Abstract
Activity of presympathetic neurons in the paraventricular nucleus (PVN) of the hypothalamus is known to play an important role in the regulation of sympathetic outflow. Sympathetic overactivity is associated with many pathophysiological conditions such as diabetes mellitus and hypertension; however, the underlying synaptic mechanisms are poorly understood. In this study, we examined the GABAergic inhibitory synaptic control of kidney-related presympathetic PVN neurons in the streptozotocin-treated type 1 diabetic mouse model, using patch-clamp slice electrophysiology in combination with retrograde labeling. Type 1 diabetes resulted in decreased frequency of miniature inhibitory postsynaptic currents (mIPSCs). Our data also demonstrated a reduction of mIPSC amplitude and mean inhibitory current without alteration of input resistance. Furthermore, our data revealed decreased tonic GABAergic inhibition of kidney-related PVN neurons in diabetic conditions, which was consistent with the observed increased excitability of the presympathetic PVN neurons. In summary, our data demonstrated decreased phasic and tonic inhibitory control of kidney-related presympathetic PVN neurons that suggest altered sympathetic circuitry in type 1 diabetes.
Keywords: paraventricular nucleus, presympathetic neurons, type 1 diabetes, pseudorabies virus, patch clamp
preautonomic neurons in the paraventricular nucleus (PVN) of the hypothalamus play a significant role in the control of autonomic nervous system, thus influencing homeostatic functions (Chen and Toney 2003; Ciriello et al. 1984; Huang and Weiss 1999; LaGrange et al. 2003; Park et al. 2009; Swanson and Sawchenko 1980; Uyama et al. 2004; Yi et al. 2010). Presympathetic neurons in the PVN, through their projections to the brain stem and spinal cord, govern sympathetic outflow (Chen and Toney 2010; Kenney et al. 2003; Li and Pan 2007a; Pyner 2009). Increased sympathetic activity is a well-known characteristic of many pathophysiological conditions including diabetes mellitus and hypertension, and impairment of presympathetic PVN neurons could contribute to the development and progression of elevated sympathetic outflow. For instance, the kidney is regulated by the renal sympathetic nerves, and any alteration in the renal sympathetic activity impacts the tone of renal vessels and thereby affects blood pressure (Mifflin 2001).
In the central nervous system, excitatory and inhibitory inputs regulate the activity of neurons. In vivo studies established that bilateral microinjection of GABAA receptor agonists into the PVN decreased baseline renal sympathetic nerve activity, heart rate, and mean arterial pressure (Li and Pan 2007b; Zhong et al. 2008). In contrast, administration of GABAA receptor antagonists into the PVN increased baseline renal sympathetic nerve activity and blood pressure (Kannan and Yamashita 1985; Li and Pan 2006; Zhong et al. 2008), indicating an existing persistent inhibition of presympathetic PVN neurons. PVN hyperactivity is known to increase sympathetic nerve activity in hypertensive rats (Ciriello et al. 1984; Herzig et al. 1991; Takeda et al. 1991), and removal of GABAergic inhibition results in augmented glutamatergic inputs within the PVN (Li and Pan 2007a, 2007b). These observations indicate the importance of GABAergic inhibition of presympathetic PVN neurons and suggest that decreased GABAergic inhibition could play a role in the elevation of sympathetic activity.
At the cellular level, excitatory and inhibitory synaptic currents could be divided into conventional phasic and persistent tonic currents (Nusser and Mody 2002; Okamoto et al. 2009). Phasic synaptic currents are outcome of neurotransmitter release at the synaptic cleft that activates ionotropic receptors, resulting in postsynaptic currents. On the other hand, neurotransmitter diffusing away from the synaptic cleft after being released can activate extrasynaptic ionotropic receptors. Continuous activation of extrasynaptic ionotropic receptors generates a persistent, tonic current. Although the role of tonic current is still largely speculative, this tonic current may play a role in synaptic integration by changing membrane input conductance and neuronal excitability (Nusser and Mody 2002; Nusser et al. 1995, 1998; Semyanov et al. 2003, 2004).
Diabetes mellitus, obesity, and metabolic syndrome are among the risk factors in the development of increased sympathetic activity that could contribute to the development and progression of hypertension. Interestingly, already in the early stages of the disease, autonomic dysfunction has been observed in diabetic patients (Spallone et al. 1994). Because the sympathetic outflow is increased in diabetic conditions and GABAergic inhibitory control of presympathetic PVN neurons plays an important role in the regulation of sympathetic outflow, we hypothesized that the inhibitory regulation of kidney-related presympathetic PVN neurons are reduced in streptozotocin-treated hyperglycemic mice.
In the present study, by using pseudorabies virus 152 (PRV-152), we identified kidney-related presympathetic PVN neurons and determined the consequences of type 1 diabetes on the GABAergic inhibitory control. Our study demonstrated increased excitation and reduced inhibitory control of kidney-related presympathetic PVN neurons in streptozotocin-treated hyperglycemic mice. This finding provides evidence that altered GABAergic regulation could contribute to the development of increased sympathetic outflow during diabetes.
MATERIALS AND METHODS
Animals.
Male CD1 mice (7–8 wk old; Harlan Laboratories) were used in all experiments. Experiments were performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Tulane University's Institutional Animal Care and Use Committee.
PRV-152 injection.
A retrogradely transported pseudorabies viral vector (PRV-152, supplied by Dr. L. W. Enquist) that expresses enhanced green fluorescent protein (EGFP) was used to identify kidney-related neurons. Under anesthesia, the left kidney was exposed and ∼4 μl of PRV-152 were injected into the left renal parenchyma (2 injections at 2 sites). A drop of adhesive “liquid bandage” was used to seal each injection to prevent the leakage of the virus. The animals were maintained in a biosafety level 2 facility up to 88–98 h postinjection.
Streptozotocin injection.
The mice were fasted overnight and then injected intraperitoneally with streptozotocin (STZ; 200 mg/kg) dissolved in 0.1 mol/l citrate buffer. Body weight and blood glucose level (OneTouch Ultra, LifeScan) were monitored before STZ injection and then daily afterward. Mice with glucose level above 300 mg/dl for at least 3 days were considered hyperglycemic and were injected with PRV-152 as described above.
Brain slices preparation.
Acute brain slices were prepared from control and STZ-treated hyperglycemic mice. After anesthetization with isoflurane, the brain was removed and immersed in ice-cold oxygenated artificial cerebrospinal fluid (aCSF) containing the following (in mM): 124 NaCl, 26 NaHCO3, 1.4 NaH2PO4, 11 glucose, 3 KCl, 1.3 MgCl2, and 1.5 CaCl2, pH 7.3–7.4. Transverse hypothalamic slices containing the PVN (300 μm) were made using a vibratome. The slices were stored in a holding chamber at 34–36°C and then transferred to a recording chamber mounted on a fixed stage under an upright microscope (Nikon FN1).
Whole cell patch-clamp recordings.
