Abstract
Background:
Instrumentation has become an integral component in the management of various spinal pathologies. The rate of infection varies from 2% to 20% of all instrumented spinal procedures. Every occurrence produces patient morbidity, which may adversely affect long-term outcome and increases health care costs.
Methods:
A comprehensive review of the literature from 1990 to 2012 was performed utilizing PubMed and several key words: Infection, spine, instrumentation, implant, management, and biofilms. Articles that provided a current review of the pathogenesis, diagnosis, prevention, and management of instrumented spinal infections over the years were reviewed.
Results:
There are multiple risk factors for postoperative spinal infections. Infections in the setting of instrumentation are more difficult to diagnose and treat due to biofilm. Infections may be early or delayed. C Reactive Protein (CRP) and Magnetic Resonance Imaging (MRI) are important diagnostic tools. Optimal results are obtained with surgical debridement followed by parenteral antibiotics. Removal or replacement of hardware should be considered in delayed infections.
Conclusions:
An improved understanding of the role of biofilm and the development of newer spinal implants has provided insight in the pathogenesis and management of infected spinal implants. This literature review highlights the mechanism, pathogenesis, prevention, and management of infection after spinal instrumentation. It is important to accurately identify and treat postoperative spinal infections. The treatment is often multimodal and prolonged.
Keywords: Biofilm, infection, instrumentation, spinal surgery
INTRODUCTION
Instrumentation, now an integral component in the treatment of numerous spinal pathologies, is correlated with a 2-20% infection rate. The ability to manage postoperative wound infections has become, therefore, more critical and challenging, as they are positively associated with extended hospitalizations, increased morbidity and healthcare costs, poorer long-term outcomes, and greater dissatisfaction with the initial operative procedure.
Nevertheless, there are no universally accepted protocols for treating deep wound infections utilizing spinal instrumentation. Traditionally, it was thought that spinal instrumentation can act as a locus minoris resistentiae for bacteria and therefore explanation of the hardware was critical. However, more current practices vary in terms of the need for implant removal. This manuscript reviews the mechanism, pathogenesis, prevention, and management of infection following the application of spinal instrumentation, and reports on how biofilms impact these infections.
COMPREHENSIVE LITERATURE REVIEW OF INSTRUMENTED SPINAL INFECTIONS
A comprehensive review of the literature from 1990 to 2012 was performed utilizing PubMed and several key words: Infection, spine, instrumentation, implant, management and biofilms. Current articles that reviewed the pathogenesis, diagnosis, prevention and management of instrumented spinal infections were identified.
Epidemiology and risk factors for spinal infections
The incidence of surgical site infections (SSIs) after adult spine surgery varies from 0.7% to 20% [Table 1].[9,10,13,15,17,22,26,27,28,41,47,56,70,79,88,89,96,104,120,150,154] Although the type of spinal surgery most significantly correlates with infection rates, there are multiple other preoperative, intraoperative, or postoperative factors that also contribute to the risk of infection following spinal fusions; age, male sex, steroid therapy, diabetes, smoking, American Society of Anesthesiology (ASA) score, obesity, malnutrition, presence of comorbidities, and previous surgery [Table 1].[5,23,38,41,45,70,76,82,84,97,98,122,123,142]
Table 1.
Risk factors for surgical site infection
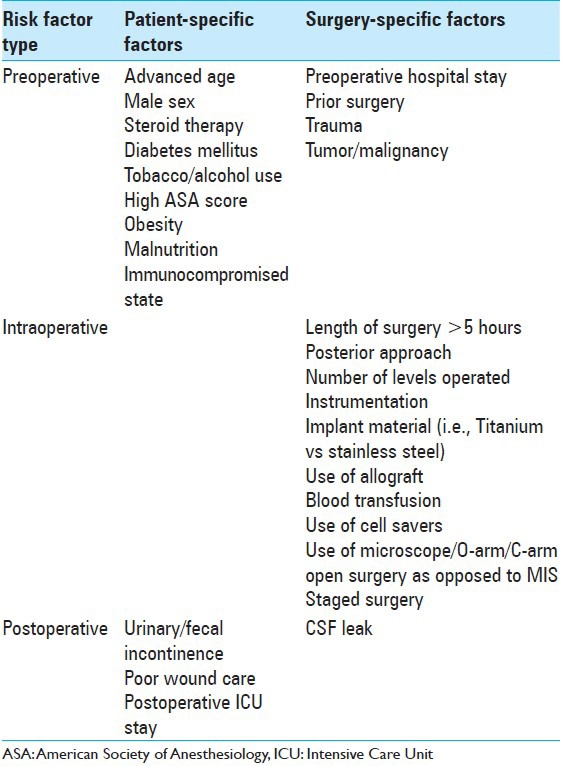
The risk of intraoperative/postoperative infection is increased by utilizing a posterior surgical approach, applying instrumentation, using allograft, requiring a blood transfusion, and longer operations. The utilization of intraoperative equipment (e.g., surgical microscopes, fluoroscopy, intraoperative computed tomography [CT]) also increases the risk of infection through breaches in sterile technique. Additional strict adherence to proper postoperative wound care is also critical in minimizing the risk of postoperative wound infections.[38]
Surgical factors contributing to spinal infections
Multiple factors increase the rates of infection following spinal surgery.[62,109,110,112,122] These include the staging of surgery (multiple sequential operations), operative time >5 hours, blood transfusions, use of allograft, and a greater number of operated levels Direct intraoperative bacterial contamination of the surgical wound from the local milieu is another important factor that contributes to early postoperative spinal infections. A higher infection rate is also related to the introduction of spinal instrumentation and is variously attributed to; increased wound exposure to air (longer surgical duration), greater soft tissue dissection, and increased muscle/skin retraction. Furthermore, the longer the implants are exposed to air the greater the risk of infection; thus the relevant instrumentation trays should not be opened until it is time to place the implants.
Attributes of closed suction drainage to limit spinal infections
The use of closed suction drainage is thought to lower the risk of SSI as even small postoperative hematomas can provide a medium for bacterial overgrowth. Although routine postoperative drainage of spinal wounds does not uniformly decrease the incidence of early postoperative spinal infections, Ho et al. established that the failure to drain wounds correlates with a significantly higher risk of delayed spinal infections.[17,18,62,72,102]
Increased infection risk with posterior spinal instrumentation
A well-recognized risk factor for the development of postoperative spinal wound infections is the utilization of posterior instrumentation. However, this finding is largely based on suboptimal retrospective analyses; only two studies actually document a clear, statistically significant increase in infection rates associated with the use of spinal instrumentation.[23,30,41,45,70,76,84,85,97] While instrumentation itself increases the likelihood of developing a SSI, its correlation with longer surgical times and more extensive posterior exposure independently contribute to higher infection rates.[81,82,97,98] Dissection and retraction of the posterior musculature also devascularizes the paraspinal muscles, increases the potential for blood loss, and results in larger dead spaces, which also contribute to the risk of infection.
Lesser risk of infection with anterior spinal instrumentation
In contrast, anterior spinal exposures are correlated with a reduced risk of infection as exposures typically traverse relatively avascular tissue planes, and avoid significant muscle dissection.[48,84,97,98,154] It has yet to be determined whether minimally invasive spine surgery (even with instrumentation) is associated with lower infection rates vs. open surgery.[96,98,101,128]
Risk of infection may vary with type of implant and susceptibility to biofilm
The risk of infection may also vary with the type of implant due to an increased susceptibility to the development of biofilm.[6,129,149,152] This topic is discussed in detail later in the manuscript.
Nonsurgical factors increase the rate of postoperative spinal infections
There are multiple nonsurgical issues that appear to increase the rate of postoperative spinal infections. Olsen et al. performed a multivariate analysis involving 2316 spinal procedures and found that the following variables significantly increased the likelihood of postoperative infections; diabetes, suboptimal timing of prophylactic antibiotic therapy, elevated pre-or postoperative serum glucose levels, obesity, and two or more residents on a case.[98]
Timing of administration of preoperative antibiotics increases postoperative infection risk
The timing of administration of preoperative antibiotics is strongly correlated with an increased risk of postoperative infection. Ideally, preoperative prophylactic antibiotics should be administered within an hour of surgery (e.g., cephalosporin except in penicillin allergic patients); administration up to 15 minutes prior to the incision may be even more effective.[10,15]
Postoperative incontinence increases postoperative infection rate
Postoperative incontinence following laminectomy and/or fusion has also been reported to be independently associated with increased risk of postoperative infection.[98]
Spinal surgery for tumor resection increases spinal infection risk
Spinal surgery for tumor resection is also independently associated with an increased risk of postoperative infection.[98]
Early postoperative spinal infections
Definition of early spinal infections
Early infections, defined as those occurring within a month of surgery, are attributed to the intraoperative inoculation of the surgical wound with the microorganism. They typically become evident within 2-3 weeks of surgery.
