Abstract
Night shift work and rapid transmeridian travel result in a misalignment between circadian rhythms and the new times for sleep, wake, and work, which has health and safety implications for both the individual involved and the general public. Entrainment to the new sleep/wake schedule requires circadian rhythms to be phase-shifted, but this is often slow or impeded. The authors show superimposed light and melatonin PRCs to explain how to appropriately time these zeitgebers to promote circadian adaptation. They review studies in which bright light and melatonin were administered to try to counteract jet lag or to produce circadian adaptation to night work. They demonstrate how jet lag could be prevented entirely if rhythms are shifted before the flight using their preflight plan and discuss the combination of interventions that they now recommend for night shift workers.
Keywords: human, circadian rhythms, shift work, jet lag, bright light, melatonin, phase response curve, phase shifts
The invention of artificial light has led to a constant drive toward a 24-h society with work at all hours of the day and night. In addition, the development of jet travel has meant that an increasing number of people move rapidly between time zones. Both situations produce a misalignment between circadian rhythms and the new sleep/wake schedule, resulting in a constellation of symptoms such as sleepiness, decreased alertness, impaired performance, difficulty initiating and maintaining sleep, irritability, and gastrointestinal distress (Boulos et al., 1995; Eastman et al., 1995a). Functioning with reduced alertness affects not only the individuals themselves but also the safety of the general public, particularly for workers who can make high-risk mistakes, such as pilots, health care workers, and nuclear power plant operators. The long-term health implications of shift work and, in particular, jet lag are not widely recognized. Frequent jet travel is associated with menstrual disturbance (Iglesias et al., 1980), temporal lobe atrophy, and cognitive defects (Cho, 2001). In addition, consuming meals at an unusual circadian phase, after transmeridian travel and during the night shift, can result in inappropriate metabolic responses that are risk factors for cardiovascular disease and type II diabetes mellitus (Hampton et al., 1996). Indeed, night shift workers have an increased prevalence of both ulcers and heart disease compared to day shift workers (Knutsson, 2003; Drake et al., 2004). There is also an increased risk of cancer associated with prolonged exposure to night shift work (Schernhammer et al., 2001) and jet travel (Reynolds et al., 2002). Both jet lag and shift work are highly prevalent in industrialized countries. In the United States alone, there are more than 15 million people flying overseas each year, to destinations other than the American continents (U.S. Department of Commerce, 2003), and 5.8 million people working full-time night or rotating shifts (Beers, 2000). Thus, there is a clear need to try to reduce the occurrence and duration of circadian misalignment.
The symptoms of jet lag dissipate as the circadian clock becomes realigned with the new light/dark (LD) cycle, but this can take several days, and in some cases the clock can get stuck in the old phase position for several days (Takahashi et al., 1999). Exposure to bright light at the wrong time can keep circadian rhythms from phase-shifting at all, despite a shifted sleep/dark period (which creates a shifted LD cycle) (Mitchell et al., 1997). Nevertheless, complete entrainment can easily occur following transmeridian travel with exposure to the zeitgebers of the new time zone. In contrast, entrainment to night shift work is rare due to exposure to the conflicting natural 24-h zeitgebers, which is especially pronounced on days off. Thus, many experts have given up on circadian adaptation (defined as phase-shifting circadian rhythms to align with the new sleep schedule), which requires permanent night shifts or slow rotations, and recommend rapid rotations instead.
Many people use stimulants and hypnotics in an attempt to maximize alertness and facilitate sleep at the desired times. Modafinil promotes wakefulness, but it cannot eliminate the intense sleepiness and performance decrements during the night. For example, the Multiple Sleep Latency Test (MSLT) showed that shift workers given 200 mg modafinil stayed awake longer than they did when given a placebo, but they still fell asleep almost instantly during the night shift (in 1.24 min after 4 weeks of treatment) (Czeisler et al., 2003). The Maintenance of Wakefulness Test (MWT) also showed that modafinil increased the time awake, but there was still the usual decline over the course of the night shift and little difference between modafinil and placebo at the end of most night shifts (Walsh et al., 2004). Hypnotics before daytime sleep can maintain baseline nighttime sleep duration but do not eliminate the increase in sleepiness across the night (e.g., Schweitzer et al., 1991). Although some people are phase tolerant (Dawson and Campbell, 1991) and have the capacity to sleep at the “wrong” time, even without sleeping pills, there is no evidence that they are phase tolerant for being awake at the wrong time (cf. Frey et al., 2004). Therefore, we think that the best solution for both shift work and jet travel is to phase-shift circadian rhythms to entrain to the new sleep/wake schedule. We believe that small, repeated shifts of the circadian clock, especially if the sleep/wake schedule is simultaneously shifted to remain in alignment (cf. Stewart et al., 1995; Burgess et al., 2003), will be healthier in the long run than sleeping, eating, working, and staying awake at the wrong circadian phase. Long-term studies comparing these alternatives may eventually become feasible.
Light and Melatonin Phase Response Curves
In humans, as in other animals, both light and exogenous melatonin can shift the circadian clock according to a PRC. The most frequently used markers of the phase of the clock are the body temperature minimum (Tmin), the dim light melatonin onset (DLMO), and the midpoint of the melatonin profile. The interval between the DLMO and the Tmin is usually about 6 to 8 h. Here we present a new, although incomplete, bright-light PRC, which shows what happens when 2-h bright-light pulses are applied to free-running subjects (Fig. 1). Bright light in the evening and beginning of the sleep period produced phase delays, whereas bright light near the end of sleep and in the morning caused phase advances. We expect that, as in many animal PRCs, there will be a dead zone during the day when light does not shift the clock. Some other human light PRCs were generated in reentrainment protocols and do not have dead zones (e.g., Jewett et al., 1997). But in these cases, when the bright light pulse occurred during the day, the sleep/dark period was shifted slightly, thus allowing dim light to fall closer to larger amplitude portions of the PRC than usual. We believe this is enough to produce the small phase shifts in the middle of the day. In fact, Dumont and Carrier (1997) convincingly demonstrated an afternoon dead zone to 5-h bright-light pulses when the sleep/dark period is not shifted. Some PRCs to light in humans have the crossover point from delays to advances at the Tmin (e.g., Czeisler et al., 1989), whereas in Figure 1 and other PRCs, it occurs slightly after the Tmin (summarized in Mitchell et al., 1997). The 0.5 mg melatonin PRC shows that maximum phase advances occur in the afternoon and maximum delays occur during sleep. The sleep rectangle in the graph is from our data but can still be used for orientation to the melatonin PRC, because our subjects went to bed about 3 h after their DLMO, which is typical (e.g., Martin and Eastman, 2002). However, we required a rather long baseline sleep schedule, so the sleep rectangle for most people should start at about the same time but end earlier.