Whole cell patch-clamp recordings were performed at 34–37°C on kidney-related neurons in the PVN identified under a ×40 water-immersion objective (NA = 0.8). Epifluorescence was used to identify EGFP-containing neurons and infrared illumination and differential interference contrast optics (IR-DIC) to target specific cells. For whole cell patch-clamp recordings, electrodes (3–7 MΩ) were filled with a solution containing the following (in mM): 130 K+- or Cs+-gluconate, 10 HEPES, 5 EGTA, 1 NaCl, 1 MgCl2, 1 CaCl2, 3 KOH or CsOH, and 2–3 Mg-ATP with 0.2% biocytin, pH 7.3–7.4. Electrophysiological signals were recorded using an Axoclamp 700B amplifier (Molecular Devices) and acquired using pClamp (Molecular Devices). Inhibitory postsynaptic currents (IPSCs) were recorded at −10 mV and excitatory postsynaptic currents (EPSCs) at −60 mV. Synaptic currents were analyzed offline using pClamp or MiniAnalysis (Synaptosoft). Tetrodotoxin (TTX; 1 μM; Tocris Bioscience) in the bath solution was used to block action potentials and monitor miniature IPSCs (mIPSCs) and EPSCs (mEPSCs). In specific experiments, in the presence of kynurenic acid (1 mM; Sigma-Aldrich), the GABAA receptor antagonist bicuculline methiodide (30 μM; Tocris Bioscience) was applied to determine tonic inhibitory current in kidney-related PVN neurons.
Statistical analysis.
Continuous recordings were conducted, and 2-min periods were analyzed with MiniAnalysis (Synaptosoft) to measure peak amplitude, frequency, decay time, and area of postsynaptic currents. The mean phasic current (Iphasic) was calculated as follows: Iphasic = frequency × charge transfer (Q), where Q was measured as the area under the IPSCs (Nusser and Mody 2002; Park et al. 2006). Curve fitting for mIPSC decay time was performed using MiniAnalysis as previously described (Gao and Smith, 2010; Yeung et al. 2003). A weighted time constant was calculated with the equation τw = ΣAiτi/ΣAi (Banks and Pearce 2000; Yeung et al. 2003) and used for comparison. Comparison between groups was made with an unpaired two-tailed Student's t-test. For all analyses, P < 0.05 was considered significance. Values are reported as means ± SE.
RESULTS
Within 3 days STZ injection resulted in hyperglycemia. The average glucose level of STZ-treated mice was 498 ± 21 mg/dl (n = 31), whereas that of controls was 172 ± 6 mg/dl. PRV-152 was used to identify presympathetic kidney-related PVN neurons in control and in a mouse model of type 1 diabetes. Our recordings were conducted at 88–98 h postinoculation, and the observed EGFP labeling indicating kidney-related PVN neurons was consistent with previously published data (Cano et al. 2004).
Membrane properties of kidney-related PVN neurons in control and type 1 diabetic mice.
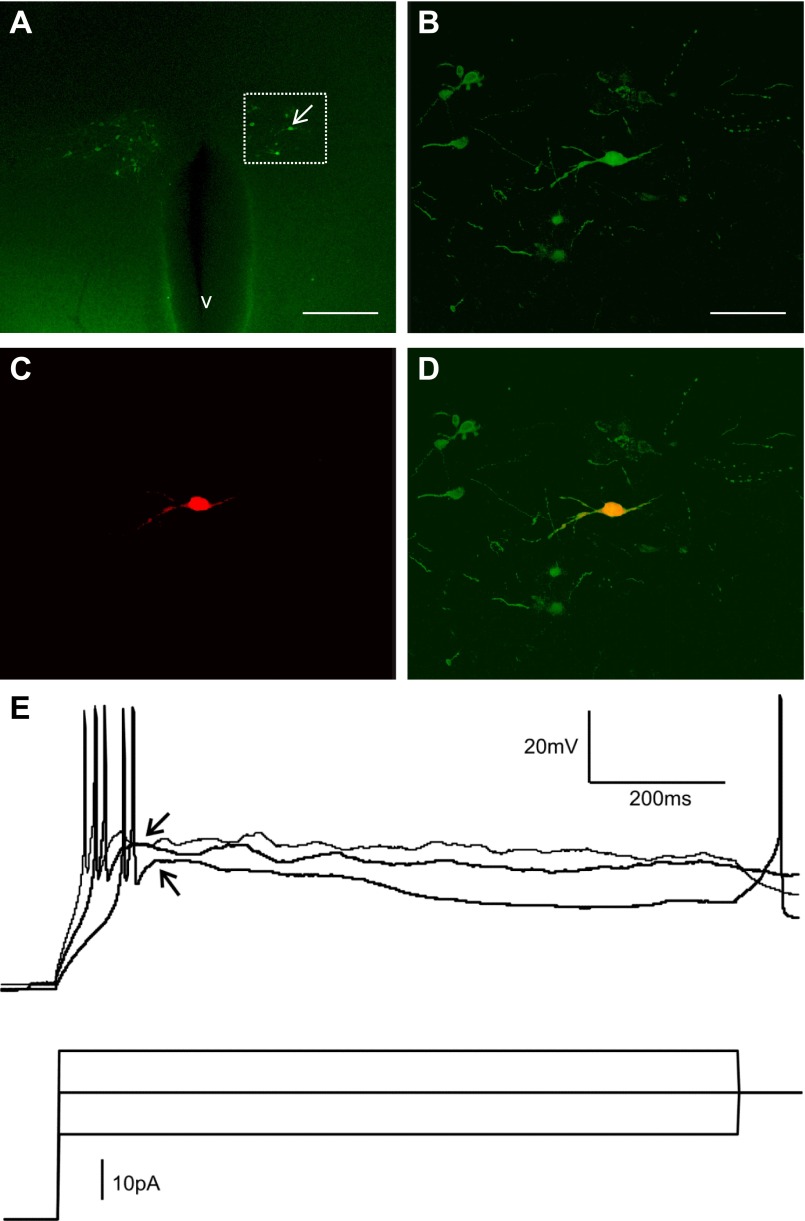
Because very little is known about the membrane properties of kidney-related PVN neurons, first we determined the basic electrophysiological characteristics of kidney-related PVN neurons in control and STZ-treated type 1 diabetic mice. Recordings were conducted from kidney-related PVN neurons. Presympathetic kidney-related PVN neurons were identified on the basis of their green fluorescence (Fig. 1). In some cases we also tested for the presence of low-threshold spikes (LTS) as a characteristic of preautonomic PVN neurons, and we were able to observe LTS in kidney-related PVN neurons that further confirmed their preautonomic nature (Fig. 1E) (Luther et al. 2002; Luther and Tasker 2000; Stern 2001). Resting membrane potentials, input resistance, and action potential frequency were determined in kidney-related PVN neurons of control and STZ-treated hyperglycemic mice.
Fig. 1.
Visualization of kidney-related presympathetic paraventricular nucleus (PVN) neurons. A: hypothalamic section (300 μm) with enhanced green fluorescent protein (EGFP)-labeled kidney-related presympathetic neurons ∼94 h after injection of the left kidney with pseudorabies virus 152 (PRV-152). The arrow points to the recorded cell. B–D: higher magnifications of the boxed area shown in A, including a fluorescence image of a recorded kidney-related PVN neuron after fixation (B), identification of the biocytin labeling with avidin-Texas red conjugate (C), and their merged image (D). E: representative current-clamp recording from a kidney-related PVN neuron that generated low-threshold spike (arrow) in response to depolarizing current pulses following hyperpolarization of the membrane. v, third ventricle. Scale bars: A, 300 μm; B, 75 μm.
In control mice, one of the nine recorded kidney-related PVN neurons was considered as an outlier due to its much higher firing rate and was excluded from the analyses. The resting membrane potential of kidney-related PVN neurons in control mice was −44.6 ± 2.5 mV (range −36 to −53.4 mV, n = 8). The membrane potential of kidney-related PVN neurons in STZ-treated mice was −47.2 ± 1.8 mV (range −40.7 to −56.0 mV, n = 11, P > 0.05), indicating no significant difference in resting membrane potentials between control and hyperglycemic mice (Fig. 2C).
