Abstract
Sensory systems must avoid saturation to encode a wide range of stimulus intensities. One way the retina accomplishes this is by using both dim-light-sensing rod and bright-light-sensing cone photoreceptor circuits. OFF cone bipolar cells are a key point in this process, as they receive both excitatory input from cones and inhibitory input from AII amacrine cells via the rod pathway. However, in addition to AII amacrine cell input, other inhibitory inputs from cone pathways also modulate OFF cone bipolar cell light signals. It is unknown how these inhibitory inputs to OFF cone bipolar cells change when switching between rod and cone pathways or whether all OFF cone bipolar cells receive rod pathway input. We found that one group of OFF cone bipolar cells (types 1, 2, and 4) receive rod-mediated inhibitory inputs that likely come from the rod-AII amacrine cell pathway, while another group of OFF cone bipolar cells (type 3) do not. In both cases, dark-adapted rod-dominant light responses showed a significant contribution of glycinergic inhibition, which decreased with light adaptation and was, surprisingly, compensated by an increase in GABAergic inhibition. As GABAergic input has distinct timing and spatial spread from glycinergic input, a shift from glycinergic to GABAergic inhibition could significantly alter OFF cone bipolar cell signaling to downstream OFF ganglion cells. Larger GABAergic input could reflect an adjustment of OFF bipolar cell spatial inhibition, which may be one mechanism that contributes to retinal spatial sensitivity in the light.
Keywords: bipolar cell, γ-aminobutyric acid, glycine, amacrine cell
sensory systems must avoid signal saturation to encode a wide dynamic range of incoming information, for example, when stepping outside into the bright sunlight from a dimly lit room. This occurs in the retina partly by using two photoreceptors with different sensitivities—rod photoreceptors that sense dim light and cone photoreceptors that sense brighter light. Light information sent to bipolar cells (BCs) forms three primary pathways. ON and OFF cone BCs respond to the onset and offset of light signals from cones, respectively, while rod BCs respond to the onset of light signals from rods. ON and OFF cone BCs synapse onto ON and OFF ganglion cells (GCs), respectively, which are the output neurons of the retina. Rod BCs are unique, however, in that they do not contact GCs directly; instead they synapse onto the AII amacrine cell (AC) (McGuire et al. 1984; Strettoi et al. 1990).
Rod BCs and AII ACs are part of a specialized rod pathway that makes use of existing cone circuitry (Fig. 1A). Light onset signals from rods are relayed from rod BCs to AII ACs, which have electrical connections with ON BCs (Chun et al. 1993; Deans et al. 2002; Strettoi et al. 1992; Trexler et al. 2001). These electrical connections can also be bidirectional, to relay cone light information to the AII AC, but are more robust in the AII AC → ON BC direction (Beaudoin et al. 2008; Manookin et al. 2008; Munch et al. 2009; Trexler et al. 2001, 2005; Veruki and Hartveit 2002). Light offset signals from rods are also relayed through the AII AC, which has anatomical connections with OFF BCs and OFF GCs (Grunert and Wässle 1996; Haverkamp et al. 2003; Strettoi et al. 1994). As the AII AC releases glycine, it inhibits the OFF pathway at light onset and the removal of inhibition at light offset serves as an “Off” signal. OFF BCs may also receive rod input through direct connections or rod-cone coupling providing alternate routes for light offset signals (DeVries and Baylor 1995; Tsukamoto et al. 2001; Volgyi et al. 2004).
Fig. 1.
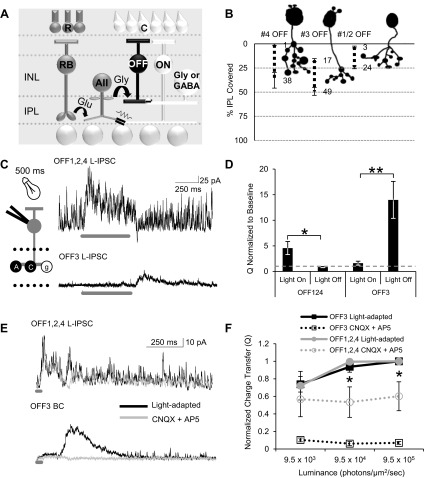
Inhibition to OFF bipolar cells in the dark-adapted retina. A: schematic of the rod pathway circuitry. Rod photoreceptors (R) activated by dim light release glutamate (Glu) onto rod bipolar cells (RB), which release glutamate onto AII amacrine cells (ACs). AII ACs release glycine onto OFF cone bipolar cells (OFF BCs) while transmitting the depolarizing signal to ON cone bipolar cells (ON BCs) via gap junctions. Cone photoreceptors (C) are activated by brighter light and release glutamate onto OFF and ON BCs. Activation of these BCs in turn releases glutamate onto other GABAergic (GABA) and glycinergic (Gly) ACs, which also have inputs onto OFF BCs. Additionally, activation of ON BCs can depolarize AII ACs through electrical gap junctions. INL, inner nuclear layer; IPL, inner plexiform layer. B: example cell morphology traces of type 4, 3, and 1/2 OFF BCs. Type 4 OFF BCs have terminals that ramify in 1–38% (n = 4) of the IPL, type 3 OFF BCs have terminals that ramify in 17–50% (n = 11) of the IPL, and type 1/2 OFF BCs have terminals that ramify in 1–16% of the IPL (n = 9). Type 1 and 2 OFF BCs are difficult to differentiate morphologically and so have been pooled. C: example light-evoked inhibitory postsynaptic currents (L-IPSCs) recorded from an OFF4 BC (representing the OFF1,2,4 group that we have pooled together) and an OFF3 BC to a 500-ms stimulus (dark gray bar). OFF1,2,4 BCs show an inhibitory response with the onset of light, while OFF3 BCs show an inhibitory response with the offset of light. Recording paradigm is shown on left (A, GABAA receptor; C, GABAC receptor; g, glycine receptor). D: OFF1,2,4 (n = 12) BCs have a significantly higher charge transfer (Q) during light onset, while OFF3 (n = 10) BCs have significantly higher charge transfer during light offset. Dashed gray line represents the normalized baseline responses. OFF1,2,4 during light offset and OFF3 during light onset were not different from baseline (P > 0.05). E: example L-IPSCs recorded from an OFF4 and a OFF3 BC to a 30-ms stimulus (dark gray bar) in the light and with the application of CNQX and AP-5. L-IPSCs remained in OFF1,2,4 BCs but were abolished in OFF3 BCs with CNQX and AP-5. F: intensity response curves of charge transfer normalized to the max light-adapted L-IPSC in OFF1,2,4 (n = 4) and OFF3 (n = 5) light-adapted BCs. CNQX and AP-5 significantly reduced OFF1,2,4 L-IPSCs at the brighter light intensities but abolished OFF3 BC L-IPSCs at all light intensities. *P < 0.05, **P < 0.01.
A recent paper (Arman and Sampath 2012) suggested that the AII AC-OFF GC connection determines the response to very dim light stimuli, signaling rod threshold responses. However, OFF BCs have a prominent localization of glycine receptors (glycineRs) (Haverkamp et al. 2003; Sassoe-Pognetto et al. 1994) showing large spontaneous (Eggers and Lukasiewicz 2006b; Ivanova et al. 2006) and light-evoked (Eggers et al. 2007) glycineR-mediated currents compared with rod BCs. Although glycinergic inputs to OFF BCs may not be crucial for determining the scotopic threshold of the retina (Arman and Sampath 2012), there are clearly significant AII AC inputs to OFF BCs that are likely mediating the significant glycinergic input that OFF BCs receive at brighter rod and potentially cone light intensities, which have not previously been tested. Additionally, OFF BCs are divided into at least four main subtypes (Ghosh et al. 2004), and previous anatomical work has suggested that at least one subtype of OFF BC does not receive input from the AII AC (Tsukamoto et al. 2001). The strength and importance of this connection therefore likely vary across OFF BC pathways, but this has not previously been determined physiologically.
OFF BC signaling to GCs is also shaped by inhibition from other ACs, which are roughly divided into narrow-field glycinergic and wide-field GABAergic groups (Menger et al. 1998; Pourcho and Goebel 1983, 1985; Vaney 1990). AC inhibitory inputs combine their signals onto BCs, which has been shown to significantly modulate the output of BCs (Eggers and Lukasiewicz 2006a, 2006b, 2010; Eggers et al. 2007; Sagdullaev et al. 2006). Since rod and cone pathways signal at different light intensities, applying a bright background light that causes rod saturation allows the two pathways to be investigated separately (Dacheux and Raviola 1986; Xin and Bloomfield 1999). It is unknown how glycinergic input to OFF BCs changes when switching between rod and cone pathways or whether there are functional differences in glycinergic inputs to multiple OFF BC subtypes (Fig. 1B) with rod vs. cone activation (Euler and Wässle 1995; Ghosh et al. 2004; Pignatelli and Strettoi 2004).