Symptoms and signs of early postoperative spinal wound infections
The signs and symptoms of early postoperative spinal wound infections may include pain, fever, erythema, swelling, warmth, tenderness, and wound drainage. Pain may herald the development of infection particularly when it is escalating in nature. Fever is an unreliable parameter, and often absent. Wound drainage is common for both superficial or deep SSI, and may be present in up to 90% of patients.[110]
Virulent pathogens responsible for early postoperative spinal infections
Early postoperative spinal infections are most frequently due to relatively virulent pathogens such as Staphylococcus aureus, beta-hemolytic streptococci, and aerobic Gram-negative bacilli. Staphylococcus aureus is the most common bacteria responsible for early postoperative infection after spinal surgery.[13,19,41,45,70,75,97,120,131,143,153] The majority of the cases are due to methicillin-sensitive Staphylococcus aureus (MSSA), however, the incidence of methicillin-resistant Staphylococcus aureus (MRSA) is escalating.[75,76] Although the majority of infections are due to a single pathogen, a polymicrobial process may involve 10-50% of cases.[81,118]
There has been an increase in the frequency of infections caused by Gram-negative bacteria, and other organisms such as Pseudomonas aeruginosa, Escherichia coli, Enterobacter, and Acinetobacter.[41,65,67,98,118]
Delayed infections
Although there is no standardized definition for delayed or chronic postoperative spinal infections, many studies have defined these as occurring between 10 days to more than a year after the index procedure/surgery.[9,78,116] Although some authors define delayed infections as those occurring once the original surgical site has healed, most accept the definition of delayed infections to mean those occurring 3-9 months postoperatively.[25,58,59,62,120,146] Furthermore, patients with delayed infections accompanied by spinal instrumentation typically present several months to years later with chronic pain, implant failure, or lack of adequate spinal fusion.[16,27,114,146,148] Although constitutional symptoms may be the only indication of infection, local findings such as increased pain at the incision site and tenderness to palpation of the soft tissue under the incision are usually present. Wound drainage can also occur in delayed spinal infections.[16,27,114,146,148]
Majority of delayed instrumented spinal infections (0.2-6.9%) Occur in scoliosis patients
The majority of delayed infections following instrumented spinal fusions (range 0.2-6.9%) occur following scoliosis surgery.[16,27,56,114,146,150] Factors promoting the risk of delayed infections after scoliosis surgery include: Failure to use a drain, the necessity for intraoperative blood transfusion, the use of bone allograft, intraoperative and hematogenous seeding, and metal fretting (leading to a sterile inflammatory response).[9,46,95,129,146] Of interest, the incidence of delayed infections does not appear to directly correlate with the number of levels fused.[61,130]
Delayed infections are often culture negative vs. Early infections
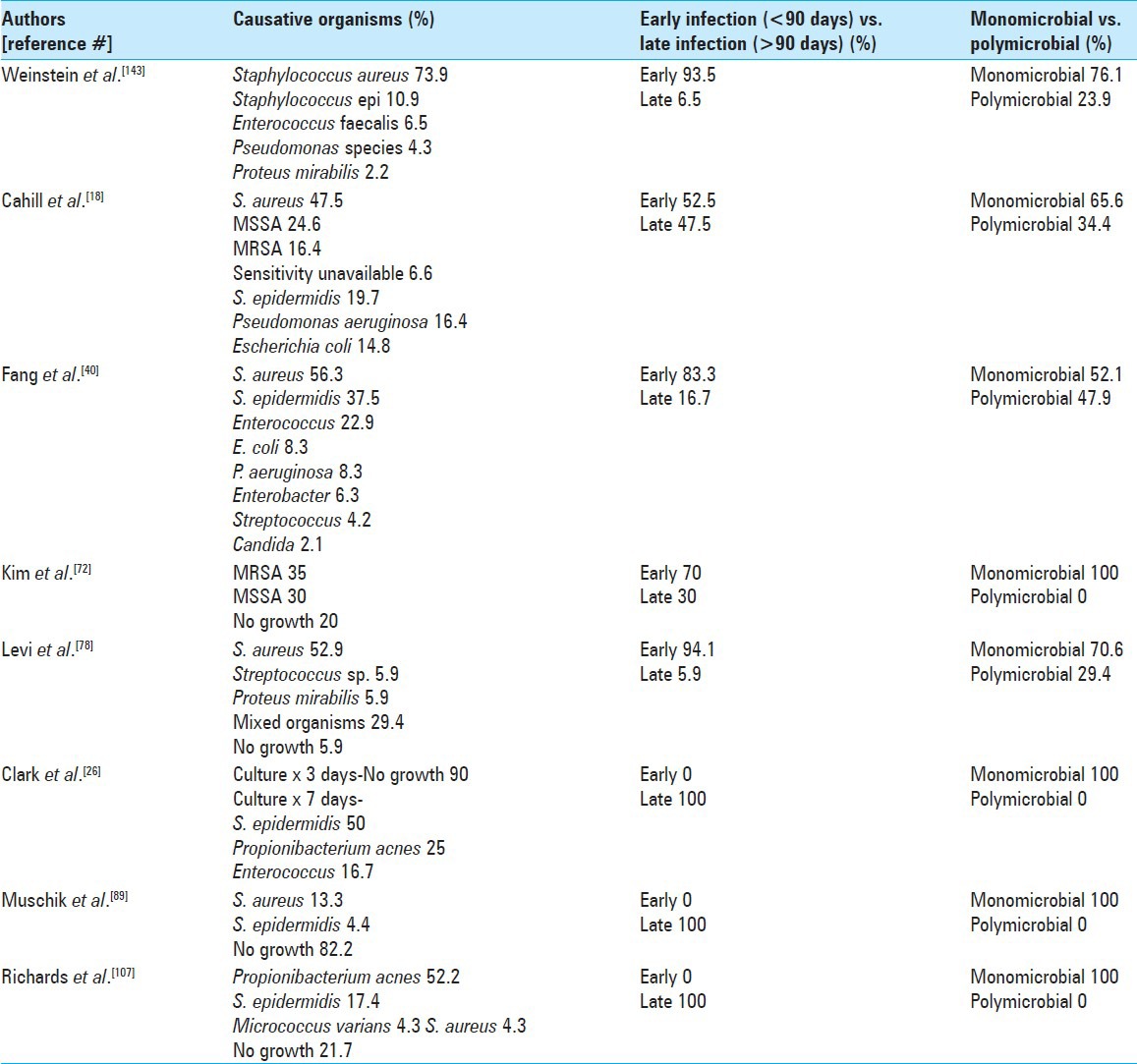
Delayed infections are more often culture negative vs. early infections because as they are frequently caused by less virulent pathogens (e.g., Propionibacterium acnes, coagulase negative Staphylococcus epidermidis, bacillus, and micrococcus species).[16,27,56,94,114,115,146,150] For some time Propionibacterium was considered a culture contaminant but now it is clear that this organism is responsible for a significant number of late infections following implantation of spinal instrumentation.[14] It has been suggested that postoperative sterile inflammatory processes may create a favorable environment for the growth of low virulence organisms such as Propionibacterium.[57] It is a facultative anaerobe and a fastidious organism that can be hard to detect unless the cultures are evaluated for a prolonged period of time.[12,16,27,114,146,150] A list of organisms responsible for early and late SSIs are summarized in Table 2.
Table 2.
Causative organisms for early and late surgical site infections
Biofilm
Certain bacteria can adhere to the surface of implants to form a biofilm, defined as a microbial derived sessile community characterized by cells that are embedded in a matrix of extracellular polymeric substances, which they produce.[51,52,53] Within biofilm, bacterial cells become irreversibly attached to the substratum and/or each other. Biofilm can, therefore, assert some protection for microbial organisms against antibiotics, phagocytes, and other cellular and humoral immune responses.[29,34] Furthermore, bacteria within biofilm often demonstrate an altered phenotype with regard to growth rate and gene transcription (both of which can impact diagnostic and management strategies).[34,35,36,37,38,39,40,41,43,44,45,46,47,51,53,63]
Common organisms have predilection for forming biofilm
Unfortunately the common organisms implicated in postoperative infections after spinal instrumentation like S. aureus, coagulase negative Staphylococcus and Propionibacterium, have a predilection for biofilm formation.[28,34,36,52,53,55,63,69,132] Biofilm-associated organisms grow more slowly than planktonic organisms, and biofilm confers a measurable decrease in antimicrobial susceptibility on the associated organisms.[28,34,36,52,53,63,69] Important prerequisites for the formation of biofilms are the inherent characteristics of the substratum (e.g., surface roughness and the relative hydrophobic tendency of instrumentation), which have a significant effect on the rate/extent of adherence, and susceptibility to the formation of biofilm by microorganisms.[11,34,36,50,55,132,137] Additionally, the presence of a seroma or hematoma can alter the surface properties of an implant thereby impacting the overall susceptibility of bacterial adherence and biofilm formation; this complicates the generalization of in vitro study findings to the clinical arena.[43,108]
Implant materials exhibit variable susceptibility to biofilm
Implants vary in their susceptibility to the development of biofilm. Bacterial adherence to the implant, a prerequisite for biofilm formation, was studied in vitro by Schildhauer et al.[121] These investigators reported that S. aureus is less likely to adhere to pure titanium as compared with titanium alloys and polished stainless steel; there was, however, no difference in adhesion based on the roughness of the metal surface.[121] Interestingly, tantalum was the least susceptible to adherence. Arens et al. reported a lower infection rate with pure titanium as opposed to stainless steel using an animal model; they concluded that this could be related to adherence.[6] A clinical study by Soultanis et al. found that the implant material directly impacted the infection rate; titanium had a lower infection rate than stainless steel.[129] Polyethyletherketone (PEEK), a polymer now widely utilized for spinal surgery, shows a relatively high propensity for biofilm formation and, therefore, infection.[149,150]
Biofilm makes identification of causative infectious organism difficult
Biofilm can increase the difficulty of identifying the causative infectious organism. Analysis of nonspinal prosthetic infections with suspected biofilm show that multiple cultures of peri-implant tissue may not be accurate, and can result in missed diagnoses.[8] Even when an organism is identified, the standard antimicrobial susceptibility testing may not correctly predict the efficacy of an agent against biofilm associated bacteria.[29,34,51,52,63,124] The major problem is that, in general, cultures of biofilm (scraped from the implant) do not grow. Sampredo et al. reported a technique of vortexing and sonification followed by culturing, which was more sensitive than peri-implant cultures obtained from removing spine implants.[119] This material may provide important information in the future using newer molecular or immunologic methods.[29]
Age of biofilm influences susceptibility to antibiotics
The age of the biofilm influences the susceptibility of instrumented fusions to antimicrobial therapy.[34] The cells within biofilm are protected from host defenses, and mature biofilm infections are even less susceptible to antimicrobial agents than recent biofilm infections. This can be clinically important as in early infections, as the immature biofilm if often less tenacious, and, therefore, can be adequately removed/debrided, thus facilitating eradication of infection while preserving the spinal instrumentation.