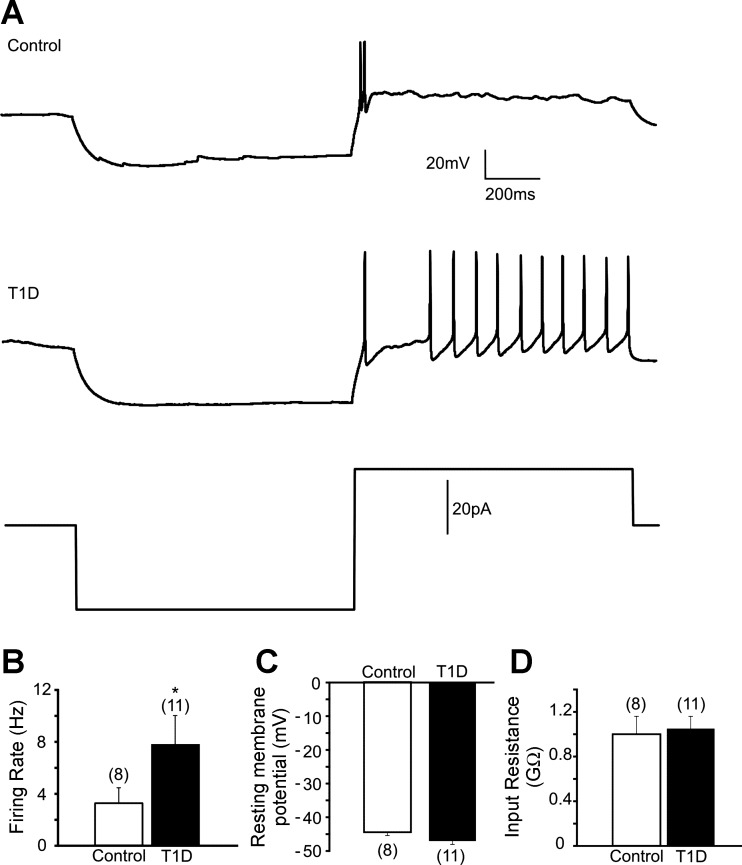
Fig. 2.
Membrane properties of kidney-related PVN neurons in control and type 1 diabetic mice. A: representative traces of action potentials in control (top trace) and type 1 diabetic mice (middle trace) after injection of 20-pA depolarizing current following hyperpolarization of the membrane (step protocol shown in bottom trace). B: combined data showing increased firing rate of action potentials in kidney-related PVN neurons of streptozotocin (STZ)-treated mice. C: combined data demonstrating resting membrane potential in kidney-related PVN neurons of control and type 1 diabetic mice. D: combined data showing input resistance of kidney-related PVN neurons in control and diabetic conditions. Values are means ± SE, with the numbers of recorded cells shown in parentheses. *P < 0.05 indicates significance. T1D, type 1 diabetic.
The input resistance is a reflection of all ionic current passing through an entire membrane surface, with the exception of capacity current. A series of current steps applied to PRV-labeled PVN neurons in current-clamp mode allowed us to identify the input resistance. The current-clamp recordings were obtained from kidney-related PVN neurons of control and type 1 diabetic mice. The input resistance of kidney-related PVN neurons in control mice was 0.98 ± 0.16 GΩ (n = 8), whereas the input resistance of kidney-related PVN neurons in STZ-treated mice was 1.05 ± 0.11 GΩ (n = 11, P > 0.05; Fig. 2D). These data indicate no significant difference in input resistance of kidney-related PVN neurons between control and hyperglycemic mice.
The firing rate of kidney-related PVN neurons of control mice was 3.2 ± 1.2 Hz (range 0–7.3 Hz, n = 8) after injection of 20-pA depolarizing current. On the other hand, in STZ-treated type 1 diabetic mice, the rate of action potential firing was 8.0 ± 1.7 Hz after 20-pA depolarizing current injection (range 0–12.7 Hz, n = 11, P < 0.05; Fig. 2, A and B). This finding indicates increased excitability of presympathetic kidney-related PVN neurons during type 1 diabetes.
Decreased phasic inhibition of kidney-related PVN neurons in type 1 diabetic mice.
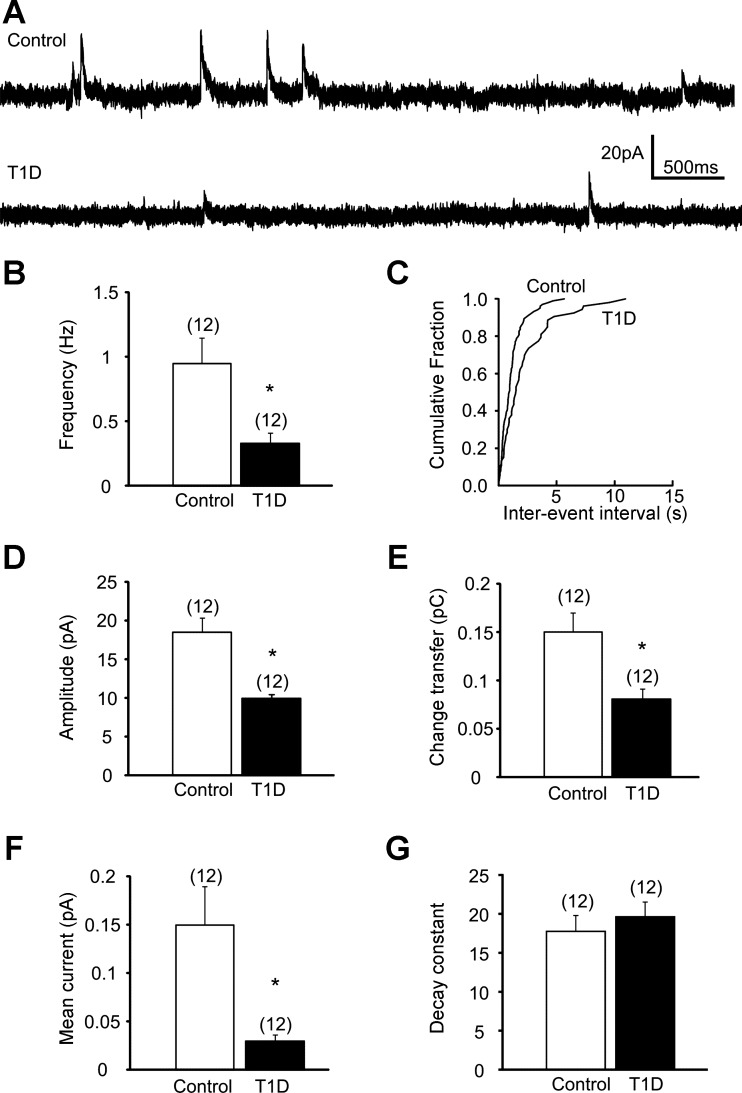
Previous observations indicated synaptic plasticity of presympathetic PVN neurons in rats with heart disease (Han et al. 2010). In this study, we have assessed the inhibitory control of kidney-related PVN neurons in control and type 1 diabetic conditions. Recordings of mIPSCs were conducted at −10 mV in aCSF containing 1 μM TTX to block action potential-dependent neurotransmitter release. The average frequency of mIPSCs of kidney-related presympathetic PVN neurons in slices from control mice was 0.95 ± 0.21 Hz (range 0.16–2.49 Hz, n = 12). In contrast, the frequency of mIPSCs in kidney-related PVN neurons of STZ-treated hyperglycemic animals was 0.33 ± 0.08 Hz (range 0.11–1.08 Hz, n = 12, P < 0.05; Fig. 3, A–C). Our data demonstrate significantly lower mIPSC frequency in STZ-treated type 1 diabetic mice compared with controls.
