Abstract
Context
Both light and melatonin can be used to phase shift the human circadian clock, but the phase advancing effect of the combination has not been extensively investigated.
Objective
The objective of the study was to determine whether phase advances induced by morning intermittent bright light and a gradually advancing sleep schedule could be increased with afternoon melatonin.
Participants
Healthy adults (25 males, 19 females, between the ages of 19 and 45 yr) participated in the study.
Design
There were 3 d of a gradually advancing sleep/dark period (wake time 1 h earlier each morning), bright light on awakening [ four 30-min bright-light pulses (∼5000 lux) alternating with 30 min room light < 60 lux] and afternoon melatonin, either 0.5 or 3.0 mg melatonin timed to induce maximal phase advances, or matching placebo. The dim light melatonin onset was measured before and after the treatment to determine the phase advance.
Results
There were significantly larger phase advances with 0.5 mg (2.5 h, n = 16) and 3.0 mg melatonin (2.6 h, n = 13), compared with placebo (1.7 h, n = 15), but there was no difference between the two melatonin doses. Subjects did not experience jet lag-type symptoms during the 3-d treatment
Conclusions
Afternoon melatonin, morning intermittent bright light, and a gradually advancing sleep schedule advanced circadian rhythms almost 1 h/d and thus produced very little circadian misalignment. This treatment could be used in any situation in which people need to phase advance their circadian clock, such as before eastward jet travel or for delayed sleep phase syndrome.
Both Light and exogenous melatonin can shift the circadian clock according to phase response curves (PRCs) in which the magnitude and direction of the phase shift varies with the stimulus administration time. In humans, bright light administered in the evening and beginning of the sleep period results in phase delays, whereas bright light administered at the end of the sleep period and in the morning produces phase advances (1). In contrast, a PRC for melatonin shows maximal phase advances in the late afternoon/early evening and maximal phase delays during the sleep period and in the morning (2) (see the superimposed light and melatonin PRCs in Ref. 3). Both bright light and melatonin have successfully been used in laboratory and field settings to phase shift circadian rhythms, accelerate reentrainment, and thus promote adaptation to night shift work or a new time zone after rapid transmeridian jet travel (e.g. Refs. 3–11), whereas melatonin has been used to entrain free-running blind individuals to the 24-h day (12–14). It is harder to phase advance than phase delay human circadian rhythms (1, 15), even with facilitating bright light (16), and this is due, at least in part, to the fact that the human circadian clock has a period slightly longer than 24 h (17, 18).
In a previous study, we showed that advancing the sleep/dark schedule by 1 h/d, and administering bright light immediately on awakening could phase advance the human circadian clock by about 30 min/d (7). The current study showed that the phase advance could be increased to about 50 min/d by adding afternoon melatonin. This is the first study to show that a combination of morning bright light and afternoon melatonin, both timed to phase advance, can produce a larger phase advance shift than bright light alone.
Subjects and Methods
Subjects
Healthy young adults (25 males, 19 females) between the ages of 19 and 45 yr (27.1 ± 5.6, mean ± SD) with normal body mass indices (<31 kg/m2) participated. They had an average morningness-eveningness score (19) of 51.8 ± 8.4. All subjects were nonsmokers; did not consume large amounts of caffeine (>500 mg/d); and reported no medical, psychiatric, or sleep disorders as assessed from interviews and questionnaires. Subjects were free from prescription medications, except for four female subjects who were taking oral contraceptives. We excluded individuals who had traveled overseas in the previous month and/or worked a night shift during the 2 months before the start of the study. The protocol was approved by the Rush University Medical Center Institutional Review Board. Subjects gave written informed consent before the study and were paid for their participation.
Protocol
This was a between subjects design with three groups (placebo or 0.5 or 3.0 mg melatonin) randomly assigned but with an attempt to balance the groups for sex and morningness-eveningness score.
Each subject was assigned a strict 8-h baseline sleep schedule that was similar to their typical sleep schedule. Scheduled bedtimes ranged from 2300 to 0200 h. Subjects had to remain in bed, in the dark, trying to sleep throughout the 8-h scheduled sleep/dark episodes. They were permitted to nap during a 6-h period centered 12 h from the midpoint of sleep. On d 7 they came to the lab for a baseline phase assessment beginning 7 h before and ending 3 h after their baseline bedtime and then slept in the laboratory and were awakened at their baseline wake time. Subjects continued their baseline sleep schedule on d 8–10. The three treatment days began on d 11 (treatment d 1). On d 14 the subjects underwent their final phase assessment beginning 10 0 before and ending 2 h after their baseline bedtime. During the 3 treatment days, wake time was successively advanced by 1 h each day (Fig. 1), and naps were not permitted.
