Summary
The time at which the dim light melatonin onset (DLMO) occurs can be used to ensure the correct timing of light and/or melatonin administration in order to produce desired circadian phase shifts. Sometimes however, measuring the DLMO is not feasible. Here we determined if the DLMO was best estimated from fixed sleep times (based on habitual sleep times) or free (ad libitum) sleep times. Young healthy sleepers on fixed (n = 60) or free (n = 60) sleep schedules slept at home for 6 days. Sleep times were recorded with sleep logs verified with wrist actigraphy. Half-hourly saliva samples were then collected during a dim light phase assessment and were later assayed to determine the DLMO. We found that the DLMO was more highly correlated with sleep times in the free sleepers than in the fixed sleepers (DLMO versus wake time, r = 0.70 and r = 0.44, both P < 0.05). The regression equation between wake time and the DLMO in the free sleepers predicted the DLMO in an independent sample of free sleepers (n = 23) to within 1.5 h of the actual DLMO in 96% of cases. These results indicate that the DLMO can be readily estimated in people whose sleep times are minimally affected by work, class and family commitments. Further work is necessary to determine if the DLMO can be accurately estimated in people with greater work and family responsibilities that affect their sleep times, perhaps by using weekend wake times, and if this method will apply to the elderly and patients with circadian rhythm disorders.
Keywords: bedtime, circadian rhythms, dim light melatonin onset, melatonin, sleep, wake
Introduction
Endogenous melatonin levels typically begin to increase prior to nighttime sleep, peak in the early hours of the morning and decrease to daytime levels after waking. The secretion of melatonin from the pineal gland is controlled by the circadian clock, which is located in the suprachiasmatic nuclei, within the anterior hypothalamus. Additionally, the secretion of melatonin is suppressed by light (Lewy et al., 1980). The onset of the endogenous melatonin rhythm in dim light (DLMO) is a widely used and reliable marker of the human circadian clock (Klerman et al., 2002; Lewy et al., 1999). The DLMO is usually readily determined from either plasma or saliva samples that are collected at frequent intervals, such as half-hourly intervals. The DLMO is determined as the point of time where melatonin levels rise above a set threshold.
Knowing the timing of the DLMO is key to utilizing the phase response curve to exogenous melatonin, as the phase response curve is often referenced to the DLMO (Lewy et al., 1998). Furthermore, as several authors have found that the DLMO occurs approximately 7 h before the core body temperature minimum (e.g. Brown et al., 1997; Cagnacci et al., 1996; Sharkey and Eastman, 2002), knowing the timing of the DLMO can also enable the use of the light phase response curves, most of which have been referenced to the temperature minimum (Czeisler et al., 1989; Eastman, 1992; Minors et al., 1991). Thus knowing the timing of the DLMO enables the correct timing of exogenous melatonin and/or bright light in order to induce desired circadian phase shifts.
However, there are occasions when collecting plasma or saliva samples in very dim light, and/or having them assayed is either too expensive or simply not feasible. Two previous studies have specifically examined the possibility of using sleep times to estimate the timing of the DLMO in young healthy people. Martin and Eastman (2002) studied 26 such subjects who were permitted to sleep whenever they wished (free sleepers) for 14 days. Following that, each subject participated in a phase assessment session, from which the DLMO was later determined. When Martin and Eastman averaged each subject's sleep times in the 5 days before the phase assessment, they found significant correlations between sleep onset and the DLMO (r = 0.74), sleep midpoint and the DLMO (r = 0.89) and also wake time and the DLMO (r = 0.85). While these correlations were high, the question remained as to whether the correlations could be further increased by requiring subjects to maintain a fixed sleep schedule. A fixed sleep schedule could stabilize the circadian system and so produce a tighter association between the DLMO and sleep times. This in turn would be likely to increase the accuracy of estimating the DLMO from sleep times.
Burgess et al. (2003b) addressed this issue by examining the relationship between sleep times and the DLMO in 16 young healthy subjects instructed to sleep at fixed times, similar to their self-reported habitual sleep times (fixed sleepers) for a week. The highest significant correlation Burgess et al. reported was between the DLMO and wake time (r = 0.77), from the day before the phase assessment session (similar to the wake times of all nights because of the fixed sleep schedule). All the correlations between the sleep times and the DLMO were lower than what Martin and Eastman reported. Thus, this result did not support the notion that fixed sleep schedules stabilized the DLMO, nor that the estimation of the DLMO from sleep times could be improved with fixing sleep times.
In the current study we re-examined this question with larger samples of both free and fixed sleepers. We have not included any data from our previous studies. We expected that with large samples the correlations between average sleep times and the DLMO would be equally as good in fixed sleepers, when compared with free sleepers, if not better. We also anticipated that the correlations would be fairly high, suggesting that the DLMO can be readily estimated from average sleep times.