Fig. 3.
Decreased phasic inhibition of kidney-related PVN neurons in type 1 diabetic mice. A: continuous whole cell patch-clamp recordings of miniature inhibitory postsynaptic currents (mIPSCs) at holding potential of −10 mV from control (top trace) and type 1 diabetic mice (bottom trace). B: combined data showing decreased mIPSC frequency in kidney-related PVN neurons of STZ-treated type 1 diabetic mice. C: cumulative event probability plots of interevent interval distribution in recordings from control and type 1 diabetic mice. D–G: combined data showing amplitude (D), charge transfer (E), mean current (F), and decay time constant (G) of mIPSCs in kidney-related presympathetic PVN neurons in control and type 1 diabetic conditions. Values are means ± SE, with the numbers of recorded cells shown in parentheses. *P < 0.05 indicates significance.
The amplitude of mIPSCs in kidney-related presympathetic PVN neurons of control mice was 18.50 ± 1.98 pA (range 10.11–32.69 pA, n = 12) and 10.00 ± 0.54 pA (range 6.05–13.85 pA, n = 12) in STZ-treated type 1 diabetic mice. The mIPSC amplitude was significantly smaller in STZ-treated mice compared with controls (P < 0.05; Fig. 3D).
The charge transfer Q measured as area under the mIPSCs was 0.15 ± 0.02 pC (range 0.05–0.35 pC, n = 12) in kidney-related PVN neurons of control mice. In type 1 diabetic mice the charge transfer was 0.08 ± 0.01 pC (range 0.03–0.18 pC, n = 12), indicating significantly smaller Q values in kidney-related PVN neurons of STZ-treated mice compared with controls (P < 0.05; Fig. 3E). We also calculated the mean current as Q × frequency of mIPSCs. In control mice the mean current was 0.15 ± 0.04 pA (range 0.02–0.46 pA, n = 12), whereas that in type 1 diabetic animals was 0.03 ± 0.006 pA (range 0.004–0.062 pA, n = 12). This observation demonstrates significantly less mean miniature inhibitory current in type 1 diabetic mice compared with controls (P < 0.05; Fig. 3F).
The decay time was fitted well with a single exponential in 7 of 12 neurons in both control and STZ-treated mice, and two exponentials were better fitted in the remaining cells. The weighted decay time constant was 17.7 ± 2.0 ms in control and 19.6 ± 1.8 ms in STZ-treated mice. These data indicate no significant difference in decay time constant between groups (P > 0.05; Fig. 3G).
Reduced GABAergic tonic inhibitory control of kidney-related PVN neurons in type 1 diabetic mice.
Tonic inhibitory current mediated by GABAA receptors has been previously identified in PVN neurons (Park et al. 2006, 2007), supporting the idea that tonic inhibition plays a significant role in the maintenance of neuronal activity. Because autonomic impairment includes neuronal hyperactivity, we reasoned that tonic inhibitory control of kidney-related PVN neurons could be altered in type 1 diabetes.
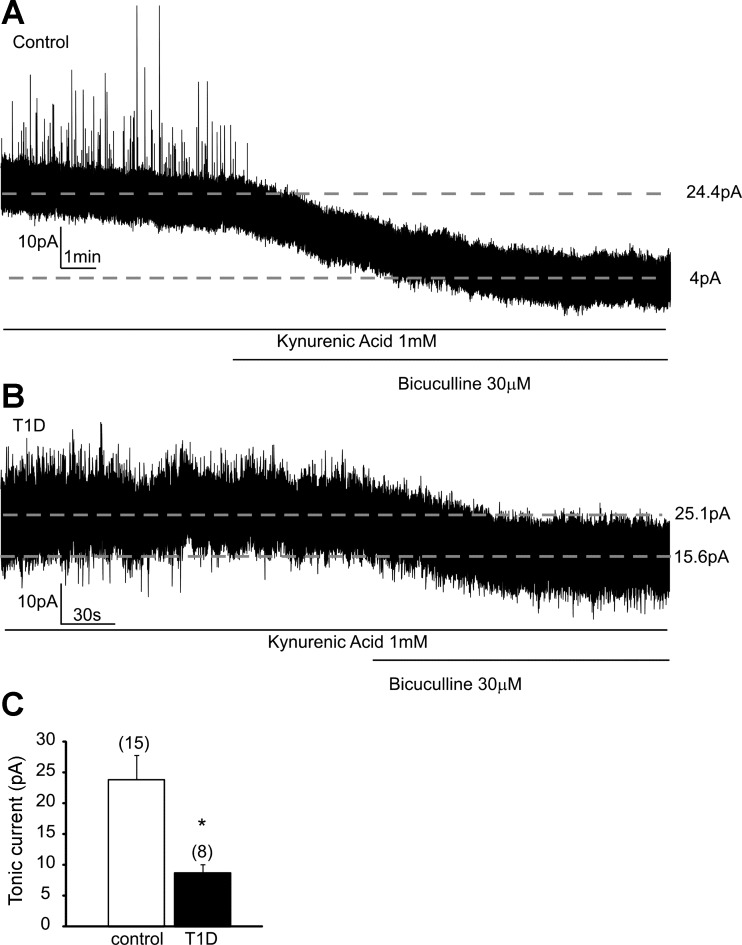
In this study, we assessed the GABAA receptors-mediated tonic inhibitory current at −10 mV holding potential. The bath solution contained kynurenic acid (1 mM) to block glutamatergic currents, and the tonic GABAA current was measured as a bicuculline-dependent inward shift of the holding current (Gao and Smith 2010; Park et al. 2007). Application of bicuculline (30 μM) revealed a tonic inhibitory current with an average amplitude of 23.9 ± 4.0 pA (range 8.7–57.1 pA, n = 15) in kidney-related PVN neurons of control mice (Fig. 4, A and C). Next, we assessed the potential effect of type 1 diabetes on tonic GABAergic inhibition of kidney-related presympathetic PVN neurons. In STZ-treated mice, administration of the same concentration of bicuculline produced a significantly smaller shift in holding current with an average amplitude of 8.8 ± 1.4 pA (range 4.1–16.3 pA, n = 8, P < 0.05; Fig. 4, B and C). This set of data further indicates decreased inhibition of kidney-related PVN neurons in type 1 diabetes.
Fig. 4.
Reduced GABAergic tonic inhibitory current in kidney-related PVN neurons in type 1 diabetic mice. A: representative trace recorded from a kidney-related PVN neuron in control mice in the presence of kynurenic acid, a glutamate receptor blocker (1 mM), before and after application of bicuculline (30 μM). Application of bicuculline revealed a tonic current calculated as an inward shift in holding current. B: representative trace recorded from a kidney-related PVN neuron in type 1 diabetic mouse. The tonic inhibition of kidney-related PVN neurons was attenuated in STZ-treated mice. C: combined data showing a significantly smaller tonic current in kidney-related PVN neurons in type 1 diabetic mice. Values are means ± SE, with the numbers of recorded cells shown in parentheses. *P < 0.05 indicates significance.