Figure 1.
The light PRC was generated from 7 subjects who free-ran through about 3 days (73.5h) of an ultradian LD cycle (2.5 h wake in dim light < 100 lux alternating with 1.5 h sleep in dark) (Eastman and Burgess, unpublished data). Subjects lived on the ultradian schedule on 2 different occasions, once with bright light pulses, about 3500 lux, for 2 h at the same time each day, and once without bright light pulses, counterbalanced. Phase shifts of the midpoint of the melatonin rhythm collected in dim light (<5 lux) before and after the 3 days were plotted against the time of the light pulse relative to each subject's baseline dim light melatonin onset (DLMO) and corrected for the free run when the bright light was not applied. Upward arrow: average baseline DLMO, rectangle: average baseline sleep schedule, triangle: estimated time of body temperature minimum (DLMO+7 h). The solid line is a smoothed curve fit to the 7 points. The melatonin PRC was calculated from the data of Lewy et al. (1998). Subjects (n = 6), living at home, took 0.5 mg melatonin at the same time each day for 4 days. Phase shifts of the DLMO were plotted against the time of melatonin administration relative to each subject's baseline DLMO. A smoothed curve was fit to the data after averaging the 70 data points into 3-h bins.
The phase shifts to melatonin (left y-axis) are smaller than to bright light (right y-axis), but this does not mean that melatonin is a weaker phase shifter. For our light PRC, subjects were free running, whereas the melatonin PRC subjects slept at home at normal times, and thus the magnitude of phase shifts was limited by the entraining LD cycle. Furthermore, we generated a partial PRC to 3.0 mg melatonin using the same ultradian protocol (unpublished data), which showed that melatonin produced phase advances of equal magnitude to light. Many researchers have jumped to the conclusion that melatonin is a weaker zeitgeber than light, which comes from an unreasonable comparison of light PRCs in which the sleep/dark period was shifted by several hours (e.g., Czeisler et al., 1989) with the melatonin PRC in which sleep was not shifted (Lewy et al., 1998). Furthermore, simulated night shift protocols that are similar to each other and in which melatonin or bright light are used to accelerate reentrainment show only a small difference in phase shift magnitude (Burgess et al., 2002, Table 1).
The amplitude of a light PRC depends not only on the protocol used for its generation but also on the stimulus, with higher light intensities and longer durations producing larger phase shifts, up to a certain limit. We predict that the magnitude of a phase shift will also depend on the light intensities experienced in the days or weeks before the light pulse, because we have shown that the magnitude of light-induced melatonin suppression depends on the individual's light history during the previous week (Hébert et al., 2002). Thus, we expect that the saturating light intensity for phase shift magnitude, that is, the upper limit, in people normally exposed to sunlight should be much higher than the 500 to 1000 lux limit observed in subjects kept indoors in dim light for several days before the phase-shifting light pulse was applied (Zeitzer et al., 2000). Data are not available to compare PRCs from different doses of melatonin, and it is possible that not only the amplitude but also the time of the maximums and crossover points may vary depending on how long different doses remain in circulation.
Jet Lag
The duration and severity of jet lag depend on the number of time zones crossed, and hence the number of hours that the clock needs to be phase-shifted, as well as the direction of travel. The human circadian clock has an endogenous period slightly longer than 24 h (Wever, 1979; Czeisler et al., 1999), which is at least partly why it is easier to phase-delay human circadian rhythms than it is to phase-advance them. Regardless of the reason, human rhythms advance more slowly than they delay, and reentrainment is often incomplete after advances (Mitchell et al., 1997; Eastman and Martin, 1999). Consequently, jet lag is typically worse after flying east, as this requires the clock to phase-advance, compared to flying west, which requires phase delays. A further problem with eastward travel is the risk of antidromic reentrainment, phase-shifting in the wrong direction, which can prolong the symptoms of jet lag (Boulos et al., 1995). Phase delays instead of advances often occur following an eastward flight and are probably due to the light exposure experienced upon landing in the destination time zone, which, due to the time of the flight, will often fall on the phase-delay portion of the PRC. However, for eastbound travel over a large number of time zones, it may be better to entrain by delays rather than advances, because human rhythms delay faster than they advance.
Attempts to minimize jet lag have focused on techniques to accelerate reentrainment after arrival in the new time zone (based on the light and melatonin PRCs), such as appropriately timed light exposure/avoidance (e.g., Houpt et al., 1996) or melatonin administration (e.g., Arendt et al., 1997). There have been relatively few studies of shaping light exposure after a flight, probably because using light boxes or exposing/avoiding outdoor light at specific times may not always fit in with the traveler's plans. However, one study used a light visor after a 6-h westward flight from Zurich to New York (Boulos et al., 2002). The visor delivered either bright white light (3000 lux) or dim red light (10 lux) for 3 h and was timed to phase-delay. The rhythms delayed more after 2 nights with the bright than the dim light (2.6 vs. 1.6 h), but the full phase shift of 6 h was not achieved, and there were no differences between the groups in jet lag. So, despite the application of bright light, the rhythms delayed slowly, 1.3 h/day.
