Abstract
Cilia are specialized organelles that extend from the surface of cells into the local environment. Airway epithelial cell cilia are motile to provide mucociliary clearance for host defense. On other cells, solitary cilia are specialized to detect chemical or mechanosensory signals. Sensory proteins in motile cilia have recently been identified that detect shear stress, osmotic force, fluid flow, bitter taste and sex hormones. The relationship of sensory function in human motile cilia to disease is now being revealed. One example is polycystin-1 and polycystin-2. As a complex, these proteins function as a flow sensor in cilia and are mutated in autosomal dominant polycystic kidney disease (ADPKD). The polycystins are also expressed in motile cilia of the airways, potentially operating as sensors in the lung. Computed tomography studies from patients with ADPKD revealed radiographic evidence for bronchiectasis, suggesting that polycystin-1 and -2 are important in lung function. The expression of this complex and sensory channel TRPV4, and bitter taste and sex hormones receptors in motile cilia indicate that the cell is wired to interpret environmental cues to regulate cilia beat frequency and other functions. Defective signaling of sensory proteins may result in a ciliopathy that includes lung disease.
Keywords: cilia, sensory, bronchiectasis, ADPKD, signaling
Introduction
Classes of cilia
Cilia are evolutionarily ancient structures, extending from the cell into the surrounding environment to provide motility or sensing. Thus cilia are often divided into two broad classes, known as motile or primary (also called sensory) cilia (1, 2). The structure of all cilia is remarkably conserved from single cell organisms to mammals. All cilia include an axoneme composed of 9 outer microtubule doublets surrounded by a membrane. In the case of motile cilia, these doublets, plus an additional inner pair, are associated with dynein motor proteins to provide movement. Dynein activity is regulated by cAMP-dependent signals and intracellular calcium levels that ultimately control cilia beat frequency (3). In the lung, beating cilia clear the airway as a first line of innate defense. Motile cilia are also present on the surface of epithelial cells of the oviduct, brain ventricles, nasal sinuses and Eustachian tubes of the upper respiratory tract. The flagellum that propels sperm is structurally identical to motile cilia. In addition, solitary motile cilia that spin are present on cells of the embryonic node to propel growth factors in a directional fashion for establishment of left-right body axis (4). Knowledge of how motile cilia sense the environment and respond with a change in cilia beat frequency and function is now being revealed based on the investigation of primary cilia.
Sensory cilia
Solitary, non-motile cilia are classified as “primary” or “sensory” (5–8). The best-known functions of the sensory cilia are to act as specialized receptors that detect light in the retina, sound waves in the ear and chemical signals in olfactory cilium in the nose (1). Primary cilia also act as mechanoreceptors in response to luminal flow in the renal tubules, biliary and pancreatic ducts and within the vasculature, via cilia on endothelial cells (9–11). Proteins on the membrane of some cilia are receptors for signaling pathways for, development and differentiation including sonic hedgehog (SHH), epidermal growth factor receptor (EGFR) and platelet derived growth factor receptor (PDGFR) (2, 12, 13). Thus, the cilium has been broadly conceptualized as an antenna protruding into the cell environment to provide the cell with feedback about the milieu, ultimately to regulate cellular responses.
The strict classification of one type of cilium as a sensory organelle and another as motile has now become blurred as proteins with sensory functions have been identified in motile cilia. Here, we summarize experimental evidence for the “overlap” of cilia functions. Furthermore, we provide new evidence for lung disease caused by putative mechanosensory defects related to mutations of genes causing autosomal dominant polycystic kidney disease (ADPKD). Finally, we provide a model by which sensory components of motile cilia are required for continuous feedback in the normal function of cilia for host defense.
Ciliopathies
Motile ciliopathies
The recognition that cilia have multiple functions was initially facilitated by the identification of cilia-specific expression of proteins found to be mutant in several diseases. Defects in cilia and related genes are now termed “ciliopathies” (1, 14, 15). Primary ciliary dyskinesia (PCD, also called immotile cilia syndrome) results from genetic mutations in the genes that code for proteins with motor function in motile cilia (16–18). PCD is an autosomal recessive disease in which motile cilia are immotile, beat slowly, or have abnormal beating patterns (18), resulting in left-right asymmetry defects, infertility, chronic ear infections and recurrent respiratory tract infections. Many of these symptoms are the result of failure of the cilia to appropriately interact with the protective layer of mucus and airway surface liquid that overlies the apical surface of the epithelial cell. Consequently, particulate matter and pathogens that enter the respiratory tract are trapped in layers of mucus and cannot be adequately cleared. Over time, chronic infection can lead to airway destruction and dilation, resulting in bronchiectasis (17, 19).
Primary cilia syndromes
In contrast, mutations in genes expressed in primary cilia are responsible for diverse syndromes that include defects in neural tube formation, sensory organs (retina, inner ear, olfactory system) and bone structure. A common feature of primary cilia syndromes is the development of polycystic kidneys. Among the best-described primary ciliopathies are ADPKD, nephronophthesis (NPHP) and Bardet-Beidl Syndrome (BBS) (14, 20). Thus the phenotype of the primary cilia-related syndromes may be distinct from the motile cilia disorder of PCD. However, increased awareness of the spectrum of ciliopathies has led to the recognition that some individuals affected with primary cilia syndromes have features of motile cilia dysfunction (e.g., bronchiectasis and situs inversus). This “overlap” of phenotypes has been confirmed in knockout mouse models. For example, Bardet-Beidl Syndrome (BBS) proteins function as a complex of 12 proteins required for the biogenesis of cilia (15). Mutations in BBS genes result in a syndrome that is clinically characterized by obesity, hypertension, retinopathy, polydactyly, hypogonadism, cystic kidneys, developmental delay and situs inversus. BBS genes 2 and 4 are expressed in airway motile cilia and BBS2 and 4 knockout mice have structural defects in the motile cilia as well as reduced ciliary beat frequency (21). Defects in primary cilia are also responsible for blindness in retinitis pigmentosa, retinal degeneration in Leber syndrome and deafness due to Usher’s syndrome. These genetically heterogeneous syndromes have similarly been associated with motile cilia structural and functional defects or the development of bronchiectasis (22–25). While the overlap of primary and motile ciliopathies are often due to shared essential structural components, the findings also suggest that there are specialized sensory components also assigned to motile cilia.