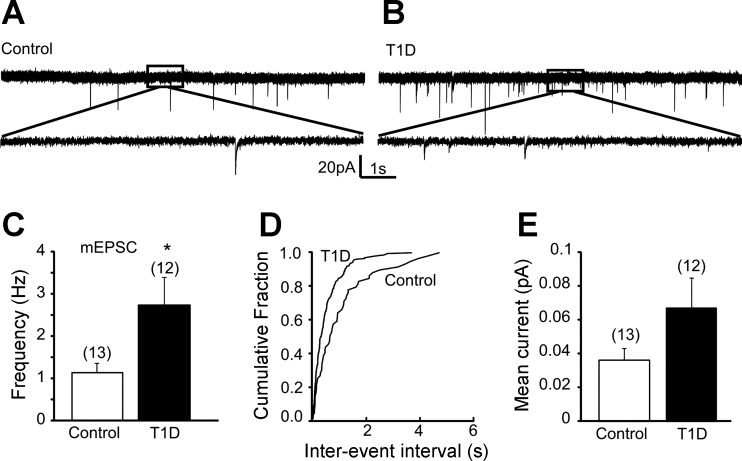
Increased mEPSC frequency in kidney-related PVN neurons of type 1 diabetic mice.
Alteration of mEPSCs can also contribute to the increased excitability of presympathetic PVN neurons; therefore we investigated the excitatory control of kidney-related PVN neurons in control and type 1 diabetic conditions. The average frequency of mEPSCs in kidney-related PVN neurons in controls was 1.13 ± 0.23 Hz (range 0.35–3.23 Hz, n = 13; Fig. 5). In contrast, the frequency of mEPSCs in kidney-related PVN neurons of STZ-treated hyperglycemic animals was 2.73 ± 0.64 Hz (range 0.73–8.19 Hz, n = 12, P < 0.05). Our data demonstrated significantly higher mEPSC frequency in type 1 diabetic mice compared with controls.
Fig. 5.
Increased frequency of miniature excitatory postsynaptic currents (mEPSCs) in kidney-related PVN neurons in type 1 diabetic mice. A and B: continuous whole cell patch-clamp recordings of mEPSCs at a holding potential of −60 mV from control (A) and type 1 diabetic mice (B). Bottom traces are enlarged portions of the respective boxed areas. C: combined data showing increased mEPSC frequency in kidney-related PVN neurons of STZ-treated mice. D: cumulative event probability plots of interevent interval distribution in recordings from control and type 1 diabetic mice. E: combined data showing mean current of mEPSCs. Values are means ± SE, with the numbers of recorded cells shown in parentheses. *P < 0.05 indicates significance.
The amplitude of mEPSCs in kidney-related presympathetic PVN neurons of control mice was 13.70 ± 0.83 pA (range 8.52–19.21 pA, n = 13), and that of STZ-treated type 1 diabetic mice was 9.57 ± 0.44 pA (range 7.02–12.39 pA, n = 12). The mean current of EPSCs in controls was 0.036 ± 0.007 pA (range 0.01–0.1 pA, n = 13), whereas that in type 1 diabetic animals was 0.067 ± 0.018 pA (range 0.008–0.202 pA, n = 12), indicating an increasing trend, but the difference did not reach significance. Similar to mIPSCs, we did not reveal a difference in decay time constant of mEPSCs between control and STZ-treated mice (3.1 ± 0.2 vs. 2.9 ± 0.3 ms, P > 0.05).
DISCUSSION
In the present study, we demonstrated altered regulation of kidney-related PVN neurons in a model of type 1 diabetes. The following novel findings emerged: 1) mIPSC frequency was reduced in kidney-related PVN neurons of STZ-treated type 1 diabetic mice; 2) the amplitude, charge transfer, and mean synaptic current of mIPSCs were decreased in kidney-related presympathetic PVN neurons of STZ-treated mice; 3) tonic inhibitory currents were attenuated in kidney-related PVN neurons of STZ-treated mice; and 4) increased frequency of mEPSCs and increased excitability of kidney-related PVN neurons were observed in type 1 diabetic mice. The demonstration of decreased inhibition and increased excitability of kidney-related presympathetic PVN neurons suggests altered central autonomic circuitry, possibly contributing to elevated sympathetic nerve activity.
Technical considerations.
In this study, PRV-152 was used to identify kidney-related presympathetic PVN neurons. PRV-152 has been shown to spread strictly retrogradely, with little or no ability to spread anterogradely or perform axo-axonal labeling (Pickard et al. 2002; Smith et al. 2000). Thus the EGFP expression, which can be observed under epifluorescence illumination, indicates kidney-related PVN neurons. Because the innervation of kidney originates from the sympathetic nervous system, we can assume that the EGFP labeling indicates kidney-related presympathetic neurons. Patch-clamp recordings were conducted between 88 and 98 h after inoculation of the left kidney. This time point has been shown sufficient to label neurons in the PVN (Cano et al. 2004). The possible harmful effects of PRV on neuronal survival and viability have been addressed in numerous articles (Cano et al. 2004; Card et al. 1993; McCarthy et al. 2009). Previous reports also showed no changes in electrical properties of PRV-infected neurons, indicating that the virus does not have adverse effects on electrical properties at this time point (Derbenev et al. 2010; Gao et al. 2012; Glatzer et al. 2003; Smith et al. 2000). Although it remains possible that PRV-152 might alter some of the labeled neurons in a later time point, our recordings were carefully designed and monitored. Nevertheless, this PRV approach provides the opportunity to identify kidney-related presympathetic PVN neurons and study their cellular properties and the consequences of diabetes.
In our experiments, mice were injected with streptozotocin to destroy pancreatic beta cells and thus induce type 1 diabetes (Like and Rossini 1976; Schein and Loftus 1968). This is a well-described, straightforward model of type 1 diabetes producing insulin deficiency and hyperglycemia. On the other hand, we have to note that STZ administration can affect renal cells and hepatic cells as well as neurons (Pabbidi et al. 2008); however, the direct effect of STZ-treatment on the PVN is negligible due to that the serum half-life of streptozotocin is ∼15 min (Like and Rossini 1976; Schein and Loftus 1968). Therefore, it is highly unlikely that STZ will have direct effects on the patch-clamp recordings.
We also have to note that based on the current experimental settings, we cannot clearly determine if the observed alteration of neuronal activity is a consequence of circulating glucose or deficiency in insulin. Elevated glucose can acutely alter cellular function (Balfour et al. 2006; Ferreira et al. 2001), and chronic hyperglycemia also alters synaptic balance. On the other hand, insulin was reported to impact neuronal function in preautonomic brain stem neurons (Blake and Smith 2012). Our previous data revealed alteration of preautonomic PVN neurons during type 1 diabetes, and in vivo insulin replacement normalized neuronal function (Gao et al. 2012); however, insulin replacement also normalized glucose levels, and thus we cannot differentiate the separate effect of insulin deficiency and/or hyperglycemia. Nevertheless, our data demonstrate that in the type 1 diabetic condition, the synaptic regulation of kidney-related presympathetic PVN neurons is altered; however, future studies are required to determine the separate effect of glucose and insulin.
Decreased inhibitory regulation of kidney-related PVN neurons in type 1 diabetes.
Autonomic dysfunction during pathophysiological conditions such as diabetes mellitus is a well-known phenomenon (Carnethon et al. 2003a, 2003b; Holl et al. 1999; Perin et al. 2001). Increased sympathetic outflow is associated with diabetes; however, the exact mechanisms driving these changes are not fully understood. Both hyperglycemia and hypoglycemia have been shown to alter cellular functions in autonomic centers of the brain (Balfour et al. 2006; Balfour and Trapp 2007; Ferreira et al. 2001; Zsombok et al. 2011), including the PVN (Gao et al. 2012). In this study, we observed reduced inhibition of kidney-related PVN neurons following 7–11 days of hyperglycemia due to STZ-treatment, even when identical normalized glucose concentrations were used in the bath solution. Our study, by using PRV-152, allowed the identification of a specific neuronal subpopulation, the kidney-related presympathetic PVN neurons, and the electrophysiological studies provided evidence that the presympathetic circuitry at least at the level of the PVN is altered during experimental type 1 diabetes. The change in circuitry may be due to increased glucose levels or lack of insulin; however, we cannot elucidate which one might count for the effect, as mentioned above. Functional plasticity of specific receptors has been previously observed in both the PVN and brain stem during type 1 diabetes (Gao et al. 2012; Zsombok et al. 2011); however, to the best of our knowledge, this is the first report indicating altered inhibitory control of kidney-related presympathetic PVN neurons in a rodent model of type 1 diabetes.