Potential future role of antimicrobial coated implants against formation of biofilms
As the role of biofilms has been increasingly recognized in implant-related infections, strategies to prevent bacterial adherence and subsequent biofilm formation are being developed and hold promise. Antimicrobial coated implants represent a potential advance, but many factors need to be addressed before this strategy is applicable to the clinic.[29]
Prevention of spinal implant infection
Perioperative antimicrobial agents utilized to limit infection of spinal implants
Identification of multiple risk factors that contribute to infections following instrumented spinal fusions helps decrease the infection risk. Barker et al.'s meta-analysis (utilizing pooled data from six randomized control trials [RCTs]) documented a lower incidence of infection following spinal surgery utilizing antibiotic prophylaxis (Odds ratio, 0.37, 95% CI 0.17-0.78, P < 0.01).[10] They recognized the efficacy of a single preoperative dose of a prophylactic antibiotic providing Gram positive coverage. Notably, no other findings proved significant (e.g., the antibiotic utilized, the dosage protocol, the schedule for redosing antibiotics, and the duration of postoperative prophylactic antibiotics).[10,32,33,60,67,71,148]
Other intraoperative adjunctive measures to prevent infection of spinal implants
Little has been published on other adjunctive measures utilized to prevent postoperative SSIs in spinal surgery. The “no shaving” data for spinal and other procedures and the use of sophisticated air filtering systems have been positive.[23,54] Betadine irrigation was deemed superior to saline irrigation in two RCTs.[24,25] Recently, dispersing powdered Vancomycin into the wound just prior to closure has been reported to significantly reduce the risk of postoperative infections; this technique has been rapidly adopted despite the absence of a well designed RCT or case control study.[92,134]
There were no sound data, other than provided by Ho et al., to support the benefit of closed suction drainage to prevent acute postoperative surgical-site infection after spine surgery.[17,62,72,102] Finally, data regarding the use of silver-impregnated dressings is sparse, and provides only low evidence to support its widespread efficacy in spinal surgery.[38]
Diagnosis of superficial vs. Deep spinal infection
Infections following instrumented spinal fusions can be superficial or deep. By definition, superficial infections are confined to the dermis and subcutaneous tissue, while deep infections are those occurring below the fascia.[120] Superficial infections generally present within the first 2 weeks after surgery, and are accompanied by fever, increased wound pain, erythema, swelling, warmth, tenderness, and/or drainage. Deep infections may present in a manner identical to superficial infections or may develop long after the surgery (e.g., >6 weeks, to months or years later).
Laboratory evaluation of infected spinal instrumentation
Laboratory studies are an important part of the evaluation of infected spinal implants. Erythrocyte sedimentation rate (ESR), C reactive protein (CRP), and total leukocyte count (TLC) are routinely ordered when there is a suspicion of a postoperative infection.[44,73,74,135,136] ESR and CRP values are, however, considered more useful than TLC in the detection of spinal infection.[90] For each variable, a rising trend in the postoperative period is more suggestive of infection than a single abnormal value as these markers are routinely elevated in the early postoperative period even without infection. Notably, postoperative CRP levels are higher after instrumented spinal surgery vs. simple decompressions. CRP is also an excellent marker for infection as it is relatively stable for each individual, has a narrow normal range, which is minimally impacted by medications and other pathologies (excluding liver failure), and is determined via a quantitative test with predictable kinetics.[44,68,103,136,139] The CRP level generally peaks 2-3 days after surgery and returns to baseline within 2-3 weeks while ESR peaks around day 5, and returns to normal more gradually over 3-6 weeks.[91,139] Despite their utility, these indices can be elevated with/without infections at any site, (e.g., surgical vs. nonsurgical) so a single abnormal value has low specificity for infection and is of little/limited clinical significance. Alternatively, appreciation of the normal kinetics (e.g., a rising trend instead of the expected fall after a postoperative peak) and recognition of abnormal elevations should raise clinical suspicion for postoperative wound infection. Conversely, normal ESR and CRP values may help confirm the absence of infection.
Diagnostic imaging of infected spinal implants
Plain radiography, CT, and magnetic resonance imaging (MRI) are routinely ordered when an infection is suspected. Early implant loosening, rapid loss of adjacent level disc height, and abnormal soft tissue swelling are indirect markers of infection on plain X-rays, but are often not seen until a few weeks after the onset of infection. CT delineates hardware position and bony changes more accurately than plain radiographs, and CT also shows fluid collections, it is not as sensitive to infection as MRI.
MRI scans with/without contrast: Great value in diagnosing infection
MRI scans without and with contrast are of great value in diagnosing discitis, osteomyelitis, and epidural abscesses after spinal surgery. However, it is not often possible to distinguish a sterile seroma from a purulent collection (e.g., differentiation between postoperative changes and infection) utilizing early contrast enhanced CT or MRI studies following the implantation of spinal instrumentation.[8,13,93,106,127]
Radionuclide imaging not primary choice for diagnosing postoperative spinal infections
The use of radionuclide imaging is not a primary imaging modality for diagnosing postoperative spinal infections as recent surgery can result in positive studies even when no infection is present. However, an early negative radionuclide scan may indicate that an infection is not likely present. Alternatively, these scans may prove an effective diagnostic technique for diagnosing delayed infections.
Radionuclide tracer imaging of infected spinal implants
Bone scintigraphy utilizing multiple radionuclide tracers
There are several means by which the skeleton may be imaged using radionuclide tracers. Bone scintigraphy is most commonly performed using technetium-99m (Tc-99m) methylene diphosphate (MDP). Three-phase imaging is the radionuclide procedure of choice for evaluating osteomyelitis in bone not affected by any underlying condition. This tracer binds to the hydroxyapetite crystal; uptake is a function of blood flow, and the rate of new bone formation. There is a dynamic sequence (e.g., the flow or perfusion phase), followed immediately by the acquisition of static images of the region of interest (e.g., the blood pool or soft tissue phase). The final phase consists of static images of the region of interest obtained 2-4 hours after the initial injection of the tracer. Focal hyperperfusion and hyperemia with increased delayed bony uptake is diagnostic for osteomyelitis. Recent surgery or the presence of hardware may result in a false positive three phase scan.[99] Images can be obtained the next day (referred to as a four phase study) to improve the specificity. Tracer uptake in normal bone usually stops after 2-4 hours but it may continue for several hours longer in the setting of osteomyelitis. Four phase studies are more specific but less sensitive that three phase scintigraphy and have an accuracy of about 85%.[4,66]
Gallium-67 citrate used to localize spinal infections
Gallium-67 citrate (Ga-67) has been used to localize spinal infections for many decades. Within 24 hours, 25% of the radionuclide is excreted by the kidneys; further excretion occurs via the large intestine. Two to three days after the injection, 75% of the tracer is still in the body where it is equally distributed in the liver, soft tissues, and bone.
Utilizing gallium-67 and bone scintigraphy to diagnose osteomyelitis
Gallium accumulates at sites of infection or inflammation via a variety of mechanisms, and osteomyelitis is usually diagnosed by combining Ga-67 with bone scintigraphy.[99] A positive test requires two factors; the two tracers are spatially incongruent, and the relative uptake of the Gallium is greater than that of the bone agent. If the study is negative for osteomyelitis when the gallium images are normal or when the distribution of the two agents is spatially congruent, but there is less uptake of gallium than the bone agent.[99] The overall accuracy of gallium/bone imaging is 65-80%, but the need for two isotopes and multiple imaging sessions make this technique difficult.[87]
Labeled leukocyte imaging of spinal infections
Labeled leukocyte imaging represents a significant advance in the ability to detect spinal infections. The uptake of labeled cells depends on several variables; intact chemotaxis, the number and type of cell labeled, and the cellular response to the infection. A total white blood cell count of 2000/mL is required to obtain satisfactory images. Usually the majority of the labeled cells are neutrophils. However, this technique is less sensitive for processes in which the predominant cellular response is not neutrophilic (e.g., tuberculosis).[42,83,100] There are additional techniques that utilize combined leukocyte/bone marrow imaging and other nuances related to the isotope used.[99]
Fluorine-18 fluorodeoxyglucose-positron emission tomography utilized to diagnose spinal infections
Fluorine-18 (F-18) fluorodeoxyglucose-positron emission tomography (FDG-PET) may also be used to identify spinal infections. However, this technology is expensive and requires sophisticated equipment thus making it not widely available. Similar to radionuclide studies, the utility of FDG-PET is limited in the acute postoperative setting, but is useful for establishing the diagnosis of delayed infections surrounding instrumentation.[133]
Ultrasound detects postoperative fluid collections and helps guide fluid aspiration
Although ultrasonography can detect postoperative fluid collections, it cannot determine whether these represent noninfectious vs. infectious processes (e.g., sonomorphological criteria such as internal echo structures, septation, demarcation from the environment, and reaction of the surrounding tissue). Ultrasound is, however, useful in guiding aspiration of fluid collections resulting in a high diagnostic accuracy.[77]
Management of infections following spinal surgery with instrumentation
The management of infection after spinal instrumentation is controversial, and requires careful consideration of the two most critical variables: The duration of antimicrobial therapy and whether or not the implants should to be removed. Treatment paradigms have evolved greatly over the past 10-15 years, and the present recommendation is to preserve rather than remove the spinal instrumentation in the majority of cases. However, the timing of infection after surgery (e.g., early vs. delayed) can be an important guiding factor determining the management choice.[9,39,77]
Surgical treatment of early deep postoperative infection following instrumented spinal fusion
The surgical treatment of early deep postoperative infection following spinal instrumentation is variable. There is a lack of consensus as to whether to utilize; irrigation/debridement alone, wound vacuums, continuous irrigation systems, antimicrobial beads, whether to revise instrumentation (e.g., instrumentation failure), whether to prophylactically remove instrumentation, and which antibiotic protocol to utilize.[7,49,78,86,118,140]
Presence of biofilm leads to recommendation to remove infected spinal instrumentation
Given the pathogenic role of prostheses-based biofilm, most infectious disease physicians now recommend removal of the underlying spinal instrumentation.[1,2,115] Removal of the instrumentation offers the advantage of eliminating hardware that may harbor biofilm-related microorganisms thus increasing the chance of eradicating the infection. Nevertheless, this potential advantage must be weighed against the risks of prematurely removing internal fixators essential for maintaining normal spinal alignment and preserving spinal stability.