Fig. 1.
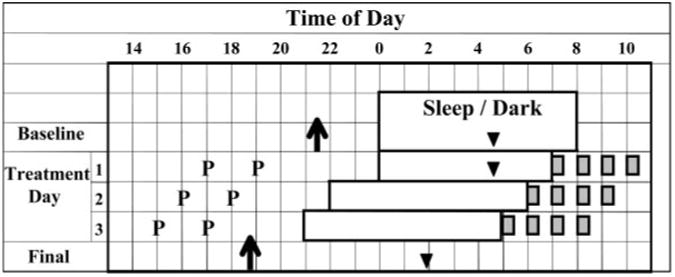
Protocol with all elements normalized to a 0800 h wake time. Rectangles represent sleep/dark episodes, and filled gray squares represent the four bright light pulses. Ps, Times of pill administration. Arrows represent the times of the average DLMOs for the 0.5-mg melatonin group determined from the baseline and final phase assessments. Triangles represent the estimated times of the temperature minima (7 h after the DLMO) for the 0.5-mg melatonin group. The arrow and triangle from the baseline phase assessment (which was conducted a few days earlier) are drawn on the last baseline day to illustrate the phase relationship between these circadian phase markers and sleep. There was no sleep/dark episode during the phase assessments. See Fig. 1 in Burgess et al. (7) for an expanded, more detailed protocol diagram.
Phase-advancing treatment
During the 3 treatment days, subjects slept at the lab in individual, dark, temperature-controlled bedrooms. Each afternoon, subjects were administered either 0.5 or 3.0 mg melatonin or matching placebo. The melatonin was timed to induce the maximal possible phase advance for each dose. The PRC for 0.5 mg melatonin shows a maximum phase advance when melatonin is administered about 3 h before the dim light melatonin onset (DLMO) (2, 3). Because the DLMO occurs about 2 h before bedtime, we gave the 0.5-mg dose 5 h before baseline bedtime on the first day of treatment. If we gave the 3.0-mg dose at the same time, then, because it would remain in the body longer, it might spill over onto the delay portion of the 0.5 mg melatonin PRC. Therefore, we generated a partial PRC for 3.0 mg melatonin using an ultradian sleep/dark, wake/light protocol that permitted free-running circadian rhythms (3). We found that the maximal phase shift occurred about 5 h before the DLMO (data not shown). Thus, we gave the 3.0-mg dose 7 h before bedtime. The half life of melatonin is about 40 min (20), and so both our doses should overlap with the endogenous melatonin levels and reach similar levels by bedtime.
To ensure the blind of the study, the subjects took two pills on each treatment day (Fig. 1). For the placebo group, both pills were placebo. For the 0.5-mg group, the first pill was placebo and the second pill was 0.5 mg melatonin. For the 3.0-mg group, the first pill was 3.0 mg melatonin and the second pill was placebo. The timing of the pills was advanced by 1 h each day to keep up with the expected phase advance in the circadian clock, and its melatonin PRCs, and to ensure that they continued to be administered near the optimal circadian phase. To confirm that the pills were correctly administered, subjects gave three saliva samples at hourly intervals for assessment of melatonin levels; the first sample was taken 1 h 10 min after administration of the first pill.
On awakening on treatment days, the subjects were exposed to four 30-min bright-light pulses produced by a single light box (54 × 54 cm diffuser screen size; Enviro-Med, Vancouver, WA) placed on a desk about 40 cm in front of the subjects' eyes. The light box contained four 54-cm-long 40-W cool white fluorescent horizontal lamps (PL-L 40 W/41/RS/IS, 4100 K; Philips, Eindhoven, The Netherlands). Light intensity ranged from 3160 to 6880 lux, depending on the angle of gaze with a mean of 4949 ± 770 lux as measured periodically during the exposure with a 401025 light meter (Extech, Waltham, MA). The irradiance (400–1064 nm) at the typical distance in the typical angle of gaze, was 2000 μW/cm2 (measured with an IL 1400 radiometer and SEL-033/F/W detector; International Light, Newburyport, MA). The irradiance in the blue part of the spectrum (400–490 nm) was 470 μW/cm2, as measured with a SCS490 sharp cut filter. The total photon density was 5.5 × 1015 photons/cm2•sec and in the blue region was 1.1 × 1015 photons/cm2.sec. The 30 min of room light in between the bright-light pulses was less than 60 lux and was produced by a ceiling light fixture containing three cool-white fluorescent lamps, controlled by a dimmer switch.