There have been more studies of melatonin for jet lag. Arendt et al. (1997) recommended taking 5 mg melatonin at local bedtime for 4 days after the flight. A review of 10 years of studies showed an overall 50% reduction in subjective assessment of jet lag symptoms with melatonin (n = 474) compared to placebo (n = 126) (Arendt et al., 1997). However, the effectiveness of the Arendt regime for facilitating phase shifts should depend on the flight direction and the number of time zones crossed. For 6 time zones east, bedtime in the new time zone is 6 h earlier, and thus taking melatonin at that time coincides with the maximum phase advance on the melatonin PRC (Fig. 1). However, as the number of time zones crossed changes, the melatonin would be administered at less optimal times according to the melatonin PRC.
Improper timing of melatonin might explain why there was no improvement in jet lag for 31 subjects flying 10 time zones east who followed the Arendt regime (Edwards et al., 2000). Better results were seen after 8-h eastward flights from Tokyo to Los Angeles. Takahashi et al. (1999, 2000) studied 6 subjects who took the trip twice and took 3 mg melatonin at bedtime for3 days after one of the flights. All subjects exhibited entrainment by phase-advancing when taking melatonin, but without melatonin, 1 subject entrained in the wrong direction and another did not shift at all, even after 5 days in Los Angeles. For the 4 subjects who phase-advanced on both trips, melatonin increased the phase shift (from about 1.1 to 1.4 h/day) so that after 5 days entrainment was almost complete with melatonin (about 7 of the 8 h) but somewhat less after placebo (about 5.5 h). In another study, subjects (n = 257, mostly physicians) flew from Oslo to New York, where they attended an educational program for 5 days and then returned home, 6 time zones east (Spitzer et al., 1999). They began their treatment on the day of travel home and continued it for 5 days after the flight (either 5.0 mg at bedtime, 0.5 mg at bedtime, 0.5 mg in a moving pattern, or placebo). There were no differences in jet lag symptoms among the groups. Melatonin was timed to coincide with the phase-advance portion of the PRC according to New York time. However, the subjects probably spent most of their time indoors, and it is possible that they had not fully shifted so that the melatonin was not administered at the optimal time.
Aside from the complications of the timing of melatonin and light in the new time zone, a major problem with only attempting interventions upon arrival is that the traveler will suffer from jet lag until the misalignment is reduced. Thus, there will be a number of “wasted” days with poor sleep, reduced productivity, and negative impacts on health and safety. Many people resort to stimulants and hypnotics to ease symptoms of jet lag while their rhythms slowly entrain to the new time zone, but as discussed earlier, these can only partially alleviate the symptoms.
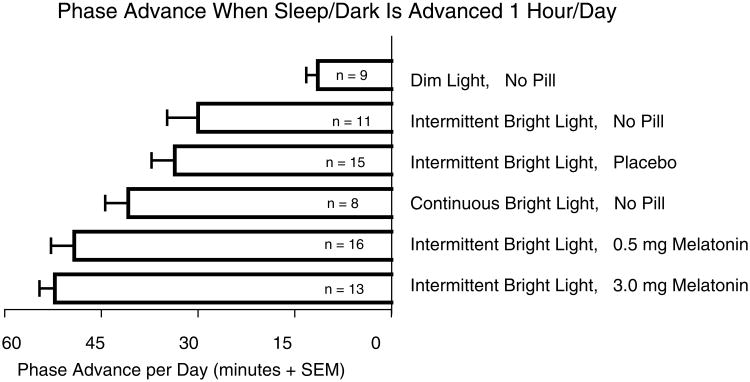
We proposed shifting the circadian clock to the destination time zone before the flight (Burgess et al., 2003; Eastman et al., 2005; Revell et al., 2005). A preflight schedule for a flight 6 time zones east (e.g., from Chicago to London) is shown in Figure 2. In this diagram, the misalignment that produces jet lag is completely eliminated, but even if the clock is only partially phase-advanced (because of fewer preflight days of treatment), there will be a reduction of jet lag. Furthermore, postflight light exposure is more likely to occur on the advance portion of the PRC, thus enhancing the rate of adaptation and reducing the risk of antidromic reentrainment. We tested this type of schedule in our lab by measuring the DLMO before and after3 days of the schedule (e.g., days –4 to –2 in Fig. 2). Our goal was to produce the maximal possible daily advance in circadian rhythms, to keep up with the advance in the sleep schedule, so that we do not produce circadian misalignment, and thus jet lag–type symptoms, before the flight. Figure 3 shows that we produced an advance of almost 1 h/day with afternoon melatonin and morning intermittent bright light. Most of the groups were tested with intermittent bright light because this is more practical for everyday use in the field, as it can easily be fitted around daily activities, and because the phase advances with continuous and intermittent bright light (in the no pill conditions) did not significantly differ from each other (Burgess et al., 2003). Although the 3.0 mg dose produced a slightly larger phase advance than the 0.5 mg dose (Fig. 3), this was not a statistically significant difference. In addition, the Stanford Sleepiness Scale administered every 30 min from the time of pill administration until bedtime showed that the ratings after 0.5 mg were identical to those after placebo, whereas there was slightly more (although not significantly more) sleepiness after the 3.0 mg dose. Therefore, we recommend the 0.5 mg dose (Fig. 2).
Figure 2.
Preflight schedule to advance circadian rhythms before an eastward flight across 6 time zones. Day –5 shows the sleep schedule and Tmin (body temperature minimum) of a typical young person. On day –4, 0.5 mg melatonin is taken 5 h before baseline bedtime and intermittent bright light pulses are used upon wakening, both timed to phase-advance. Each day the schedule is advanced by 1 h, to keep up with the expected phase advance in the clock, shown by the Tmin, and to ensure that the light and melatonin continue to be administered at optimal times to achieve the maximal phase advance. On the 1st night after the flight, the Tmin would already be advanced to within the sleep period. The flight, as is the case with most flights to Europe from the United States, departs in the afternoon and arrives in the morning. As the clock would already be phase-advanced, all the light exposure upon arrival will occur after the Tmin, thus producing phase advances and completing reentrainment.
