Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disease, wherein a progressive loss of cholinergic synapses occurs in hippocampus and neocortex. Decreased concentration of the neurotransmitter, acetylcholine (ACh), appears to be critical element in the development of dementia, and the most appropriate therapeutic approach to treat AD and other form of dementia is to restore acetylcholine levels by inhibiting both major form of cholinesterase: Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Consequently, researches have focused their attention towards finding cholinesterase inhibitors from natural products. A large number of such inhibitors have been isolated from medicinal plants. This review presents a comprehensive account of the advances in field of cholinesterase inhibitor phytoconstituents. The structures of some important phytoconstituents (collected through www.Chemspider.com) are also presented and the scope for future research is discussed.
Keywords: Acetylcholinesterase, alkaloids, alzheimer's disease, butyrylcholinesterase, buxaceae
INTRODUCTION
Alzheimer's disease (AD) is a neurodegenerative disease of the central nervous system, wherein cholinergic neurons projecting to the neocortex and hippocampus are predominantly affected causing profound memory impairment, emotional disturbance, and personality changes in late stages.[1,2] According to cholinergic hypothesis, memory impairment in Alzheimer's disease is due to the deficit of cholinergic function in the brain, thereby, reducing hippocampal and cortical levels of the neurotransmitter acetylcholine (ACh) and associated enzyme choline transferase.[3,4] In the healthy brain acetylcholinesterase (AChE) is the most important enzyme regulating the level of ACh, while butyrylcholinesterase (BChE) plays a minor role. In patients with AD, the level of AChE activity declines and the activity of BChE increases and the ratio between BChE and AChE can change from 0.6 in the normal brain to as high as 11 in cortical areas affected by the disease.[5] Therefore, inhibition of AChE and BChE is the most effective therapeutic approach to treat the symptoms of AD.[5,6] Consequently, cholinesterase inhibitors are the only approved drugs for treating patients with mild to moderately severe Alzheimer's disease.[3,4,7]
Although, synthetic drugs such donepezil, neostigmine, and rivastigmine are available for the symptomatic treatment of AD, search for newer molecules from natural products has gained much attention by the researchers worldwide. As a result, a number of botanicals used in various traditional systems of medicines as memory enhancers have been tested for anticholinesterase activity. Bacopa monniera, Ginkgo biloba, Acorus calamus, Epimedium koreanum, Rhododendron ponticum, Rhododendron luteum, Corydalis solida, Glaucium corniculatum, and Buxus sempervirens are some of the medicinal plants used as cognitive enhancers by traditional healers which have been found to posses moderate to excellent anticholinesterase activity.[8,9,10,11,12] Further, a number of active compounds with good cholinesterase activity have been isolated from medicinal plants. With this background, the present review was planned to comprehend the fragmented information available on the cholinesterase inhibitors from medicinal plants.
ANTICHOLINESTERASE PHYTOCHEMICAL CLASSES
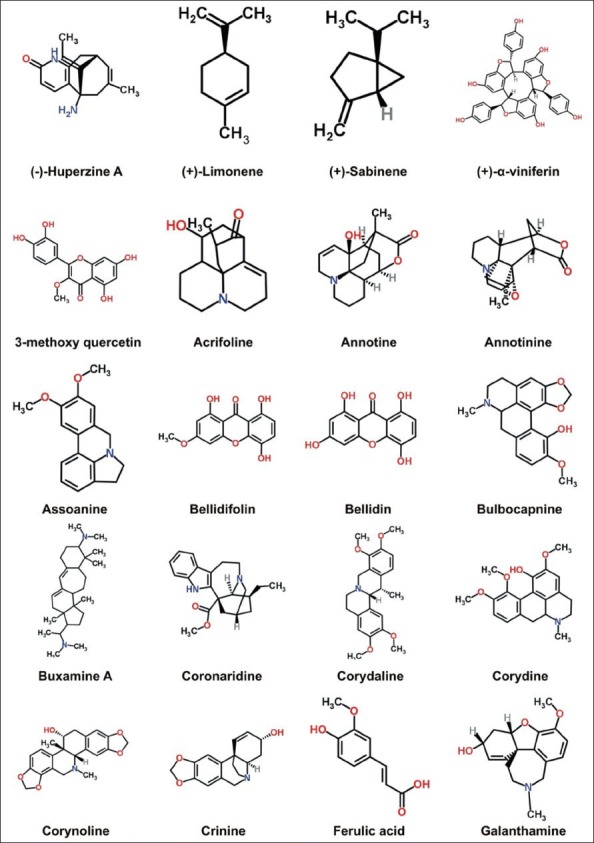
In this review, 119 compounds having anti-AChE activity [Table 1] and 67 compounds having anti-BChE activity are presented [Table 2]. The structures of some important anticholinesterase compounds are presented in Figures 1a–c. Majority of these phytochemicals with potential AChE and BChE inhibitory activity are alkaloids followed by terpenes, sterols, flavanoids, and glycosides. Triterpenoid alkaloids, steroidal alkaloids, indole alkaloids, isoquinoline alkaloid, and lycopodane-type alkaloid are the major types of alkaloids having significant anticholinesterase activity making them promising candidates to be used as cholinesterase inhibitors in clinical practice. Most of the compounds having potential anticholinesterase activity are isolated from Buxaceae, Amaryllidaceae, Lycopodiaceae, Lamiaceae, Chenopodiaceae, Papaveraceae, Apocynaceae, and Labiatae species.[13,17,18,25,27,29,45,59] Following are three of the imporant families having potential compounds to be used as anticholonesterase inhibitors.