Increased sympathetic activity is linked with many pathophysiological conditions, including heart failure, hypertension, and diabetes mellitus (Anderson et al. 1989; Li and Patel 2003; Mancia et al. 2007; Spallone et al. 1994). Clinical studies have demonstrated that during the early course of type 1 diabetes, blood pressure is elevated compared with that of healthy subjects (Holl et al. 1999); however, the observations from animal studies are controversial (Bunag et al. 1982; Hayashi et al. 1983; Hicks et al. 1998; Katovich et al. 1995). Gradual increase of blood pressure along with hyperglycemia has also been shown in STZ-treated C57bl/sv129 mice (Wichi et al. 2007), and systolic blood pressure tends to be higher in STZ-treated mice compared with nontreated mice; however, the difference was significant only after a prolonged period (Nadarajah et al. 2012). Our data demonstrate altered control of presympathetic PVN neurons in the type 1 diabetic condition, which could play a role in the development of elevated sympathetic outflow. Sympathetic overactivity, for example, in heart failure, has been associated with reduced inhibition of rostral ventrolateral medulla (RVLM)-projecting presympathetic PVN neurons due to decrease of GABA release (Han et al. 2010). Hypertensive rats also exhibited higher basal firing rate and smaller GABAA-mediated current in presympathetic PVN neurons compared with normotensive rats (Li and Pan 2006). Furthermore, the GABAA blocker-dependent increase of firing rate in PVN neurons was also blunted in hypertensive rats (Li and Pan 2006).
In STZ-treated diabetic rats, inhibition of GABAergic neurotransmission in the PVN produced a smaller renal sympathetic nerve discharge than in control animals (Reynolds et al. 1996), indicating reduced inhibitory mechanisms in the PVN during type 1 diabetes. PVN receives inputs from the brain stem and a variety of forebrain areas. It is generally accepted that presympathetic PVN neurons integrate information and regulate sympathetic outflow through direct projections to the spinal cord and RVLM, and they may also regulate sympathetic outflow indirectly via the nucleus tractus solitarii (NTS) or parabrachial nucleus (Dampney 1994; Swanson and Sawchenko 1983). Numerous neurotransmitters and neuromodulators influence the level of SNA; however, evidence has indicated that GABA plays a tonic inhibitory role in the PVN (Martin and Haywood 1993; Martin et al. 1991). It has been suggested that increased renal SNA (RSNA) is due to increased activity of PVN sympathoexcitatory neurons as a consequence of reduced GABAergic inhibition, but increased excitation by NMDA or AT1 receptors may also contribute (LaGrange et al. 2003; Li and Patel 2003; Zucker et al. 2001). The source and location of GABAergic neurons is still debated; however, GABAergic neurons have been described in surrounding hypothalamic areas, including the lateral hypothalamic area, anterior hypothalamic area, medial preoptic area, dorsomedial hypothalamic nucleus, and suprachiasmatic nucleus (Boudaba et al. 1996). Previous in vivo data demonstrated that inhibiting GABAA receptors by administration of bicuculline increases the level of RSNA (Zhang and Patel 1998); therefore, on the basis of our data, we can speculate that decreased GABAergic regulation of kidney-related presympathetic PVN neurons may lead to an increase of RSNA. Our data showing reduced phasic inhibitory currents suggest less GABA release to kidney-related PVN neurons. The reduced phasic current together with decreased tonic inhibition contributes to the increased excitability of kidney-related PVN neurons that could upregulate the activity of presympathetic RVLM and/or IML neurons and thus contribute to the elevated renal sympathetic outflow.
Our data demonstrating decreased mIPSC frequency, amplitude, and mean inhibitory current suggest reduction in GABA release during type 1 diabetes. Furthermore, the greater firing rate in kidney-related PVN neurons of STZ-treated type 1 diabetic mice after depolarizing current injection indicates increased excitability of the neurons. This could be due to reduced inhibitory control of PVN neurons during type 1 diabetic conditions as suggested by Reynolds et al. (1996). On the other hand, our data did not provide evidence for alteration of input resistance; however, a decrease in inhibition and an increase in excitation might counterbalance the changes in input resistance. This is supported by our findings demonstrating increased frequency of mEPSCs in STZ-treated mice. Presympathetic PVN neurons receive excitatory, glutamatergic inputs from the brain stem including the NTS (Affleck et al. 2012) and from forebrain areas including the surrounding hypothalamic nuclei (Boudaba et al. 1997; Ulrich-Lai et al. 2011). Within the hypothalamus the dorsomedial hypothalamus and the perifornical region have been identified electrophysiologically as major excitatory sites projecting to the PVN (Boudaba et al. 1997). Immunostaining studies revealed glutamatergic inputs to the posterior PVN, which contains large population of preautonomic neurons, from the ventromedial hypothalamic nucleus, posterior hypothalamic nucleus, medial amygdala, the dorsomedial hypothalamic nucleus, and lateral hypothalamic area (Ulrich-Lai et al. 2011). The higher frequency of mEPSC in type 1 diabetic mice suggests that the identified kidney-related PVN neurons may receive more excitatory regulation that could also contribute to the increased sympathetic outflow. On the other hand, no change in overall phasic current in the diabetic condition could indicate minor contribution of excitatory regulation to increased sympathetic outflow. Nevertheless, the identification of excitatory mechanisms will be the subject of future investigations.
Similar to our findings, there was no change in the input resistance and resting membrane potential in neurosecretory PVN neurons even when a shift of inhibitory-excitatory synaptic balance (Potapenko et al. 2011) was observed in presympathetic PVN neurons during heart failure (Han et al. 2010; Stern et al. 2012). Furthermore, during myocardial infarction, the spontaneous firing activity of presympathetic PVN neurons increased and both the spontaneous and miniature IPSC frequencies were reduced (Han et al. 2010). These changes were observed in the RVLM-projecting subpopulation of presympathetic PVN neurons, whereas the authors did not observe changes in the spinally projecting presympathetic PVN population (Han et al. 2010). The above-mentioned findings, including our current data, support the hypothesis that the inhibition of presympathetic PVN neurons is suppressed in pathophysiological conditions involving sympathetic overactivity.
On the other hand, no change in spontaneous and miniature IPSC frequency in dorsal horn neurons during diabetes has also been reported (Wang et al. 2007); however, the authors have demonstrated reduced presynaptic GABAB receptor function in STZ-treated rats (Wang et al. 2007). The difference between our data and theirs could originate from the difference between spinal and central neurons, or it could be that our recordings were conducted in an early stage of diabetes (∼7–11 days), whereas Wang and coworkers conducted their experiments 4 wk after STZ treatment (Wang et al. 2007).
Taken together, our data support the hypothesis that pathophysiological conditions associated with elevated sympathetic activity such as diabetes reduce the inhibitory control of presympathetic PVN neurons and increase excitability. Therefore, we can speculate that the decreased inhibition of presympathetic PVN neurons contributes to the development of sympathetic overactivity.
Reduced GABAergic tonic inhibitory control of kidney-related PVN neurons during type 1 diabetes.