Here we investigated how inhibition to OFF BC subtypes changes with light adaptation. To elucidate how light adaptation is affecting the different OFF BC circuits, we examined the physiological connections that the rod pathway has with the different OFF BC subtypes. We found that one type of OFF BC is not contacted by the rod BC-AII AC, while the other three types are. A decrease in AII AC activity, due to a decrease in rod pathway signaling with light adaptation, might result in a decrease in inhibitory input to OFF BCs. Surprisingly, we found that a compensatory switch from glycine- to GABA-mediated OFF BC inhibition occurs with light adaptation that may alter OFF BC spatial inhibition and sensitivity.
METHODS
Mouse retinal slice preparation.
Animal protocols were approved by the University of Arizona Institutional Animal Care and Use Committee (IACUC). As described previously (Eggers and Lukasiewicz 2006a; Eggers et al. 2013), male mice (C57BL/6J strain, Jackson Laboratories, Bar Harbor, ME) 35–60 days of age were euthanized with carbon dioxide. Their eyes were enucleated, and the cornea and lens were removed. The eyecup was incubated for 20 min in cold extracellular solution (see Solutions and drugs) with 800 U/ml of hyaluronidase to dissolve the remaining vitreous humor. The hyaluronidase solution was then replaced with ice-cold, oxygenated extracellular solution, and the retina was dissected out of the eyecup. After removal, the retina was trimmed down into one large flat rectangle by removing the peripheral retina and leaving only the central retina surrounding the optic disk. A nitrocellulose membrane filter paper (0.45-μm pore size, Millipore) was placed on the retina section, which was transferred to a hand chopper. An average of six 250-μm slices were cut, rotated 90°, and mounted onto glass coverslips with vacuum grease. Cells used from these slices were never more than 700 μm away from the center of the retina, and only cells near the center of each slice were used for recordings. In this way, much of the differential input due to the dorsal-ventral cone opsin gradient reported in mice was mitigated (Applebury et al. 2000; Haverkamp et al. 2005). The tissue was maintained in oxygenated extracellular solution at room temperature. All dissection procedures were performed under infrared illumination to preserve the light sensitivity of the preparations.
Solutions and drugs.
The extracellular recording solution used for dissection and to examine light-evoked currents contained (in mM) 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 2 CaCl2, 20 glucose, and 26 NaHCO3 and was bubbled with 95% O2-5% CO2. The intracellular solution contained (in mM) 120 CsOH, 120 gluconic acid, 1 MgCl2, 10 HEPES, 10 TEA-Cl, 10 phosphocreatine-Na2, 4 Mg-ATP, 0.5 Na-GTP, and 10 EGTA with 50 μM Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and was adjusted to pH 7.2 with CsOH. To isolate the inhibitory receptor inputs, SR-95531 (20 μM) to block GABAARs, (1,2,5,6-tetrahydropyridine-4-yl)methylphosphinic acid (TPMPA, 50 μM) to block GABACRs, and strychnine (1 μM) to block glycineRs were used. Strychnine was washed on first, followed by TPMPA and finally SR-95531 to prevent serial inhibitory effects (Eggers and Lukasiewicz 2006a, 2010). To block synapse-activated inhibition, CNQX (25 μM) was used to block AMPARs and kainateRs and AP-5 (50 μM, Santa Cruz Biotechnology, Santa Cruz, CA) was used to block NMDARs. All drug solutions were washed on the slice for 5 min before recordings began with a gravity-driven superfusion system (Cell Microcontrols, Norfolk, VA) at a rate of ∼1–2 ml/min. Unless otherwise indicated, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Whole cell recordings.
Whole cell patch-clamp recordings, sampled at 10 kHz, were made from BCs and ACs from retinal slices. Light-evoked inhibitory postsynaptic currents (L-IPSCs) and spontaneous (s)IPSCs were recorded from retinal BCs voltage-clamped to 0 mV, the reversal potential of nonselective cation channel currents. BC recordings were stable and no rundown of the light response was observed over the recording period. Light-evoked excitatory postsynaptic currents (L-EPSCs) and sEPSCs were recorded from ACs voltage-clamped to −60 mV, the reversal potential for chloride channel-mediated currents. Liquid junction potentials of 20 mV were corrected at the beginning of each recording. Electrodes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) on a P-97 Flaming/Brown puller (Sutter Instruments, Novato, CA) and had resistances of 5–7 MΩ. Mice were dark-adapted overnight, and all recording procedures were performed in the dark under infrared illumination to preserve the light sensitivity of the slices. Recordings were made in extracellular solution heated to 32°C, using thin-stage and inline heaters (Cell Microcontrols). Responses were filtered at 6 kHz with the four-pole Bessel filter on a Multi-clamp 700B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA) and digitized with a Digidata 1140 data acquisition system (Molecular Devices).
Morphological identification of cell subtypes.
Alexa Fluor 488 included in the recording pipette was used to label OFF BC and AC subtypes. They were classified as either type 1/2, 3, or 4 OFF BCs or AII ACs on the basis of their axonal morphologies and stratification within the inner plexiform layer (IPL) and the position of their somas in the inner nuclear layer (Ghosh et al. 2004). Type 1 and 2 BCs were difficult to distinguish on the basis of morphology, so their results were pooled. Additionally, although subtypes of the OFF type 3 BC have been described (Mataruga et al. 2007; Wassle et al. 2009), their morphologies were quite similar, so all OFF type 3 cells were pooled. The cells were imaged with Nikon Digital Sight camera with Elements software and a Nikon Intensilight C-HGFIE Fluorescent lamp (Nikon Instruments, Tokyo, Japan). Photoshop (Adobe, Seattle, WA) and Elements software were used to measure and trace the cells. Detailed analysis of axon terminal morphology was performed on a subset of all recorded OFF BCs (n = 24) to determine anatomical differences between OFF BC types, and these criteria were subsequently used to identify OFF BCs. Cell tracings are provided for easy quantification as well as visualization since the fluorescent images of OFF BC terminal morphologies were often at multiple depths vertically in the slice preparation.
Light stimuli.
Full-field light stimuli were evoked with a light-emitting diode (LED; Agilent HLMP-3950, λpeak = 525 nm, Palo Alto, CA) projected through the camera port of the microscope, which elicited a strong response in both dark- and light-adapted conditions. We focused our recordings on cells located within the regions of mixed green/UV cone opsin input (Applebury et al. 2000; Haverkamp et al. 2005), where there was little difference between BC and GC cell responses to green light (Breuninger et al. 2011; Wang et al. 2011). Thus 525-nm light should stimulate both rods (peak sensitivity at 500 nm) and cones robustly. The stimulus intensity (max of 9.5 × 105 photons·μm−2/s−1), background rod-saturating light (950 photons·μm−2/s−1), and duration (30 ms) were controlled with Clampex software by varying the current through the LED. The background intensity was chosen as it was shown to maximally activate rods (Wang and Kefalov 2009). A long light stimulus (500 ms) was used to determine the type of inhibition to all recorded BCs (ON or OFF) as well as the type of excitation of the ACs. A rod-saturating background light was applied for 5 min to light-adapt the retina slice and was sustained throughout all light-adapted recordings.
Data analysis and statistics.
L-IPSC and L-EPSC traces from a given response condition were averaged with Clampfit software (Molecular Devices), and the charge transfer (Q), peak amplitude, and decay to 37% of the peak (D37) were measured in each condition. Because of the significant amount of spontaneous activity, it was difficult to measure a peak from OFF BC L-IPSCs. Therefore, to estimate the peak and D37, average traces were decimated (100 fold) and each point was replaced with the average of those data points to limit variations due to spontaneous activity. To determine changes in total current, the Q was measured, which represents the magnitude of the response. Q was measured in Clampfit over the length of the response, typically 1–2 s, using the same time parameters in each condition for the same cell. All example response traces show responses to the max light flash intensity of 9.5 × 105 photons·μm−2/s−1. For intensity-response curves, light-evoked responses were normalized to the maximal response in the dark-adapted condition. The normalized data were plotted against the log10 of the stimulus intensity.
sIPSC and sEPSC data were analyzed with Clampfit software. A sIPSC or sEPSC template was calculated for each data file with the average of ∼10 prototypical events from the recording. The software used this template to automatically detect spontaneous events, which were manually accepted or rejected on the basis of strict criteria: events used to calculate the frequency were rejected if they appeared to be noise, and events used to calculate the average peak amplitude were rejected if they appeared to be noise or consisted of two or more overlapping events. Frequency was calculated by dividing the number of events by the recording time. Peak amplitude and interevent interval histogram distributions were normalized to the number of events. The signal-to-noise ratio (SNR), which gives a measurement of the actual response signaled over the background noise, was calculated by dividing peak amplitude of the L-IPSC by the variance of the baseline.