Circadian phase assessments
During phase-assessment sessions, subjects remained awake, sitting in recliners in less than 5 lux. Saliva samples were collected using Salivettes (Sarstedt, Newton, NC) at 30-min intervals, centrifuged immediately after collection, and frozen. Melatonin was measured by RIA (Pharmasan Labs, Osceola, WI). The intraassay and interassay variability was 12.1 and 13.2% respectively; the sensitivity of the assay was 0.7 pg/ml (for more details about the phase assessment session, see Ref. 21).
Measures of sleep, other procedures, and compliance
Subjects completed daily sleep logs with their bedtime, estimated sleep onset, any nighttime wake awakenings longer than 5 min, their final wake time, and the time they got out of bed. Subjects wore activity monitors on their nondominant wrist and a photosensor around their necks as a medallion (Actiwatch-L, MiniMitter Inc., Bend, OR). Every 2–3 d, the subjects visited the laboratory where the data from their wrist actigraph, sleep logs, bed and wake time phone calls, and medallion photosensor were examined and compared in their presence to verify compliance.
Subjects were permitted to consume caffeine (up to 300 mg) during the first 3 h of wake each day. Nonsteroidal antiinflammatory drugs were not permitted after d 3, and alcohol was not permitted after d 4.
Symptom questionnaires
Subjects completed the Columbia Jet Lag Scale (22) each day at bed-time and the How Are You Feeling Right Now? (HAYFRN) questionnaire, which includes the Stanford Sleepiness Scale (SSS) (23) five times a day, as described in our previous study (21). On each treatment day, subjects rated themselves on the SSS every 30 min, from 30 min before the first pill was administered until bedtime.
Data analysis
Circadian Phase
A threshold was determined from each melatonin profile as the average of the five lowest continuous daytime data points from the beginning of the session plus 15% of the average of the five highest continuous data points. The threshold mean ± SD was 2.9 ± 1.6 pg/ml. Then the melatonin profiles were smoothed, using the high level of smoothing, with the Prism software program (GraphPad, Inc., San Diego, CA), which used locally weighted regression scatter plot smoothing, (LOWESS). The DLMO was defined as the first minute of the smoothed curve that exceeded and stayed above the threshold. We added 7 h to each DLMO to estimate the temperature minimum (Tmin) because this is the typical interval (e.g. Refs. 8 and 24–26).
Sleep parameters and symptom questionnaires
For sleep logs, total sleep time (TST) was the time from sleep onset to final awakening minus any awakenings longer than 5 min, sleep onset latency was the time between bedtime and sleep onset, and sleep efficiency was TST divided by time in bed. Sleep from actigraphy was calculated with the Actiware-Sleep 3.1 program (MiniMitter); TST, sleep onset latency, and sleep efficiency were determined. The analysis program was set to the highest sensitivity for detecting movement, which will result in an underestimation of sleep time because any movement that occurs during sleep is considered wake. For each subject and each item, the five HAYFRN questionnaires per day were averaged.
Statistical analysis
The phase advance in the DLMO (from baseline to final phase assessment) for the three groups was analyzed with a one-way ANOVA, followed by Tukey's honestly significant difference post hoc test. The 30-min SSS ratings during the evenings of the treatment days were analyzed with a two-way repeated-measures ANOVA (time × group). The sleep parameters (from wrist actigraphy and sleep logs) were analyzed with a two-way multivariate analysis of variance (MANOVA) with one within subjects factor day with four levels (baseline mean of d 2–6, treatment d 1–3) and one between-subjects factor group (placebo and 0.5 and 3.0 mg). When significant, Greenhouse-Geisser corrected degrees of freedom were used for univariate analyses. The Columbia Jet Lag score and SSS from the HAYRFN questionnaires were each analyzed with a two-way ANOVA (days × group). The remaining items on the HAYFRN questionnaires were analyzed with a two-way MANOVA (days × group). Significant univariate analyses were followed by pair-wise comparisons. Data are presented as mean ± SD, unless otherwise stated.
Results
The three groups did not differ significantly in age, sex, or morningness-eveningness score. Table 1 (baseline rows) shows that they did not differ in DLMO, sleep schedules, or the interval between the Tmin and wake time and thus the circadian time of light exposure. On the first treatment day, the 0.5-mg dose was administered 2.4 ± 1.2 h before the baseline DLMO and the 3.0-mg dose 4.8 ± 0.7 h before, both close to our targets of 3 and 5 h. Melatonin levels for 1 h 10 min, 2 h 10 min, and 3 h 10 min after the first pill was administered were 260 ± 47, 216 ± 89, and 94 ± 97 pg/ml, respectively, for the 3.0-mg group and 2 ± 2, 79 ± 58, and 59 ± 44 pg/ml, respectively, for the 0.5-mg group.