Evidence for sensory function in motile cilia
Evolutionary considerations
The structure of motile cilia is nearly identical to the flagellum, an evolutionary ancient structure, used for single cell motility (26). One of the best models for the study of motile cilia is the algae, Chlamydomonas reinhartii, a biflagylate single cell organism whose cilia express a set of proteins that are highly conserved in the motile human cilia (27). Evolutionary events leading to overlap of cilia types likely resulted from expression of sensory receptors on the motile cilia to obtain information from the environment. It has been posited that enhanced specialization of cilia lead to the development of complex sensory organs such as the retina (26). How the switch in cilia types occurred evolutionarily is not known, however new findings suggest that an epigenetic mechanism could be at play. This appears to be the case for hair cells of the ear that require an otolith positioned on top of the cilia for hearing. Roy and colleagues investigated the development of hair cells in the otic vesicle of Danio rerio (zebrafish) and showed that these cilia must initially be motile to provide flow to form the otolith (28). The program for motility occurs by the expression of the motile cilia master gene, Foxj1. Following the tethering of a mature otolith at the tips of the stereocilium, Foxj1 is turned off in the mature, non-motile stereocilia, possibly through epigenetic mechanisms. Thus both evolutionary and molecular evidence indicate a close relationship between motile and sensory cilia types.
Shared motor and sensory functions in motile invertebrate cilia
Mechano- and chemosensory function in motile cilia has been observed in invertebrates for over a century (29, 30). The dual function of a cilium is illustrated by the behavior of the Paramecium, a unicellular ciliated protozoa enveloped by a border of cilia for directional motility. When the Paramecium strikes an object, the direction of cilia stroke and thus movement reverses, to avoid further contact. The contact and reversed swimming is found to be associated with a change in intracellular calcium, though the mechanism governing this response has not been established (Reviewed in (30). However, in Chlamydomonas, a sensor within the flagella required for mating, has been identified (31). The sensor is the TRPP2 cation channel, an orthologue of the human PKD2 gene (Polycystin-2), also expressed in the mechanosensing, non-motile primary cilium of the renal tubule (32).
Experimental evidence for shared motile and sensory function in vertebrates
Reports over the past two decades have described the expression of sensory-associated proteins in motile cilia of the respiratory tract, oviduct and brain (Table 1). Summarized below is current knowledge related to the small and diverse group of specialized receptors expressed on motile cilia that share the capacity to detect and respond to specific signals in the cell environment (Figure 1). It is expected that this list will expand significantly. As it currently stands, the effect of activation of sensory receptors has been identified as a regulation in the cilia beat, though it is likely that these functions will also multiply with future investigation.
Table 1.
Expression of putative sensory proteins in motile cilia
| Gene name | Receptor class | Location of motile cilia | Stimulus or Sensor | Effect | References |
|---|---|---|---|---|---|
| TRPV4 | Transient receptor potential (vanilloid) calcium channel | Airway, oviduct | Osmotic, viscosity, fluid flow | Increase Ca(i), regulate CBF | (34–36) |
| PR | Sex hormone receptor | Oviduct | Ligand binding | Increase Ca(i) Regulate CBF | (43–46, 71) |
| VANGL2 | Planar cell polarity | Ependyma | Fluid flow | Orient basal bodies | (50) |
| T2R | Bitter taste receptors, | Airway | Bitter substance | Increase Ca(i) regulate CBF | (39) |
| PKD1 | Polycystin trans-membrane protein | Airway, ependymal oviduct | Putative mechanosensory | Increase Ca(i) | (57, 59) |
| PKD2 | Transient receptor potential calcium channel | Airway, oviduct | Putative mechanosensory | Increase Ca(i) | (58) |
CBF, cilia beat frequency; Ca(i), intracellular calcium
Figure 1. Sensory functions of receptor molecules on motile cilia.
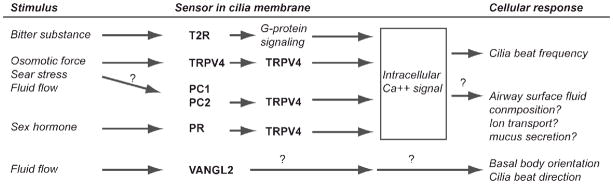
Integration of experimental evidence for receptors identified on motile cilia, putative signaling pathways and function. Functions indicated by (?) are not specifically identified in motile cilia but are identified in primary cilia, or are not yet defined, as in the case of Vangl2.