Student's t-test (2-tailed, paired) was used to compare response characteristics before and after drug application or light adaptation. Standard Student's t-test (2-tailed, unequal variance) was used to compare values between different cells. Differences were considered significant when P < 0.05 and P < 0.01. All averaged data are reported as means ± SE. The distributions of sIPSC and sEPSC amplitude and interevent interval values were compared with the Kolmogorov-Smirnov (K-S) test, with significance being P < 0.05.
RESULTS
OFF BC subtypes receive different sources of inhibition in the dark.
Previous studies have shown that OFF BCs receive glycineR-, GABAAR-, and GABACR-mediated inhibition (Eggers et al. 2007; Euler and Wässle 1998; Ivanova et al. 2006) that likely includes both rod pathway connections from rod BC-AII AC inputs and cone pathway AC input driven by ON and OFF cone BCs (Fig. 1A). However, these previous studies did not determine whether OFF BCs receive rod BC-AII AC-mediated inputs or whether the light-evoked inhibitory inputs vary between OFF BC subtypes. To resolve this, we identified three OFF BC subtypes by the stratification of their axon terminals within the IPL (Ghosh et al. 2004). We quantified the percentage of the IPL in which OFF BC axon terminals ramify (0% = outer nuclear layer/IPL border) and found that type 4 OFF BCs have terminals that ramify in 1 ± 1% to 38 ± 8% (n = 4) of the IPL, type 3 OFF BCs have terminals that cover 17 ± 2% to 50 ± 4% (n = 11) of the IPL, and both type 1 and type 2 OFF BCs have terminals that cover 1 ± 1% to 16 ± 4% of the IPL (n = 9) (Fig. 1B). Because of their similar morphology, type 1 and type 2 OFF BCs were difficult to differentiate, and their data were pooled together in these measurements.
It is not known whether all of these OFF BC subtypes receive input from the rod-rod BC-AII AC circuit. A previous study suggested that one type of OFF BC (likely type 4) had significant anatomical contacts from AII ACs, while another OFF BC (likely type 3) did not (Tsukamoto et al. 2001). To test for physiological differences in potential AII AC inputs, we recorded L-IPSCs from dark-adapted retinas, where rod pathways are active, in response to long light stimuli to categorize the inhibition as ON, OFF, or ON/OFF. If an OFF BC only receives inhibition at the offset of light, then it could not be receiving rod BC-AII AC-mediated inhibition that is activated at light onset. However, if an OFF BC receives ON inhibition, then it may be due in part to the rod pathway and coming from the AII AC. We found that the responses of the OFF BC subtypes to long light stimuli differ. OFF BC types 1, 2, and 4 (OFF1,2,4) have an ON inhibitory response, while OFF BC type 3 (OFF3) has an OFF inhibitory response (Fig. 1C). This suggests that OFF3 BCs do not receive rod pathway input from AII ACs. To quantify this, we measured the Q 500 ms before, during, and after the long light stimulus. OFF1,2,4 BCs had a significantly higher Q only during the light stimulus (normalized to the baseline Q; Fig. 1D). OFF3 BCs had a significantly higher normalized Q only after light offset (Fig. 1D). OFF1,2,4 responses during light offset and OFF3 responses during light onset were not different from the baseline (P > 0.05). We found no significant difference between the light responses of OFF BC types 1 and 2 and OFF BC type 4, so these data have been pooled as OFF BC types that all receive potential input from AII ACs.
To confirm our results on connections between AII ACs and OFF BCs, we used the fact that AII ACs are activated both by rod pathway activation and by ON cone BC activation through gap junction connections. Since the ON BC-AII activation requires only metabotropic glutamateRs, we recorded OFF BC L-IPSCs in light-adapted conditions with CNQX and AP-5 to block AMPAR/kainateR and NMDAR. Blocking these receptors blocks both OFF BC activation as well as all chemical synapse-activated inhibition so that only ON BC pathways are active. We found that OFF3 BCs are not contacted by the AII ACs, as their inhibition is abolished in light-adapted conditions with CNQX and AP-5 (Fig. 1, E and F; n = 5). In contrast, OFF1,2,4 BCs had significant inhibition remaining after block of all ionotropic glutamateRs (Fig. 1, E and F; 53 ± 13% of total inhibition at the maximum light intensity), suggesting significant contribution from ON BC-activated AII ACs (n = 4), similar to the input from AII ACs to OFF GCs shown previously (Manookin et al. 2008; Munch et al. 2009; Murphy and Rieke 2008). This inhibition was blocked by strychnine (n = 2, data not shown), showing that it was coming from glycinergic ACs, presumably the AII. From these results we can confirm that only OFF1,2,4 BCs are contacted by an ON pathway mediated by rod-AII AC. The remaining inhibition in OFF1,2,4 BCs also suggests that cones are being robustly stimulated with a 525-nm LED in a light-adapted condition.
As seen in the examples in Fig. 1, C and D, the sIPSCs from OFF1,2,4 and OFF3 BCs also appear to have different amplitudes and frequency, with OFF1,2,4 BCs receiving much more spontaneous input. If inhibitory input to the OFF1,2,4 and OFF3 BCs is coming from different sources, we would expect that the spontaneous activity might also be different, as spontaneous activity varies between neurons. We measured sIPSCs in the absence of light stimuli from dark-adapted retinas. The sIPSC peak amplitude was significantly lower in OFF3 BCs (16 ± 1 pA, n = 11) than in OFF1,2,4 BCs (38 ± 7 pA, n = 13) (P < 0.01). Additionally, the sIPSC frequency was also significantly lower in OFF3 BCs (7 ± 3 Hz, n = 12) than in OFF1,2,4 BCs (25 ± 6 Hz, n = 15) (P < 0.05). The lower amplitude and frequency of sIPSCs suggest that OFF3 and OFF1,2,4 BCs are receiving inputs from distinct sources.
Dark-adapted L-IPSCs are mostly glycinergic in OFF1,2,4 but not OFF3 BCs.
The differences in OFF1,2,4 and OFF3 BC L-IPSCs also suggest that they are receiving distinct inhibitory inputs from different sources. The majority of the dark-adapted inhibition to OFF1,2,4 BCs likely comes from the rod BC-AII AC pathway, leading to the large glycinergic light-evoked currents previously observed by Eggers et al. (2007), since the rod pathway should be the most activated in the dark-adapted state. However, our results from Fig. 1 suggest that the OFF3 BCs are not receiving AII input and thus may not have large glycinergic inputs. To test this, we recorded L-IPSCs and sIPSCs from dark-adapted OFF BCs in control conditions and with application of strychnine, a glycineR antagonist, to separate glycinergic and GABAergic contributions (Eggers et al. 2007). There was a significant decrease in OFF1,2,4 BC L-IPSCs (n = 6) with application of strychnine (Fig. 2, A and C) at all light intensities used (P < 0.01). In contrast, OFF3 BCs (n = 4) only had significant decreases in L-IPSCs at the brighter light intensities (Fig. 2, B and D), while at the two dimmest light intensities glycineR blockade produced no significant change. In dark-adapted conditions, OFF1,2,4 L-IPSCs were dominated by glycine (80 ± 5% of the total inhibition at the maximum light intensity), in contrast to OFF3 BC responses (51 ± 4%, P < 0.05). These results suggest that in the dark the main inhibition to OFF1,2,4 BCs is glycinergic mainly mediated by the rod BC-AII AC pathway, while only half of the inhibition to OFF3 BCs was due to glycine input from OFF cone-activated ACs. Since glycinergic and GABAergic inhibition have distinct time courses (Eggers and Lukasiewicz 2006b; Eggers et al. 2007), this could contribute to further differences between signaling of OFF BC subtypes.
Fig. 2.

Dark-adapted L-IPSCs are mostly mediated by glycinergic input in OFF1,2,4 but not OFF3 BCs. A and B: example L-IPSCs recorded from an OFF1/2 and an OFF3 BC, respectively, in response to a 30-ms flash of light (dark gray bar below L-IPSC). The dark-adapted L-IPSC was greatly reduced by application of strychnine. Recording paradigm is shown on left with glycine receptors blocked. C and D: intensity-response curves of normalized charge transfer of OFF1,2,4 (n = 6) and OFF3 (n = 4) BCs, respectively, to the max dark-adapted L-IPSCs. Strychnine significantly reduced OFF1,2,4 L-IPSCs at all light intensities, while only the 3 highest light intensities were reduced in OFF3 BCs. Glycinergic inhibition in OFF3 BCs appears to be a smaller component than in OFF1,2,4 BCs (P < 0.05). *P < 0.05, **P < 0.01.
Dark-adapted sIPSCs are primarily glycinergic in both OFF1,2,4 and OFF3 BCs.