Table 1. Circadian phase marked by the DLMO, the estimated Tmin,a and scheduled sleep times.
| Placebo | 0.5 mg melatonin | 3.0 mg melatonin | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Mean | SD (h) | Mean | SD (h) | Mean | SD (h) | ||||
| Phase advance of DLMO (h) | 1.7 | 0.7 | 2.5b | 0.7 | 2.6b | 0.3 | |||
| Shift/day (min)c | 34 | 50 | 52 | ||||||
| Baseline | |||||||||
| DLMO | 21:41 | 1.0 | 21:25 | 1.2 | 21:51 | 0.8 | |||
| Bedtime | 24:04 | 0.8 | 24:04 | 0.7 | 24:05 | 0.6 | |||
| Wake time | 8:04 | 0.8 | 8:04 | 0.7 | 8:05 | 0.6 | |||
| Estimated Tmin | 4:41 | 1.0 | 4:25 | 1.2 | 4:51 | 0.8 | |||
| Tmin to wake interval (h) | 3.4 | 0.9 | 3.6 | 1.2 | 3.2 | 0.7 | |||
| Treatment d 1 | |||||||||
| Tmin to wake interval (h) | 2.4 | 0.9 | 2.6 | 1.2 | 2.2 | 0.6 | |||
| Treatment d 3 | |||||||||
| Bedtime | 21:04 | 0.8 | 21:04 | 0.7 | 21:05 | 0.6 | |||
| Wake time | 5:04 | 0.8 | 5:04 | 0.7 | 5:05 | 0.6 | |||
| Estimated Tmina | 3:34 | 1.3 | 2:47 | 1.0 | 3:06 | 0.9 | |||
| Tmin to wake interval (h) | 1.5 | 1.0 | 2.3 | 0.9 | 2.0 | 0.7 | |||
| Final | |||||||||
| DLMO | 20:01 | 1.4 | 18:58 | 1.0 | 19:14 | 0.9 | |||
| Estimated Tmina | 3:01 | 1.4 | 1:58 | 1.0 | 2:14 | 0.9 | |||
Calculated by adding 7 h to the DLMO.
Significantly different from placebo (P < 0.01).
Calculated by dividing cumulative phase advance of DLMO after 3 treatment days by 3.
Circadian phase advances
Figure 2 shows that larger phase shifts were observed with melatonin, compared with placebo, and Fig. 3 shows that, overall, the largest advance occurred in the 3.0-mg group. The mean phase advances (Table 1) were significantly different among the groups [F (2,41) = 9.5, P < 0.001]. Post hoc analysis showed significantly larger phase shifts in the 0.5-mg (P = 0.003) and 3.0-mg (P = 0.001) conditions, compared with placebo, but there was no difference between the two melatonin groups (P = 0.79). Figure 1 shows the time of the mean DLMOs for the 0.5-mg group.
Fig. 2.
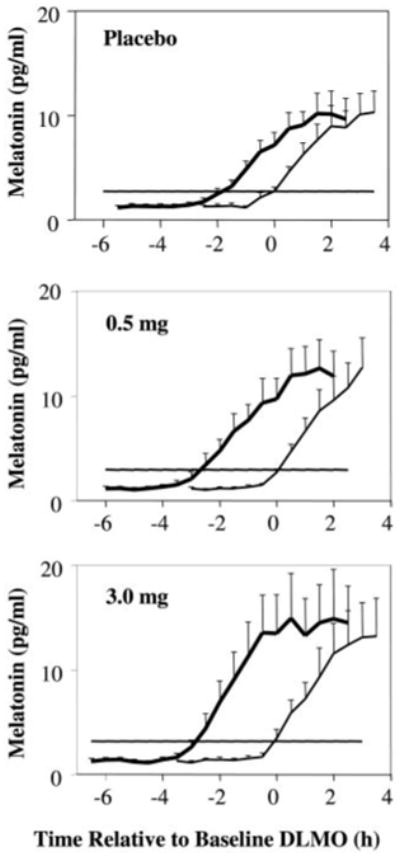
Melatonin profiles for the three treatment groups: placebo (n = 15), 0.5 mg (n = 16), and 3.0 mg (n = 13). Error bars, se. In each graph, the thinner line shows the mean profile during the baseline phase assessment, and the bold line shows the profile during the final phase assessment. Horizontal lines indicate the average DLMO threshold for each group. The mean melatonin profiles were constructed referencing each subject's data to the time of his/her DLMO from the baseline phase assessment.