Figure 3.
Phase shifts in the dim light melatonin onset (DLMO) during 3 days of a preflight schedule similar to Figure 2. For 3.5 h after waking each morning, subjects were either kept in ordinary dim room light (<60 lux) or intermittent bright light (30 min ∼5000 lux alternating with 30 min <60 lux) or continuous bright light (∼5000 lux). Some groups received melatonin or placebo in the afternoon. The 0.5 mg dose of melatonin was administered 5 h before baseline bedtime, timed to coincide with the maximum phase advance of the 0.5 mg melatonin PRC (see Fig. 1). The 3.0 mg was administered 7 h before baseline bedtime because our partial PRC (unpublished data) showed that this is when the maximal phase advances occur with 3.0 mg. Data are from Burgess et al. (2003) and Revell et al. (2005).
Although more research needs to be done to perfect the light pattern, the schedule shown in Figure 2 could already be used by real travelers. Some people might think that it requires a lot of time and effort and that it would disrupt the traveler's routine before the flight. However, we believe that it merely requires a rearrangement of time and that the possible loss of a few hours of socializing in the evenings before the flight should be balanced against the benefit of no jet lag, or substantially reduced jet lag, after the flight. The traveler would be getting up early each day and so could begin work and be alert and productive at this early hour, as his or her clock will have advanced. Intermittent light can be fitted around typical morning activities such as showering, dressing, and eating breakfast. In addition, the traveler could also work in front of the light box, and thus no time is lost. To plan your own preflight schedule for flying east, draw in your home and destination time lines as in Figure 2. For flights from the United States, see Figure 6 in Eastman et al. (2005) because the main European and all 4 U.S. time zones are shown. For flying west, we suggest delaying the sleep schedule by 2 h/day and using bright light before bed each night (cf. Eastman and Miescke, 1990). Also consider using a 2-h delaying schedule before flying east across 8 or more time zones, because shifting to the new time zone will take the same or fewer days than witha1-hadvancing schedule. Obviously, a delaying schedule is difficult for people who are not free to schedule their own work hours. Nevertheless, the more circadian rhythms are delayed before the flight, the less adjustment is needed afterward.
Future work should determine the most effective pattern of intermittent light. In addition, the human circadian system is short wavelength (blue light) sensitive, and thus it should be possible to reduce the intensity of the light treatment, while retaining phase-shifting efficacy, if the light contains more power at short wavelengths. Indeed, it has already been shown that dim blue light (∼8 lux) is as effective as bright white light (∼12,000 lux) at inducing phase advances (Warman et al., 2003). As many people find small, bright, white light boxes aversive, this would clearly be beneficial. Future work should also test these interventions in real travelers to determine their effectiveness in the field.
Shift Work: Light and Dark
Most night shift workers go to sleep after the night shift (rather than before), so circadian adaptation to night work requires a phase delay of the circadian clock. Eastman and Martin (1999) proposed that a reasonable goal is to delay the Tmin, the time of maximum sleepiness, at least into daytime sleep, and thus away from the night shift, to improve sleep and nocturnal alertness. Subsequent work with young adults showed that delaying the Tmin into either the 1st or 2nd half of daytime sleep produced similar improvements in both alertness and performance during the night shift (Crowley et al., 2004). However, although middle-aged and older workers can be delayed as much with bright light, some of them may require a greater realignment of circadian rhythms with sleep because of reduced phase tolerance (e.g., Campbell, 1995; Dijk et al., 1999; Klerman et al., 2001). Circadian rhythms would naturally delay to entrain to the shifted LD cycle produced by sleeping during the day and working at night if the schedule produced a clear and maintained shift in the LD cycle. We believe that the best strategy for facilitating a delay is to shape and strengthen the shifted LD cycle by making the daytime sleep period as dark as possible, increasing the intensity of light during the wake period, while also making sure that bright artificial or outdoor light does not fall on the advance portion of the light PRC. In addition, bright light pulses during the night shift can facilitate entrainment, but they should coincide primarily with the delay portion of the light PRC and not overlap too much with the advance portion (cf. Eastman and Martin, 1999, Fig. 2).
In a series of simulated night shift studies, we shifted the LD cycle by shifting the sleep period and covering the bedroom windows of subjects with black plastic to make it “darkroom dark.” In one study (Mitchell et al., 1997), we advanced or delayed the sleep/dark period by 9 h. When daily 3-h bright light pulses (∼5000 lux) during the night shift were timed to coincide with the appropriate portion of the light PRC, phase shifts were facilitated, but when they coincided with the opposite portion of the PRC, in many cases phase shifts were prevented. This study showed that the shift of the sleep/dark period and bright light during the night shift could exert equal and opposite forces, which in effect canceled each other out. Thus, when trying to phase-shift circadian rhythms, the shift of the sleep/dark period is as important as the bright light.
In some studies, we gave our subjects dark sunglasses to wear outside during the day. Sunglasses are especially important for the commute home from the night shift because bright outdoor morning light can occur during the phase-advance portion of the PRC (Fig.1) and can keep the circadian clock from delaying (Eastman et al., 1994). Over the years, we used a variety of sunglasses with top and side shields and with lenses ranging from very dark welder's lenses with 0.35% light transmission to normal sunglass lenses with 15% transmission. Although we showed that very dark lenses (about 2% transmission) were better for facilitating phase delays than lighter lenses (15% transmission) (Crowley et al., 2003), those dark lenses can impair traffic signal recognition. We are currently using new “espresso” lenses (Uvex) with about 12% to 15% transmission because they are approved for driving and in addition are “blue-blockers,” transmitting less of the short-wavelength light to which the circadian system is most sensitive.