A key factor in deciding whether or not to remove spinal instrumentation relates to biofilm. In vitro laboratory investigations document that biofilms may develop within 5-6 hours after bacterial inoculation, and the age of the biofilm has major clinical implications related to its tenaciousness and antimicrobial susceptibility.[35] Early surgical intervention of acute infections with wound irrigation/debridement are more readily able to disrupt biofilm formation and facilitate penetration of systemic antimicrobials to allow for resolution of the infection while preserving the instrumentation/stability. This concept is supported by the clinical experience, which demonstrates that expedient treatment of early postoperative infections results in higher rates of infection resolution, preservation of instrumentation, and better clinical outcomes.[2,39,49,90,113,125]
Delayed wound infections often require instrumentation removal/replacement
Although acute infections may be adequately treated by surgical debridement and antimicrobial therapy, the development of a delayed wound infection often requires removal or replacement of the instrumentation.[9,20,78,114,117,141,151] Late-onset infections are caused primarily by organisms known to produce biofilm (e.g., coagulase-negative Staphylococci and Propionibacter acnes). Similar to the management of other bone and joint infections involving prostheses, this makes the eradication of infection difficult without foreign body removal.[58]
More morbidity with retention of spinal instrumentation after delayed infection
Retention of spinal instrumentation after delayed infection is fraught with more morbidity and less success. Ho et al. reported the strong propensity for recurrence of infection (up to 50%) in the absence of implant removal. They found that treating infected retained spinal implants with irrigation and debridement was associated with multiple procedures irrespective of the type of organism and graft.[61]
Advocacy of prophylactic removal of infected spinal instrumentation
Prophylactic removal of spinal instrumentation is advocated by some authors to minimize the risk of developing infection relapses or if fastidious organisms like Propionibacter are identified.[28,94] Implant removal in this population subset allows for more thorough debridement, and thus reduces the risk of infection relapse.[27,58,78,94] Concern for destabilizing the spine in delayed infections is less of an issue than in the acute postoperative infections since the fusion has often matured, or there is, at least, a stiff fibrous union. It is possible, however, that even if there appears to be radiographic evidence of fusion, removal of hardware can be associated with pseudoarthosis or loss of correction; thus these patients must be followed carefully.[31,58,94,107] One stage revision of infected instrumentation may be an option as opposed to instrumentation removal for these patients.[94]
General operative treatment
Surgical debridement and irrigation of infected spinal instrumentation
Surgical debridement and irrigation (frequently with a wound drain) have been an important means of treating early postoperative infections following the implantation of spinal instrumentation.[2,39,105] Multiple debridements may be required for successful eradication of infection. Poorly vascularized surgical sites or significant wound defects may mandate the use of complex flaps for reconstruction.[37,147] In addition to surgical debridement and postoperative antimicrobial therapy, the use of suction and/or irrigation systems, antimicrobial beads, or the vacuum-assisted closure (VAC) devices may also improve the outcomes of early infection after the placement of spinal instrumentation in selected patients. Closed suction drainage usually negates the need for secondary wound closures; excellent results have been reported for these irrigation systems.[49,65,81,118,138,143,153]
Antibiotic impregnated beads and suction/irrigation devices utilized to treat infected spinal instrumentation
Glassman et al. described the successful treatment of infection following the implantation of spinal instrumentation by placing antibiotic impregnated beads and utilizing close suction irrigation techniques.[49] The efficacy of suction irrigation using antibiotics to treat acute and delayed spinal instrumented infections has also been reported.[65,81,118,143] The duration of treatment with this latter technique ranges from 5 days to 2 weeks. Some surgeons stop the irrigation when the CRP and ESR are normalized; others wait until the outflow drainage is clear.
Vacuum-assisted closure facilitates wound healing and eradicates spinal infections
VAC is a useful adjunct that facilitates wound healing and eradication of complex postoperative bacterial spinal infections.[20,21,64,67,80,86,141,144,145,155,156] VAC is a relatively new technique that utilizes controlled negative pressure to evacuate wound edema fluid, thereby increasing regional blood flow, decreasing the bacterial load, and promoting the formation of granulation tissue.[7,144] The efficacy of the VAC that utilizes a porous polyurethane foam sponge, which is cut to fit into or over the wound, has been validated in animal studies.[93] The foam is covered by a sealant film that extends several centimeters beyond the margins of the wound to create an air-tight barrier. Continuous or intermittent negative pressure is applied to the sponge via a tube, which leads to a collection container.[144,155] The VAC is applied only after the wound is thoroughly debrided. The sponge is changed or removed 2-7 days after application (e.g., until the wound is clean and can be closed over drains). One of the earliest reports was by Yuan-Innes et al., who successfully treated two patients with infected and exposed spinal hardware; others have had similar positive experiences.[20,86,155] Conversely, there have been reports of less than optimal outcomes (multiple debridements, need for instrumentation removal or replacement) using the VAC particularly when dealing with MRSA or multibacterial infections.[80,106] Additionally severe complications (e.g., uncontrolled sepsis and severe blood loss) have been reported to be associated with utilizing the VAC; patients undergoing this therapy, therefore, should be carefully monitored.[67]
Antimicrobial therapy
Duration of antimicrobial therapy
Another unresolved aspect of postoperative infections after spinal instrumentation relates to the duration of pharmacological treatment. It is optimal to base antimicrobial choice on the culture results, and antibiotic sensitivity of the organisms. Although there is general agreement on the need for 6-8 weeks of parental therapy, data addressing the need for and duration of long-term oral suppressive antibiotic therapy are lacking.[111,126] The mean duration of antibiotic therapy may be much longer as reported in the study by Kowalski et al., who found that with early postoperative infections, treatment with longer-term suppressive antibiotic therapy was associated with higher chances (80% vs. 33%) of eradicating infections and retaining implants vs. those who did not receive suppressive therapy.[78] Additionally, hyperbaric oxygen therapy was reported to be a useful adjunctive therapy for treating instrumented spinal fusions, especially in patients who have failed primary antimicrobial therapy.[3]
CONCLUSION
It is important to recognize the clinical symptoms and signs of postoperative spinal infections, and confirm the diagnosis with appropriate laboratory and imaging studies. Prompt, aggressive debridement coupled with utilizing the correct antibiotic therapy (typically 6-8 weeks of intravenous antibiotics) and, in some cases, chronic suppressive antibiotic treatment (e.g., for up to 1 year), have yielded the most successful results. Instrumentation can usually be preserved in patients with early infections (e.g., <6 weeks), but instrumentation removal should be considered for infections presenting in a delayed fashion (e.g., >6 weeks to even years). Patients should be adequately followed for one postoperative year, to ensure that the infection has been fully eradicated. Emerging techniques are increasingly preventing the formation of biofilm on instrumentation, facilitate the removal of biofilm, and increase the culture yield of biofilms on implant surfaces. For example, implant sonication provides cultures for direct identification of active and/or persistent biofilm, while the introduction of enzymes that dissolve the biofilm matrix (e.g., DNase and alginate lyase) and quorum-sensing inhibitors that increase biofilm susceptibility to antibiotics may further help manage postoperative infection. These and other techniques may further enhance the potential for successfully salvaging instrumentation, while eradicating spinal infections. Additionally, changes in antibiotic prophylaxis to prevent postoperative infections following spinal instrumentation remain active areas for further investigation.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/6/392/120783
Disclaimer: The authors of this article has no conflict of interest to disclose, and have adhered to SNI's policies regarding human/animal rights, and informed consent. Advertisers in SNI did not ask for, nor did they receive access to this article prior to publication.
Contributor Information
Manish K. Kasliwal, Email: manish_kasliwal@rush.edul.
Lee A. Tan, Email: lee_tan@rush.edu.
Vincent C. Traynelis, Email: vincent_traynelis@rush.edu.