Methods
Subjects
A total of 120 young healthy subjects participated. There were 60 ‘free sleepers’ and 60 ‘fixed sleepers’ (see Protocol below). Two subjects participated twice – once as a free sleeper and once as a fixed sleeper. All subjects except five were non-smokers and all reported no medical, psychiatric or sleep disorders as assessed from a telephone interview, in-person interview, medical history and several screening questionnaires (Minnesota Multiphasic Personality Inventory-2, Pittsburgh Sleep Quality Index (Buysse et al., 1989), and part of a general health questionnaire (Tasto et al., 1978). All subjects were also free from prescription medications, except for 16 female subjects who were taking an oral contraceptive. The menstrual phase of the female subjects during the study was not controlled as menstrual phase does not affect the melatonin rhythm (Parry et al., 1997). We excluded individuals who had worked a night shift or traveled overseas in the month prior to the start of the study. All subjects completed the Horne and Ostberg morningness–eveningness questionnaire (MEQ) (Horne and Ostberg, 1976).
Protocol
During the baseline of a night shift study (Crowley et al., 2003), the free sleepers slept at times of their own choice. We analyzed sleep data from the 6 days (Sunday to Friday) before their saliva was collected during a phase assessment session. Of the 60 subjects, 41 were students, 12 were unemployed and seven had jobs with flexible hours. All subjects participated in the summer months of July, August and September. All subjects were told not to stay up all night, and 38 of them were instructed to ensure their bedtime occurred before 2:00 hours. Napping was freely permitted.
During the baseline of two jet lag studies (Burgess et al., 2003a; Eastman et al., 2005) and another study (currently unpublished), 60 subjects slept on a fixed sleep schedule. Again, we analyzed sleep data of the 6 days (either Friday to Wednesday or Wednesday to Monday) before their saliva was collected during a phase assessment session. Twenty-eight subjects were students, 25 were employed and seven were unemployed. Subjects participated in the study year round. Each subject was instructed to adhere to a strict baseline sleep schedule that was tailored to match to within 1 h of their self-reported typical sleep onset and wake times, as confirmed with sleep logs, completed for at least a week before the start of the study. Napping was permitted in 47 of these 60 subjects, but only during a 6-h zone centered 12 h from the midpoint of the scheduled nocturnal sleep periods. Sleep/dark at this time should not alter circadian rhythms as neither afternoon sleep/dark episodes (Buxton et al., 2000) nor afternoon bright light episodes (Dumont and Carrier, 1997) phase shift circadian rhythms.
All protocols were approved by the Rush University Medical Center Institutional Review Board, and all subjects gave written informed consent prior to participation. Subjects were reimbursed for their participation.
Sleep
Bedtime, estimated sleep onset time, wake times of greater than 5 min during the sleep period, final morning wake time, cause of awakening (spontaneous, alarm or other) and naps were reported by subjects using daily sleep logs throughout the study. During their sleep periods, the fixed sleepers were instructed to lie in bed in the dark and try to sleep. They were not permitted to read, watch TV, listen to music or talk on the telephone at this time. All subjects were required to call the lab voice mail (time and date of call was recorded) before turning out their lights at night and at their wake time in the morning each day. Each subject wore a wrist actigraphy monitor (Actiwatch-64 or Actiwatch-L; Mini-Mitter, Bend, OR, USA) on their non-dominant wrist that recorded their activity every minute. The wrist monitor was only removed during showers and baths. Every 1–3 days subjects were required to come to the lab so that the data from the wrist actigraph and reported times on the sleep logs could be examined for accuracy in their presence.
Phase assessment
During the phase assessment sessions, subjects remained awake in dim light (<10 l×), as verified at the level of the subjects' eyes and in the direction of gaze, with a Minolta TL-1 light meter (Ramsey, Minolta, NJ, USA). The subjects sat in comfortable recliners and were only allowed to stand up for trips to the bathroom. The first 22 free sleepers experienced their phase assessment session in our old laboratory, and so when they requested, they were placed in a wheelchair and propelled to a neighboring bathroom while wearing dark welder's goggles (No. 5 lenses, approximately 2% light transmission, Cricket frames; Uvex Safety Inc., Smithfield, RI, USA). The remaining subjects experienced the phase assessment session in our new laboratory, in which the bathroom is adjacent to the phase assessment room and the bathroom and hall to bathroom were also dimly lit (<10 l×). In both laboratories, bathroom trips were discouraged during the 10 min before each saliva sample.
During the phase assessment sessions, subjects watched movies, read, played games, and talked with each other and the supervising staff. Subjects gave a 2 mL saliva sample every 30 min using Salivettes (Sarstedt, NC, USA). Subjects were not permitted to consume any alcohol in the 48 h prior to the start of a phase assessment session and were breathalyzed on arrival. Non-steriodal anti-inflammatory drugs were not permitted on the 3 days before and during the phase assessment session, as these drugs have been shown to suppress melatonin (Murphy et al., 1996). No caffeine, chocolate, bananas, or lipstick were allowed in the 5 h before and during the phase assessment session. No toothpaste or mouthwash was allowed during the phase assessment session. Small snacks and fluids were permitted, except in the 10 min before each sample, and subjects were required to rinse and brush their teeth with water while remaining seated 10 min before each sample if they had consumed food or drink. Saliva samples were centrifuged immediately after collection and frozen. The samples were later packed in dry ice and shipped to Pharmasan Labs (WI, USA) to be radio-immunoassayed for melatonin. All samples from an individual subject were assayed in the same batch. The sensitivity of the assay was 0.7 pg mL−1, and intra- and interassay coefficient of variabilities were 12.1 and 13.2% respectively.