We have also tested a variety of patterns and intensities of bright light designed to coincide with the phase-delay portion of the light PRC. We designed “moving” or “nudging” patterns, which occur later and later on successive days (Eastman, 1992; Mitchell et al., 1997; Martin and Eastman, 1998; Crowley et al., 2003). One advantage of moving patterns is that they keep up with the delaying clock and its PRC so that the bright light continues to be applied near the optimal circadian phase. Furthermore, these patterns can simultaneously benefit a group of people with a range of baseline circadian phases; the bright light can start far enough away from the light PRC crossover points of all the people and gradually move closer to avoid the problem of too much falling on the wrong side of the crossover point. As the light moves later and later, it will eventually delay even the individuals with later circadian phases while continuing to delay the earlier people. We used moving bright-light patterns at NASA to adjust the circadian clocks of astronauts and ground crew to the night shift before space shuttle launches (Stewart et al., 1995; Stewart and Eastman, 1996).
For bright light to be used during the night shift of real workers, it must fit in with the work being carried out. Long durations may not always be possible or practical, particularly if sitting in front of bright-light boxes is required. Intermittent exposures may be most practical, as workers could periodically move near the light sources as their work allows. We initially used 6-h exposures of ∼5000 lux (Eastman, 1992; Eastman et al., 1994) but reduced this to 3 h (Mitchell et al., 1997; Martin and Eastman, 1998) when we found that this shorter duration light, when appropriately timed, was almost as effective (Eastman et al., 1995b). We subsequently tested intermittent bright-light pulses (once/h) of 40 min (Baehr et al., 1999) and most recently have used 20-min pulses (once/h) in a moving pattern (1 h later/day), which, when combined with only normal sunglass lenses for the commute home, proved to be a powerful phase delayer (Crowley et al., 2003). In addition, we demonstrated that medium intensity night shift light (∼1200 lux) was almost as effective as bright light (∼6000 lux) in producing phase shifts (Martin and Eastman, 1998). Thus, bright light does not need to be of long duration or high intensity to have a beneficial effect on circadian adaptation to the night shift. It is possible that the intensity of the light might be further reduced, while retaining its phase-shifting efficacy, if the light is enhanced with short-wavelength, blue light to which the circadian system is most sensitive (e.g., Warman et al., 2003).
Because of family demands, some workers do not go to bed as soon as possible after the night shift; instead they may go shopping, take their children to school, or perform other household tasks. They do not realize that going to sleep at the earliest possible circadian phase would allow them to get the most sleep and thus, in the long run, better cope and spend more quality time with their family. Postponing the daytime sleep episode will not only force sleep to occur at an even more inappropriate circadian phase but will also result in increased exposure to phase-advancing light, which will inhibit phase delays. In our studies, we specified the time from the end of the night shift until bed, which, depending on the study, ranged from 1 to 2 h, because we thought this would be reasonable for real workers. In a simulated night shift study, Horowitz et al. (2001) compared a similar interval (night shifts from 2300 to 0700 h, sleep from 0800 to 1600 h (±30 min) = fixed sleep) to letting subjects go to bed whenever they wanted (free sleep). In addition, subjects were either exposed to bright light (∼2500 lux) for the 1st 6 h of the shift or remained in dim room light (∼150 lux). Thus, there were 4 groups of subjects: Bright Fixed, Bright Free, Room Fixed, and Room Free. After 3 night shifts and day sleeps, about half of the subjects in the Bright Fixed group had delayed enough to move the midpoint of their melatonin profile into daytime sleep. Very few subjects in the other groups phase-delayed this much. Thus, even if a lot of bright light is administered during the night shift, circadian adaptation will be impeded if an early, regular, dark sleep period is not required.
In our latest simulated night shift study (Crowley et al., 2003), we tested various combinations of interventions: 1) a fixed, dark early period for sleep after the night shift; 2) a moving pattern of four to five 20-min bright-light pulses (once/h) during the night shift; 3) sunglasses (2% or 15% transmission) worn outside during daylight; and 4) morning melatonin before day sleep. Two important points about the design were that during baseline subjects were free to sleep when they chose so that they would begin the night shifts with a range of phase positions to simulate the situation in real night shift workers. Second, the study was run during the summers, so the commute home always occurred in bright daylight. Figure 4 shows the time of the Tmin for all the subjects before (baseline) and after (final) 5 night shifts. We estimated that the later subjects had their Tmins within daytime sleep on practically all 5 days (see diagonal lines in Fig. 4). In contrast, the earlier subjects achieved this goal much less often. Thus, the most influential factor in determining whether subjects achieved circadian adaptation to night work was baseline phase position.
Figure 4.
Circadian phase during a simulated night shift study in which subjects worked 5 consecutive night shifts (2300 to 0700 h), slept at home in the dark (0830 to 1530 h), and were exposed to various other interventions, such as bright light during the night shifts, to enhance phase delays. The dim light melatonin onset (DLMO) for each subject was determined before (baseline) and after the night shift, and the body temperature minimums (Tmins) (triangles) were estimated by adding 7 h to the DLMO. Earlier subjects (baseline Tmin ≤ 0700 h) showed a range of phase delays depending on the intervention. Later subjects (baseline Tmin > 0700 h) phase-delayed their Tmins into the daytime sleep period regardless of the intervention combination. Data are from Crowley et al. (2003).
We categorized the degree of reentrainment of each subject according to when their final Tmin occurred relative to the previous daytime sleep period: not reentrained (Tmin before sleep), partially reentrained (Tmin in the 1st half of sleep), and completely reentrained (Tmin in the 2nd half of sleep). All the later subjects exhibited complete reentrainment, even those who were only exposed to dim light during the nightshift. For the later subjects, light during the commute home at 0700 h actually occurred before or around the Tmin, which according to our light PRC (Fig. 1) is in the delay portion. Thus, we predict that people about to start night work who have Tmins after the end of their company's night shift only need to have a regular, fixed, early daytime dark period and wear normal sunglasses for maximal circadian adaptation. The Tmin can be estimated from wakeup time in people who are free to sleep naturally; it occurs ∼ 3 to 4 h earlier (Baehr et al., 2000; Martin and Eastman, 2002).