REFERENCES
- 1.Abbey DM, Turner DM, Warson JS, Wirt TC, Scalley RD. Treatment of postoperative wound infections following spinal fusion with instrumentation. J Spinal Disord. 1995;8:278–83. doi: 10.1097/00002517-199508040-00003. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed R, Greenlee JD, Traynelis VC. Preservation of spinal instrumentation after development of postoperative bacterial infections in patients undergoing spinal arthrodesis. J Spinal Disord Tech. 2012;25:299–302. doi: 10.1097/BSD.0b013e31821fbf72. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R, Severson MA, Traynelis VC. Role of hyperbaric oxygen therapy in the treatment of bacterial spinal osteomyelitis. J Neurosurg Spine. 2009;10:16–20. doi: 10.3171/2008.10.SPI08606. [DOI] [PubMed] [Google Scholar]
- 4.Alazraki N, Dries D, Datz F, Lawrence P, Greenberg E, Taylor A., Jr Value of a 24-hour image (four-phase bone scan) in assessing osteomyelitis in patients with peripheral vascular disease. J Nucl Med. 1985;26:711–7. [PubMed] [Google Scholar]
- 5.Apisarnthanarak A, Jones M, Waterman BM, Carroll CM, Bernardi R, Fraser VJ. Risk factors for spinal surgical-site infections in a community hospital: A case-control study. Infect Control Hosp Epidemiol. 2003;24:31–6. doi: 10.1086/502112. [DOI] [PubMed] [Google Scholar]
- 6.Arens S, Schlegel U, Printzen G, Ziegler WJ, Perren SM, Hansis M. Influence of materials for fixation implants on local infection. An experimental study of steel versus titanium DCP in rabbits. J Bone Joint Surg Br. 1996;78:647–51. [PubMed] [Google Scholar]
- 7.Argenta LC, Morykwas MJ. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann Plast Surg. 1997;38:563–7. [PubMed] [Google Scholar]
- 8.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–9. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aydinli U, Karaeminogullari O, Tiskaya K. Postoperative deep wound infection in instrumented spinal surgery. Acta Orthop Belg. 1999;65:182–7. [PubMed] [Google Scholar]
- 10.Barker FG., 2nd Efficacy of prophylactic antibiotic therapy in spinal surgery: A meta-analysis. Neurosurgery. 2002;51:391–400. [PubMed] [Google Scholar]
- 11.Barth E, Myrvik QM, Wagner W, Gristina AG. In vitro and in vivo comparative colonization of Staphylococcus aureus and Staphylococcus epidermidis on orthopaedic implant materials. Biomaterials. 1989;10:325–8. doi: 10.1016/0142-9612(89)90073-2. [DOI] [PubMed] [Google Scholar]
- 12.Bayston R, Nuradeen B, Ashraf W, Freeman BJ. Antibiotics for the eradication of Propionibacterium acnes biofilms in surgical infection. J Antimicrob Chemother. 2007;60:1298–301. doi: 10.1093/jac/dkm408. [DOI] [PubMed] [Google Scholar]
- 13.Beiner JM, Grauer J, Kwon BK, Vaccaro AR. Postoperative wound infections of the spine. Neurosurg Focus. 2003;15:E14. doi: 10.3171/foc.2003.15.3.14. [DOI] [PubMed] [Google Scholar]
- 14.Bemer P, Corvec S, Tariel S, Asseray N, Boutoille D, Langlois C, et al. Significance of Propionibacterium acnes-positive samples in spinal instrumentation. Spine (Phila Pa 1976) 2008;33:E971–6. doi: 10.1097/BRS.0b013e31818e28dc. [DOI] [PubMed] [Google Scholar]
- 15.Blam OG, Vaccaro AR, Vanichkachorn JS, Albert TJ, Hilibrand AS, Minnich JM, et al. Risk factors for surgical site infection in the patient with spinal injury. Spine (Phila Pa 1976) 2003;28:1475–80. doi: 10.1097/01.BRS.0000067109.23914.0A. [DOI] [PubMed] [Google Scholar]
- 16.Bose B. Delayed infection after instrumented spine surgery: Case reports and review of the literature. Spine J. 2003;3:394–9. doi: 10.1016/s1529-9430(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 17.Brown EM, Pople IK, de Louvois J, Hedges A, Bayston R, Eisenstein SM, et al. Spine update: Prevention of postoperative infection in patients undergoing spinal surgery. Spine (Phila Pa 1976) 2004;29:938–45. doi: 10.1097/00007632-200404150-00023. [DOI] [PubMed] [Google Scholar]
- 18.Brown MD, Brookfield KF. A randomized study of closed wound suction drainage for extensive lumbar spine surgery. Spine (Phila Pa 1976) 2004;29:1066–8. doi: 10.1097/00007632-200405150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Cahill PJ, Warnick DE, Lee MJ, Gaughan J, Vogel LE, Hammerberg KW, et al. Infection after spinal fusion for pediatric spinal deformity: Thirty years of experience at a single institution. Spine (Phila Pa 1976) 2010;35:1211–7. doi: 10.1097/BRS.0b013e3181c212d1. [DOI] [PubMed] [Google Scholar]
- 20.Canavese F, Gupta S, Krajbich JI, Emara KM. Vacuum-assisted closure for deep infection after spinal instrumentation for scoliosis. J Bone Joint Surg Br. 2008;90:377–81. doi: 10.1302/0301-620X.90B3.19890. [DOI] [PubMed] [Google Scholar]
- 21.Canavese F, Krajbich JI. Use of vacuum assisted closure in instrumented spinal deformities for children with postoperative deep infections. Indian J Orthop. 2010;44:177–83. doi: 10.4103/0019-5413.62067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capen DA, Calderone RR, Green A. Perioperative risk factors for wound infections after lower back fusions. Orthop Clin North Am. 1996;27:83–6. [PubMed] [Google Scholar]
- 23.Celik SE, Kara A. Does shaving the incision site increase the infection rate after spinal surgery? Spine (Phila Pa 1976) 2007;32:1575–7. doi: 10.1097/BRS.0b013e318074c39f. [DOI] [PubMed] [Google Scholar]
- 24.Chang FY, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Can povidone-iodine solution be used safely in a spinal surgery? Eur Spine J. 2006;15:1005–14. doi: 10.1007/s00586-005-0975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976) 2005;30:1689–93. doi: 10.1097/01.brs.0000171907.60775.85. [DOI] [PubMed] [Google Scholar]
- 26.Christodoulou AG, Givissis P, Symeonidis PD, Karataglis D, Pournaras J. Reduction of postoperative spinal infections based on an etiologic protocol. Clin Orthop Relat Res. 2006;444:107–13. doi: 10.1097/01.blo.0000201174.10506.cc. [DOI] [PubMed] [Google Scholar]
- 27.Clark CE, Shufflebarger HL. Late-developing infection in instrumented idiopathic scoliosis. Spine (Phila Pa 1976) 1999;24:1909–12. doi: 10.1097/00007632-199909150-00008. [DOI] [PubMed] [Google Scholar]
- 28.Collins I, Wilson-MacDonald J, Chami G, Burgoyne W, Vineyakam P, Berendt T, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J. 2008;17:445–50. doi: 10.1007/s00586-007-0559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin Orthop Relat Res. 2005;437:7–11. doi: 10.1097/00003086-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206–10. doi: 10.1001/archsurg.1973.01350200078018. [DOI] [PubMed] [Google Scholar]
- 31.Deckey JE, Court C, Bradford DS. Loss of sagittal plane correction after removal of spinal implants. Spine (Phila Pa 1976) 2000;25:2453–60. doi: 10.1097/00007632-200010010-00006. [DOI] [PubMed] [Google Scholar]
- 32.Dimick JB, Lipsett PA, Kostuik JP. Spine update: Antimicrobial prophylaxis in spine surgery: Basic principles and recent advances. Spine (Phila Pa 1976) 2000;25:2544–8. doi: 10.1097/00007632-200010010-00020. [DOI] [PubMed] [Google Scholar]
- 33.Dobzyniak MA, Fischgrund JS, Hankins S, Herkowitz HN. Single versus multiple dose antibiotic prophylaxis in lumbar disc surgery. Spine (Phila Pa 1976) 2003;28:E453–5. doi: 10.1097/01.BRS.0000090839.61893.BE. [DOI] [PubMed] [Google Scholar]
- 34.Donlan RM. Biofilm formation: A clinically relevant microbiological process. Clin Infect Dis. 2001;33:1387–92. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 35.Donlan RM, Costerton JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donlan RM, Forster T, Murga R, Brown E, Lucas C, Carpenter J, et al. Legionella pneumophila associated with the protozoan Hartmannella vermiformis in a model multi-species biofilm has reduced susceptibility to disinfectants. Biofouling. 2005;21:1–7. doi: 10.1080/08927010500044286. [DOI] [PubMed] [Google Scholar]
- 37.Dumanian GA, Ondra SL, Liu J, Schafer MF, Chao JD. Muscle flap salvage of spine wounds with soft tissue defects or infection. Spine (Phila Pa 1976) 2003;28:1203–11. doi: 10.1097/01.BRS.0000067260.22943.48. [DOI] [PubMed] [Google Scholar]
- 38.Epstein NE. Do silver-impregnated dressings limit infections after lumbar laminectomy with instrumented fusion? Surg Neurol. 2007;68:483–5. doi: 10.1016/j.surneu.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 39.Falavigna A, Righesso Neto O, Fonseca GP, Nervo M. Management of deep wound infections in spinal lumbar fusions. Arq Neuropsiquiatr. 2006;64:1001–4. doi: 10.1590/s0004-282x2006000600022. [DOI] [PubMed] [Google Scholar]
- 40.Falavigna A, Righesso O, Traynelis VC, Teles AR, da Silva PG. Effect of deep wound infection following lumbar arthrodesis for degenerative disc disease on long-term outcome: A prospective study: Clinical article. J Neurosurg Spine. 2011;15:399–403. doi: 10.3171/2011.5.SPINE10825. [DOI] [PubMed] [Google Scholar]
- 41.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976) 2005;30:1460–5. doi: 10.1097/01.brs.0000166532.58227.4f. [DOI] [PubMed] [Google Scholar]