Inhibitory currents mediated by GABAA receptors can be divided into phasic and tonic currents. Phasic currents occur when a high concentration of neurotransmitter is released from presynaptic terminal and bind with GABA receptors located in the postsynaptic cell. On the other hand, extracellular GABA, diffusing away from the synaptic cleft or originating from nonsynaptic GABA-release by glia can also activate extrasynaptic or perisynaptic GABA receptors (Nusser et al. 1998; Park et al. 2007). This low concentration of extracellular GABA continuously binds with extrasynaptic receptors, leading to the generation of tonic inhibitory currents (Gao and Smith 2010; Nusser et al. 1998; Park et al. 2007). Therefore, in addition to the conventional phasic neurotransmission, persistent tonic regulation of neurons plays a significant role in determining the activity of the neurons (Nusser and Mody 2002; Nusser et al. 1998; Park et al. 2006, 2007).
Tonic GABAA-receptor-mediated currents were first found in cerebellar granule cells (Brickley et al. 1996) and then in many other brain areas including autonomic areas, such as the presympathetic neuronal population of the PVN (Park et al. 2007, 2009). We have demonstrated the presence of tonic GABAA receptor-mediated inhibitory current in kidney-related presympathetic PVN neurons. The magnitude of this inhibitory current was larger in kidney-related presympathetic PVN neurons than in presympathetic RVLM-projecting PVN neurons (∼24 vs. ∼10 pA; Park et al. 2007). We can speculate that this difference could be due to a greater level of tonic inhibition of kidney-related PVN neurons compared with the overall RVLM-projecting presympathetic PVN population or to different concentrations of bicuculline (30 vs. 20 μM) or a species difference (mouse vs. rat) (Park et al. 2007). Regardless of the magnitude of the tonic inhibitory current, our data also demonstrate the existence of GABAA-dependent persistent tonic inhibition of presympathetic PVN neurons, and thereby confirm and even further extend the previous findings.
As important additional information, our work revealed that in type 1 diabetic conditions, the tonic GABAergic inhibitory current in kidney-related presympathetic PVN neurons was significantly smaller than in control mice. Because sympathetic outflow from the PVN is restrained by a GABAergic inhibitory tone, we can assume that decreased tonic inhibition during diabetic conditions could lead to hyperactivity of PVN neurons and could be associated with increased renal sympathetic outflow; however, future experiments are required to prove this possibility. This also could be supported by previous findings demonstrating blunted renal sympathetic responses in diabetic rats following bicuculline injection into the PVN (Reynolds et al. 1996).
In summary, the current study extends our understanding of synaptic regulation of kidney-related presympathetic PVN neurons. Furthermore, we have demonstrate increased excitability and reduced inhibition of kidney-related PVN neurons in type 1 diabetic conditions. These data have valuable implication given that they provide a possible mechanism associated with increased sympathetic outflow during pathophysiological conditions such as diabetes mellitus.
GRANTS
This work was supported by National Institute of General Medical Sciences (NIGMS) Grant P20GM103629 [Tulane Aging Center of Biomedical Research Excellence (COBRE)], American Heart Association Award 10GRNT4540000, and a Tulane University Neuroscience Bridge Grant (to A. Zsombok), and NIGMS Grant P30GM103337 (Tulane Hypertension COBRE; to A. V. Derbenev).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.J., H.G., A.M.K., and A.V.D. performed experiments; Y.J. and H.G. analyzed data; Y.J. prepared figures; Y.J., A.V.D., and A.Z. drafted manuscript; H.G., A.V.D., and A.Z. interpreted results of experiments; A.V.D. and A.Z. edited and revised manuscript; A.Z. conception and design of research; A.Z. approved final version of manuscript.
REFERENCES
- Affleck VS, Coote JH, Pyner S. The projection and synaptic organisation of NTS afferent connections with presympathetic neurons, GABA and nNOS neurons in the paraventricular nucleus of the hypothalamus. Neuroscience 219: 48–61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 14: 177–183, 1989 [DOI] [PubMed] [Google Scholar]
- Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol 570: 469–484, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour RH, Trapp S. Ionic currents underlying the response of rat dorsal vagal neurones to hypoglycaemia and chemical anoxia. J Physiol 579: 691–702, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Kinetic differences between synaptic and extrasynaptic GABAA receptors in CA1 pyramidal cells. J Neurosci 20: 937–948, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake CB, Smith BN. Insulin reduces excitation in gastric-related neurons of the dorsal motor nucleus of the vagus. Am J Physiol Regul Integr Comp Physiol 303: R807–R814, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol 77: 3396–3400, 1997 [DOI] [PubMed] [Google Scholar]
- Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci 16: 7151–7160, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol 497: 753–759, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunag RD, Tomita T, Sasaki S. Streptozotocin diabetic rats are hypertensive despite reduced hypothalamic responsiveness. Hypertension 4: 556–565, 1982 [DOI] [PubMed] [Google Scholar]
- Cano G, Card JP, Sved AF. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J Comp Neurol 471: 462–481, 2004 [DOI] [PubMed] [Google Scholar]
- Card JP, Rinaman L, Lynn RB, Lee BH, Meade RP, Miselis RR, Enquist LW. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci 13: 2515–2539, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987–1998. Circulation 107: 2190–2195, 2003a [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Jacobs DR, Jr, Sidney S, Liu K. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: the CARDIA study. Diabetes Care 26: 3035–3041, 2003b [DOI] [PubMed] [Google Scholar]
- Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol 103: 4–15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 285: R1231–R1239, 2003 [DOI] [PubMed] [Google Scholar]
- Ciriello J, Kline RL, Zhang TX, Caverson MM. Lesions of the paraventricular nucleus alter the development of spontaneous hypertension in the rat. Brain Res 310: 355–359, 1984 [DOI] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- Derbenev AV, Duale H, Rabchevsky AG, Smith BN. Electrophysiological characteristics of identified kidney-related neurons in adult rat spinal cord slices. Neurosci Lett 474: 168–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M, Jr, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol 536: 141–152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Miyata K, Bhaskaran MD, Derbenev AV, Zsombok A. Transient receptor potential vanilloid type 1-dependent regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes 61: 1381–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Smith BN. Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol 103: 904–914, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzer NR, Hasney CP, Bhaskaran MD, Smith BN. Synaptic and morphologic properties in vitro of premotor rat nucleus tractus solitarius neurons labeled transneuronally from the stomach. J Comp Neurol 464: 525–539, 2003 [DOI] [PubMed] [Google Scholar]
- Han TH, Lee K, Park JB, Ahn D, Park JH, Kim DY, Stern JE, Lee SY, Ryu PD. Reduction in synaptic GABA release contributes to target-selective elevation of PVN neuronal activity in rats with myocardial infarction. Am J Physiol Regul Integr Comp Physiol 299: R129–R139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Senba S, Saito I, Kitajima W, Saruta T. Changes in blood pressure, urinary kallikrein, and urinary prostaglandin E2 in rats with streptozotocin-induced diabetes. Naunyn Schmiedebergs Arch Pharmacol 322: 290–294, 1983 [DOI] [PubMed] [Google Scholar]
- Herzig TC, Buchholz RA, Haywood JR. Effects of paraventricular nucleus lesions on chronic renal hypertension. Am J Physiol Heart Circ Physiol 261: H860–H867, 1991 [DOI] [PubMed] [Google Scholar]