Transient receptor potential cation channel subfamily V member 4 (TRPV4)
Seminal observations by Verdugo’s lab that cilia beat frequency was regulated by changes of viscosity in the apical fluid layer (33) was subsequently explained by the function of calcium channel, TRPV4 (34–36). TPRV4, expressed on the membrane of motile cilia in the oviduct and airway epithelium, is a vanilliod receptor subfamily member of the transient receptor potential family sensitive to osmotic force, mechanical stimuli and temperature. TRPV4 within motile cilia responds to mechanical loading, shear stress and osmotic pressure by a change in cilia beat frequency (34, 36). Thus conceptually, TRPV4 functions in response to changes in the viscosity of mucus in the airway surface liquid to regulate mucociliary clearance. Activation of TRPV4 in primary cilia of renal tubule cells and cholangiocytes has been found to also regulate potassium and bicarbonate secretion (9, 37), but similar responses contributing to the ionic composition of the airway surface liquid are not described. Interestingly, polymorphisms of the TRPV4 gene were identified in patients with COPD, suggesting a role in airway disease (38). To date, TRPV4 appears to be a key sensor for the regulation of cilia beat frequency and could regulate the composition of fluid within the motile cilia environment.
Bitter taste receptors (T2R)
Motile cilia of human respiratory epithelia were recently shown to express several members of the family of bitter taste receptors, identical to those in the tongue and nose (39). The report revealed that signaling molecules known to be components of the T2R cascade are also expressed within the cilia, including the GTP binding protein α-gustducin. Of note, different T2R receptors and G proteins were located within discrete zones on the ciliary shaft, possibly providing an organization for sensing within different regions of the airway surface liquid. Exposure of cilia to different noxious compounds caused an increase in intracellular calcium ion concentration and stimulated increased ciliary beat frequency, confirming that the pathway was functional. This cell-autonomous system is hypothesized to help epithelial cells propel harmful inhaled material out of the lung.
Progesterone receptor (PR)
Sensory receptors responsive to the hormonal milieu have been identified on human motile cilia of the fallopian tube. Here, both progesterone receptor and estrogen receptor beta are expressed in the proximal region of the cilia (40, 41). Several studies showed that progesterone treatment inhibited cilia beat frequency in human and mouse fallopian tube preparations (42–45). Treatment with estradiol alone had no impact on cilia beat frequency, while concomitant treatment of estradiol with progesterone prevented the progesterone effect on cilia (43). The physiologic impact of the effect is thought to be slowing movement of the oocyte through the fallopian tubes as uterine mucosa is optimized for implantation (43–45). The effect of progesterone is rapid in onset in the oviduct suggesting a non-genomic mechanism (42, 45). Interestingly, progesterone receptor is shown to regulate the expression and function of TRPV4, providing a potential mechanism for the hormone effect (46). The expression or role of progesterone or estrogen receptors in motile cilia of other organs has not been identified.
Vang-like 2 (VANGL2, Van Gogh like 2)
Vangl2 is a planar cell polarity (PCP) protein, a member of a family that functions to establish patterning in early development by signaling to align cells within an epithelial layer. Relative to cilia organization, VANGL2 was first found to be required to properly orient the stereociliary bundles of the hair cells in the cochlea (47) and later, the orientation of the motile ciliated cells of the Xenopus larvae to provide directional beating (48, 49). Cilia orientation required fluid flow across the motile cilia, provided by the beating of the cilia themselves, to generate a signal for the adjustment of basal body orientation and hence directionality for cilia force (48). How this occurs was uncovered when Vangl2 expression was detected asymmetrically along the cilia and the apical membrane of the cell. Analysis of cells rendered genetically incapable of cilia biogenesis showed that the expression of Vangl2 in the cilia (and not in the cell surface) was specifically required to orient the basal bodies (50). These findings suggest that Vangl2 within the cilia is acting as a senor for normal cilia orientation by signaling to refine the positioning of the basal body relative to the position of Vangl2 on the ciliary shaft. Humans with disoriented basal bodies and bronchiectasis have been reported, though it is not yet known if specific Vangl2 mutations or another disruption of the Vangl2 pathway is responsible for this defect (51).
Polycystin-1 (PKD1) and polycystin-2 (PKD2)
Mutations in genes PKD1 and PKD2 coding for polycystin-1 (PC-1) and polycystin-2 (PC-2; TRPP2), respectively are responsible for ADPKD and polycystic kidneys in animal models (reviewed in (20). PC-1 is located within the membrane of the renal tubule cilia and binds PC-2, a member of the transient receptor potential family that functions as a calcium permeable channel. The complex of PC-1 and PC-2 are present in the membrane of primary cilia in the epithelial cells of the renal tubules to sense the flow of urine. A mutation in either of the genes results in cyst formation within the kidney and, over time, the development of renal failure (20, 52).
Key studies by Praetorius and Spring have uncovered the mechanosensory capability of the solitary cilium to sense flow (53–55). Their studies showed that direct bending or fluid flow over a cilium generated an increase in intracellular calcium. This flow signal required intact function of the proteins PC-1 and PC-2 for signaling (10). Later work showed that the sensory function of PC-2 is dependent on the function of TRPV4. TRPV4 and PC2 have been co-localized on the primary cilia and shown to have functional interactions for the generation of calcium-dependent currents in Xenopus laevis oocytes (56). In the same report, flow dependent calcium signaling in mammalian cells was shown to require TRPV4. While further studies are required in vivo, these findings further support a central role for TRPV4 in motile cilia sensing (56).
PKD1 and PKD2 expression in motile cilia
Our microarray profiling of primary culture mouse airway epithelial cells indicated the expression of PKD1 and PKD2. We then demonstrated that both PC-1 and PC-2 are expressed in motile cilia of the airways (57, 58). Both proteins have also been identified on motile cilia in the oviduct (35) and the ependymal cells of the brain (59). Expression in motile cilia led us to consider that PC-1 and PC-2 might have a mechanosensory function in the lung important for mucociliary clearance. We hypothesized that the mechanosensory function of PC-1 and PC-2 in the motile cilia are required for optimal cilia function for mucociliary clearance and if mutated, as in ADPKD, would result in airways disease that presents as bronchiectasis.
