Abstract
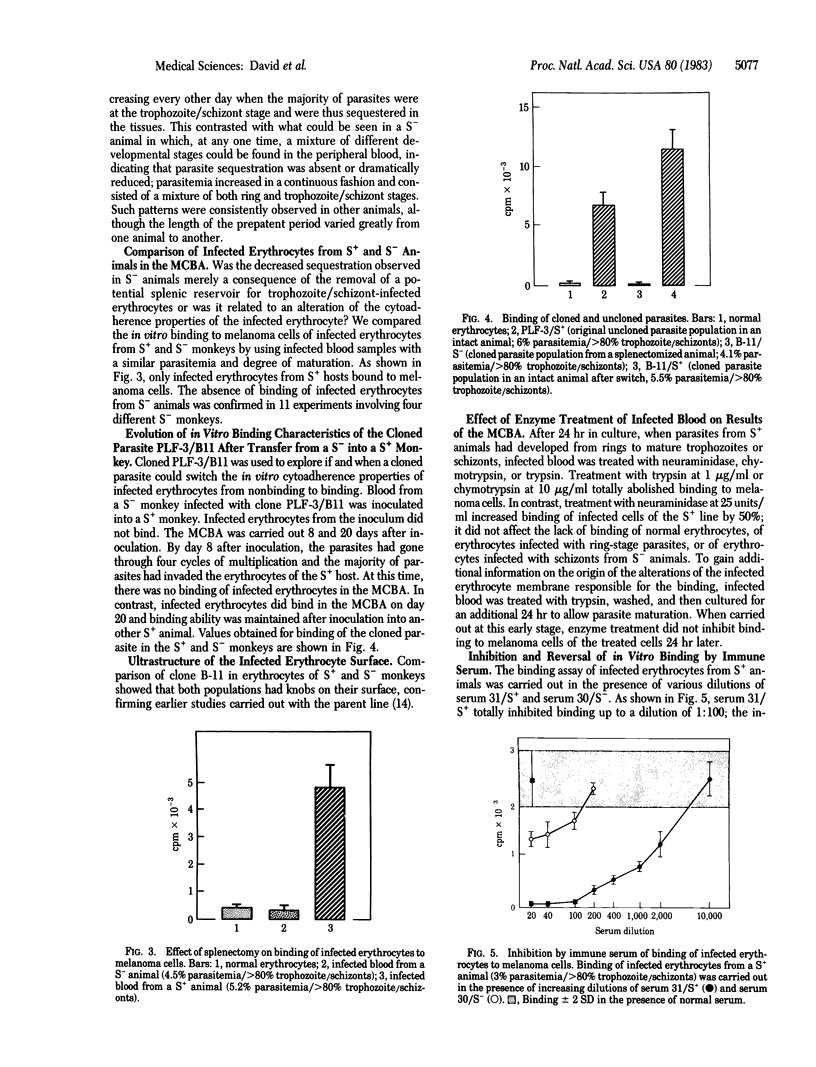
Sequestration, the adherence of infected erythrocytes containing late developmental stages of the parasite (trophozoites and schizonts) to the endothelium of capillaries and venules, is characteristic of Plasmodium falciparum infections. We have studied two host factors, the spleen and antibody, that influence sequestration of P. falciparum in the squirrel monkey. Sequestration of trophozoite/schizont-infected erythrocytes that occurs in intact animals is reduced in splenectomized animals; in vitro, when infected blood is incubated with monolayers of human melanoma cells, trophozoite/schizont-infected erythrocytes from intact animals but not from splenectomized animals bind to the melanoma cells. The switch in cytoadherence characteristics of the infected erythrocytes from nonbinding to binding occurs with a cloned parasite. Immune serum can inhibit and reverse in vitro binding to melanoma cells of infected erythrocytes from intact animals. Similarly, antibody can reverse in vivo sequestration as shown by the appearance of trophozoite/schizont-infected erythrocytes in the peripheral blood of an intact animal after inoculation with immune serum. These results indicate that the spleen modulates the expression of parasite alterations of the infected erythrocyte membrane responsible for sequestration and suggest that the prevention and reversal of sequestration could be one of the effector mechanisms involved in antibody-mediated protection against P. falciparum malaria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnwell J. W., Desowitz R. S. Studies on parasitic crisis in malaria: I. Signs of impending crisis in Plasmodium berghei infections of the white rat. Ann Trop Med Parasitol. 1977 Dec;71(4):429–433. doi: 10.1080/00034983.1977.11687208. [DOI] [PubMed] [Google Scholar]
- David P. H., Hommel M., Oligino L. D. Interactions of Plasmodium falciparum infected erythrocytes with ligand-coated agarose beads. Mol Biochem Parasitol. 1981 Dec;4(3-4):195–204. doi: 10.1016/0166-6851(81)90018-9. [DOI] [PubMed] [Google Scholar]
- Diggs C., Joseph K., Flemmings B., Snodgrass R., Hines F. Protein synthesis in vitro by cryopreserved Plasmodium falciparum. Am J Trop Med Hyg. 1975 Sep;24(5):760–763. doi: 10.4269/ajtmh.1975.24.760. [DOI] [PubMed] [Google Scholar]
- Garnham P. C. The role of the spleen in protozoal infections with special reference to splenectomy. Acta Trop. 1970;27(1):1–14. [PubMed] [Google Scholar]