Fig. 3.
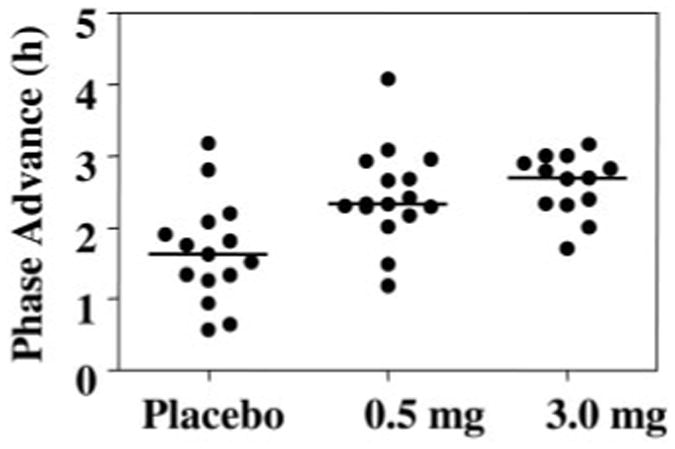
Phase advances in the DLMO for all subjects. Horizontal lines represent the medians, which were 1.6, 2.3, and 2.7 h.
Sleep
All of our subjects slept quite well (Fig. 4). The decrease in TST on the first treatment day was because subjects were put to bed at their usual time but were awakened 1 h earlier (Fig. 1). On the other days, subjects slept almost all of the allotted 8 h (Fig. 4). The 3.0-mg group had slightly longer sleep latencies during the treatment days and correspondingly poorer sleep efficiencies. However, there were no significant group differences in any of the sleep measures. The sleep MANOVA indicated a nonsignificant main effect of group, a nonsignificant group by days interaction, but a significant main effect of days [F (18, 23) = 570, P < 0.001]. Univariate analysis indicated that this was due to the reduction in TST on treatment d 1.
Fig. 4.
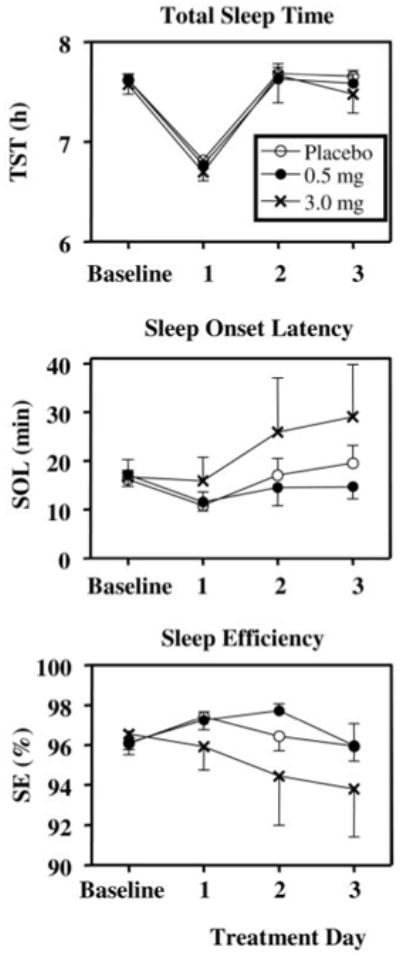
Sleep parameters from sleep logs during baseline (d 2–6) and treatment days for the three groups. Total sleep time was reduced on treatment d 1 as a function of the protocol (Fig. 1). Values are means + or − SE.
Symptom questionnaires
The SSS ratings (Fig. 5, top) showed a slight increase on the first day of treatment relative to baseline that then decreased on treatment d 2 and 3. There was a significant main effect of days [F (3,113) = 5.60, P = 0.002] with treatment d 1 and 2 being significantly higher than both baseline (P < 0.01) and treatment d 3 (P < 0.02). There was no significant main effect of group or days by group interaction.
Fig. 5.