Radiographic bronchiectasis in ADPKD
Multisystem features of ADPKD
ADPKD is a systemic disease with a range of intra-abdominal cystic (e.g., renal, hepatic, pancreatic cysts) and non-cystic features (e.g., abdominal wall hernias) (Reviewed in (60). Previously reported thoracic manifestations of ADPKD are cardiac valve defects (e.g., mitral valve prolapse) and aortic root dilation (60). Since the pathogenesis is due to mutations in either PKD1 or PKD2, and the proteins are expressed in multiple tissues, extra-renal abnormalities are likely related to the genes mutated in ADPKD. Our recognition that the polycystins are expressed in airway cilia led us to recently uncover the first pulmonary manifestation of ADPKD, radiographic bronchiectasis (57). Computed tomography (CT) is the major imaging modality used for diagnosing bronchiectasis (61). Owing to the large number of abdominal CT scans obtained for the assessment of complications related to cystic structures in kidney and liver (62), we were able to take advantage of thoracic information from these CT scans. In a study of 95 patients with ADPKD, we demonstrated a 3-fold increase in prevalence of radiographic bronchiectasis in ADPKD (35%) compared to a cohort of individuals with non-ADPKD renal disease (13%) (57). However, this report had several limitations and led to questions of severity, risk factors and clinical features. We therefore expanded our original cohort of patients and extended the analysis to provide further evidence for a relationship between motile cilia sensory function and the development of lung disease.
ADPKD cohort for study of bronchiectasis
We furthered our findings of bronchiectasis in ADPKD by assessing the radiographic severity, risk factors and clinical features of bronchiectasis as well as the prevalence of other thoracic manifestations of ADPKD. Furthermore, we assessed a group of renal transplant recipients with ADPKD, uncovering a high prevalence bronchiectasis that was previously not reported. We identified 163 patients with adequate evidence of ADPKD by CT scan and a chart diagnosis of ADPKD. The group included 121 non-transplanted and 42 post-renal transplant subjects. For the 121 non-transplanted ADPKD subjects, 38 were found to have radiographic bronchiectasis (“Bronchiectasis” cohort) and the remaining 83 did not (“No Bronchiectasis” cohort) (Table 2). Demographics including gender, age, race, smoking status and severity of renal disease at the time of CT scan were compared. Most parameters did not significantly differ between groups. The mean age in the Bronchiectasis group was older (52 versus 61 years old, p < 0.001).
Table 2.
Characteristics of ADPKD subjects
| No Bronchiectasis n = 83 | Bronchiectasis n = 38 | p-value | |
|---|---|---|---|
| Gender, n (%) | 0.10a | ||
| Female | 46 (55.4) | 15 (39.5) | |
| Male | 37 (44.6) | 23 (60.5) | |
| Age, mean years ± SD | 52.46 ± 12.45 | 60.79 ± 13.21 | <0.001c |
| Race or ethnicity, n (%) | 0.11b | ||
| White | 61 (76.3) | 26 (68.4) | |
| Black | 14 (17.5) | 12 (31.6) | |
| Other | 5 (6.3) | 0 | |
| Smoking, n (%) | 0.10a | ||
| Yes | 36 (44.4) | 14 (36.8) | |
| No | 27 (33.3) | 21 (55.3) | |
| Smoking, pack years, mean ± SD, n = 56 and 32 | 10.02 ± 13.0 | 10.6 ± 20.8 | 0.87c |
| BUN, mean ± SD, n = 78 and 36 | 34.1 ± 21.8 | 36.9 ± 21.5 | 0.52c |
| CR, mean ± SD, n = 79 and 37 | 3.78 ± 3.46 | 4.08 ± 4.23 | 0.69c |
| GFR, mean ± SD, n = 78 and 36 | 40.07 ± 34.83 | 37.16 ± 29.46 | 0.66c |
| ALB, mean ± SD, n = 78 and 36 | 3.84 ± 0.71 | 3.92 ± 0.55 | 0.53c |
| Dialysis, n (%) | 0.68a | ||
| Yes | 24 (30) | 10 (26.3) | |
| No | 56 (70) | 28 (73.7) | |
| Family history of PKD, n (%) | 0.93a | ||
| Yes | 47 (75.8) | 21 (75) | |
| No | 15 (24.2) | 7 (25) | |
| Hypertension, n (%) | 0.49a | ||
| Yes | 60 (75.9) 19 | 31 (81.6) | |
| No | (24.1) | 7 (18.4) |
Chi-squared test for categorical variables.
Fisher’s exact test for categorical variables.
Independent groups t-test for continuous variables.
Abbreviations: BUN, blood urea nitrogen; CR, creatinine; GFR, glomerular filtration rate; ALB, albumin; PKD, polycystic kidney disease;
Scoring radiographic bronchiectasis in ADPKD
An important consideration was to determine the severity of bronchiectasis associated with ADPKD. The criteria used for diagnosis of bronchiectasis were those established in the field (61). Bronchiectasis severity was scored using a simplified Brody Scoring System with three main categories (63): ratio of the diameter of bronchus to the adjacent vessel diameter, bronchial wall thickness compared to adjacent vessel, and the presence of atelectasis. The sum of these three components was used as the severity score (Table 2). No subjects received a grade higher than 3, indicating that the phenotype is generally mild. Evidence of bronchiectasis was observed in all lung lobes, but due to the preponderance of abdominal CT scans included, most observed cases were in the lower lobes. Many included wall thickening suggestive of inflammation (Figure 2) but dilated airways alone were more commonly noted (Table 3). Only cylindrical bronchiectasis was observed rather than cystic bronchiectasis commonly found in cystic fibrosis lung disease.