For the earlier subjects, that is, their Tmins were before the travel home time (like the subjects in our previous studies), the degree of reentrainment depended on the interventions. All but 1 of the 12 earlier subjects who received bright light during the night shift were completely reentrained. This demonstrates that all that is usually needed to achieve complete circadian adaptation, even if you have an earlier phase position, is bright light during the night shift, normal sunglasses on the commute home, and, of course, a fixed, dark period for sleep. Earlier subjects who were kept in ordinary room light during the night shift and wore normal sunglasses did not usually reentrain (70% of these subjects did not get their Tmins into sleep). However, simply wearing very dark glasses (2% transmission) on the commute home was beneficial: 90% of the earlier subjects who did not receive bright light but who wore the dark sunglasses got their Tmins into sleep. Thus, if there is no bright light during the night shift, it is still possible to adapt if workers wear very dark sunglasses on the commute home and adhere to the daytime dark schedule. However, the dark lenses in this study are not good for driving and therefore would not be suitable for all workers. Thus, the most reliable way to achieve circadian adaptation for all workers, regardless of circadian phase before night work, is to use bright light during the night shift. However, we believe that very bright light is not necessary as long as the light during the night shift is more intense than any light received during the commute home or before daytime sleep (Eastman et al., 1994), because the circadian system distinguishes night from day by comparing relative rather than absolute light intensities (e.g., Meyer and Millam, 1991). The importance of the entire 24-h LD pattern to adaptation was demonstrated in permanent night nurses (Dumont et al., 2001). The majority (22/30) of these nurses were nonshifters, and their light exposure pattern was like that of day workers, with more light during the daytime than at night. In contrast, the nurses that exhibited phase advances or delays had a reversed pattern, with the darkest part of the LD cycle occurring during the day.
Although simulated night shift studies are important in the development of interventions, ultimately it is important to test these techniques in actual shift workers to establish their effectiveness and practicality. In a study of night nurses (Boivin and James, 2002), 1 group was exposed to intermittent bright light (∼2000 lux) for the 1st 6 h of the night shift provided by light boxes in the nursing station and wore sunglasses (15% transmission) on the morning commute home. A 2nd group worked in their habitual light environments and did not wear sunglasses. Both groups had a fixed 8-h dark period for sleep beginning 2 h after the end of the night shift. Almost all the subjects in the bright light, sunglasses group achieved circadian adaptation (Tmin delayed into daytime sleep),despite intermittent days off, but only about half of the subjects in dim light, no sunglasses group achieved that goal. This study shows that a fixed, early daytime sleep period in darkened bedrooms is not always enough to achieve circadian adaptation, that bright light during the night shift is practical for use in work settings, and that circadian adaptation to the night shift can be attained even when workers are free to sleep whenever they want on days off.
Shift Work: Melatonin
Melatonin has been used in simulated and real night shift studies, both for its phase-shifting and soporific effects. In our recent study (Crowley et al., 2003), subjects took 1.8 mg melatonin or placebo before daytime sleep at 0830 h. This time of day coincides with the phase-delay portion of the melatonin PRC (Fig. 1), so we hoped the melatonin would accelerate reentrainment. We used a sustained-release formula, which we hoped would also promote sleep during the day when sleep occurred at the wrong circadian phase. However, melatonin did not increase the phase delay (Crowley et al., 2003) or have much of a soporific effect (Smith et al., 2004). In another study (Sack and Lewy, 1997), melatonin (0.5 mg) or placebo was administered at bedtime to a group of nurses and staff working 7 consecutive night shifts alternating with 7 days off. Seven of 24 subjects were termed melatonin responders; they delayed at least 3 h more with melatonin than with placebo. The others shifted as much with both or did not shift with either treatment. Thus, melatonin only helped a minority of the subjects.
In one of our simulated night shift studies (Sharkey and Eastman, 2002), subjects went to bed in the afternoon before, rather than after, the night shift. Therefore, they needed to advance their circadian rhythms for adaptation. They took 0.5 mg or 3.0 mg melatonin or placebo 30 min before daytime sleep, which was 7.5 h before baseline bedtime and thus on the phase-advance portion of the melatonin PRC (Fig. 1). Melatonin significantly increased the phase advance compared to placebo; 73% of the subjects given 3.0 mg and 56% of the subjects given 0.5 mg got their Tmins into daytime sleep compared to 0% with placebo. One possible reason why melatonin helped phase-advance rhythms in this study and not phase-delay them in the other (Crowley et al., 2003) could be that melatonin coincided with a higher amplitude portion of the phase-advance region of the melatonin PRC than it did with the delay region (Fig. 1).
We also tested melatonin in a simulated night shift study in which we kept circadian rhythms from delaying so we could study the soporific effect of melatonin separately from its phase-shifting effect (Sharkey et al., 2001). Subjects took 1.8 mg sustained-release melatonin or placebo before two 8-h daytime sleep periods. Melatonin produced a slight increase in sleep compared to placebo on the 1st, but not the 2nd, day sleep period. The reason that the effect wore off could be due to tolerance to melatonin, but more research is necessary to confirm this.
There have been a few studies of emergency medical technicians and physicians working night shifts and taking melatonin before daytime sleep, but there was little benefit from melatonin (e.g., Jockovich et al., 2000). This could be due to the short time allotted for daytime sleep, since the greatest difficulty with sleep after night work is maintaining it rather than initiating it. In addition, light exposure was not controlled and thus phase-advancing light, on the commute or in their bedrooms, could have attenuated any phase delays induced by melatonin. Indeed, the ability of melatonin to improve self-rated daytime sleep in police officers working nights (Folkard et al., 1993) could be because the night shift ended at 0600 h, perhaps reducing exposure to morning phase-advancing light, combined with 7 consecutive night shifts, which would also facilitate delays. However, without circadian phase measures, we do not know if the benefit was due to a phase delay or a soporific effect or both.