- 42.Fineman DS, Palestro CJ, Kim CK, Needle LB, Vallabhajosula S, Solomon RW, et al. Detection of abnormalities in febrile AIDS patients with In-111-labeled leukocyte and Ga-67 scintigraphy. Radiology. 1989;170:677–80. doi: 10.1148/radiology.170.3.2783783. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher M, Loeb GI. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl Environ Microbiol. 1979;37:67–72. doi: 10.1128/aem.37.1.67-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foglar C, Lindsey RW. C-reactive protein in orthopedics. Orthopedics. 1998;21:687–91. doi: 10.3928/0147-7447-19980601-11. [DOI] [PubMed] [Google Scholar]
- 45.Friedman ND, Sexton DJ, Connelly SM, Kaye KS. Risk factors for surgical site infection complicating laminectomy. Infect Control Hosp Epidemiol. 2007;28:1060–5. doi: 10.1086/519864. [DOI] [PubMed] [Google Scholar]
- 46.Gaine WJ, Andrew SM, Chadwick P, Cooke E, Williamson JB. Late operative site pain with isola posterior instrumentation requiring implant removal: Infection or metal reaction? Spine (Phila Pa 1976) 2001;26:583–7. doi: 10.1097/00007632-200103010-00027. [DOI] [PubMed] [Google Scholar]
- 47.Gaynes RP, Culver DH, Horan TC, Edwards JR, Richards C, Tolson JS. Surgical site infection (SSI) rates in the United States, 1992-1998: The National Nosocomial Infections Surveillance System basic SSI risk index. Clin Infect Dis. 2001;33(Suppl 2):S69–77. doi: 10.1086/321860. [DOI] [PubMed] [Google Scholar]
- 48.Ghanayem AJ, Zdeblick TA. Cervical spine infections. Orthop Clin North Am. 1996;27:53–67. [PubMed] [Google Scholar]
- 49.Glassman SD, Dimar JR, Puno RM, Johnson JR. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine (Phila Pa 1976) 1996;21:2163–9. doi: 10.1097/00007632-199609150-00021. [DOI] [PubMed] [Google Scholar]
- 50.Gracia E, Fernandez A, Conchello P, Lacleriga A, Paniagua L, Seral F, et al. Adherence of Staphylococcus aureus slime-producing strain variants to biomaterials used in orthopaedic surgery. Int Orthop. 1997;21:46–51. doi: 10.1007/s002640050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gristina AG. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science. 1987;237:1588–95. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 52.Gristina AG, Costerton JW. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg Am. 1985;67:264–73. [PubMed] [Google Scholar]
- 53.Gristina AG, Jennings RA, Naylor PT, Myrvik QN, Webb LX. Comparative in vitro antibiotic resistance of surface-colonizing coagulase-negative staphylococci. Antimicrob Agents Chemother. 1989;33:813–6. doi: 10.1128/aac.33.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gruenberg MF, Campaner GL, Sola CA, Ortolan EG. Ultraclean air for prevention of postoperative infection after posterior spinal fusion with instrumentation: A comparison between surgeries performed with and without a vertical exponential filtered air-flow system. Spine (Phila Pa 1976) 2004;29:2330–4. doi: 10.1097/01.brs.0000142436.14735.53. [DOI] [PubMed] [Google Scholar]
- 55.Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976) 2005;30:38–43. doi: 10.1097/01.brs.0000147801.63304.8a. [DOI] [PubMed] [Google Scholar]
- 56.Hahn F, Zbinden R, Min K. Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J. 2005;14:783–8. doi: 10.1007/s00586-004-0854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haidar R, Najjar M, Der Boghossian A, Tabbarah Z. Propionibacterium acnes causing delayed postoperative spine infection: Review. Scand J Infect Dis. 2010;42:405–11. doi: 10.3109/00365540903582459. [DOI] [PubMed] [Google Scholar]
- 58.Hedequist D, Haugen A, Hresko T, Emans J. Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine (Phila Pa 1976) 2009;34:60–4. doi: 10.1097/BRS.0b013e31818ed75e. [DOI] [PubMed] [Google Scholar]
- 59.Heggeness MH, Esses SI, Errico T, Yuan HA. Late infection of spinal instrumentation by hematogenous seeding. Spine (Phila Pa 1976) 1993;18:492–6. [PubMed] [Google Scholar]
- 60.Hellbusch LC, Helzer-Julin M, Doran SE, Leibrock LG, Long DJ, Puccioni MJ, et al. Single-dose vs multiple-dose antibiotic prophylaxis in instrumented lumbar fusion-a prospective study. Surg Neurol. 2008;70:622–7. doi: 10.1016/j.surneu.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 61.Ho C, Skaggs DL, Weiss JM, Tolo VT. Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine (Phila Pa 1976) 2007;32:2739–44. doi: 10.1097/BRS.0b013e31815a5a86. [DOI] [PubMed] [Google Scholar]
- 62.Ho C, Sucato DJ, Richards BS. Risk factors for the development of delayed infections following posterior spinal fusion and instrumentation in adolescent idiopathic scoliosis patients. Spine (Phila Pa 1976) 2007;32:2272–7. doi: 10.1097/BRS.0b013e31814b1c0b. [DOI] [PubMed] [Google Scholar]
- 63.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–32. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 64.Horn PL, Ruth B, Kean JR. Use of wound V.A.C therapy in pediatric patients with infected spinal wounds: A retrospective review. Orthop Nurs. 2007;26:317–22. doi: 10.1097/01.NOR.0000295960.94450.69. [DOI] [PubMed] [Google Scholar]
- 65.Ido K, Shimizu K, Nakayama Y, Shikata J, Matsushita M, Nakamura T. Suction/irrigation for deep wound infection after spinal instrumentation: A case study. Eur Spine J. 1996;5:345–9. doi: 10.1007/BF00304351. [DOI] [PubMed] [Google Scholar]
- 66.Israel O, Gips S, Jerushalmi J, Frenkel A, Front D. Osteomyelitis and soft-tissue infection: Differential diagnosis with 24 hour/4 hour ratio of Tc-99m MDP uptake. Radiology. 1987;163:725–6. doi: 10.1148/radiology.163.3.3575722. [DOI] [PubMed] [Google Scholar]
- 67.Jones GA, Butler J, Lieberman I, Schlenk R. Negative-pressure wound therapy in the treatment of complex postoperative spinal wound infections: Complications and lessons learned using vacuum-assisted closure. J Neurosurg Spine. 2007;6:407–11. doi: 10.3171/spi.2007.6.5.407. [DOI] [PubMed] [Google Scholar]
- 68.Jonsson B, Soderholm R, Stromqvist B. Erythrocyte sedimentation rate after lumbar spine surgery. Spine (Phila Pa 1976) 1991;16:1049–50. doi: 10.1097/00007632-199109000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Kajiyama S, Tsurumoto T, Osaki M, Yanagihara K, Shindo H. Quantitative analysis of Staphylococcus epidermidis biofilm on the surface of biomaterial. J Orthop Sci. 2009;14:769–75. doi: 10.1007/s00776-009-1405-0. [DOI] [PubMed] [Google Scholar]
- 70.Kanafani ZA, Dakdouki GK, El-Dbouni O, Bawwab T, Kanj SS. Surgical site infections following spinal surgery at a tertiary care center in Lebanon: Incidence, microbiology, and risk factors. Scand J Infect Dis. 2006;38:589–92. doi: 10.1080/00365540600606440. [DOI] [PubMed] [Google Scholar]
- 71.Kanayama M, Hashimoto T, Shigenobu K, Oha F, Togawa D. Effective prevention of surgical site infection using a Centers for Disease Control and Prevention guideline-based antimicrobial prophylaxis in lumbar spine surgery. J Neurosurg Spine. 2007;6:327–9. doi: 10.3171/spi.2007.6.4.7. [DOI] [PubMed] [Google Scholar]
- 72.Kanayama M, Oha F, Togawa D, Shigenobu K, Hashimoto T. Is closed-suction drainage necessary for single-level lumbar decompression?: Review of 560 cases. Clin Orthop Relat Res. 2010;468:2690–4. doi: 10.1007/s11999-010-1235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang BU, Lee SH, Ahn Y, Choi WC, Choi YG. Surgical site infection in spinal surgery: Detection and management based on serial C-reactive protein measurements. J Neurosurg Spine. 2010;13:158–64. doi: 10.3171/2010.3.SPINE09403. [DOI] [PubMed] [Google Scholar]
- 74.Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6:311–5. doi: 10.1016/j.spinee.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 75.Kim JI, Suh KT, Kim SJ, Lee JS. Implant removal for the management of infection after instrumented spinal fusion. J Spinal Disord Tech. 2010;23:258–65. doi: 10.1097/BSD.0b013e3181a9452c. [DOI] [PubMed] [Google Scholar]
- 76.Klekamp J, Spengler DM, McNamara MJ, Haas DW. Risk factors associated with methicillin-resistant staphylococcal wound infection after spinal surgery. J Spinal Disord. 1999;12:187–91. [PubMed] [Google Scholar]
- 77.Korge A, Fischer R, Kluger P, Puhl W. The importance of sonography in the diagnosis of septic complications following spinal surgery. Eur Spine J. 1994;3:303–7. doi: 10.1007/BF02200141. [DOI] [PubMed] [Google Scholar]
- 78.Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Mandrekar JN, Osmon DR. The management and outcome of spinal implant infections: Contemporary retrospective cohort study. Clin Infect Dis. 2007;44:913–20. doi: 10.1086/512194. [DOI] [PubMed] [Google Scholar]
- 79.Labbe AC, Demers AM, Rodrigues R, Arlet V, Tanguay K, Moore DL. Surgical-site infection following spinal fusion: A case-control study in a children's hospital. Infect Control Hosp Epidemiol. 2003;24:591–5. doi: 10.1086/502259. [DOI] [PubMed] [Google Scholar]