As may be seen in Figs. 1 and 2, OFF BCs have a significant amount of background spontaneous activity, which reflects their inputs and may affect signal transmission of light-evoked responses. Although the light-evoked inputs to the classes of OFF BCs differ, sIPSCs for both OFF1,2,4 and OFF3 BCs in the dark were mostly due to glycineR activation. Strychnine abolished sIPSCs in half of the OFF1,2,4 and a quarter of the OFF3 BCs. Of the spontaneous events that remained, there was a significant reduction in the sIPSC peak amplitude for OFF1,2,4 (K-S P < 0.01, n = 3) and OFF3 (K-S P < 0.01, n = 3) BCs (Fig. 3) but no change in the decay τ of the sIPSCs in either group (OFF1,2,4, n = 3, P = 0.88; OFF3 BCs, n = 3, P = 0.79; data not shown). Although small differences in the decay of GABAA- and glycineR-mediated sIPSCs have previously been shown (Eggers et al. 2007), the lack of a significant change in the kinetics of the sIPSCs that remain after strychnine, presumably due to GABAAR input (Eggers et al. 2007), could be due to the very small sample of sIPSCs that remained in only a handful of cells. There was also a significant decrease in sIPSC frequency with strychnine application for both OFF1,2,4 (n = 6) and OFF3 (n = 4) BCs (P < 0.01). These results suggest that in the dark most of the tonic spontaneous inhibition to OFF BCs is due to glycinergic-mediated currents likely coming from both the AII AC and other ON ACs for OFF1,2,4 BCs and only OFF glycinergic ACs for OFF3 BCs.
Fig. 3.
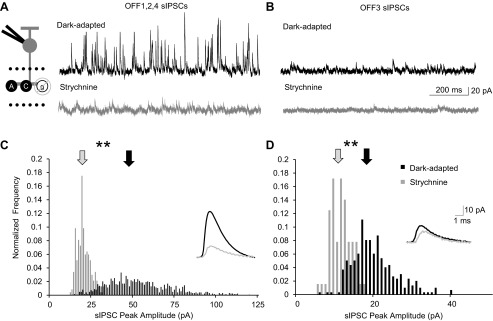
Dark-adapted spontaneous (s)IPSCs are mediated mainly by glycinergic input in both OFF1,2,4 and OFF3 cells. A and B: example traces showing the sIPSCs from an OFF4 and an OFF3 BC, respectively, in dark-adapted conditions and with application of strychnine. Recording paradigm is shown on left with glycine receptors blocked. C and D: sIPSC peak amplitude histogram distributions (normalized to number of events) of the OFF1,2,4 and OFF3 BCs from A and B, respectively. Application of strychnine significantly reduced the sIPSC peak amplitude for OFF1,2,4 BCs (K-S P < 0.01) and OFF3 BCs (K-S P < 0.01). Arrows show the average amplitude of the sIPSC. Insets are averages of dark-adapted (black line) and strychnine application (gray line) sIPSCs; scale bars apply to both insets. **P < 0.01.
Light adaptation alters timing and charge transfer of AII AC L-EPSCs.
We have found that dark-adapted inhibition to OFF1,2,4 and OFF3 BCs varies, likely due to AII AC versus non-AII AC inputs. However, OFF BCs function in both rod- and cone-dominant conditions, switching from rod to cone pathways that could modulate inhibition to the OFF BC subtypes differently. Light adaptation has been used to separate the rod and cone pathways by applying a maximally rod-activating background light to the retina (Xin and Bloomfield 1999). If the rod pathway is saturated via light adaptation, then the amount of excitation the rod BC gives to the AII AC should decrease. However, AII ACs can also be directly activated in cone-dominant conditions by ON BCs through electrical connections (Strettoi et al. 1994; Trexler et al. 2001; Volgyi et al. 2004). To confirm that activation of AII ACs declines when switching from rod to cone activation with our light stimulus, we recorded L-EPSCs from AII ACs in dark- and light-adapted (maximal rod pathway activation of 950 photons/μm2; Wang and Kefalov 2009) conditions.
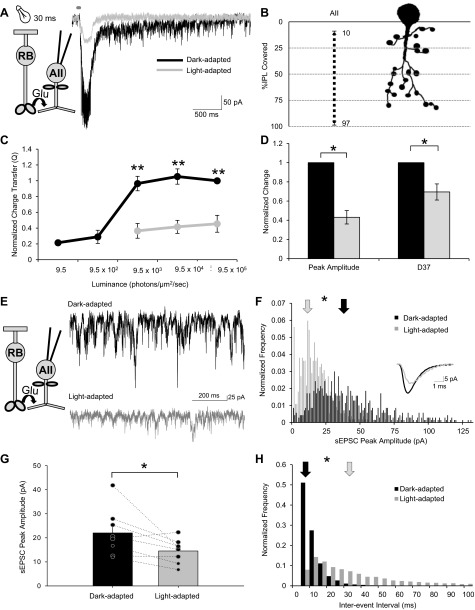
AII ACs were identified prior to recording by their characteristic soma shape and large proximal dendrite and labeled with a fluorescent dye, Alexa Fluor 488, during the recording to quantify their morphology after the recording. We found that AII AC processes covered between 10 ± 2% and 97 ± 4% of the IPL (n = 6; Fig. 4B). With light adaptation, there was a significant decrease in the Q of the AII L-EPSCs at each light intensity (n = 9; Fig. 4, A and C). In addition, there was a significant decrease in the normalized peak amplitude (to 43 ± 7%) and D37 (to 69 ± 8%) of the L-EPSC (Fig. 4D). The remaining excitatory input is most likely due to cone activation via electrical connections of the AII AC and ON BCs, which is smaller than the high-gain rod BC-AII AC synapse (Pang et al. 2004; Volgyi et al. 2004; Xin and Bloomfield 1999). Although previous studies show that AII ACs do function during light-adapted cone-dominant conditions by receiving rod input via rod-cone coupling and ON BC-AII AC electrical coupling (Demb and Singer 2012; Manookin et al. 2008; Munch et al. 2009), our results, in combination with Fig. 1, E and F, and other studies, show that this drive is less robust than AII AC activation in the dark. AII AC sEPSCs were also recorded under both dark- and light-adapted conditions. With light adaptation, the sEPSC peak amplitude significantly decreased from 22 ± 3 pA to 14.5 ± 2 pA (n = 8; Fig. 4, E–G). In addition, the normalized sEPSC frequency significantly decreased to 45 ± 10% of control with light adaptation (n = 9) and the interevent interval significantly increased (487% of dark-adapted interevent interval average) (Fig. 4H). Overall, these data suggest that AII AC inhibition to OFF BCs decreases with our light-adapting stimulus.
Fig. 4.
Light adaptation alters the timing and the charge transfer of the AII light-evoked excitatory postsynaptic current (L-EPSC) and the magnitude and frequency of spontaneous (s)EPSCs. A: L-EPSCs from an AII AC in response to a 30-ms flash of light (dark gray bar above the L-EPSC). There was a significant reduction of the dark-adapted L-EPSC with light adaptation. Recording paradigm is shown on left. B: example cell morphology traces of an AII AC. AII ACs have terminals that cover 10–97% (n = 6) of the IPL. They have lobular terminals in the OFF BC sublamina of the IPL, responsible for glycine release, and longer, thinner processes in the ON sublamina that make gap junction connections to ON BCs. C: intensity-response curves of the charge transfer normalized to the max dark-adapted L-EPSC in AII dark- and light-adapted conditions (n = 9). There was a significant difference in the charge transfer with light adaptation at all intensities measured. D: normalized change in L-EPSC response parameters of AII ACs (n = 9) with light adaptation. In the light, the peak amplitude and decay time [decay to 37% of peak (D37)] significantly decreased. E: example traces showing the sEPSCs of AII ACs in dark-adapted and light-adapted conditions. Recording paradigm is shown on left. F: sEPSC peak amplitude histogram distribution (normalized to number of events) of the AII AC seen in E. Each pair of data points in G shows the change in the response of a single BC. Light adaptation significantly reduced the sEPSC peak amplitude for the AII AC (K-S P < 0.01). Arrows show the average amplitude of the sEPSCs. Inset is an average of dark-adapted (black line) and light-adapted (gray line) sEPSCs. G: the peak amplitude of sEPSCs was significantly decreased in AII ACs after light adaptation (n = 8). H: sEPSC interevent interval histogram distribution (normalized to number of events) of the AII AC seen in E. Light adaptation significantly increased the sEPSC interevent interval for the AII AC (K-S P < 0.01). Arrows show the average interval between the sEPSCs. *P < 0.05, **P < 0.01.
Magnitudes of OFF BC L-IPSCs are unaffected by switching from rod to cone circuits.