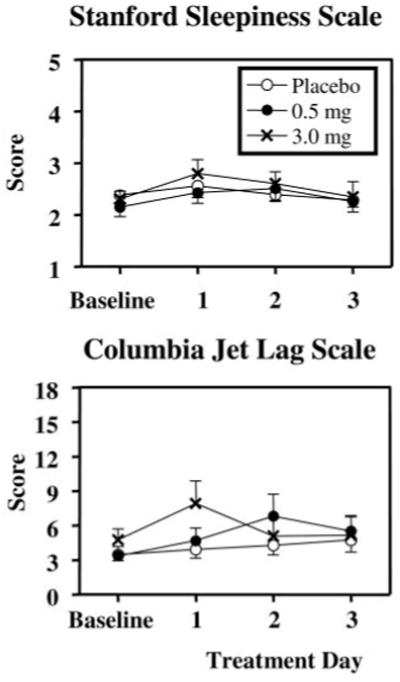
Symptom questionnaires during baseline (d 2–6) and treatment days for the three groups. The SSS was administered five times per day, and the scale maximum (most sleepy) is 7. The Columbia Jet Lag Scale was administered at bedtime and the scale maximum is 36. Values are means + or − SE.
The Columbia Jet Lag scores changed very little during the study (Fig. 5). There were no significant main effects of group or days and no significant group by days interaction.
The ratings for the six symptoms on the HAYFRN questionnaire were mild and evenly distributed across study groups and days. The MANOVA indicated no significant main effects of group or days and no significant days by group interaction.
There was a significant increase in sleepiness in the hours before bedtime during the treatment days for all groups (Fig. 6) [significant main effect of time, F (4,175) = 92.5, P < 0.001]. However, there was no significant main effect of group or time by group interaction.
Fig. 6.
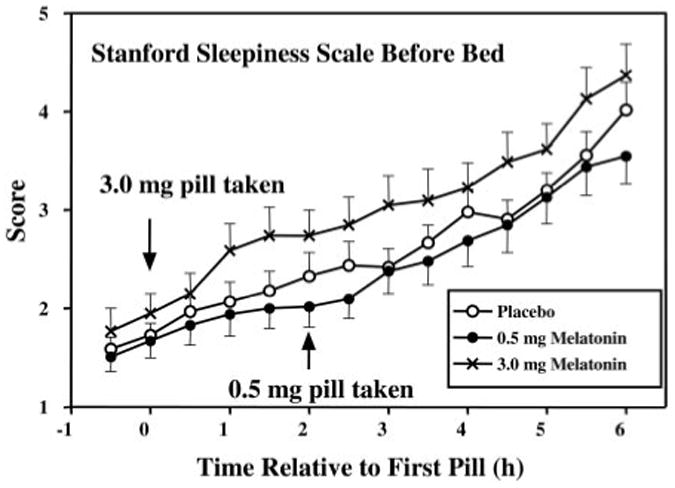
SSS on treatment days from 30 min before administration of the first pill until bedtime. Values are means + or − SE.
Discussion
We demonstrated that the phase advances produced with a gradually advancing sleep/dark schedule and morning intermittent bright light can be significantly increased by afternoon melatonin. In addition, this treatment produced relatively little misalignment between circadian rhythms and, the sleep schedule and thus did not produce side effects or jet lag-type symptoms.
Although slightly larger phase shifts were obtained with the 3.0-mg dose, compared with 0.5 mg, this difference was not statistically significant. In addition, the higher melatonin dose made subjects slightly, although not significantly, more sleepy in the evenings after taking the pill. Therefore, we recommend using the 0.5-mg dose in combination with morning intermittent bright light and an advancing sleep schedule in any situation in which people need to advance their circadian rhythms, such as for people with delayed sleep phase syndrome (27) or before eastward jet travel. Most people would be able to take the 3.0-mg dose without decrements in alertness, especially if, they were active after taking the pill because the soporific effects are posture dependent (28). We advanced wake time by 1 h but not bedtime on the first day of treatment, cutting sleep short by 1 h, which was probably responsible for the slight increase in sleepiness on treatment d 1. Therefore, we advise also advancing bedtime and keeping the sleep opportunity the same on all treatment days.
Although appropriately timed bright light and melatonin may be able to hasten reentrainment after an abruptly advanced sleep schedule, as occurs with early morning shifts or after eastward jet travel, people will suffer several days of circadian misalignment and the associated jet lag-type symptoms until reentrainment is complete. Arendt et al.(4, 29) recommended taking 5.0 mg melatonin in the afternoon for 1–3 d before departure for a little preflight advance and then at bedtime at the destination to maximize both the phase-advancing and sleep-inducing effects. This regimen is ideal with the doses we tested when bedtime is advanced 5–7 h, but there will still be several days of circadian misalignment. With our treatment, a gradually advancing sleep schedule, gradually advancing morning bright light, and gradually advancing afternoon melatonin are used preflight and so more of the phase shifting is done ahead of time. However, it is not necessary to follow the treatment for a number of days equivalent to the number of time zones crossed. Partially advancing rhythms preflight will reduce the severity and duration of misalignment and will also reduce the risk of antidromic reentrainment because light is less likely to fall on the phase-delay portion of the light PRC (for further explanation, see Refs. 3 and 21).