Future work should focus on one of the major problems faced by night shift workers—days off! Even “permanent” night shift work is “rotating” in that the sleep period shifts back and forth between day sleep after night work and night sleep on days off. Thus, without several consecutive night shifts and several consecutive days off, it would be difficult to fully adapt to either situation. We have proposed a compromise whereby the Tmin is delayed enough to occur during the beginning of daytime sleep after night shifts but will still occur during nighttime sleep on days off if workers maintain a relatively delayed sleep schedule. In our previous study (Crowley et al., 2004), we demonstrated that partial reentrainment(delaying the Tmin in to the beginning of day time sleep) was sufficient to enhance performance and alertness during the night shift, thus showing that a compromise phase position is sufficient. We have designed schedules that could produce and maintain such a compromise phase position (Eastman and Martin, 1999, Fig. 5; Burgess et al., 2002, Fig. 4) and are currently testing a similar schedule.
Acknowledgments
This work was supported by grants R01 NR07667 from NINR and R01 OH003954 from NIOSH and the Centers for Disease Control and Prevention. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
References
- Arendt J, Skene DJ, Middleton B, Lockley SW, Deacon S. Efficacy of melatonin treatment in jet lag, shift work, and blindness. J Biol Rhythms. 1997;12:604–617. doi: 10.1177/074873049701200616. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Fogg LF, Eastman CI. Intermittent bright light and exercise to entrain human circadian rhythms to night work. Am J Physiol. 1999;277:R1598–R1604. doi: 10.1152/ajpregu.1999.277.6.R1598. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Beers TM. Flexible schedules and shift work: replacing the 9-to-5workday? Monthly Labor Rev. 2000 Jun;:33–40. [Google Scholar]
- Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002;17:556–567. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Campbell SS, Lewy AJ, Terman M, Dijk DJ, Eastman CI. Light treatment for sleep disorders: consensus report. VII. Jet lag. J Biol Rhythms. 1995;10:167–176. doi: 10.1177/074873049501000209. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Macchi MM, Sturchler MP, Stewart KT, Brainard GC, Suhner A, Wallace G, Steffen R. Light visor treatment for jet lag after westward travel across six time zones. Aviat Space Environ Med. 2002;73:953–963. [PubMed] [Google Scholar]
- Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6:407–420. [PubMed] [Google Scholar]
- Campbell SS. Effects of times bright-light exposure on shift-work adaptation in middle-aged subjects. Sleep. 1995;18:408–416. doi: 10.1093/sleep/18.6.408. [DOI] [PubMed] [Google Scholar]
- Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nature Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18:513–523. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Complete or partial circadian re-entrainment improves performance, alertness, and mood during night shift work. Sleep. 2004;27:1077–1087. doi: 10.1093/sleep/27.6.1077. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Dinges DF, Walsh JK, Roth T, Niebler G. Modafinil for the treatment of excessive sleepiness in chronic shift work sleep disorder. Sleep. 2003;26:A114. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Dawson D, Campbell SS. Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep. 1991;14:511–516. doi: 10.1093/sleep/14.6.511. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(pt 2):611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- Dumont M, Benhaberou-Brun D, Paquet J. Profile of 24-h light exposure and circadian phase of melatonin secretion in night workers. J Biol Rhythms. 2001;16:502–511. doi: 10.1177/074873001129002178. [DOI] [PubMed] [Google Scholar]
- Dumont M, Carrier J. Daytime sleep propensity after moderate circadian phase shifts induced with bright light exposure. Sleep. 1997;20:11–17. doi: 10.1093/sleep/20.1.11. [DOI] [PubMed] [Google Scholar]
- Eastman CI. High intensity light for circadian adaptation to a 12-h shift of the sleep schedule. Am J Physiol. 1992;263:R428–R436. doi: 10.1152/ajpregu.1992.263.2.R428. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Boulos Z, Terman M, Campbell SS, Dijk DJ, Lewy AJ. Light treatment for sleep disorders: consensus report. VI. Shift work. J Biol Rhythms. 1995a;10:157–164. doi: 10.1177/074873049501000208. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Gazda CJ, Burgess HJ, Crowley SJ, Fogg LF. Advancing circadian rhythms before eastward flight: a strategy to prevent or reduce jet lag. Sleep. 2005;28:33–44. doi: 10.1093/sleep/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Liu L, Fogg LF. Circadian rhythm adaptation to simulated night shift work: effect of nocturnal bright-light duration. Sleep. 1995b;18:399–407. doi: 10.1093/sleep/18.6.399. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 1999;31:87–98. doi: 10.3109/07853899908998783. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Miescke KJ. Entrainment of circadian rhythms with 26-hr bright light and sleep-wake schedules. Am J Physiol. 1990;259:R1189–R1197. doi: 10.1152/ajpregu.1990.259.6.R1189. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Stewart KT, Mahoney MP, Liu L, Fogg LF. Dark goggles and bright light improve circadian rhythm adaptation to night-shift work. Sleep. 1994;17:535–543. doi: 10.1093/sleep/17.6.535. [DOI] [PubMed] [Google Scholar]
- Edwards BJ, Atkinson G, Waterhouse J, Reilly T, Godfrey R, Budgett R. Use of melatonin in recovery from jet-lag following an eastward flight across 10 time-zones. Ergonomics. 2000;43:1501–1513. doi: 10.1080/001401300750003934. [DOI] [PubMed] [Google Scholar]