We have shown that in the dark OFF1,2,4 BCs receive mostly glycinergic input, likely from the AII ACs, while only about half of OFF3 BC L-IPSCs consist of glycineR-mediated currents from other AC types. However, it is unknown how inhibition to either OFF1,2,4 or OFF3 BCs will change when the cone pathways are dominant. To determine whether there are changes in inhibition to OFF BCs with light adaptation, L-IPSCs were recorded from OFF1,2,4 and OFF3 BCs under a rod-adapting background light. If OFF1,2,4 BCs are receiving primarily rod-mediated inhibition, we would expect there to be a significant reduction in L-IPSCs after light adaptation, while we might not expect OFF3 BC L-IPSCs to change significantly since they are receiving cone pathway inhibition. Surprisingly, under these conditions, there was no significant difference in the Q in either OFF1,2,4 (n = 13, P > 0.05) or OFF3 (n = 12, P > 0.05) BCs with light adaptation at any stimulus intensity (Fig. 5, A–C). The absence of any change in the Q of the L-IPSC in OFF1,2,4 BCs suggests that cone-pathway mediated inhibition is compensating for the reduction in rod pathway-mediated inhibition from the AII AC.
Fig. 5.

Light adaptation alters the timing of the L-IPSC in only OFF1,2,4 BCs, while the magnitude of L-IPSCs remains unaffected for each BC group. A and B: example L-IPSCs from an OFF1/2 and an OFF3 BC, respectively, in response to a 30-ms flash of light (dark gray bar below L-IPSC trace). There was no significant reduction of the dark-adapted L-IPSC with light adaptation. Recording paradigm is shown on left. C: intensity-response curves of charge transfer normalized to the max dark-adapted L-IPSC in OFF1,2,4 (n = 13) and OFF3 (n = 12) dark- and light-adapted L-IPSCs. There was no significant difference in the charge transfer between OFF1,2,4 and OFF3 BCs with light adaptation. D: normalized change in L-IPSC response parameters of OFF1,2,4 (n = 13) and OFF3 (n = 10) BCs to max dark-adapted response with light adaptation. The peak amplitude was significantly reduced, while the decay time was significantly increased in OFF1,2,4 BCs. In OFF3 BCs, neither the peak amplitude nor the decay time changed significantly. *P < 0.05, **P < 0.01.
A change in the source of inhibition to OFF1,2,4 BCs from AII ACs to cone pathway ACs was also suggested by the significant decrease in the normalized peak amplitude (63 ± 9%, P < 0.01) and a significant increase in the normalized D37 (193 ± 30%, P < 0.05) (Fig. 5D). In dark-adapted conditions there was no significant difference in D37 between the OFF1,2,4 BC L-IPSCs and the AII AC L-EPSCs (AII D37 of 147 ± 40 ms, OFF1,2,4 BC D37 of 142 ± 45 ms, P > 0.05), while in the light they differed significantly (AII D37 of 104 ± 29 ms, OFF1,2,4 BC D37 of 195 ± 34 ms, P = 0.05), further suggesting different sources of input. There was no difference in the time to peak between dark- and light-adapted conditions for either OFF BC group (P > 0.05, data not shown). In contrast, the lack of change in the Q or timing parameters (D37 107 ± 27, P = 0.8) of OFF3 BCs agrees with our previous conclusion that their inhibition comes from cone pathways.
Additionally, there were differences in L-IPSC timing parameters between OFF1,2,4 and OFF3 BCs in the dark and in the light. There was a significant difference in the time to peak of L-IPSCs between OFF1,2,4 and OFF3 BCs in the dark (OFF1,2,4 average of 125 ± 24 ms, OFF3 average of 213 ± 23 ms, P < 0.05) and in the light (OFF1,2,4 average of 124 ± 33 ms, OFF3 average of 222 ± 21 ms, P < 0.05). It makes sense for OFF3 BCs to have longer response delays, as they begin to receive inhibitory input only when the light turns off. Finally, the L-IPSC D37 of OFF1,2,4 BCs was significantly longer than that of OFF3 BCs only in the light (OFF1,2,4 average of 195 ± 38 ms, OFF3 average of 90 ± 16 ms, P < 0.05). The timing differences of L-IPSCs between the two groups of BCs highlight their different circuitry connections and pathways.
Light adaptation differentially modulates OFF1,2,4 and OFF3 BC sIPSCs.
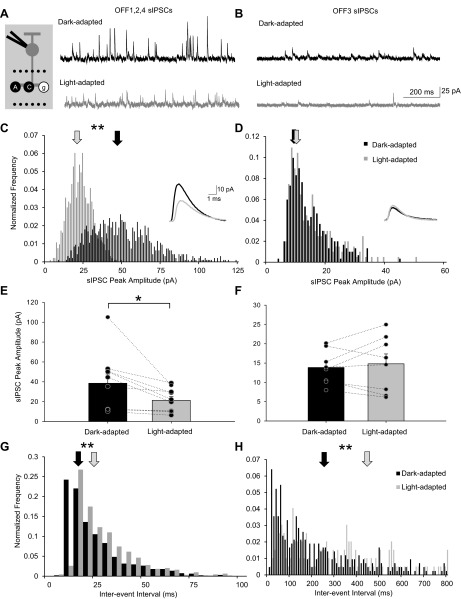
sIPSCs in OFF BCs form the background inhibition they are receiving from all AC inputs with no external stimulus involved. Thus any modulation of the sIPSCs would change the baseline level of inhibition the cells are receiving and, in turn, alter their glutamate release onto GCs. In contrast to the L-IPSCs, the sIPSCs of OFF1,2,4 and OFF3 BCs were differentially changed with a switch to cone pathways under light-adapted conditions. The sIPSC peak amplitude of OFF1,2,4 BCs decreased significantly (38 ± 9 to 21 ± 4 pA, K-S P < 0.01; Fig. 6, A, C, and E). This is consistent with the idea that a majority of these glycinergic sIPSCs are coming from the rod BC-AII AC-mediated pathway, as we show in Fig. 4 that spontaneous AII AC activity decreases significantly with light. There was no change in the sIPSC peak amplitude in OFF3 BCs (13.58 ± 1.54 to 15 ± 3 pA, K-S P > 0.05; Fig. 6, B, D, and F), further suggesting that the sIPSCs of OFF3 BCs are coming from cone-dependent OFF pathways. The decay τ of the sIPSCs did not change significantly with light adaptation in OFF1,2,4 (n = 8, P = 0.68) or OFF3 (n = 7, P = 0.93) BCs (data not shown). However, the sIPSC frequency normalized to the dark-adapted condition significantly decreased in the light in both OFF1,2,4 (to 69 ± 11%, n = 10, P < 0.05) and OFF3 (to 51 ± 10%, n = 9, P < 0.01) BCs. As a result, the interevent intervals of the sIPSCs also significantly increased in both OFF BC groups (119% of dark-adapted interevent interval average in OFF1,2,4 BCs and 166% of dark-adapted interevent interval average in OFF3 BCs) (Fig. 6, G and H). Taken together, these results suggest that with light adaptation OFF1,2,4 BCs are losing a main source of spontaneous inhibition (rod BC-AII AC pathway) because of a decrease in both peak amplitude and frequency, whereas OFF3 BCs may just be receiving fewer overall sIPSCs from the same ACs.
Fig. 6.
Light adaptation decreased sIPSC peak amplitude and frequency in OFF1,2,4 BCs but only frequency in OFF3 BCs. A and B: example traces showing the sIPSCs from an OFF1/2 and an OFF3 BC, respectively, in dark-adapted and light-adapted conditions. Recording paradigm is shown on left. The sIPSC peak amplitude histogram distributions (normalized to number of events) of the OFF1,2,4 and OFF3 BCs are shown in C and D, respectively. Light adaptation significantly reduced the sIPSC amplitude for OFF1,2,4 BCs (K-S P < 0.01) but not OFF3 BCs (K-S P > 0.05). Arrows show the average peak amplitude of the sIPSCs. Insets are averages of dark-adapted (black line) and light-adapted (gray line) sIPSCs; scale bars apply to both insets. E and F: the peak amplitude of sIPSCs was significantly decreased in OFF1,2,4 BCs (E, n = 10) after light adaptation and did not change in OFF3 BCs (F, n = 8). G and H: sEPSC interevent interval histogram distributions (normalized to number of events) of the OFF1/2 and OFF3 BCs seen in A and B, respectively. Each pair of data points in E and F shows the change in the response of a single BC. Light adaptation significantly increased the sIPSC interevent interval for both cell groups (K-S P < 0.01). Arrows show the average interval between the sIPSCs. *P < 0.05, **P < 0.01.
Changes in signal-to-noise ratio suggest distinct sources of input.