- Hicks KK, Seifen E, Stimers JR, Kennedy RH. Effects of streptozotocin-induced diabetes on heart rate, blood pressure and cardiac autonomic nervous control. J Auton Nerv Syst 69: 21–30, 1998 [DOI] [PubMed] [Google Scholar]
- Holl RW, Pavlovic M, Heinze E, Thon A. Circadian blood pressure during the early course of type 1 diabetes. Analysis of 1,011 ambulatory blood pressure recordings in 354 adolescents and young adults. Diabetes Care 22: 1151–1157, 1999 [DOI] [PubMed] [Google Scholar]
- Huang J, Weiss ML. Characterization of the central cell groups regulating the kidney in the rat. Brain Res 845: 77–91, 1999 [DOI] [PubMed] [Google Scholar]
- Kannan H, Yamashita H. Connections of neurons in the region of the nucleus tractus solitarius with the hypothalamic paraventricular nucleus: their possible involvement in neural control of the cardiovascular system in rats. Brain Res 329: 205–212, 1985 [DOI] [PubMed] [Google Scholar]
- Katovich MJ, Hanley K, Strubbe G, Wright BE. Effects of streptozotocin-induced diabetes and insulin treatment on blood pressure in the male rat. Proc Soc Exp Biol Med 208: 300–306, 1995 [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Weiss ML, Mendes T, Wang Y, Fels RJ. Role of paraventricular nucleus in regulation of sympathetic nerve frequency components. Am J Physiol Heart Circ Physiol 284: H1710–H1720, 2003 [DOI] [PubMed] [Google Scholar]
- LaGrange LP, Toney GM, Bishop VS. Effect of intravenous angiotensin II infusion on responses to hypothalamic PVN injection of bicuculline. Hypertension 42: 1124–1129, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 49: 916–925, 2007a [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol 290: H1110–H1119, 2006 [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Role of γ-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther 320: 615–626, 2007b [DOI] [PubMed] [Google Scholar]
- Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand 177: 17–26, 2003 [DOI] [PubMed] [Google Scholar]
- Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 193: 415–417, 1976 [DOI] [PubMed] [Google Scholar]
- Luther JA, Daftary SS, Boudaba C, Gould GC, Halmos KC, Tasker JG. Neurosecretory and non-neurosecretory parvocellular neurones of the hypothalamic paraventricular nucleus express distinct electrophysiological properties. J Neuroendocrinol 14: 929–932, 2002 [DOI] [PubMed] [Google Scholar]
- Luther JA, Tasker JG. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol 523: 193–209, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, Reid J, Van Zwieten PA. The sympathetic nervous system and the metabolic syndrome. J Hypertens 25: 909–920, 2007 [DOI] [PubMed] [Google Scholar]
- Martin DS, Haywood JR. Hemodynamic responses to paraventricular nucleus disinhibition with bicuculline in conscious rats. Am J Physiol Heart Circ Physiol 265: H1727–H1733, 1993 [DOI] [PubMed] [Google Scholar]
- Martin DS, Segura T, Haywood JR. Cardiovascular responses to bicuculline in the paraventricular nucleus of the rat. Hypertension 18: 48–55, 1991 [DOI] [PubMed] [Google Scholar]
- McCarthy KM, Tank DW, Enquist LW. Pseudorabies virus infection alters neuronal activity and connectivity in vitro. PLoS Pathog 5: e1000640, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin SW. What does the brain know about blood pressure? News Physiol Sci 16: 266–271, 2001 [DOI] [PubMed] [Google Scholar]
- Nadarajah R, Milagres R, Dilauro M, Gutsol A, Xiao F, Zimpelmann J, Kennedy C, Wysocki J, Batlle D, Burns KD. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int 82: 292–303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87: 2624–2628, 2002 [DOI] [PubMed] [Google Scholar]
- Nusser Z, Roberts JD, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci 15: 2948–2960, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18: 1693–1703, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Vincent Chen HS, Tong G, Hayden MR, Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med 15: 1407–1413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabbidi RM, Cao DS, Parihar A, Pauza ME, Premkumar LS. Direct role of streptozotocin in inducing thermal hyperalgesia by enhanced expression of transient receptor potential vanilloid 1 in sensory neurons. Mol Pharmacol 73: 995–1004, 2008 [DOI] [PubMed] [Google Scholar]
- Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial GABA transporters. J Physiol 587: 4645–4660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Skalska S, Son S, Stern JE. Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J Physiol 582: 539–551, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Skalska S, Stern JE. Characterization of a novel tonic gamma-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology 147: 3746–3760, 2006 [DOI] [PubMed] [Google Scholar]
- Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens 23: 45–55, 2001 [DOI] [PubMed] [Google Scholar]
- Pickard GE, Smeraski CA, Tomlinson CC, Banfield BW, Kaufman J, Wilcox CL, Enquist LW, Sollars PJ. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J Neurosci 22: 2701–2710, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapenko ES, Biancardi VC, Florschutz RM, Ryu PD, Stern JE. Inhibitory-excitatory synaptic balance is shifted toward increased excitation in magnocellular neurosecretory cells of heart failure rats. J Neurophysiol 106: 1545–1557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat 38: 197–208, 2009 [DOI] [PubMed] [Google Scholar]
- Reynolds AY, Zhang K, Patel KP. Renal sympathetic nerve discharge mediated by the paraventricular nucleus is altered in STZ induced diabetic rats. Nebr Med J 81: 419–423, 1996 [PubMed] [Google Scholar]
- Schein PS, Loftus S. Streptozotocin: depression of mouse liver pyridine nucleotides. Cancer Res 28: 1501–1506, 1968 [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci 6: 484–490, 2003 [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci 27: 262–269, 2004 [DOI] [PubMed] [Google Scholar]
- Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci USA 97: 9264–9269, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallone V, Gambardella S, Maiello MR, Barini A, Frontoni S, Menzinger G. Relationship between autonomic neuropathy, 24-h blood pressure profile, and nephropathy in normotensive IDDM patients. Diabetes Care 17: 578–584, 1994 [DOI] [PubMed] [Google Scholar]
- Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537: 161–177, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Sonner PM, Son SJ, Silva FC, Jackson K, Michelini LC. Exercise training normalizes an increased neuronal excitability of NTS-projecting neurons of the hypothalamic paraventricular nucleus in hypertensive rats. J Neurophysiol 107: 2912–2921, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6: 269–324, 1983 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410–417, 1980 [DOI] [PubMed] [Google Scholar]
- Takeda K, Nakata T, Takesako T, Itoh H, Hirata M, Kawasaki S, Hayashi J, Oguro M, Sasaki S, Nakagawa M. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res 543: 296–300, 1991 [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol 519: 1301–1319, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol 280: 808–820, 2004 [DOI] [PubMed] [Google Scholar]
- Wang XL, Zhang HM, Chen SR, Pan HL. Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol 579: 849–861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichi RB, Farah V, Chen Y, Irigoyen MC, Morris M. Deficiency in angiotensin AT1a receptors prevents diabetes-induced hypertension. Am J Physiol Regul Integr Comp Physiol 292: R1184–R1189, 2007 [DOI] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol 63: 2–8, 2003 [DOI] [PubMed] [Google Scholar]
- Yi CX, la Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta 1802: 416–431, 2010 [DOI] [PubMed] [Google Scholar]
- Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol 275: R728–R734, 1998 [DOI] [PubMed] [Google Scholar]
- Zhong MK, Shi Z, Zhou LM, Gao J, Liao ZH, Wang W, Gao XY, Zhu GQ. Regulation of cardiac sympathetic afferent reflex by GABAA and GABAB receptors in paraventricular nucleus in rats. Eur J Neurosci 27: 3226–3232, 2008 [DOI] [PubMed] [Google Scholar]
- Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci 31: 14024–14031, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP. The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann NY Acad Sci 940: 431–443, 2001 [PubMed] [Google Scholar]