- Gysin J., Hommel M., da Silva L. P. Experimental infection of the squirrel monkey (Saimiri sciureus) with Plasmodium falciparum. J Parasitol. 1980 Dec;66(6):1003–1009. [PubMed] [Google Scholar]
- Hommel M., David P. H., Oligino L. D., David J. R. Expression of strain-specific surface antigens on Plasmodium falciparum-infected erythrocytes. Parasite Immunol. 1982 Nov;4(6):409–419. doi: 10.1111/j.1365-3024.1982.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Hommel M., David P. H., Oligino L. D. Surface alterations of erythrocytes in Plasmodium falciparum malaria. Antigenic variation, antigenic diversity, and the role of the spleen. J Exp Med. 1983 Apr 1;157(4):1137–1148. doi: 10.1084/jem.157.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieda C. M., Bowles D. J., Ravid A., Sharon N. Lectins in lymphocyte membranes. FEBS Lett. 1978 Oct 15;94(2):391–396. doi: 10.1016/0014-5793(78)80985-5. [DOI] [PubMed] [Google Scholar]
- Kilejian A., Abati A., Trager W. Plasmodium falciparum and Plasmodium coatneyi: immunogenicity of "knob-like protrusions" on infected erythrocyte membranes. Exp Parasitol. 1977 Jun;42(1):157–164. doi: 10.1016/0014-4894(77)90073-x. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Reese R. T. Antigenicity of the infected-erythrocyte and merozoite surfaces in Falciparum malaria. J Exp Med. 1979 Nov 1;150(5):1241–1254. doi: 10.1084/jem.150.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luse S. A., Miller L. H. Plasmodium falciparum malaria. Ultrastructure of parasitized erythrocytes in cardiac vessels. Am J Trop Med Hyg. 1971 Sep;20(5):655–660. [PubMed] [Google Scholar]
- Miller L. H. Distribution of mature trophozoites and schizonts of Plasmodium falciparum in the organs of Aotus trivirgatus, the night monkey. Am J Trop Med Hyg. 1969 Nov;18(6):860–865. doi: 10.4269/ajtmh.1969.18.860. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Udeinya I. J., Leech J. H., Hay R. J., Aikawa M., Barnwell J., Green I., Miller L. H. Plasmodium falciparum malaria. An amelanotic melanoma cell line bears receptors for the knob ligand on infected erythrocytes. J Clin Invest. 1982 Aug;70(2):379–386. doi: 10.1172/JCI110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Theakston R. D. Comments on the ultrastructure of human erythrocytes infected with Plasmodium malariae. Ann Trop Med Parasitol. 1970 Sep;64(3):329–329. doi: 10.1080/00034983.1970.11686700. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W., Rudzinska M. A., Bradbury P. C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35(6):883–885. [PMC free article] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]