Data analysis
Sleep parameters
For each of the 6 days prior to the phase assessment session, we determined bedtime, estimated sleep onset time, dark midpoint, final morning wake time and total sleep time from the sleep logs. The dark midpoint was halfway between bedtime and wake time. Total sleep time was the time from sleep onset to wake time minus any awakenings during the night, plus any naps taken during the day. For each subject, each of these variables was calculated for each of the 6 days, and these were then averaged to produce a mean bedtime, sleep onset time, dark midpoint, wake time and total sleep time for each subject. We also calculated the standard deviation (SD) of bedtime, sleep onset time and wake time across the 6 days for each subject, in order to obtain a measure of the variability in their sleep times during the 6 days.
Dim light melatonin onset
To determine each subject's DLMO, a threshold was calculated as twice the mean of the first three low daytime values (Voultsios et al., 1997). Each subject's DLMO was the point in time (as determined with linear interpolation) when the melatonin concentration exceeded the threshold. The threshold ranged from 0.7 to 10.6 pg mL−1 (mean ± SD = 2.3 ± 1.5 pg mL−1).
Statistical analysis
Data are presented as mean ± SD unless otherwise specified. A two-way manova (sex versus group) was used to assess differences between the free and fixed sleepers and males and females. Univariate anovaS were planned if the manova was significant. Chi-square tests were run on discrete variables. Pearson's product moment correlations and associated linear regressions were calculated to determine the relationship between the DLMO, sleep parameters and MEQ. Differences between correlations were tested with the Fisher z-test. Statistical significance was determined at P < 0.05.
Results
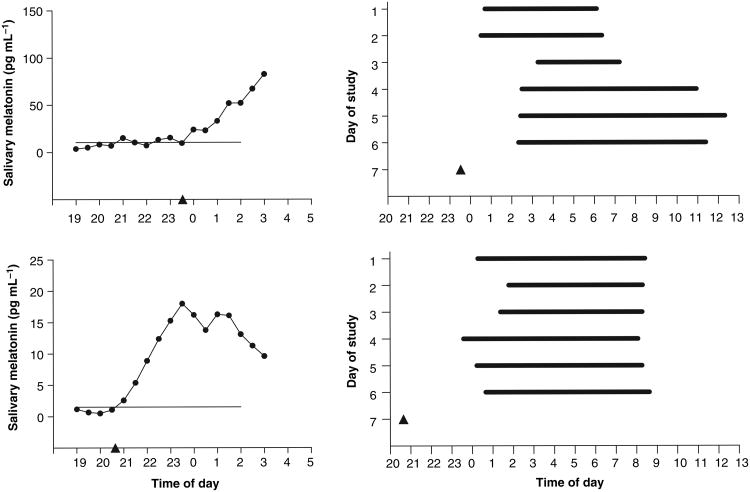
The manova was significant and thus the results from the univariate anovas are reported in Tables 1 and 2. There were no significant interactions but there were several significant main effects of group and of sex. Table 1 shows that the free sleepers were significantly younger than the fixed sleepers and also had a lower MEQ score (more eveningness). Table 2 shows that all the sleep times and the DLMO were significantly later in the free sleepers than in the fixed sleepers. As expected, the free sleepers' total sleep time was significantly longer than in the fixed sleepers. The free sleepers were also significantly more variable in their bedtime, sleep onset time and wake time than the fixed sleepers, as shown by the mean SD (Table 2). On average each free sleeper varied their bedtime by 2.1 ± 1.2 h and their wake time by 2.8 ± 1.5 h over the 6 days (the variation in the fixed sleepers was negligible). The sleep times and melatonin profiles of the two free sleepers with the highest and lowest variability in wake time are shown in Fig. 1. The DLMO to bedtime and DLMO to sleep onset intervals did not differ significantly between the fixed and free groups, but the wake time to DLMO interval was close to significantly shorter in the free sleepers (Table 2). In the free sleepers the earliest bedtime (lights out) was 21:19 hours and the latest was 4:40 hours. The earliest wake time (lights on) was 2:30 hours and the latest was 13:51 hours. On 123 of a total of 360 mornings (34%) the free sleepers reported the use of an alarm to wake up. The fixed sleepers were instructed to use an alarm every morning. In the fixed sleepers, compliance to the fixed sleep schedules was good. Only five subjects slept at times more than 30 min from their sleep and nap schedule: two woke late, one fell asleep early and two napped before their nap zone. In all five subjects, each had only one incident of non-compliance. The earliest bedtime in the fixed sleepers was 21:45 hours and the latest was 1:30 hours. The earliest wake time in the fixed sleepers was 5:10 hours and the latest was 10:30 hours.