Changes in the background activity of OFF BC inhibition with light adaptation suggested that the strength of signal over background noise might also be changing. This can be estimated by calculating the SNR of a response. The SNR was calculated by dividing the peak amplitude of the L-IPSC by the variance of 100 ms of baseline, which accurately represented baseline variation for the given cell. Given that OFF1,2,4 BCs have significantly more inhibitory spontaneous activity than OFF3 BCs, we predicted that the SNR for their responses might be different as well, which would affect the efficiency of OFF BC signal transmission to OFF GCs. For this reason, we calculated the SNR of OFF1,2,4 (n = 13) and OFF3 (n = 11) BCs in both dark- and light-adapted conditions to determine whether there was a change in the relationship between the L-IPSC and the baseline sIPSCs. The SNR significantly increased for both OFF1,2,4 and OFF3 BCs with light adaptation (P < 0.05), suggesting that the decrease in the sIPSCs helps to magnify the L-IPSC (Fig. 7, A and B). In the light, when the cone pathways are active, the light-evoked inhibitory input to OFF BCs is larger relative to the dark-adapted state as a result of the decrease in background noise. However, the SNR was significantly larger in OFF3 BCs in both dark- and light-adapted conditions (Fig. 7C). This is most likely a result of the significantly fewer sIPSCs in OFF3 BCs (Fig. 1). Our results further support the idea that OFF1,2,4 and OFF3 BCs are receiving inhibitory input from distinct sources and suggest that, in general, cone-mediated inputs have a higher SNR than rod-mediated inputs. A previous study (Arman and Sampath 2012) showed that glycinergic inputs do not significantly modulate the SNR (calculated with the unequal variance model of d′ or da) of very dim light voltage responses in OFF BCs. Our results, however, suggest that at brighter rod and cone light intensities this may be an important mechanism for determining the gain of inhibition to OFF BCs, possibly allowing for larger light-evoked modulation of the OFF BC excitatory signal.
Fig. 7.
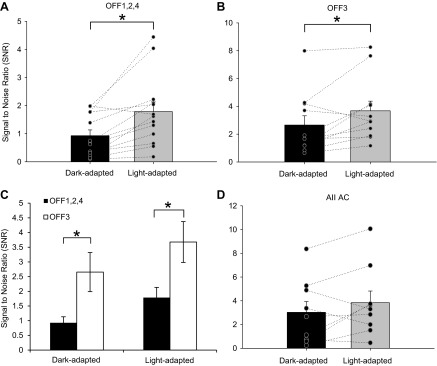
The signal-to-noise ratio (SNR) increased in both OFF BC groups with light adaptation but did not change in AII ACs. A and B: SNRs in OFF1,2,4 (n = 13) and OFF3 (n = 11) BCs, respectively. There was a significant increase in the SNR in the OFF1,2,4 as well as OFF3 BCs with light adaptation. C: SNR comparing OFF1,2,4 (n = 13) and OFF3 (n = 11) BCs in dark- and light-adapted conditions. In both instances, there was a significantly higher SNR in OFF3 BCs. SNR was calculated by dividing the peak amplitude of the L-IPSC by the variance of 100 ms of the baseline current. D: SNR of AII AC light responses in dark- and light-adapted conditions. There was no significant difference between the conditions (n = 9). Each pair of data points in A, B, and D shows the change in the response of a single BC. *P < 0.05.
Dark-adapted AII ACs receive a large amount of spontaneous activity (Fig. 4), which sets the background for light-evoked signals. Thus any changes in the sEPSCs could change the light signal to postsynaptic OFF BCs. As a result of the decrease in the peak amplitude and frequency of spontaneous activity, the SNR of the AII AC did not change (n = 9, P = 0.18; Fig. 7D). The increase in SNR of OFF BCs with light adaptation, contrasted to the unchanged SNR of the AII AC, suggests that OFF1,2,4 BCs that receive primarily AII AC input in the dark must be getting input from other sources in the light. The smaller AII AC signal, presumably due to both less quantal release from RBCs and rod saturation, could not be providing the drive for the increase of the OFF BC signal relative to spontaneous noise. Taken together, these findings indicate that light adaptation increases the SNR, allowing for more sensitive higher visual responses in the light-adapted condition.
Increased GABAergic input provides compensatory light-adapted inhibition to OFF BCs.
In dark-adapted conditions, the L-IPSCs and sIPSCs in OFF1,2,4 BCs were due mostly to glycinergic input, presumably from AII ACs. However, because there was no change in the L-IPSCs of OFF1,2,4 BCs with light adaptation, while the AII AC activation significantly decreased, it is necessary to determine what input is mediating the compensatory inhibition. To investigate the inhibition OFF BCs are receiving in the light, strychnine was applied to the preparation to block glycineRs, followed by TPMPA and finally SR-95531 to block GABACRs and GABAARs, respectively. In OFF1,2,4 BCs, strychnine significantly decreased the L-IPSCs at all light intensities (n = 11) and application of SR-95531 and TPMPA abolished all light-evoked responses (n = 5; Fig. 8, A and C). There was no significant reduction in L-IPSCs after TPMPA application (data not shown; 37 ± 7% of the total initial Q after strychnine to 50 ± 25% after TPMPA, P > 0.05), but the additional application of SR-95531 abolished the light responses in all cells tested (n = 4). This suggests that light-adapted OFF BCs, like dark-adapted OFF BCs (Eggers et al. 2007), receive very little GABACR-mediated input. The OFF1,2,4 BC L-IPSCs switched to significantly more GABA input mediated by GABAARs (43 ± 10% glycine, 57 ± 10% GABA, P < 0.05) when going from dark- to light-adapted conditions (Fig. 8E). The decrease in glycinergic input to OFF1,2,4 BCs (37 ± 11%) with light adaptation correlates with the decrease in excitatory input to AII ACs (54 ± 10%) in the light, suggesting that the majority of the glycinergic input comes from this connection (Welch's t-test, P = 0.08).
Fig. 8.
With light adaptation, both OFF BC groups received compensatory GABAergic inhibition. A and B: example light-adapted L-IPSCs from an OFF1/2 and an OFF3 BC, respectively, in response to a 30-ms flash of light (dark gray bar below the L-IPSC). The light-adapted L-IPSC was unchanged with isolation of GABAergic input (strychnine) in OFF3 BCs only. OFF1,2,4 light-adapted L-IPSCs decreased when GABAergic input was isolated. All L-IPSCs were absent when both glycine and GABA receptors were blocked (TPMPA + SR-95531). Recording paradigm is shown on left with GABA and glycine receptors blocked. C and D: intensity-response curves of charge transfer normalized to the max light-adapted L-IPSC in OFF1,2,4 (n = 11) and OFF3 (n = 8) BCs, respectively. Addition of strychnine had no effect on the L-IPSCs of OFF3 BCs but significantly decreased the L-IPSCs of OFF1,2,4 BCs. Asterisks indicate significant difference between the light-adapted control and strychnine application; daggers indicate significant difference between the strychnine treatment and the GABA receptor blockade (SR-95531 + TPMPA). E: relative proportions of glycinergic and GABAergic input to OFF BCs under dark- and light-adapted conditions for OFF1,2,4 (n = 5 and 11, respectively) and OFF3 (n = 5 and 8, respectively) subtypes. With light adaptation, OFF1,2,4 BCs had a significant decrease in glycinergic input and OFF3 BCs had virtually no glycine response. OFF1,2,4 BCs had significantly more glycinergic input than OFF3 BCs in both light conditions. Asterisks indicate significant difference between the dark- and light-adapted conditions within an OFF BC group; pound signs indicate significant difference between OFF1,2,4 and OFF3 BCs in each light condition. *,†,#P < 0.05, **,††P < 0.01.
Although OFF3 BCs receive no input from AII ACs and have much less glycinergic input in the dark than OFF1,2,4 BCs (Fig. 8E; P < 0.05), the rod pathway input eventually is distributed across at least some cone BC pathways via electrical synapses. This could mean that the activation of the OFF cone BCs and OFF ACs that send input to the OFF3 BCs could vary between rod- and cone-dominant conditions. In OFF3 BCs, strychnine had no significant effects on the light-adapted L-IPSCs (n = 8) and application of SR-95531 and TPMPA abolished all light-evoked responses (n = 5) (Fig. 8, B and D). Similar to OFF1,2,4 BCs, TPMPA application had no effect (data not shown; 93 ± 14% of the total initial Q after strychnine to 79 ± 19% after TPMPA, P > 0.05) but the additional application of SR-95531 virtually eliminated the light responses in all cells tested (n = 4, 12 ± 4% after SR-95531, P < 0.05). The OFF3 BC L-IPSCs switched to solely GABAAR-mediated input (P < 0.05) in the light. There was a significantly smaller percentage of light-evoked glycineR input to OFF3 than OFF1,2,4 BCs in both dark- and light-adapted conditions (Fig. 8E; P < 0.05), likely because of remaining AII AC inputs to OFF 1,2,4 BCs in the light. These results suggest that cone pathway activation significantly alters the proportions of inhibition to switch in favor of GABAergic input to OFF1,2,4 and OFF3 BCs, effectively altering the type and source of inhibition that the OFF BCs receive.
