2776(08)60444-7. [DOI] [PubMed] [Google Scholar]
- Jarvis M. R., Casperson G. F., Kranz D. M., Voss E. W., Jr Affinity maturation of NZB and BALB/cV mice anti-fluorescyl response. Mol Immunol. 1982 Apr;19(4):525–533. doi: 10.1016/0161-5890(82)90220-6. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Herron J. N., Giannis D. E., Voss E. W., Jr Kinetics and mechanism of deuterium oxide-induced fluorescence enhancement of fluorescyl ligand bound to specific heterogeneous and homogeneous antibodies. J Biol Chem. 1981 May 10;256(9):4433–4438. [PubMed] [Google Scholar]
- Kranz D. M., Herron J. N., Voss E. W., Jr Mechanisms of ligand binding by monoclonal anti-fluorescyl antibodies. J Biol Chem. 1982 Jun 25;257(12):6987–6995. [PubMed] [Google Scholar]
- Kranz D. M., Voss E. W., Jr Partial elucidation of an anti-hapten repertoire in BALB/c mice: comparative characterization of several monoclonal anti-fluorescyl antibodies. Mol Immunol. 1981 Oct;18(10):889–898. doi: 10.1016/0161-5890(81)90012-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loh E., Black B., Riblet R., Weigert M., Hood J. M., Hood L. Myeloma proteins from NZB and BALB/c mice: structural and functional differences. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1395–1399. doi: 10.1073/pnas.76.3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin D. E., Voss E. W., Jr Fluorescein. Hapten and antibody active-site probe. Biochemistry. 1971 Jan 19;10(2):208–213. doi: 10.1021/bi00778a003. [DOI] [PubMed] [Google Scholar]
- Portmann A. J., Levison S. A., Dandliker W. B. Anti-fluorescein antibody of high affinity and restricted heterogeneity as characterized by fluorescence polarization and quenching equilibrium techniques. Biochem Biophys Res Commun. 1971 Apr 2;43(1):207–212. doi: 10.1016/s0006-291x(71)80108-0. [DOI] [PubMed] [Google Scholar]
- Rodwell J. D., Karush F. Restriction in IgM expression--I. The VH regions of equine anti-lactose antibodies. Mol Immunol. 1980 Dec;17(12):1553–1561. doi: 10.1016/0161-5890(80)90181-9. [DOI] [PubMed] [Google Scholar]
- Saulk P. H., Clem W. Studies on the cryoprecipitation of a human IGG3 cryoglobulin: the effects of temperature-induced comformational changes on the primary interaction. Immunochemistry. 1975 Jan;12(1):29–37. doi: 10.1016/0019-2791(75)90046-4. [DOI] [PubMed] [Google Scholar]
- Shirai T., Hayakawa K., Okumura K., Tada T. Differential cytotoxic effect of natural thymocytotoxic autoantibody of NZB mice on functional subsets of T cells. J Immunol. 1978 Jun;120(6):1924–1929. [PubMed] [Google Scholar]
- Shirai T., Mellors R. C. Natural thymocytotoxic autoantibody and reactive antigen in New Zealand black and other mice. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1412–1415. doi: 10.1073/pnas.68.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Voss E. W., Jr, Eschenfeldt W., Root R. T. Fluorescein: a complete antigenic group. Immunochemistry. 1976 May;13(5):447–453. doi: 10.1016/0019-2791(76)90382-7. [DOI] [PubMed] [Google Scholar]
- Voss E. W., Jr, Watt R. M., Weber G. Solvent perturbation of the fluorescence of fluorescyl ligand bound to specific antibody. Fluorescence enhancement of antibody bound fluorescein (hapten) in deuterium oxide. Mol Immunol. 1980 Apr;17(4):505–517. doi: 10.1016/0161-5890(80)90090-5. [DOI] [PubMed] [Google Scholar]
- Watt R. M., Herron J. N., Voss E. W., Jr First order dissociation rates between a subpopulation of high affinity rabbit anti-fluorescyl IgG antibody and homologous ligand. Mol Immunol. 1980 Oct;17(10):1237–1243. doi: 10.1016/0161-5890(80)90020-6. [DOI] [PubMed] [Google Scholar]
- Watt R. M., Voss E. W., Jr Characterization of affinity-labeled fluorescyl ligand to specifically-purified rabbit IgG antibodies. Immunochemistry. 1978 Dec;15(12):875–882. doi: 10.1016/0161-5890(78)90121-9. [DOI] [PubMed] [Google Scholar]
- Watt R. M., Voss E. W., Jr Mechanism of quenching of fluorescein by anti-fluorescein IgG antibodies. Immunochemistry. 1977 Jul;14(7):533–551. doi: 10.1016/0019-2791(77)90308-1. [DOI] [PubMed] [Google Scholar]