Table 1. Mean (SD) age, body mass index (BMI), morningness–eveningness score (MEQ), number of nappers and time and duration of naps in the various categories.
| Fixed | Free | Group | Sex | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Male (n = 28) |
Female (n = 32) |
Male (n = 29) |
Female (n = 31) |
Fixed (n = 60) |
Free (n = 60) |
Male (n = 57) |
Female (n = 63) |
|
| Age (years) | 29.8 (6.5) | 26.3 (3.7) | 24.0 (6.6) | 23.4 (5.4) | 27.9 (5.4) | 23.7** (5.9) | 26.9 (7.1) | 24.9* (4.8) |
| BMI (kg m−2) | 25.5 (3.0) | 22.1 (2.5) | 25.3 (3.7) | 23.9 (3.5) | 23.7 (3.2) | 24.6 (3.6) | 25.4 (3.3) | 22.9** (3.1) |
| MEQ | 52.6 (8.2) | 52.0 (7.6) | 47.3 (8.4) | 47.5 (7.7) | 52.3 (7.8) | 47.4** (8.0) | 49.9 (8.7) | 49.8 (7.9) |
| % Nappers | 32 | 50 | 24 | 52 | 42 | 38 | 28 | 51*** |
| Time of nap midpoint (hours) | 14:49 (1.7) | 15:41 (1.2) | 14:25 (2.2) | 15:33 (2.6) | 15:22 (1.4) | 15:12 (2.5) | 14:38 (1.9) | 15:37 (2.0) |
| Nap duration (h) | 0.8 (0.3) | 1.1 (0.7) | 1.4 (0.8) | 1.3 (0.7) | 1.0 (0.6) | 1.3 (0.7) | 1.1 (0.6) | 1.2 (0.7) |
There were no significant sex by group interactions.
P < 0.05 main effect of anovas.
P < 0.01 main effect of anovas.
P < 0.01 determined by chi-square test.
Table 2. The mean (SD) sleep parameters, DLMO, and intervals between the DLMO and sleep parameters in the male and female fixed and free sleepers.
| Fixed | Free | Group | Sex | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Male (n = 28) |
Female (n = 32) |
Male (n = 29) |
Female (n = 31) |
Fixed (n = 60) |
Free (n = 60) |
Male (n = 57) |
Female (n = 63) |
|
| Bedtime (hours) | 23:08 (0.7) | 23:25 (0.9) | 0:51 (1.1) | 1:02 (1.2) | 23:17 (0.9) | 0:57** (1.1) | 0:00 (1.3) | 0:12 (1.3) |
| Sleep onset time (hours) | 23:22 (0.8) | 23:43 (0.9) | 1:16 (1.1) | 1:25 (1.2) | 23:33 (0.9) | 1:21** (1.2) | 0:20 (1.3) | 0:33 (1.4) |
| Dark midpoint (hours) | 3:03 (0.8) | 3:18 (1.0) | 5:06 (1.1) | 5:16 (1.2) | 3:11 (0.9) | 5:11** (1.2) | 4:06 (1.4) | 4:16 (1.5) |
| Wake time (hours) | 6:59 (0.9) | 7:12 (1.1) | 9:20 (1.4) | 9:29 (1.3) | 7:06 (1.0) | 9:25** (1.3) | 8:11 (1.7) | 8:20 (1.6) |
| Total sleep time (h) | 7.5 (0.6) | 7.6 (0.6) | 8.1 (0.8) | 8.1 (0.6) | 7.6 (0.6) | 8.1** (0.7) | 7.8 (0.8) | 7.9 (0.7) |
| Bedtime SD (h) | 0.0 (0.1) | 0.0 (0.0) | 0.8 (0.5) | 0.8 (0.4) | 0.0 (0.0) | 0.8** (0.4) | 0.4 (0.5) | 0.4 (0.5) |
| Sleep onset SD (h) | 0.1 (0.1) | 0.2 (0.1) | 0.7 (0.4) | 0.8 (0.5) | 0.1 (0.1) | 0.8** (0.5) | 0.4 (0.4) | 0.5 (0.5) |
| Wake time SD (h) | 0.1 (0.2) | 0.1 (0.1) | 1.1 (0.6) | 1.1 (0.6) | 0.1 (0.1) | 1.1** (0.6) | 0.6 (0.7) | 0.6 (0.7) |
| DLMO (hours) | 20:46 (1.1) | 20:46 (1.2) | 22:56 (1.4) | 22:27 (1.6) | 20:46 (1.1) | 22:41** (1.5) | 21:52 (1.7) | 21:36 (1.6) |
| DLMO-bedtime interval (h) | 2.4 (1.0) | 2.6 (1.1) | 1.9 (1.1) | 2.6 (1.3) | 2.5 (1.1) | 2.3 (1.2) | 2.1 (1.1) | 2.6* (1.2) |
| DLMO-sleep onset interval (h) | 2.6 (1.0) | 2.9 (1.0) | 2.3 (1.0) | 3.0 (1.2) | 2.8 (1.0) | 2.7 (1.1) | 2.5 (1.0) | 3.0* (1.1) |
| Wake time-DLMO interval (h) | 13.8 (1.1) | 13.6 (1.2) | 13.6 (1.0) | 13.0 (1.1) | 13.7 (1.1) | 13.3*** (1.1) | 13.7 (1.1) | 13.3* (1.2) |
There were no significant sex by group interactions.
P < 0.05 main effect of anovas
P < 0.01 main effect of anovas.
P = 0.055 main effect of anovas.
Figure 1.
Melatonin profiles and sleep times during the 6 days before the phase assessment session for the free sleeper with the highest variability in wake time (top) and free sleeper with the lowest variability in wake time (bottom). On each melatonin profile, the horizontal line represents the DLMO threshold. The dark period times (bedtime to wake time) of each subject on each day are represented by the thick horizontal black bars. Neither subject napped during the day. The phase assessment occurred on day 7, and the DLMO determined from this is illustrated by the triangle.