Unlike the L-IPSCs, the sIPSCs in light-adapted conditions were mostly mediated by glycinergic input for both OFF1,2,4 and OFF3 BCs (data not shown). For most cells, application of strychnine eliminated all sIPSCs, causing a significant decrease in frequency in OFF1,2,4 (to 20 ± 16% of total frequency, n = 8) and OFF3 (to 21 ± 16% of total frequency, n = 6) BCs (P < 0.01). For the cells where some sIPSCs remained, there was no significant difference in the sIPSC peak amplitude of OFF1,2,4 (n = 3, P = 0.25) and OFF3 (n = 2, P = 0.54) BCs. The decay τ of the sIPSCs did not significantly change in the light when glycineRs were blocked in OFF1,2,4 (n = 3, P = 0.14) or OFF3 (n = 3, P = 0.14) BCs. Despite the switch of the L-IPSCs to larger GABAergic input, the sIPSCs of both OFF1,2,4 and OFF3 BCs in the light are still mediated by glycine release, suggesting that glycinergic ACs' high basal rate of neurotransmitter release plays the role of setting background inhibition to OFF BCs.
DISCUSSION
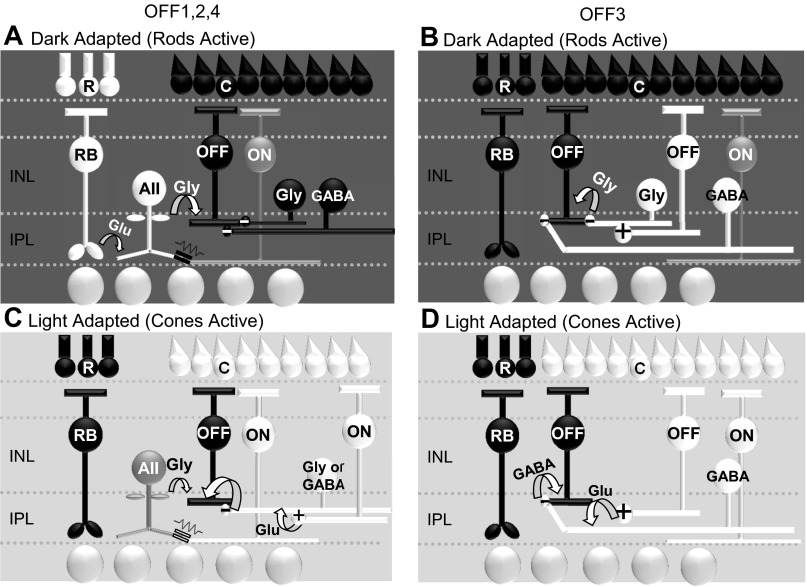
In natural visual situations, light levels may activate both rods and cones simultaneously and changes in background light levels may emphasize rod or cone activation. Several studies have analyzed retinal signaling across different background light levels and have shown that gain and noise in the rod pathway change in turn (Dunn et al. 2006; Dunn and Rieke 2008). Here we show that the ambient light state modulates the source and noise level of OFF BC inhibition when switching between rod and cone pathways. When a rod-saturating background was applied glycinergic input to OFF BCs was reduced, but this reduction was compensated by an increase in GABAergic input. In the dark OFF1,2,4 BCs are receiving substantial glycinergic input from the rod-AII AC pathway, while in the light they receive equal amounts of glycine and GABAergic input from ON cone pathways (Fig. 9, A and C). In contrast, OFF3 BCs, which do not receive input from the rod pathway AII AC, receive much more GABAergic input in both the dark- and light-adapted conditions from OFF cone pathways (Fig. 9, B and D). Although the total amount of inhibition to OFF BCs did not change, the SNR increased with light adaptation because of a decrease in spontaneous baseline inhibition to OFF BCs and was different between the rod- and cone-activated pathways. Collectively, these suggest a specialization among OFF BC pathways for light information from the rods and cones.
Fig. 9.
Summary schematics showing inhibitory input to OFF1,2,4 and OFF3 BCs under dark- and light-adapted conditions. A: under dark-adapted conditions, OFF1,2,4 BCs receive large light-evoked glycinergic inhibition from the rod-AII AC pathway in addition to other glycinergic and GABAergic inputs. This is done through crossover inhibition via excitation of the AII AC with dim light and serves as an ON inhibitory signal to the OFF pathway. B: under dark-adapted conditions, OFF3 BCs receive large light-evoked glycinergic and GABAergic inhibition from OFF cone pathways activated by brighter light intensities. C: under light-adapted conditions OFF1,2,4 BCs receive compensatory GABAergic and glycinergic inhibition from cone-activated ACs. The AII AC may receive depolarizing signals through gap junction connections with ON BCs and partly mediate inhibitory currents in the OFF1,2,4 BCs (Fig. 1, E and F). However, there is an overall higher proportion of GABAergic input in the light. D: in light-adapted conditions, OFF3 BCs receive compensatory inhibition solely mediated by GABAergic input from OFF cone-activated ACs.
AII ACs target specific OFF BC pathways.
The AII AC-OFF BC synapses are prominent morphologically (McGuire et al. 1984; Strettoi et al. 1990) but had not been directly investigated physiologically, A previous report concluded that AII AC-OFF GC connections, but not AII AC-OFF BC connections, signal the threshold of rod responses (Arman and Sampath 2012) and did not find large glycinergic inputs in response to the dim light intensities tested or changes in the SNR when blocking glycinergic inputs. However, our data show that with the use of brighter rod light stimuli, OFF BCs receive robust AII AC input, which is targeted to three subtypes (OFF 1,2,4) of OFF BCs (Fig. 1). This supports a previous morphological study that showed that OFF4 BCs, but not OFF3 BCs, received AII AC inputs (Tsukamoto et al. 2001). The potential differences in sensitivity between OFF BCs and OFF GCs to AII input may be due to differential synapse structure between the AII and OFF BCs and OFF GCs.
Increased GABAergic inhibition with light adaptation may modulate spatial sensitivity of OFF pathway.
We show that decreases in glycinergic OFF BC inhibition are compensated by increases in GABAergic input under light-adapted conditions. Glycinergic ACs such as the AII AC are narrow-field cells and thus may not mediate wide spatial inhibition, whereas GABAergic AC processes span long lengths of the retina (Menger et al. 1998; Pourcho and Goebel 1983, 1985; Vaney 1990). Although in the dark AII ACs are connected via gap junctions that increase their spatial range to ∼200–400 μm in very dim light, they are uncoupled with light adaptation and their spatial spread is much lower than potentially coupled wide-field GABAergic ACs (1,000–4,000 μm; Bloomfield et al. 1997; Xin and Bloomfield 1997, 1999). Thus the switch from glycinergic to GABAergic inhibition has the potential to widen the spatial inhibitory surrounds of OFF BCs, which may change their spatial sensitivity.
Many previous studies have shown that GC spatial sensitivity increases when moving from dim to bright light conditions (Barlow et al. 1957; Kuffler 1953; Maffei et al. 1971; Troy et al. 1999), which has been suggested to be due to changes in inner retinal circuitry (Dedek et al. 2008). Our findings suggest that changing inhibition to BCs may be one signaling mechanism to downstream GCs to enable more differentiation between spatially distinct light signals. It may be more important to have narrow-field inhibition in the dark where there is no need for high spatial sensitivity, as shown by the large convergence of rod signals (Vaney 1991). In light-adapted conditions, cone vision mediates the highest spatial acuity in order to differentiate novel light signals from the background luminance. This is formed from GABAergic input modulating center-surround organization, which is an important feature for GC spatial tuning (Cook and McReynolds 1998; Flores-Herr et al. 2001; O'Brien et al. 2003; Volgyi et al. 2002). Increasing GABAergic inhibition to OFF BCs could be one way of adjusting the inhibitory surrounds of BCs to affect the spatial center signals that GCs receive.
One potential mechanism for modulation of OFF BC inhibition is the increase in dopamine levels in the light (Doyle et al. 2002). Dopamine was found to potentiate GABAAR currents, possibly through phosphorylation by the cAMP-PKA signaling cascade (Feigenspan and Bormann 1994). This mechanism could cause circuitry changes such that cone-activated GABAergic ACs are potentiated in the light while glycinergic AC outputs are decreased. A logical progression of this idea is that the increase in GABAergic inhibition could be partly inhibiting AII AC glycinergic release in addition to inhibiting OFF BCs. However, several studies have shown that AII ACs in the light receive primarily glycine-mediated inhibition and not GABAergic inhibition (Gill et al. 2006; Weiss et al. 2008). Thus it is likely that inhibition of the AII AC is not the direct cause of the inhibitory switch and dopamine may be acting through other means. Additionally, the increase in the inhibitory SNR could modulate the relative output of OFF BCs to filter out unneeded background light information. Further examination is necessary to determine what role the increase in the SNR plays in OFF BC signaling and whether there is a change in the spatial signaling to these cells. This would be an interesting future expansion of the present study.
OFF BC subtypes are receiving and sending different types of signals.