- 80.Labler L, Keel M, Trentz O, Heinzelmann M. Wound conditioning by vacuum assisted closure (V.A.C.) in postoperative infections after dorsal spine surgery. Eur Spine J. 2006;15:1388–96. doi: 10.1007/s00586-006-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86:975–80. doi: 10.3171/jns.1997.86.6.0975. [DOI] [PubMed] [Google Scholar]
- 82.Linam WM, Margolis PA, Staat MA, Britto MT, Hornung R, Cassedy A, et al. Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure. Infect Control Hosp Epidemiol. 2009;30:109–16. doi: 10.1086/593952. [DOI] [PubMed] [Google Scholar]
- 83.Love C, Palestro CJ. Radionuclide imaging of infection. J Nucl Med Technol. 2004;32:47–57. [PubMed] [Google Scholar]
- 84.Maragakis LL, Cosgrove SE, Martinez EA, Tucker MG, Cohen DB, Perl TM. Intraoperative fraction of inspired oxygen is a modifiable risk factor for surgical site infection after spinal surgery. Anesthesiology. 2009;110:556–62. doi: 10.1097/ALN.0b013e3181974be7. [DOI] [PubMed] [Google Scholar]
- 85.Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;284:99–108. [PubMed] [Google Scholar]
- 86.Mehbod AA, Ogilvie JW, Pinto MR, Schwender JD, Transfeldt EE, Wood KB, et al. Postoperative deep wound infections in adults after spinal fusion: Management with vacuum-assisted wound closure. J Spinal Disord Tech. 2005;18:14–7. doi: 10.1097/01.bsd.0000133493.32503.d3. [DOI] [PubMed] [Google Scholar]
- 87.Meyers SP, Wiener SN. Diagnosis of hematogenous pyogenic vertebral osteomyelitis by magnetic resonance imaging. Arch Intern Med. 1991;151:683–7. [PubMed] [Google Scholar]
- 88.Milstone AM, Maragakis LL, Townsend T, Speck K, Sponseller P, Song X, et al. Timing of preoperative antibiotic prophylaxis: A modifiable risk factor for deep surgical site infections after pediatric spinal fusion. Pediatr Infect Dis J. 2008;27:704–8. doi: 10.1097/INF.0b013e31816fca72. [DOI] [PubMed] [Google Scholar]
- 89.Mirovsky Y, Floman Y, Smorgick Y, Ashkenazi E, Anekstein Y, Millgram MA, et al. Management of deep wound infection after posterior lumbar interbody fusion with cages. J Spinal Disord Tech. 2007;20:127–31. doi: 10.1097/01.bsd.0000211266.66615.e5. [DOI] [PubMed] [Google Scholar]
- 90.Mok JM, Guillaume TJ, Talu U, Berven SH, Deviren V, Kroeber M, et al. Clinical outcome of deep wound infection after instrumented posterior spinal fusion: A matched cohort analysis. Spine (Phila Pa 1976) 2009;34:578–83. doi: 10.1097/BRS.0b013e31819a827c. [DOI] [PubMed] [Google Scholar]
- 91.Mok JM, Pekmezci M, Piper SL, Boyd E, Berven SH, Burch S, et al. Use of C-reactive protein after spinal surgery: Comparison with erythrocyte sedimentation rate as predictor of early postoperative infectious complications. Spine (Phila Pa 1976) 2008;33:415–21. doi: 10.1097/BRS.0b013e318163f9ee. [DOI] [PubMed] [Google Scholar]
- 92.Molinari RW, Khera OA, Molinari WJ., 3rd Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J. 2012;21(Suppl 4):S476–82. doi: 10.1007/s00586-011-2104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: A new method for wound control and treatment: Animal studies and basic foundation. Ann Plast Surg. 1997;38:553–62. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 94.Muschik M, Luck W, Schlenzka D. Implant removal for late-developing infection after instrumented posterior spinal fusion for scoliosis: Reinstrumentation reduces loss of correction. A retrospective analysis of 45 cases. Eur Spine J. 2004;13:645–51. doi: 10.1007/s00586-004-0694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Naderi S, Acar F, Mertol T. Is spinal instrumentation a risk factor for late-onset infection in cases of distant infection or surgery? Case report. Neurosurg Focus. 2003;15:E15. doi: 10.3171/foc.2003.15.3.15. [DOI] [PubMed] [Google Scholar]
- 96.O’Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine. 2009;11:471–6. doi: 10.3171/2009.5.SPINE08633. [DOI] [PubMed] [Google Scholar]
- 97.Olsen MA, Mayfield J, Lauryssen C, Polish LB, Jones M, Vest J, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98(2 Suppl):149–55. [PubMed] [Google Scholar]
- 98.Olsen MA, Nepple JJ, Riew KD, Lenke LG, Bridwell KH, Mayfield J, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90:62–9. doi: 10.2106/JBJS.F.01515. [DOI] [PubMed] [Google Scholar]
- 99.Palestro CJ, Love C. Radionuclide imaging of musculoskeletal infection: Conventional agents. Semin Musculoskelet Radiol. 2007;11:335–52. doi: 10.1055/s-2008-1060336. [DOI] [PubMed] [Google Scholar]
- 100.Palestro CJ, Torres MA. Radionuclide imaging of nonosseous infection. Q J Nucl Med. 1999;43:46–60. [PubMed] [Google Scholar]
- 101.Parker SL, Adogwa O, Witham TF, Aaronson OS, Cheng J, McGirt MJ. Post-operative infection after minimally invasive versus open transforaminal lumbar interbody fusion (TLIF): Literature review and cost analysis. Minim Invasive Neurosurg. 2011;54:33–37. doi: 10.1055/s-0030-1269904. [DOI] [PubMed] [Google Scholar]
- 102.Payne DH, Fischgrund JS, Herkowitz HN, Barry RL, Kurz LT, Montgomery DM. Efficacy of closed wound suction drainage after single-level lumbar laminectomy. J Spinal Disord. 1996;9:401–3. [PubMed] [Google Scholar]
- 103.Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perry JW, Montgomerie JZ, Swank S, Gilmore DS, Maeder K. Wound infections following spinal fusion with posterior segmental spinal instrumentation. Clin Infect Dis. 1997;24:558–61. doi: 10.1093/clind/24.4.558. [DOI] [PubMed] [Google Scholar]
- 105.Picada R, Winter RB, Lonstein JE, Denis F, Pinto MR, Smith MD, et al. Postoperative deep wound infection in adults after posterior lumbosacral spine fusion with instrumentation: Incidence and management. J Spinal Disord. 2000;13:42–5. doi: 10.1097/00002517-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 106.Ploumis A, Mehbod AA, Dressel TD, Dykes DC, Transfeldt EE, Lonstein JE. Therapy of spinal wound infections using vacuum-assisted wound closure: Risk factors leading to resistance to treatment. J Spinal Disord Tech. 2008;21:320–3. doi: 10.1097/BSD.0b013e318141f99d. [DOI] [PubMed] [Google Scholar]
- 107.Potter BK, Kirk KL, Shah SA, Kuklo TR. Loss of coronal correction following instrumentation removal in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2006;31:67–72. doi: 10.1097/01.brs.0000192721.51511.fe. [DOI] [PubMed] [Google Scholar]
- 108.Pringle JH, Fletcher M. Influence of substratum wettability on attachment of freshwater bacteria to solid surfaces. Appl Environ Microbiol. 1983;45:811–7. doi: 10.1128/aem.45.3.811-817.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976) 2009;34:1422–8. doi: 10.1097/BRS.0b013e3181a03013. [DOI] [PubMed] [Google Scholar]
- 110.Pull ter Gunne AF, Mohamed AS, Skolasky RL, van Laarhoven CJ, Cohen DB. The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine (Phila Pa 1976) 2010;35:1323–8. doi: 10.1097/BRS.0b013e3181bcde61. [DOI] [PubMed] [Google Scholar]
- 111.Quinones-Hinojosa A, Jun P, Jacobs R, Rosenberg WS, Weinstein PR. General principles in the medical and surgical management of spinal infections: A multidisciplinary approach. Neurosurg Focus. 2004;17:E1. doi: 10.3171/foc.2004.17.6.1. [DOI] [PubMed] [Google Scholar]
- 112.Rathjen K, Wood M, McClung A, Vest Z. Clinical and radiographic results after implant removal in idiopathic scoliosis. Spine (Phila Pa 1976) 2007;32:2184–8. doi: 10.1097/BRS.0b013e31814b88a5. [DOI] [PubMed] [Google Scholar]
- 113.Rayes M, Colen CB, Bahgat DA, Higashida T, Guthikonda M, Rengachary S, et al. Safety of instrumentation in patients with spinal infection. J Neurosurg Spine. 2010;12:647–59. doi: 10.3171/2009.12.SPINE09428. [DOI] [PubMed] [Google Scholar]
- 114.Richards BR, Emara KM. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis: Revisited. Spine (Phila Pa 1976) 2001;26:1990–6. doi: 10.1097/00007632-200109150-00009. [DOI] [PubMed] [Google Scholar]
- 115.Richards BS. Delayed infections following posterior spinal instrumentation for the treatment of idiopathic scoliosis. J Bone Joint Surg Am. 1995;77:524–9. doi: 10.2106/00004623-199504000-00004. [DOI] [PubMed] [Google Scholar]
- 116.Rihn JA, Lee JY, Ward WT. Infection after the surgical treatment of adolescent idiopathic scoliosis: Evaluation of the diagnosis, treatment, and impact on clinical outcomes. Spine (Phila Pa 1976) 2008;33:289–94. doi: 10.1097/BRS.0b013e318162016e. [DOI] [PubMed] [Google Scholar]
- 117.Robertson PA, Taylor TK. Late presentation of infection as a complication of Dwyer anterior spinal instrumentation. J Spinal Disord. 1993;6:256–9. doi: 10.1097/00002517-199306030-00013. [DOI] [PubMed] [Google Scholar]
- 118.Rohmiller MT, Akbarnia BA, Raiszadeh K, Raiszadeh K, Canale S. Closed suction irrigation for the treatment of postoperative wound infections following posterior spinal fusion and instrumentation. Spine. 2010;35:642–6. doi: 10.1097/BRS.0b013e3181b616eb. [DOI] [PubMed] [Google Scholar]