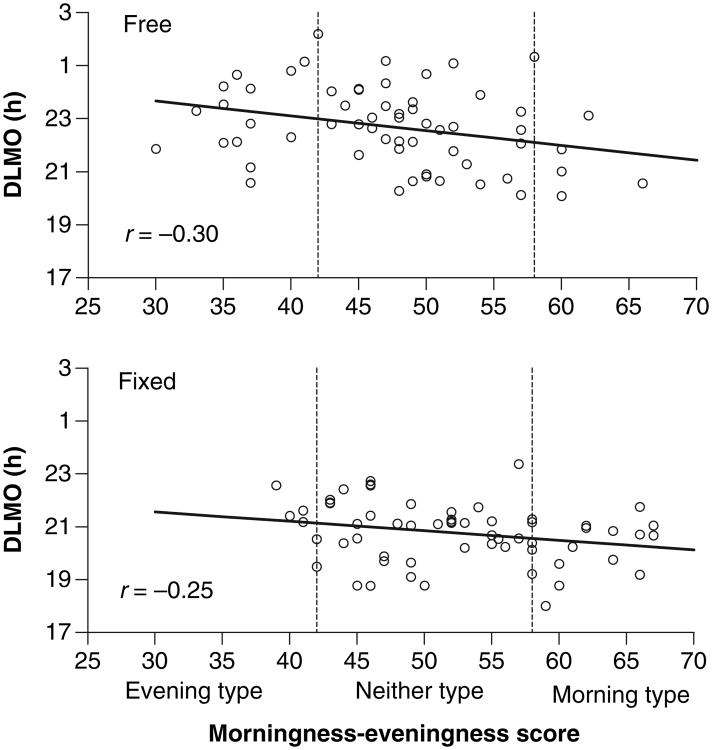
Table 3 shows the correlations between the DLMO and sleep parameters, and the DLMO and the MEQ score. All correlations in the free sleepers were of greater magnitude than in the fixed sleepers. The highest correlation in the free sleepers was between the DLMO and wake time (r = 0.70), and this correlation was significantly greater than the same correlation in the fixed sleepers (r = 0.44). These relationships are illustrated in Fig. 2. In the fixed sleepers there are at least two clusters of points that can be identified – from those subjects who woke at 6:00 hours and at 7:00 hours. Within each of these clusters there is great variability in the timing of the DLMO. In contrast, in the free sleepers there is a fairly uniform distribution of wake times and DLMOs. Figure 3 illustrates the weaker relationships between the DLMO and MEQ.
Table 3. The Pearson product moment correlations between the DLMO and sleep parameters and morningness–eveningness (MEQ) in the free and fixed sleepers.
| Correlation | |||
|---|---|---|---|
|
|
|||
| Fixed sleepers (n = 60) |
Free sleepers (n = 60) |
P-value (z-test) |
|
| DLMO versus bedtime | 0.47** | 0.60** | NS |
| DLMO versus sleep onset time | 0.49** | 0.66** | NS |
| DLMO versus dark midpoint | 0.47** | 0.69** | <0.05 |
| DLMO versus wake time | 0.44** | 0.70** | <0.05 |
| DLMO versus total sleep time | 0.10 | 0.29* | NS |
| DLMO versus MEQ | −0.25*** | −0.30* | NS |
The Fisher z-test determines the significance of the difference between two correlation coefficients.
P < 0.05.
P ≤ 0.001.
P = 0.056.
Figure 2.
The scatterplots and associated linear regressions between the dim light melatonin onset (DLMO) and wake time in the free sleepers (top) and fixed sleepers (bottom). The regression equation for the relationship between the DLMO and wake time in the free sleepers is: DLMO (decimal time) = 0.80 × wake time (decimal time) −8.83.
Figure 3.
The scatterplots and associated linear regressions between the dim light melatonin onset (DLMO) and MEQ score in the free sleepers (top) and fixed sleepers (bottom).
We applied the regression equation predicting the DLMO from wake time in our free sleepers (Fig. 2) to an independent sample of free sleepers from our previous study (Martin and Eastman, 2002). The DLMOs in the previous sample were originally calculated with a threshold equal to 20% of the maximum point. We re-analyzed them using the current method (we lost three DLMOs in the process – in all three there were no low daytime points). We found that the regression equation was able to predict the DLMOs in our previous sample to within 1.0 h of when it actually occurred in 17 of 23 subjects (74% of cases). Additionally, we found that the regression equation was able to predict the DLMOs in our previous sample to within 1.5 h of when it actually occurred in 22 of 23 subjects (96% of cases).
We also examined the data for sex differences. As shown in Table 1, the female subjects were slightly but significantly younger, had a significantly lower BMI and were more likely to nap than the male subjects. Table 2 shows that in the female subjects the DLMO to bedtime and DLMO to sleep onset interval were longer than in the males, and the wake time to DLMO interval was correspondingly shorter in the females than in the males.