Figure 2. Representative examples of radiographic bronchiectasis in patients with ADPKD.

Computed tomography sections obtained approximately at the level of the mid lung fields. A. Lack of airway tapering (arrow) and dilated airways, grades 1 and 2, in the right and left lungs (arrowheads); B. Grade 1 wall thickening (arrow); C. Grade 1 airway dilation (arrows).
Table 3.
Radiologic and Clinical Severity of Bronchiectasis in ADPKD
| No Bronchiectasis n = 83 | Bronchiectasis n = 38 | p-value | |
|---|---|---|---|
| Radiographic bronchiectasis severity, n (%) | 83 (100) | 0 | |
| 0 | 0 | 13 (34.2) | |
| 1 | 0 | 16 (42.1) | |
| 2 | 0 | 9 (23.7) | |
| 3 | 0 | 0 | |
| 4 | 0 | 0 | |
| 5 | 0 | 0 | |
|
| |||
| Bronchiectasis features, n (%) | |||
| Ratio of bronchus to vessel | |||
| 0 (<1X) | 3 (7.9) | ||
| 1 (1-2X) | 35 (92.1) | ||
| 2 (2-3X) | 0 | ||
| 3 (> 3X) | 0 | ||
| Thickness of bronchial wall to vessel diameter | |||
| 0 (no thickening) | 23 (60.5) | ||
| 1 (thickened but less than vessel diameter) | 15 (39.5) | ||
| 2 (1-2X vessel) | 0 | ||
| Presence of atelectasis | |||
| 0 (none) | 15 (39.5) | ||
| 1 (present) | 23 (60.5) | ||
|
| |||
| Clinical features | |||
| Bronchiectasis symptoms (n = 58, 33) c | 0.45a | ||
| Yes | 15 (25.9) | 11 (33.3) | |
| No | 43 (74.1) | 22 (67.7) | |
Chi-squared test for categorical variables.
Fisher’s exact test for categorical variables.
n = adequate data for no bronchiectasis and bronchiectasis cohorts
Clinical features and risk factors for bronchiectasis in ADPKD
The radiographic bronchiectasis score was compared to the prevalence of symptoms typically related to bronchiectasis obtained from retrospective review of clinical charts. Similar numbers of subjects had radiographic severity scores of 1, 2, and 3 in the symptomatic and asymptomatic groups (p = 0.33), suggesting that bronchiectasis symptoms did not increase with radiographic severity. No association was detected between radiographic bronchiectasis score and measures of renal function or number of dialysis-dependent years using Pearson correlation. Logistic regression analysis was used to determine if any of the demographic, clinical, or radiographic characteristics were associated with an increased risk of bronchiectasis. Univariate analysis revealed that age was the only significant clinical characteristic associated with presence of radiographic bronchiectasis (OR 1.05, 95% CI 1.02–1.09, p = 0.002). Multivariable analysis that included age, gender and smoking history revealed age as a significant risk factor for development of bronchiectasis (p = 0.002).
We also considered that other thoracic or abdominal (non-renal) manifestations of ADPKD may be related to the development of bronchiectasis. We found that 45.7% subjects (54 of 118) had pericardial effusions comparable to the 35% prevalence previously reported (64). There was no significant association between renal function, cardiac abnormalities or pericardial effusion and bronchiectasis. There was also no relationship between intra-abdominal extra-renal cysts (hepatic, pancreatic) and bronchiectasis.
Bronchiectasis in ADPKD patients following renal transplantation
Additionally, we evaluated bronchiectasis in a transplanted cohort of ADPKD patients, who may be at increased risk due to immunosuppressant agents. Their baseline demographics were not significantly different from those of non-transplanted individuals except that these patients were generally older than the non-transplanted cohort (mean age 62.6 versus 55.1 yrs, p = 0.001). The prevalence of bronchiectasis was significantly increased in the transplanted ADPKD population (52.4% versus 31.4%, p = 0.02). Furthermore, compared to the non-transplanted group, this population exhibited more severe radiographic changes, where 9.5% of patients scored as 1, 23.8% as 2, 16.7% as 3 and 2.4% as 5 (p = 0.03). The transplanted group specifically displayed relatively increased airway size (45% versus 29% with airway score ≥ 1, p = 0.003) and a higher frequency of atelectasis (38% versus 19%, p = 0.01) compared to the non-transplanted group.
Lung disease and sensory function in motile cilia
The etiology of bronchiectasis in ADPKD
Our study of bronchiectasis in an expanded cohort of patients with ADPKD indicates a prevalence of radiographic bronchiectasis of approximately 31% in those undergoing CT scan, confirming our prior report (57). In that study, we found bronchiectasis in 35% of patients with ADPKD compared to 13% of a control group with chronic kidney disease attributed to hypertension and diabetes. This is consistent with the prevalence of bronchiectasis in the general population as determined by changes on CT scan, which has been reported to be about 12.5% (65). The extent of dilation (bronchoarterial ratio of 2 or greater) was also higher than expected based on changes occurring with age alone (66). Importantly, renal transplanted ADPKD patients had an even higher incidence of bronchiectasis (52.4%). Our findings strongly suggest that a defect in the function of the PC-1, PC-2 complex results in airway remodeling characteristic of bronchiectasis. Evidence for bronchiectasis was also suggested by autopsy evidence (57) and thick bronchial walls on CT scan (e.g., Figure 2). We speculate that bronchiectasis is due to impaired mucociliary clearance as a result of interrupted flow sensing.