- Folkard S, Arendt J, Clark M. Can melatonin improve shift workers' tolerance of the night shift? Some preliminary findings. Chronobiol Int. 1993;10:315–320. doi: 10.3109/07420529309064485. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Badia P, Wright KP. Inter- and intra- individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004;13:305–315. doi: 10.1111/j.1365-2869.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- Hampton SM, Morgan LM, Lawrence N, Anastasiadou T, Norris F, Deacon S, Ribeiro D, Arendt J. Postprandial hormone and metabolic responses in simulated shift work. J Endocrinol. 1996;151:259–267. doi: 10.1677/joe.0.1510259. [DOI] [PubMed] [Google Scholar]
- Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Efficacy of bright light and sleep/darkness scheduling in alleviating circadian maladaptation to night work. Am J Physiol Endocrinol Metab. 2001;281:E384–E391. doi: 10.1152/ajpendo.2001.281.2.E384. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Boulos Z, Moore-Ede MC. Midnightsun: software for determining light exposure and phase-shifting schedules during global travel. Physiol Behav. 1996;59:561–568. doi: 10.1016/0031-9384(95)02111-6. [DOI] [PubMed] [Google Scholar]
- Iglesias R, Terres A, Chavarria A. Disorders of the menstrual cycle in airline stewardesses. Aviat Space Environ Med. 1980;51:518–520. [PubMed] [Google Scholar]
- Jewett ME, Rimmer DW, Duffy JF, Klerman EB, Kronauer RE, Czeisler CA. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol. 1997;273:R1800–R1809. doi: 10.1152/ajpregu.1997.273.5.r1800. [DOI] [PubMed] [Google Scholar]
- Jockovich M, Cosentino D, Cosentino L, Wears RL, Seaberg DC. Effect of exogenous melatonin on mood and sleep efficiency in emergency medicine residents working night shifts. Acad Emerg Med. 2000;7:955–958. doi: 10.1111/j.1553-2712.2000.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Duffy JF, Dijk DJ, Czeisler CA. Circadian phase resetting in older people by ocular bright light exposure. J Invest Med. 2001;49:30–40. doi: 10.2310/6650.2001.34088. [DOI] [PubMed] [Google Scholar]
- Knutsson A. Health disorders of shift workers. Occup Med. 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- Martin SK, Eastman CI. Medium-intensity light produces circadian rhythm adaptation to simulated night-shift work. Sleep. 1998;21:154–165. [PubMed] [Google Scholar]
- Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- Meyer WE, Millam JR. Plasma melatonin levels in Japanese quail exposed to dim light are determined by subjective interpretation of day and night, not light intensity. Gen Comp Endocrinol. 1991;82:377–385. doi: 10.1016/0016-6480(91)90313-u. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Hoese EK, Liu L, Fogg LF, Eastman CI. Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms. 1997;12:5–15. doi: 10.1177/074873049701200103. [DOI] [PubMed] [Google Scholar]
- Revell VL, Burgess HJ, Gazda CJ, Eastman CI. Afternoon melatonin and morning intermittent bright light can help you prepare for eastward jet travel. Sleep. 2005;28:A55. [Google Scholar]
- Reynolds P, Cone J, Layefsky M, Goldberg DE, Hurley S. Cancer incidence in California flight attendants (United States) Cancer Causes Control. 2002;13:317–324. doi: 10.1023/a:1015284014563. [DOI] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ. Melatonin as a chronobiotic: treatment of circadian desynchrony in night workers and the blind. J Biol Rhythms. 1997;12:595–603. doi: 10.1177/074873049701200615. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- Schweitzer PK, Koshorek G, Muehlbach MJ, Morris DD, Roehrs T, Walsh JK, Roth T. Effects of estazolam and triazolam on transient insomnia associated with phase-shifted sleep. Hum Psychopharmacol. 1991;6:99–107. [Google Scholar]
- Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol Regul Integrat Comp Physiol. 2002;282:R454–R463. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Fogg LF, Eastman CI. Effects of melatonin administration on daytime sleep after simulated night shift work. J Sleep Res. 2001;10:181–192. doi: 10.1046/j.1365-2869.2001.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Lee C, Crowley SJ, Eastman CI. Can melatonin help the daytime sleep of night shift workers? Abstr Ninth Meeting Soc Res Biol Rhythms. 2004:126. [Google Scholar]
- Spitzer RL, Terman M, Williams JB, Terman JS, Malt UF, Singer F, Lewy AJ. Jet lag: clinical features, validation of a new syndrome-specific scale, and lack of response to melatonin in a randomized, double-blind trial. Am J Psychiatry. 1999;156:1392–1396. doi: 10.1176/ajp.156.9.1392. [DOI] [PubMed] [Google Scholar]
- Stewart KT, Eastman CI. The light stuff: shiftwork, circadian rhythms, and manned spaceflight. In: Holick MF, Jung EG, editors. Biologic Effects of Light 1995. Walter de Gruyter & Co.; Berlin/New York: 1996. pp. 340–347. [Google Scholar]
- Stewart KT, Hayes BC, Eastman CI. Light treatment for NASAshiftworkers. Chronobiol Int. 1995;12:141–151. doi: 10.3109/07420529509064509. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Sasaki M, Itoh H, Ozone M, Yamadera W, Hayshida K, Ushijima S, Matsunaga N, Obuchi K, Sano H. Effect of 3 mg melatonin on jet lag syndrome in an 8-h eastward flight. Psychiatr Clin Neurosci. 2000;54:377–378. doi: 10.1046/j.1440-1819.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Sasaki M, Itoh H, Sano H, Yamadera W, Ozone M, Obuchi K, Nishimura H, Matsunaga N. Re-entrainment of circadian rhythm of plasma melatonin on an 8-h eastward flight. Psychiatr Clin Neurosci. 1999;53:257–260. doi: 10.1046/j.1440-1819.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Commerce, ITA Office of Travel & Tourism Industries 2003. Profile of US Resident Traveler Visiting Overseas Destinations. 2003 Retrieved from Survey of International Air Travelers, www.tinet.ita.doc.gov/view/f-2003-101-001/index.html.
- Walsh JK, Randazzo AC, Stone KL, Schweitzer PK. Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep. 2004;27:434–439. doi: 10.1093/sleep/27.3.434. [DOI] [PubMed] [Google Scholar]
- Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 2003;342:37–40. doi: 10.1016/s0304-3940(03)00223-4. [DOI] [PubMed] [Google Scholar]
- Wever RA. The Circadian System of Man. New York: Springer-Verlag; 1979. [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]