Discussion
This study examined how well the DLMO can be estimated from sleep times in large samples of fixed and free sleepers, and the results do not support the idea that fixed sleep schedules stabilize the DLMO or improve estimations of the DLMO from sleep times. The correlations between sleep times and the DLMO were all higher in the free sleepers than in the fixed sleepers. For the free sleepers, the two highest correlations were between wake time and the DLMO and between dark midpoint and the DLMO (r = 0.70 and r = 0.69). The higher correlations in the free sleepers confirm the results from two previous studies – the correlations from a study of free sleepers (Martin and Eastman, 2002) were higher than those from a study of fixed sleepers (Burgess et al., 2003b). Additionally, a recent abstract reported that the correlations between sleep times and the DLMO were higher in adolescents who slept freely on their summer break versus when they had more set sleep times during the school year (Crowley et al., 2004). The findings of all of these studies suggest that maintaining a fixed sleep schedule based on usual sleep times for 5–7 days does not stabilize the DLMO such that it becomes more tightly associated with sleep times than observed following free sleep. Indeed in Fig. 2 it can be seen that the fixed sleepers who had a wake time at 6:00 or 7:00 hours, showed great variability in the timing of their DLMOs. Thus the higher correlations in free sleepers appears likely to be due to them being able to sleep at times promoted by the circadian clock, whereas fixed sleepers are forced to sleep at times dictated by their work, class and/or family commitments. The fixed sleepers tended to wake earlier than they should have as seen in the trend for them to have a longer wake time to DLMO interval, or shorter DLMO to wake interval, indicating that they woke at an earlier circadian phase than the free sleepers.
It is possible that we might have seen higher correlations in the fixed sleepers if their sleep schedules had been based on their weekend sleep times, instead of their average sleep times which were largely influenced by their earlier weekday schedule. If study participants are not able to sleep freely, then the DLMO is probably best estimated from their weekend sleep times, which are likely to be less affected by work, class and/or family commitments. Indeed, a high correlation (r = 0.80) between the temperature minimum and wake time was found in fixed sleepers whose sleep schedules were based on their later weekend sleep times (Baehr et al., 2000).
The higher correlations in the free sleepers in our sample may be, in part, because of a wider range of wake times in the free sleepers, which is obvious when the two groups in Fig. 2 are compared. Larger ranges of any variable can produce higher correlations. However, when we recalculated the correlation between the DLMO and wake time in subjects who woke between 7:00 and 9:00 hours only, we found that the correlation was still greater in the free sleepers (r = 0.56, P < 0.01) than in the fixed sleepers (r = 0.31, P = 0.10). The higher correlations in the free sleepers may also be due to there being more evening types in the sample of free sleepers. As evening types wake at an earlier circadian phase (Baehr et al., 2000; Duffy et al., 1999), morning light falls closer to the crossover point of their light phase response curve, and so may have a greater influence on determining the timing of their circadian clocks. However, when we recalculated the correlation between the DLMO and wake time in only neither types, we found that the correlation was still greater in the free sleepers (r = 0.71, P < 0.001) than in the fixed sleepers (r = 0.40, P < 0.01). It is conceivable that seasonal effects account for the higher correlations in the free sleepers (who participated only in summer). However, circadian phase in humans remains stable across seasons at temperature latitudes (Wehr et al., 2001b), humans are not usually exposed to much external light (Hebert et al., 1998) and the circadian system habituates to regular bright light exposure (Hebert et al., 2002). Indeed, despite their subjects participating in the fall and winter, Martin and Eastman (2002) still reported higher correlations in their free sleepers than we report here.
The correlations in the free sleepers in the Martin and Eastman study were all higher than what we observed in our free sleepers, but not significantly so (Fisher's test P > 0.05). We examined several possible reasons for the difference, but none appeared to explain the difference. For example, Martin and Eastman's slightly higher correlations could be because they determined the threshold for calculating the DLMO in a different way, as 20% of the single maximum point. This threshold often leads to later DLMOs as the threshold is usually higher than the thresholds determined with our method. We used our method to calculate the DLMO because we did not collect entire melatonin profiles and so could not be sure of the maximum point. We have found in practice that our low threshold typically hits much closer to where we would visually identify the DLMO. Furthermore, our threshold was easy to use – in only two of 136 melatonin profiles examined for inclusion in this study was a DLMO visually obvious but the calculated threshold too low. Nonetheless, we cannot think of a reason why a lower threshold would systematically reduce the correlations between the DLMO and sleep times.
We also examined the Martin and Eastman (2002) sleep logs to evaluate other possible reasons for their slightly higher correlations. As the range of wake times and DLMOs in our free sleepers are slightly greater than in the Martin and Eastman sample, a restriction of range effect cannot account for our lower correlations. Furthermore, their subjects were not freer of social and work commitments than our free sleepers, as 51% of the awakenings were due to an alarm, a higher percentage than in our free sleepers (34%). We also considered the possibility that naps (dark pulses) may have phase shifted the circadian clock (Buxton et al., 2000), thereby reducing the association between nighttime sleep times and the DLMO. Indeed, our data support this idea, because when we correlated wake time and DLMO in the 37 free sleepers who did not nap, the correlation increased from r = 0.70 to r = 0.79, and when we performed the same correlation in the 35 fixed sleepers who did not nap the correlation increased from r = 0.44 to r = 0.52. However, we found that proportionally, more free sleepers napped in Martin and Eastman's sample (54%) than in our sample (38%), and so napping cannot explain the slightly lower correlations in our free sleepers compared with their free sleepers.