Specific factors related to the etiology of radiographic bronchiectasis in ADPKD are not yet understood and clinical features are unclear. Bronchiectasis may be due to a combination of underlying genetic predisposition and environmental insults (16, 19, 67, 68). Although expression of PC1 and PC-2 in cilia of airway epithelium may be critical, these proteins are also expressed in airway smooth muscle (69). A defect in these proteins at either site could result in lung remodeling. ADPKD has been described as a “multi-hit” process due to genetic predisposition plus injury, thus age may increase prevalence simply due to increased time for respiratory tract infection and injury (70). Additionally, bronchiectasis is a progressive disease that may be present long before debilitating symptoms are recognized. The patient population under investigation in this study was relatively young, with an average age of 55 years. The limited clinical manifestations observed may, in fact, reflect the age-dependence of this disease and suggest that patients within this cohort were studied too early in the disease process to detect symptoms, despite radiographic changes. Future studies must include comprehensive evaluation of clinical respiratory symptoms and pulmonary function testing. Given that the progression of bronchiectasis may be prevented by early intervention with airway clearance therapies, more rigorous screening and early detection of such a finding in this patient population may help prevent future respiratory complications (68).
A shared role for cilia sensory signaling to regulate beat frequency
The experimental evidence presented for PC1, PC-2 and other proteins noted in Table 1, strongly suggests that the role for sensory function in motile cilia is the regulation of cilia beat frequency and direction (Figure 1). A common pathway for TPRV4, bitter taste receptor, PC1 and -2, and progesterone receptor signaling is modulation of intracellular calcium concentration. Functional interactions of PC-2 and PR with TRPV4 place TPRV4 in a central position for regulating signaling. It has not yet been shown that polycystin signaling regulates intracellular calcium concentration or cilia beat control, but these are potential responses. The pathway for progesterone-mediated slowing of cilia beat frequency has likewise not been determined, however, the progesterone receptor has been shown to modulate TPRV4 and ATP calcium dependent signaling, providing candidates for future investigation (46, 71). One could further speculate that activation of progesterone receptors, slowing of cilia beat frequency in the airway and subsequent decreased mucociliary clearance could play a role in gender disparity in lung disease, causing a female disadvantage in bronchiectasis and in asthma (67, 72).
Modeling cilia sensory feedback in lung health and disease
Based on the current knowledge of sensory receptors in motile cilia, we propose that a continuous cell-autonomous or regional response maintains mucociliary clearance required for normal lung homeostasis (Figure 3). At least four critical functions for lung health are regulated by motile cilia sensors: (1) establishment of unidirectional cilia beating through orientation of basal bodies by Vangl2; (2) adjustment of cilia beat frequency in response to shear stress or flow of airway surface liquid, mucus and possibly air by TPRV and PC-1, PC-2; (3) host defense against noxious inhaled particles via bitter taste receptors; and (4) possibly regulation of the ionic or mucus composition of the airway surface fluid, based on known roles of PC2 in other tissues (9, 37). Following infection or a change in the environment resulting in an increase in mucous cell metaplasia, there must be enhanced signaling to maintain airway clearance. Interruption of these pathways due to a failure of ciliated repair or a genetic or epigenetic event would impair these pathways, leading to failure of mucociliary clearance, increased inflammation, lung injury and eventually airway remodeling and bronchiectasis. Future studies directed toward understanding these mechanisms are predicted to be fruitful.
Figure 3. Model of sensory function in motile cilia in the airway.

Cilia sensors act to provide continuous feedback to regulate cilia beat function, cilia orientation following cilia biogenesis during post-injury repair and potentially, ion and mucin components of airway surface liquid (ASL) (left). In response to environmental factors (center), there is enhanced activation of specialized sensors to trigger host response pathways. Impaired or mutated sensory protein or pathway results in features of disease (right).
Acknowledgments
Supported by NIH awards T32 HL07317 (RJ), KL2 RR024994/UL1RR024992 (RJ) and an ATS Career Development Award (RJ) and R01 HL56244 (SLB).