In this study we have investigated the potential role of the primary rod pathway that transfers dim light information through the rod BC-AII AC circuit to OFF BCs (Chun et al. 1993; Deans et al. 2002; McGuire et al. 1984; Sassoe-Pognetto et al. 1994; Strettoi et al. 1990; Trexler et al. 2001). However, there are two other rod pathways: the secondary pathway, activated by moderate light that signals OFF BCs through electrical connections between rods and cones (DeVries and Baylor 1995; Volgyi et al. 2004), and the tertiary pathway, where brighter light causes direct rod activation of OFF BCs (Tsukamoto et al. 2001; Volgyi et al. 2004). Since there are multiple OFF BC pathways that convey distinct portions of the light signal, it is possible that the inputs from these three rod pathways will vary among OFF BC types. Our conclusions in this study support previous anatomical work showing that OFF3 BCs do not have AII AC connections (Tsukamoto et al. 2001). Several studies have shown that OFF3-like BCs contact both rods and cones and may be the BC that mediates the alternative rod pathway from rods directly to BCs (Mataruga et al. 2007; Tsukamoto et al. 2001). Furthermore, it has recently been shown that OFF BCs (types 1 and 2) may receive rod information via rod-cone coupling in scotopic conditions (Pang et al. 2012) whose input may also activate cone inhibitory circuits that provide inhibition to OFF BCs. This pathway may be responsible for additional inhibition to OFF BCs but requires a different set of investigations to elucidate specific connections and its role in the inhibitory switch with light adaptation. We have shown here that OFF1,2,4 subtypes may receive input from the primary rod pathway (rod BC-AII AC-OFF BC) while OFF3 BCs instead may be getting brighter light information from the secondary and tertiary rod pathways. Our data support the idea that portions of rod pathway signals are specialized to different OFF BC pathways, although more work is required to determine how the dynamic range and timing of rod pathway input to OFF BCs vary.
In summary, we found that L-IPSCs in OFF BCs change from primarily glycine mediated to GABA mediated between dark- and light-adapted conditions. This occurs in a pathway-specific manner that may serve to help retinal fine-tuning of spatial sensitivity during daytime vision so that the visual scene can be accurately signaled. This type of switch allows the retina to make efficient use of available neurons and to use them in multiple signaling modes, a mechanism that may be common across many brain systems.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant EY-018131 (E. D. Eggers), University of Arizona NIH Graduate Training in Systems and Integrative Physiology grant 5T32GM008400 (R. E. Mazade), and Science Foundation Arizona (R. E. Mazade).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.E.M. and E.D.E. performed experiments; R.E.M. analyzed data; R.E.M. and E.D.E. interpreted results of experiments; R.E.M. prepared figures; R.E.M. drafted manuscript; R.E.M. and E.D.E. edited and revised manuscript; R.E.M. and E.D.E. approved final version of manuscript; E.D.E. conception and design of research.
ACKNOWLEDGMENTS
We thank Dan Stutman for cell tracing and measurements, Drs. Johnnie Moore-Dotson and Regina Nobles for helpful comments on this manuscript, and Adam Bernstein for technical assistance.
REFERENCES
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27: 513–523, 2000 [DOI] [PubMed] [Google Scholar]
- Arman AC, Sampath AP. Dark-adapted response threshold of OFF ganglion cells is not set by OFF bipolar cells in the mouse retina. J Neurophysiol 107: 2649–2659, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Fitzhugh R, Kuffler SW. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol 137: 338–354, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin DL, Manookin MB, Demb JB. Distinct expressions of contrast gain control in parallel synaptic pathways converging on a retinal ganglion cell. J Physiol 586: 5487–5502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Vis Neurosci 14: 565–576, 1997 [DOI] [PubMed] [Google Scholar]
- Breuninger T, Puller C, Haverkamp S, Euler T. Chromatic bipolar cell pathways in the mouse retina. J Neurosci 31: 6504–6517, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MH, Han SH, Chung JW, Wassle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol 332: 421–432, 1993 [DOI] [PubMed] [Google Scholar]
- Cook PB, McReynolds JS. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nat Neurosci 1: 714–719, 1998 [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci 6: 331–345, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron 36: 703–712, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek K, Pandarinath C, Alam NM, Wellershaus K, Schubert T, Willecke K, Prusky GT, Weiler R, Nirenberg S. Ganglion cell adaptability: does the coupling of horizontal cells play a role? PLoS One 3: e1714, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Singer JH. Intrinsic properties and functional circuitry of the AII amacrine cell. Vis Neurosci 29: 51–60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Baylor DA. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proc Natl Acad Sci USA 92: 10658–10662, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci 19: 593–601, 2002 [DOI] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the mammalian retina. J Neurosci 26: 3959–3970, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Rieke F. Single-photon absorptions evoke synaptic depression in the retina to extend the operational range of rod vision. Neuron 57: 894–904, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABAA, GABAC and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci 26: 9413–9425, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol 103: 25–37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Mazade RE, Klein JS. Inhibition to retinal rod bipolar cells is regulated by light levels. J Neurophysiol 110: 153–161, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol 79: 1384–1395, 1998 [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol 361: 461–478, 1995 [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proc Natl Acad Sci USA 91: 10893–10897, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA, Wässle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci 21: 4852–4863, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70–82, 2004 [DOI] [PubMed] [Google Scholar]
- Gill SB, Veruki ML, Hartveit E. Functional properties of spontaneous IPSCs and glycine receptors in rod amacrine (AII) cells in the rat retina. J Physiol 575: 739–759, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunert U, Wässle H. Glycine receptors in the rod pathway of the macaque monkey retina. Vis Neurosci 13: 101–115, 1996 [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Muller U, Harvey K, Harvey RJ, Betz H, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the alpha3 subunit. J Comp Neurol 465: 524–539, 2003 [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci 25: 5438–5445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Muller U, Wassle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci 23: 350–364, 2006 [DOI] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol 16: 37–68, 1953 [DOI] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A, Cervetto L. Homeostasis in retinal receptive fields. J Neurophysiol 34: 579–587, 1971 [DOI] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci 28: 4136–4150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataruga A, Kremmer E, Muller F. Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. J Comp Neurol 502: 1123–1137, 2007 [DOI] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci 4: 2920–2938, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger N, Pow DV, Wässle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol 401: 34–46, 1998 [DOI] [PubMed] [Google Scholar]
- Munch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci 12: 1308–1316, 2009 [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nat Neurosci 11: 318–326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien BJ, Richardson RC, Berson DM. Inhibitory network properties shaping the light evoked responses of cat alpha retinal ganglion cells. Vis Neurosci 20: 351–361, 2003 [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Paul DL, Wu SM. Rod, M-cone and M/S-cone inputs to hyperpolarizing bipolar cells in the mouse retina. J Physiol 590: 845–854, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol 558: 897–912, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli V, Strettoi E. Bipolar cells of the mouse retina: a gene gun, morphological study. J Comp Neurol 476: 254–266, 2004 [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol 233: 473–480, 1985 [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist, (3H)muscimol: a combined Golgi and autoradiographic study. J Comp Neurol 219: 25–35, 1983 [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron 50: 923–935, 2006 [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Wässle H, Grunert U. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the alpha1 subunit of the glycine receptor. J Neurosci 14: 5131–5146, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Cone bipolar cells as interneurons in the rod pathway of the rabbit retina. J Comp Neurol 347: 139–149, 1994 [DOI] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol 295: 449–466, 1990 [DOI] [PubMed] [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol 325: 152–168, 1992 [DOI] [PubMed] [Google Scholar]
- Trexler EB, Li W, Massey SC. Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. J Neurophysiol 93: 1476–1485, 2005 [DOI] [PubMed] [Google Scholar]
- Trexler EB, Li W, Mills SL, Massey SC. Coupling from AII amacrine cells to ON cone bipolar cells is bidirectional. J Comp Neurol 437: 408–422, 2001 [DOI] [PubMed] [Google Scholar]
- Troy JB, Bohnsack DL, Diller LC. Spatial properties of the cat X-cell receptive field as a function of mean light level. Vis Neurosci 16: 1089–1104, 1999 [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci 21: 8616–8623, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney D. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Lett 125: 187–190, 1991 [DOI] [PubMed] [Google Scholar]
- Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog Retin Res 9: 49–100, 1990 [Google Scholar]
- Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci 22: 10558–10566, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. J Neurosci 24: 11182–11192, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Xin D, Bloomfield SA. Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina. J Physiol 539: 603–614, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Kefalov VJ. An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol 19: 1665–1669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YV, Weick M, Demb JB. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J Neurosci 31: 7670–7681, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci 29: 106–117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, O'Sullivan GA, Heinze L, Chen HX, Betz H, Wassle H. Glycinergic input of small-field amacrine cells in the retinas of wildtype and glycine receptor deficient mice. Mol Cell Neurosci 37: 40–55, 2008 [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis Neurosci 16: 653–665, 1999 [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. J Comp Neurol 383: 512–528, 1997 [DOI] [PubMed] [Google Scholar]