Both wake time and dark midpoint correlated slightly higher with the DLMO than bedtime and sleep onset in the free sleepers. Dark midpoint is a derivative of both bedtime and wake time, and so the results suggest that wake time (lights on) is somewhat more strongly associated with circadian phase than bedtime or sleep onset. Indeed all but one of the studies examining correlations between sleep times and the DLMO in healthy subjects report higher correlations with wake time than bedtime or sleep onset time (Burgess et al., 2003b; Crowley et al., 2004; Martin and Eastman, 2002; Yoon et al., 2003). The single exception comes from a sample of 12 morning and 12 evening types (Mongrain et al., 2004) – the correlations are likely affected by the presence of two groups in the data set and may not apply to the general population. Wake time also correlated more highly with the DLMO than MEQ (Table 3; Figs 2 and 3). All correlations in young healthy subjects reported between the MEQ and the DLMO (Boulos et al., 2002; Griefahn, 2002; Martin and Eastman, 2002), MEQ and the temperature minimum (Baehr et al., 2000; Duffy et al., 1999) and MEQ and melatonin peak (Liu et al., 2000) were lower in magnitude than what we found here between free wake time and DLMO.
Ongoing work in our laboratory also points to the dependence of circadian phase on wake time. While keeping wake time constant, we observed only a 0.6-h delay in the DLMO when we delayed bedtime by 3 h (Burgess and Eastman, 2004), but when we held bedtime constant and delayed wake time by 3 h, we found a 2.5-h delay in the DLMO (Burgess and Eastman, 2005). The greater association between wake time and the DLMO versus bedtime and the DLMO is probably because light after waking in the morning is typically of much greater intensity than light before bedtime in the evening (Burgess and Eastman, 2004; Hebert et al., 1998), and morning light occurs closer to the crossover point of the light phase response curve, thereby having a greater effect on the circadian clock. Some have theorized that the circadian clock is composed of a morning and evening oscillator, which respond to dawn and dusk respectively (Pittendrigh and Daan, 1976; Wehr et al., 2001a). This model would predict that the DLMO would be expected to be more closely linked to bedtime than to wake time, which is clearly not the case. Perhaps this is due to the putative morning and evening oscillators being tightly coupled.
When we used the regression equation from the wake times in the free sleepers to predict the timing of the DLMOs from the Martin and Eastman study, we were able to predict 74% of the DLMOs to within 1 h and 96% of the DLMOs to within 1.5 h of when they actually occurred. This result and the results of the previous two studies confirms that when determining the DLMO from a phase assessment session is too expensive or not feasible, it may be estimated from wake times of free sleepers. Field studies may use this method to estimate the DLMO when the regular collection of samples is too difficult and/or keeping subjects in dim light is not possible. Furthermore, being able to estimate the DLMO will help people who want to use light or exogenous melatonin to phase shift their circadian clocks towards destination time before flight, in order to reduce their subsequent jet lag (Burgess et al., 2003a; Eastman et al., 2005). Importantly however, all the studies that have so far examined the possibility of estimating the DLMO from sleep times have examined young healthy subjects, and in adults, they show that the DLMO occurs approximately 2.5 h before bedtime and approximately 13.5 h after wake time. Therefore our regression equations may not readily apply to the elderly, patients with circadian rhythm disorders, jet-lagged travelers or night workers with altered phase relationships between sleep and the circadian clock.
We did not find clear sex differences in sleep times, MEQ or the timing of the DLMO. While Martin and Eastman did report earlier sleep onset, wake and sleep midpoint times in females, others using larger samples have not found significant sex differences in sleep times (Hume et al., 1998; Monk et al., 2000). In our free sleepers, the DLMO was slightly earlier in the females than in males, although the difference was not significant. Our group has previously reported a similar trend for an earlier temperature minimum (Kattapong et al., 1995) or DLMO (Martin and Eastman, 2002) in females than males, and we and others using larger sample sizes report a significantly earlier temperature minimum (Baehr et al., 2000) and more morningness (Adan and Natale, 2002) in females. In the current study, we did find significantly longer DLMO to bedtime and DLMO to sleep onset intervals and a shorter wake time to DLMO interval in females. Others have also reported this (Mongrain et al., 2004). Our group has also reported a longer Tmin to wake interval in females (Baehr et al., 2000). Thus, females go to bed and wake up at a later phase of their circadian cycle.
In conclusion, the results from this study indicate that the DLMO can be estimated from wake time to within 1.5 h of the actual DLMO in young healthy sleepers who sleep relatively freely. Future work needs to compare free sleepers with fixed sleepers whose schedules are fixed at later times, such as their weekend sleep times, as these fixed sleep times may be better for estimating the DLMO. Within subjects designs in which subjects are measured on both fixed and free sleep times would also be useful. Future studies should also determine whether the correlations between sleep times and the DLMO become weaker if people nap during the day at times shown to shift circadian phase (Buxton et al., 2000). The influence of nonphotic zeitgebers such as meal times on the association between sleep times and the DLMO should also be investigated.