References
- 1.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–93. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 2.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–22. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 4.Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207–30. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- 6.Wheatley DN. Primary cilia in normal and pathological tissues. Pathobiology. 1995;63:222–38. doi: 10.1159/000163955. [DOI] [PubMed] [Google Scholar]
- 7.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–10. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 8.Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol. 2005;289:F1159–69. doi: 10.1152/ajprenal.00118.2005. [DOI] [PubMed] [Google Scholar]
- 9.Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci US A. 2007;104:19138–43. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 11.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–71. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–78. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008;85:261–301. doi: 10.1016/S0070-2153(08)00810-7. [DOI] [PubMed] [Google Scholar]
- 14.Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:281–95. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- 15.Quinlan RJ, Tobin JL, Beales PL. Modeling ciliopathies: Primary cilia in development and disease. Curr Top Dev Biol. 2008;84:249–310. doi: 10.1016/S0070-2153(08)00605-4. [DOI] [PubMed] [Google Scholar]
- 16.Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:459–67. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 17.Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, Knowles MR, Zariwala MA. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med. 2009;11:473–87. doi: 10.1097/GIM.0b013e3181a53562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirst RA, Rutman A, Williams G, O’Callaghan C. Ciliated air-liquid cultures as an aid to diagnostic testing of primary ciliary dyskinesia. Chest. 2010;138:1441–7. doi: 10.1378/chest.10-0175. [DOI] [PubMed] [Google Scholar]
- 19.Javidan-Nejad C, Bhalla S. Bronchiectasis. Radiol Clin North Am. 2009;47:289–306. doi: 10.1016/j.rcl.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–68. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah AS, Farmen SL, Moninger TO, Businga TR, Andrews MP, Bugge K, Searby CC, Nishimura D, Brogden KA, Kline JN, Sheffield VC, Welsh MJ. Loss of Bardet-Biedl syndrome proteins alters the morphology and function of motile cilia in airway epithelia. Proc Natl Acad Sci US A. 2008;105:3380–5. doi: 10.1073/pnas.0712327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Brauwer PJ, Blaise P, Hermans G, Boniver V, Bartsch P, Rakic JM. Retinitis pigmentosa and bronchiectasis: a case report on a rare association suggestive of a common underlying primary ciliary dyskinesia (PCD) Bull Soc Belge Ophtalmol. 2010:9–14. [PubMed] [Google Scholar]
- 23.Krawczynski MR, Dmenska H, Witt M. Apparent X-linked primary ciliary dyskinesia associated with retinitis pigmentosa and a hearing loss. J Appl Genet. 2004;45:107–10. [PubMed] [Google Scholar]
- 24.Moore A, Escudier E, Roger G, Tamalet A, Pelosse B, Marlin S, Clement A, Geremek M, Delaisi B, Bridoux AM, Coste A, Witt M, Duriez B, Amselem S. RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J Med Genet. 2006;43:326–33. doi: 10.1136/jmg.2005.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papon JF, Perrault I, Coste A, Louis B, Gerard X, Hanein S, Fares-Taie L, Gerber S, Defoort-Dhellemmes S, Vojtek AM, Kaplan J, Rozet JM, Escudier E. Abnormal respiratory cilia in non-syndromic Leber congenital amaurosis with CEP290 mutations. J Med Genet. 2010;47:829–34. doi: 10.1136/jmg.2010.077883. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell DR. The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv Exp Med Biol. 2007;607:130–40. doi: 10.1007/978-0-387-74021-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–52. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 28.Yu X, Lau D, Ng CP, Roy S. Cilia-driven fluid flow as an epigenetic cue for otolith biomineralization on sensory hair cells of the inner ear. Development. 2011;138:487–94. doi: 10.1242/dev.057752. [DOI] [PubMed] [Google Scholar]
- 29.Wiederhold ML. Mechanosensory transduction in “sensory” and “motile” cilia. Annu Rev Biophys Bioeng. 1976;5:39–62. doi: 10.1146/annurev.bb.05.060176.000351. [DOI] [PubMed] [Google Scholar]
- 30.Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci. 2010;123:505–9. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- 31.Solter KM, Gibor A. Evidence for role of flagella as sensory transducers in mating of Chlamydomonas reinhardi. Nature. 1977;265:444–5. doi: 10.1038/265444a0. [DOI] [PubMed] [Google Scholar]
- 32.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J Cell Biol. 2007;179:501–14. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson NT, Villalon M, Royce FH, Hard R, Verdugo P. Autoregulation of beat frequency in respiratory ciliated cells. Demonstration by viscous loading. Am Rev Respir Dis. 1991;144:1091–4. doi: 10.1164/ajrccm/144.5.1091. [DOI] [PubMed] [Google Scholar]
- 34.Andrade YN, Fernandes J, Vazquez E, Fernandez-Fernandez JM, Arniges M, Sanchez TM, Villalon M, Valverde MA. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol. 2005;168:869–74. doi: 10.1083/jcb.200409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teilmann SC, Byskov AG, Pedersen PA, Wheatley DN, Pazour GJ, Christensen ST. Localization of transient receptor potential ion channels in primary and motile cilia of the female murine reproductive organs. Mol Reprod Dev. 2005;71:444–52. doi: 10.1002/mrd.20312. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzo IM, Liedtke W, Sanderson MJ, Valverde MA. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci U S A. 2008;105:12611–6. doi: 10.1073/pnas.0803970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taniguchi J, Tsuruoka S, Mizuno A, Sato J, Fujimura A, Suzuki M. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol. 2007;292:F667–73. doi: 10.1152/ajprenal.00458.2005. [DOI] [PubMed] [Google Scholar]
- 38.Zhu G, Gulsvik A, Bakke P, Ghatta S, Anderson W, Lomas DA, Silverman EK, Pillai SG. Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease. Hum Mol Genet. 2009;18:2053–62. doi: 10.1093/hmg/ddp111. [DOI] [PubMed] [Google Scholar]