Ideally, a treatment that produces even larger phase advances would be even better as long as the circadian rhythms remained aligned with the sleep schedule, as they did in the current study. One way to increase the phase advance would be to use light that is enriched with blue wavelengths to which the circadian system is most sensitive (e.g. Refs. 30-33). It is also possible that a different timing of melatonin administration could produce larger phase advances because when it comes to producing phase shifts, the timing is as critical as the dose. In the current study, the two melatonin doses were administered at different circadian phases to coincide with the maximum phase-advance regions of their respective PRCs. However, the timing of the 0.5-mg dose was based on a PRC generated from subjects living at home in relatively uncontrolled conditions, and there was a large degree of scatter in the data points contributing to the PRC (2). In addition, these subjects were entrained to 24-h zeitgebers and thus the magnitude of the phase shifts was limited. The timing of the 3.0-mg dose was based on our own only preliminary partial PRC in a study in which subjects free-ran through an ultradian light-wake/dark-sleep schedule. PRCs to different doses of melatonin using identical protocols need to be generated to pinpoint the optimal circadian times for the administration of different doses.
Two previous studies comparing different doses of melatonin for producing phase shifts showed a dose-dependent response (8, 34). In a study from our laboratory (8), the sleep/dark schedule was abruptly advanced 7 h, and 0.5 or 3.0 mg melatonin was administered 7.5 h before baseline bedtime on four consecutive days. Larger phase shifts were observed with the 3.0-mg dose, compared with the 0.5-mg dose (3.9 vs. 3.0 h)., Another group (34) observed larger phase advances with a 5.0-mg dose (1.4 h), compared with 0.5-mg (0.7 h) and 0.05-mg (0.4 h) doses, all administered approximately 7 h before bed-time. In both studies, the different doses were administered at the same time, and so the greater phase shifts with the larger doses may have been, at least in part, due to a more ideal timing for these doses rather than the increased amount of melatonin present.
When the individual phase shifts are considered (Fig. 3), there appears to be a ceiling for maximum phase shift of about 3 h, but the minimum shift increased from placebo to 0.5 to 3.0 mg. This implies that there is a limit in the magnitude of phase shift that can be attained and that melatonin increases the chances of attaining this limit. In another study from our laboratory (8), a similar pattern was observed with a maximum advance of approximately 4–5 h and the minimum increasing from placebo to the 0.5- to 3.0-mg dose (see Fig. 3 in Ref. 8). There is probably an upper limit to the size of phase shift that can be induced in 1 d; dose-dependent curves for phase shifting with light exhibit a plateau (35), and in studies with large abrupt advances of sleep/dark, the maximum phase advances achieved were never more than 2 h/d (for a detailed review, see Ref. 21). We can speculate that if with light alone the daily maximum shift is not achieved, then coadministration of melatonin may push the system to the upper limit. Alternatively, daily limits with melatonin alone and light alone may exist such that the combination can produce larger cumulative phase shifts than either can individually.
Acknowledgments
Melatonin and matching placebos were provided by Ecological Formulas (a division of Cardiovascular Research Ltd., Concord, CA). Light boxes were donated by Enviro-Med (Vancouver, WA). Authors H.J.B., C.J.G., and M.R.S. are listed alphabetically. They all made important contributions to this work. We are grateful to Young Cho, Meredith Durkin, Valerie Ellois, Cynthia Hiltz, Hyungsoo Kim, Clara Lee, Katy Lenz, Tom Molina, and Jonathan Swisher for all their assistance with data collection. We thank Erin Lensch for help with the sleep analysis and figures. We thank our medical director, Keith L. Callahan, M.D.
This work was supported by National Institutes of Health Grant R01 NR007677.