Acknowledgments
This work was supported by NIH grants to C. Eastman: NINR RO1 NR07677, CDC NIOSH RO1 OH03954. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIOSH or the CDC. We thank Karen Chuang, Stephanie Crowley, Meredith Durkin, Clifford Gazda, Cynthia Hiltz, Caroline Kang, Hyungsoo Kim, Clara Lee, Kathryn Lenz, Jim Lyda, Tom Molina, Mark Smith, Natalie Stroupe, Barbara Trzop and Christine Tseng for their assistance. We also thank Dr Alfred Lewy for his interesting discussions on the dependence of DLMO on wake time.
References
- Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int. 2002;19:709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Macchi MM, Sturchler MP, Stewart KT, Brainard GC, Suhner A, Wallace G, Steffen R. Light visor treatment for jet lag after westward travel across six time zones. Aviat Space Environ Med. 2002;73:953–963. [PubMed] [Google Scholar]
- Brown EN, Choe Y, Shanahan TL, Czeisler CA. A mathematical model of diurnal variations in human plasma melatonin levels. Am J Physiol. 1997;272:E506–E516. doi: 10.1152/ajpendo.1997.272.3.E506. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Waking up late phase delays the human circadian clock more than staying up late. Sleep. 2005;28:A55. [Google Scholar]
- Burgess HJ, Eastman CI. Early versus late bedtimes phase shift the human dim light melatonin rhythm despite a fixed morning lights on time. Neurosci Lett. 2004;356:115–118. doi: 10.1016/j.neulet.2003.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003a;18:318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS, Dawson D. The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav Sleep Med. 2003b;1:102–114. doi: 10.1207/S15402010BSM0102_3. [DOI] [PubMed] [Google Scholar]
- Buxton OM, L'Hermite-Baleriaux M, Turek FW, Van Cauter E. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol. 2000;278:R373–R382. doi: 10.1152/ajpregu.2000.278.2.R373. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Soldani R, Laughlin GA, Yen SSC. Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J Appl Physiol. 1996;80:25–29. doi: 10.1152/jappl.1996.80.1.25. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18:513–523. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. Predicting melatonin onset phase in adolescents on summer and school schedules. Sleep. 2004;27:A78. doi: 10.1093/sleep/29.12.1632. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Carrier J. Daytime sleep propensity after moderate circadian phase shifts induced with bright light exposure. Sleep. 1997;20:11–17. doi: 10.1093/sleep/20.1.11. [DOI] [PubMed] [Google Scholar]
- Eastman CI. High intensity light for circadian adaptation to a 12-h shift of the sleep schedule. Am J Physiol. 1992;263:R428–R436. doi: 10.1152/ajpregu.1992.263.2.R428. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Gazda CJ, Burgess HJ, Crowley SJ, Fogg LF. Advancing circadian rhythms before eastward flight: a strategy to prevent or reduce jet lag. Sleep. 2005;28:33–44. doi: 10.1093/sleep/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griefahn B. The validity of the temporal parameters of the daily rhythm of melatonin levels as an indicator of morningness. Chronobiol Int. 2002;19:561–577. doi: 10.1081/cbi-120004226. [DOI] [PubMed] [Google Scholar]
- Hebert M, Dumont M, Paquet J. Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol Int. 1998;15:59–70. doi: 10.3109/07420529808998670. [DOI] [PubMed] [Google Scholar]
- Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Hume KI, Van F, Watson A. A field study of age and gender differences in habitual adult sleep. J Sleep Res. 1998;7:85–94. doi: 10.1046/j.1365-2869.1998.00103.x. [DOI] [PubMed] [Google Scholar]
- Kattapong KR, Fogg LF, Eastman CI. Effect of sex, menstrual cycle phase and oral contraceptive use on circadian temperature rhythms. Chronobiol Int. 1995;12:257–266. [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker of circadian phase position. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Liu X, Uchiyama M, Shibui K, Kim K, Kudo Y, Tagaya H, Suzuki H, Okawa M. Diurnal preference, sleep habits, circadian sleep propensity and melatonin rhythm in healthy human subjects. Neurosci Lett. 2000;280:199–202. doi: 10.1016/s0304-3940(00)00793-x. [DOI] [PubMed] [Google Scholar]
- Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. J Biol Rhythms. 2004;19:248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Rose LR, Hall JA, Kupfer DJ. The sleep of healthy people – a diary study. Chronobiol Int. 2000;17:49–60. doi: 10.1081/cbi-100101031. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–139. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Parry BL, Berga SL, Mostofi N, Klauber MR, Resnick A. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms. 1997;12:47–64. doi: 10.1177/074873049701200107. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker Structure: a clock for all seasons. J Comp Physiol. 1976;106:333–355. [Google Scholar]
- Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol. 2002;282:R454–R463. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasto DL, Colligan MJ, Skjei EW, Polly SJ. Health Consequences of Shift Work. 1978 NIOSH Publication No. 78–154, Cincinnati. [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Aeschbach D, Duncan WC. Evidence for a biological dawn and dusk in the human circadian timing system. J Physiol. 2001a;535:937–951. doi: 10.1111/j.1469-7793.2001.t01-1-00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA, Duncan WC, Sher L, Aeschbach D, Schwartz PJ, Turner EH, Postolache TT, Rosenthal NE. A circadian signal of change of season in patients with seasonal affective disorder. Arch Gen Psychiatry. 2001b;58:1108–1114. doi: 10.1001/archpsyc.58.12.1108. [DOI] [PubMed] [Google Scholar]
- Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51:1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]