- 39.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–4. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teilmann SC, Clement CA, Thorup J, Byskov AG, Christensen ST. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol. 2006;191:525–35. doi: 10.1677/joe.1.06565. [DOI] [PubMed] [Google Scholar]
- 41.Shao R, Weijdegard B, Fernandez-Rodriguez J, Egecioglu E, Zhu C, Andersson N, Thurin-Kjellberg A, Bergh C, Billig H. Ciliated epithelial-specific and regional-specific expression and regulation of the estrogen receptor-beta2 in the fallopian tubes of immature rats: a possible mechanism for estrogen-mediated transport process in vivo. Am J Physiol Endocrinol Metab. 2007;293:E147–58. doi: 10.1152/ajpendo.00101.2007. [DOI] [PubMed] [Google Scholar]
- 42.Bylander A, Nutu M, Wellander R, Goksor M, Billig H, Larsson DG. Rapid effects of progesterone on ciliary beat frequency in the mouse fallopian tube. Reprod Biol Endocrinol. 2010;8:48. doi: 10.1186/1477-7827-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmood T, Saridogan E, Smutna S, Habib AM, Djahanbakhch O. The effect of ovarian steroids on epithelial ciliary beat frequency in the human Fallopian tube. Hum Reprod. 1998;13:2991–4. doi: 10.1093/humrep/13.11.2991. [DOI] [PubMed] [Google Scholar]
- 44.Paltieli Y, Eibschitz I, Ziskind G, Ohel G, Silbermann M, Weichselbaum A. High progesterone levels and ciliary dysfunction--a possible cause of ectopic pregnancy. J Assist Reprod Genet. 2000;17:103–6. doi: 10.1023/A:1009465900824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wessel T, Schuchter U, Walt H. Ciliary motility in bovine oviducts for sensing rapid non-genomic reactions upon exposure to progesterone. Horm Metab Res. 2004;36:136–41. doi: 10.1055/s-2004-814336. [DOI] [PubMed] [Google Scholar]
- 46.Jung C, Fandos C, Lorenzo IM, Plata C, Fernandes J, Gene GG, Vazquez E, Valverde MA. The progesterone receptor regulates the expression of TRPV4 channel. Pflugers Arch. 2009;459:105–13. doi: 10.1007/s00424-009-0706-7. [DOI] [PubMed] [Google Scholar]
- 47.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–7. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 2009;19:924–9. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, Mirzadeh Z, Cremer H, Montcouquiol M, Sawamoto K, Spassky N. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–50. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 51.De Iongh R, Rutland J. Orientation of respiratory tract cilia in patients with primary ciliary dyskinesia, bronchiectasis, and in normal subjects. J Clin Pathol. 1989;42:613–9. doi: 10.1136/jcp.42.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens. 2009;18:99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol. 2003;191:193–200. doi: 10.1007/s00232-002-1055-z. [DOI] [PubMed] [Google Scholar]
- 54.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–9. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 55.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 56.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–47. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Driscoll JA, Bhalla S, Liapis H, Ibricevic A, Brody SL. Autosomal dominant polycystic kidney disease is associated with an increased prevalence of radiographic bronchiectasis. Chest. 2008;133:1181–8. doi: 10.1378/chest.07-2147. [DOI] [PubMed] [Google Scholar]
- 58.Jain R, Pan J, Driscoll JA, Wisner JW, Huang T, Gunsten SP, You Y, Brody SL. Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2010;43:731–9. doi: 10.1165/rcmb.2009-0328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wodarczyk C, Rowe I, Chiaravalli M, Pema M, Qian F, Boletta A. A novel mouse model reveals that polycystin-1 deficiency in ependyma and choroid plexus results in dysfunctional cilia and hydrocephalus. PLoS One. 2009;4:e7137. doi: 10.1371/journal.pone.0007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:173–80. doi: 10.1053/j.ackd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Naidich DP, Webb WR, Müller NL, Vlahos I, Krinsky GA, Srichai MB. Computed tomography and magnetic resonance of the thorax. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- 62.Hogan MC, Norby SM. Evaluation and management of pain in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:e1–e16. doi: 10.1053/j.ackd.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brody AS, Kosorok MR, Li Z, Broderick LS, Foster JL, Laxova A, Bandla H, Farrell PM. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging. 2006;21:14–21. doi: 10.1097/01.rti.0000203937.82276.ce. [DOI] [PubMed] [Google Scholar]
- 64.Qian Q, Hartman RP, King BF, Torres VE. Increased occurrence of pericardial effusion in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2007;2:1223–7. doi: 10.2215/CJN.01920507. [DOI] [PubMed] [Google Scholar]
- 65.Gupta S, Siddiqui S, Haldar P, Raj JV, Entwisle JJ, Wardlaw AJ, Bradding P, Pavord ID, Green RH, Brightling CE. Qualitative analysis of high-resolution CT scans in severe asthma. Chest. 2009;136:1521–8. doi: 10.1378/chest.09-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsuoka S, Uchiyama K, Shima H, Ueno N, Oish S, Nojiri Y. Bronchoarterial ratio and bronchial wall thickness on high-resolution CT in asymptomatic subjects: correlation with age and smoking. AJR Am J Roentgenol. 2003;180:513–8. doi: 10.2214/ajr.180.2.1800513. [DOI] [PubMed] [Google Scholar]
- 67.Morrissey BM, Harper RW. Bronchiectasis: sex and gender considerations. Clin Chest Med. 2004;25:361–72. doi: 10.1016/j.ccm.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 68.Zoumot Z, Wilson R. Respiratory infection in noncystic fibrosis bronchiectasis. Curr Opin Infect Dis. 2010;23:165–70. doi: 10.1097/QCO.0b013e328335af91. [DOI] [PubMed] [Google Scholar]
- 69.Wu J, Du H, Wang X, Mei C, Sieck GC, Qian Q. Characterization of primary cilia in human airway smooth muscle cells. Chest. 2009;136:561–70. doi: 10.1378/chest.08-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med. 2001;7:151–6. doi: 10.1016/s1471-4914(01)01953-0. [DOI] [PubMed] [Google Scholar]
- 71.Lee KL, Dai Q, Hansen EL, Saner CN, Price TM. Modulation of ATP-induced calcium signaling by progesterone in T47D-Y breast cancer cells. Mol Cell Endocrinol. 2010;319:109–15. doi: 10.1016/j.mce.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCallister JW, Mastronarde JG. Sex differences in asthma. J Asthma. 2008;45:853–61. doi: 10.1080/02770900802444187. [DOI] [PubMed] [Google Scholar]