Abbreviations
- DLMO
Dim light melatonin onset
- HAYFRN
How Are You Feeling Right Now? Questionnaire
- MANOVA
multivariate analysis of variance
- PRC
phase-response curve
- SSS
Stanford Sleepiness Scale
- Tmin
temperature minimum
- TST
total sleep time
References
- 1.Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 1999;31:87–98. doi: 10.3109/07853899908998783. [DOI] [PubMed] [Google Scholar]
- 2.Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 3.Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–365. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arendt J, Skene DJ, Middleton B, Lockley SW, Deacon S. Efficacy of melatonin treatment in jet lag, shift work, and blindness. J Biol Rhythms. 1997;12:604–617. doi: 10.1177/074873049701200616. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Sasaki M, Itoh H, Ozone M, Yamadera W, Hayshida K, Ushijima S, Matsunaga N, Obuchi K, Sano H. Effect of 3 mg melatonin on jet lag syndrome in an 8-h eastward flight. Psychiatry Clin Neurosci. 2000;54:377–378. doi: 10.1046/j.1440-1819.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- 6.Samel A, Wegmann HM, Vejvoda M, Maab H, Gundel A, Schutz M. Influence of melatonin treatment on human circadian rhythmicity before and after a simulated 9-hr time shift. J Biol Rhythms. 1991;6:235–248. doi: 10.1177/074873049100600304. [DOI] [PubMed] [Google Scholar]
- 7.Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol Regul Integr Comp Physiol. 2002;282:R454–R463. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18:513–523. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- 10.Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. 1990;322:1253–1259. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- 11.Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002;17:556–567. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- 12.Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- 13.Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 14.Hack LM, Lockley SW, Arendt J, Skene DJ. The effects of low-dose 0.5-mg melatonin on the free-running circadian rhythms of blind subjects. J Biol Rhythms. 2003;18:420–429. doi: 10.1177/0748730403256796. [DOI] [PubMed] [Google Scholar]
- 15.Aschoff J, Hoffmann K, Pohl H, Wever R. Re-entrainment of circadian rhythms after phase shifts of the zeitgeber. Chronobiologia. 1975;2:23–78. [PubMed] [Google Scholar]
- 16.Mitchell PJ, Hoese EK, Liu L, Fogg LF, Eastman CI. Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms. 1997;12:5–15. doi: 10.1177/074873049701200103. [DOI] [PubMed] [Google Scholar]
- 17.Wever RA. The circadian system of man. New York-Heidelberg-Berlin: Springer-Verlag; 1979. [Google Scholar]
- 18.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period ofthe human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 19.Horne JA, Ostberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 20.Waldhauser F, Waldhauser M, Lieberman HR, Deng MH, Lynch HJ, Wurtman RJ. Bioavailability of oral melatonin in humans. Neuroendocrinology. 1984;39:307–313. doi: 10.1159/000123997. [DOI] [PubMed] [Google Scholar]
- 21.Eastman CI, Gazda CJ, Burgess HJ, Crowley SJ, Fogg LF. Advancing circadian rhythms before eastward flight: a strategy to prevent or reduce jet lag. Sleep. 2005;28:33–44. doi: 10.1093/sleep/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spitzer RL, Terman M, Williams JB, Terman JS, Malt UF, Singer F, Lewy AJ. Jet lag: clinical features, validation of a new syndrome-specific scale, and lack of response to melatonin in a randomized, double-blind trial. Am J Psychiatry. 1999;156:1392–1396. doi: 10.1176/ajp.156.9.1392. [DOI] [PubMed] [Google Scholar]
- 23.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 24.Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. J Biol Rhythms. 2004;19:248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- 25.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–188. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 26.Eastman CI, Martin SK, Hebert M. Failure of extraocular light to facilitate circadian rhythm reentrainment in humans. Chronobiol Int. 2000;17:807–826. doi: 10.1081/cbi-100102116. [DOI] [PubMed] [Google Scholar]
- 27.Wyatt JK. Delayed sleep phase syndrome: pathophysiology and treatment options. Sleep. 2004;27:1195–1203. doi: 10.1093/sleep/27.6.1195. [DOI] [PubMed] [Google Scholar]
- 28.Cajochen C, Krauchi K, Wirz-Justice A. The acute soporific action of daytime melatonin administration: effects onthe EEG during wakefulness and subjective alertness. J Biol Rhythms. 1997;12:636–643. doi: 10.1177/074873049701200619. [DOI] [PubMed] [Google Scholar]
- 29.Arendt J, Aldhous M, Marks V. Alleviation of jet lag by melatonin: preliminary results of controlled double blind trial. Br Med J. 1986;292:1170. doi: 10.1136/bmj.292.6529.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 2003;342:37–40. doi: 10.1016/s0304-3940(03)00223-4. [DOI] [PubMed] [Google Scholar]
- 33.Smith M, Revell V, Eastman C. Blue-enriched light enhances circadian phase advances. Soc Light Treatment Biol Rhythms Abstracts. 2005;17:52. [Google Scholar]
- 34.Deacon S, Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 1995;688:77–85. doi: 10.1016/0006-8993(95)96872-i. [DOI] [PubMed] [Google Scholar]
- 35.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Part 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