- 119.Sampedro MF, Huddleston PM, Piper KE, Karau MJ, Dekutoski MB, Yaszemski MJ, et al. A biofilm approach to detect bacteria on removed spinal implants. Spine (Phila Pa 1976) 2010;35:1218–24. doi: 10.1097/BRS.0b013e3181c3b2f3. [DOI] [PubMed] [Google Scholar]
- 120.Sasso RC, Garrido BJ. Postoperative spinal wound infections. J Am Acad Orthop Surg. 2008;16:330–7. doi: 10.5435/00124635-200806000-00005. [DOI] [PubMed] [Google Scholar]
- 121.Schildhauer TA, Robie B, Muhr G, Koller M. Bacterial adherence to tantalum versus commonly used orthopedic metallic implant materials. J Orthop Trauma. 2006;20:476–84. doi: 10.1097/00005131-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 122.Schimmel JJ, Horsting PP, de Kleuver M, Wonders G, van Limbeek J. Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010;19:1711–9. doi: 10.1007/s00586-010-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schuster JM, Rechtine G, Norvell DC, Dettori JR. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: A systematic review. Spine (Phila Pa 1976) 2010;35(9 Suppl):S125–37. doi: 10.1097/BRS.0b013e3181d8342c. [DOI] [PubMed] [Google Scholar]
- 124.Schwank S, Rajacic Z, Zimmerli W, Blaser J. Impact of bacterial biofilm formation on in vitro and in vivo activities of antibiotics. Antimicrob Agents Chemother. 1998;42:895–8. doi: 10.1128/aac.42.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sheehan E, McKenna J, Mulhall KJ, Marks P, McCormack D. Adhesion of Staphylococcus to orthopaedic metals, an in vivo study. J Orthop Res. 2004;22:39–43. doi: 10.1016/S0736-0266(03)00152-9. [DOI] [PubMed] [Google Scholar]
- 126.Sierra-Hoffman M, Jinadatha C, Carpenter JL, Rahm M. Postoperative instrumented spine infections: A retrospective review. South Med J. 2010;103:25–30. doi: 10.1097/SMJ.0b013e3181c4e00b. [DOI] [PubMed] [Google Scholar]
- 127.Smith AS, Blaser SI. Infectious and inflammatory processes of the spine. Radiol Clin North Am. 1991;29:809–27. [PubMed] [Google Scholar]
- 128.Smith JS, Shaffrey CI, Sansur CA, Berven SH, Fu KM, Broadstone PA, et al. Rates of infection after spine surgery based on 108,419 procedures: A report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2011;36:556–63. doi: 10.1097/BRS.0b013e3181eadd41. [DOI] [PubMed] [Google Scholar]
- 129.Soultanis KC, Pyrovolou N, Zahos KA, Karaliotas GI, Lenti A, Liveris I, et al. Late postoperative infection following spinal instrumentation: Stainless steel versus titanium implants. J Surg Orthop Adv. 2008;17:193–9. [PubMed] [Google Scholar]
- 130.Sponseller PD, LaPorte DM, Hungerford MW, Eck K, Bridwell KH, Lenke LG. Deep wound infections after neuromuscular scoliosis surgery: A multicenter study of risk factors and treatment outcomes. Spine (Phila Pa 1976) 2000;25:2461–6. doi: 10.1097/00007632-200010010-00007. [DOI] [PubMed] [Google Scholar]
- 131.Stambough JL, Beringer D. Postoperative wound infections complicating adult spine surgery. J Spinal Disord. 1992;5:277–85. doi: 10.1097/00002517-199209000-00005. [DOI] [PubMed] [Google Scholar]
- 132.Stoodley P, Kathju S, Hu FZ, Erdos G, Levenson JE, Mehta N, et al. Molecular and imaging techniques for bacterial biofilms in joint arthroplasty infections. Clin Orthop Relat Res. 2005;437:31–40. doi: 10.1097/01.blo.0000175129.83084.d5. [DOI] [PubMed] [Google Scholar]
- 133.Strobel K, Stumpe KD. PET/CT in musculoskeletal infection. Semin Musculoskelet Radiol. 2007;11:353–64. doi: 10.1055/s-2008-1060337. [DOI] [PubMed] [Google Scholar]
- 134.Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: Efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976) 2011;36:2084–8. doi: 10.1097/BRS.0b013e3181ff2cb1. [DOI] [PubMed] [Google Scholar]
- 135.Takahashi J, Ebara S, Kamimura M, Kinoshita T, Itoh H, Yuzawa Y, et al. Early-phase enhanced inflammatory reaction after spinal instrumentation surgery. Spine (Phila Pa 1976) 2001;26:1698–704. doi: 10.1097/00007632-200108010-00014. [DOI] [PubMed] [Google Scholar]
- 136.Takahashi J, Shono Y, Hirabayashi H, Kamimura M, Nakagawa H, Ebara S, et al. Usefulness of white blood cell differential for early diagnosis of surgical wound infection following spinal instrumentation surgery. Spine (Phila Pa 1976) 2006;31:1020–5. doi: 10.1097/01.brs.0000214895.67956.60. [DOI] [PubMed] [Google Scholar]
- 137.Teixeira P, Trindade AC, Godinho MH, Azeredo J, Oliveira R, Fonseca JG. Staphylococcus epidermidis adhesion on modified urea/urethane elastomers. J Biomater Sci Polym Ed. 2006;17:239–46. doi: 10.1163/156856206774879072. [DOI] [PubMed] [Google Scholar]
- 138.Thalgott JS, Cotler HB, Sasso RC, LaRocca H, Gardner V. Postoperative infections in spinal implants. Classification and analysis: A multicenter study. Spine (Phila Pa 1976) 1991;16:981–4. doi: 10.1097/00007632-199108000-00020. [DOI] [PubMed] [Google Scholar]
- 139.Thelander U, Larsson S. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. Spine (Phila Pa 1976) 1992;17:400–4. doi: 10.1097/00007632-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 140.Tibbs PA. Closed irrigation-suction technic in lumbar laminectomy infections. Neurosurgery. 1980;6:120. doi: 10.1097/00006123-198001000-00020. [DOI] [PubMed] [Google Scholar]
- 141.van Rhee MA, de Klerk LW, Verhaar JA. Vacuum-assisted wound closure of deep infections after instrumented spinal fusion in six children with neuromuscular scoliosis. Spine J. 2007;7:596–600. doi: 10.1016/j.spinee.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 142.Veeravagu A, Patil CG, Lad SP, Boakye M. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976) 2009;34:1869–72. doi: 10.1097/BRS.0b013e3181adc989. [DOI] [PubMed] [Google Scholar]
- 143.Vender JR, Hester S, Houle PJ, Choudhri HF, Rekito A, McDonnell DE. The use of closed-suction irrigation systems to manage spinal infections. J Neurosurg Spine. 2005;3:276–82. doi: 10.3171/spi.2005.3.4.0276. [DOI] [PubMed] [Google Scholar]
- 144.Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) Device: A review. Am J Clin Dermatol. 2005;6:185–94. doi: 10.2165/00128071-200506030-00005. [DOI] [PubMed] [Google Scholar]
- 145.Vicario C, de Juan J, Esclarin A, Alcobendas M. Treatment of deep wound infections after spinal fusion with a vacuum-assisted device in patients with spinal cord injury. Acta Orthop Belg. 2007;73:102–6. [PubMed] [Google Scholar]
- 146.Viola RW, King HA, Adler SM, Wilson CB. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine (Phila Pa 1976) 1997;22:2444–50. doi: 10.1097/00007632-199710150-00023. [DOI] [PubMed] [Google Scholar]
- 147.Vitaz TW, Oishi M, Welch WC, Gerszten PC, Disa JJ, Bilsky MH. Rotational and transpositional flaps for the treatment of spinal wound dehiscence and infections in patient populations with degenerative and oncological disease. J Neurosurg. 2004;100(1 Suppl Spine):46–51. doi: 10.3171/spi.2004.100.1.0046. [DOI] [PubMed] [Google Scholar]
- 148.Watters WC, 3rd, Baisden J, Bono CM, Heggeness MH, Resnick DK, Shaffer WO, et al. Antibiotic prophylaxis in spine surgery: An evidence-based clinical guideline for the use of prophylactic antibiotics in spine surgery. Spine J. 2009;9:142–6. doi: 10.1016/j.spinee.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 149.Webster TJ, Patel AA, Rahaman MN, Sonny Bal B. Anti-infective and osteointegration properties of silicon nitride, poly (ether ether ketone), and titanium implants. Acta Biomater. 2012;8:4447–54. doi: 10.1016/j.actbio.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 150.Weinstein MA, McCabe JP, Cammisa FP., Jr Postoperative spinal wound infection: A review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13:422–6. doi: 10.1097/00002517-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 151.Widmer AF. New developments in diagnosis and treatment of infection in orthopedic implants. Clin Infect Dis. 2001;33(Suppl 2):S94–106. doi: 10.1086/321863. [DOI] [PubMed] [Google Scholar]
- 152.Williams DL, Woodbury KL, Haymond BS, Parker AE, Bloebaum RD. A modified CDC biofilm reactor to produce mature biofilms on the surface of peek membranes for an in vivo animal model application. Curr Microbiol. 2011;62:1657–63. doi: 10.1007/s00284-011-9908-2. [DOI] [PubMed] [Google Scholar]
- 153.Wimmer C, Gluch H. Management of postoperative wound infection in posterior spinal fusion with instrumentation. J Spinal Disord. 1996;9:505–8. [PubMed] [Google Scholar]
- 154.Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: A survey of 850 spinal procedures. J Spinal Disord. 1998;11:124–8. [PubMed] [Google Scholar]
- 155.Yuan-Innes MJ, Temple CL, Lacey MS. sVacuum-assisted wound closure: A new approach to spinal wounds with exposed hardware. Spine (Phila Pa 1976) 2001;26:E30–3. doi: 10.1097/00007632-200102010-00006. [DOI] [PubMed] [Google Scholar]
- 156.Zehnder SW, Place HM. Vacuum-assisted wound closure in postoperative spinal wound infection. Orthopedics. 2007;30:267–72. doi: 10.3928/01477447-20070401-19. [DOI] [PubMed] [Google Scholar]