Table 1.
Acetylcholinesterase inhibitors from medicinal plants
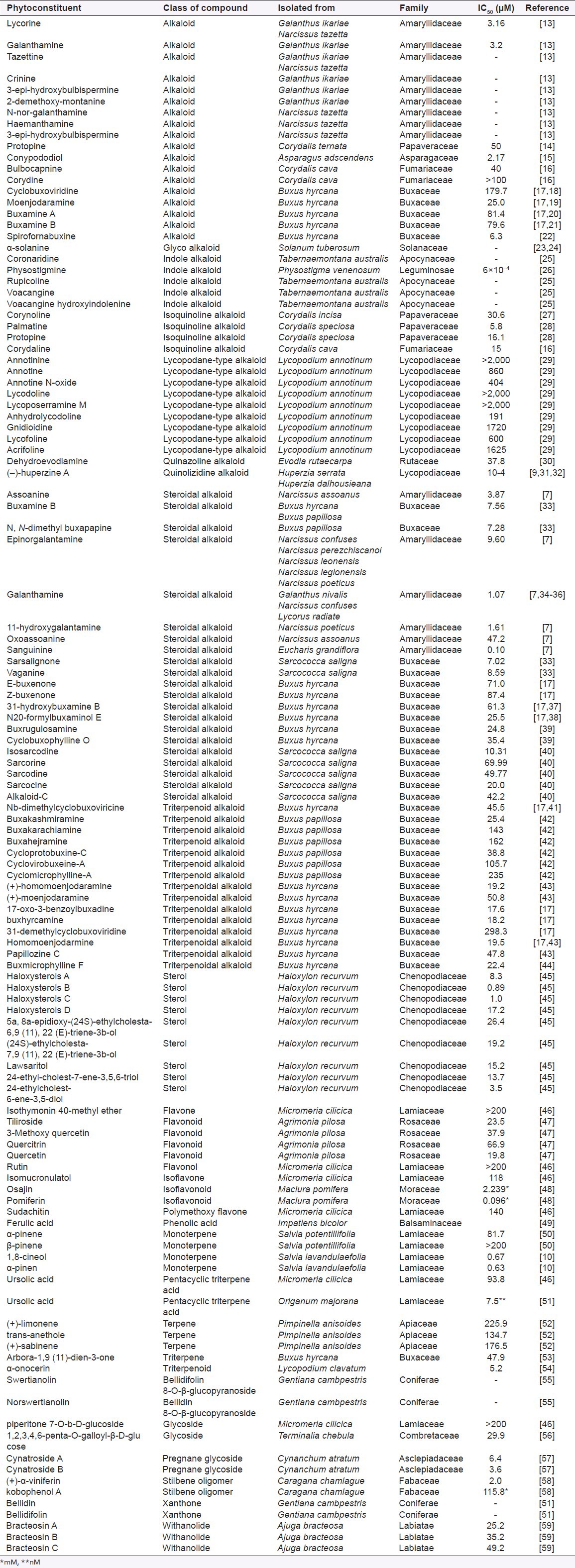
Table 2.
Butyrylcholinesterase inhibitors from medicinal plants
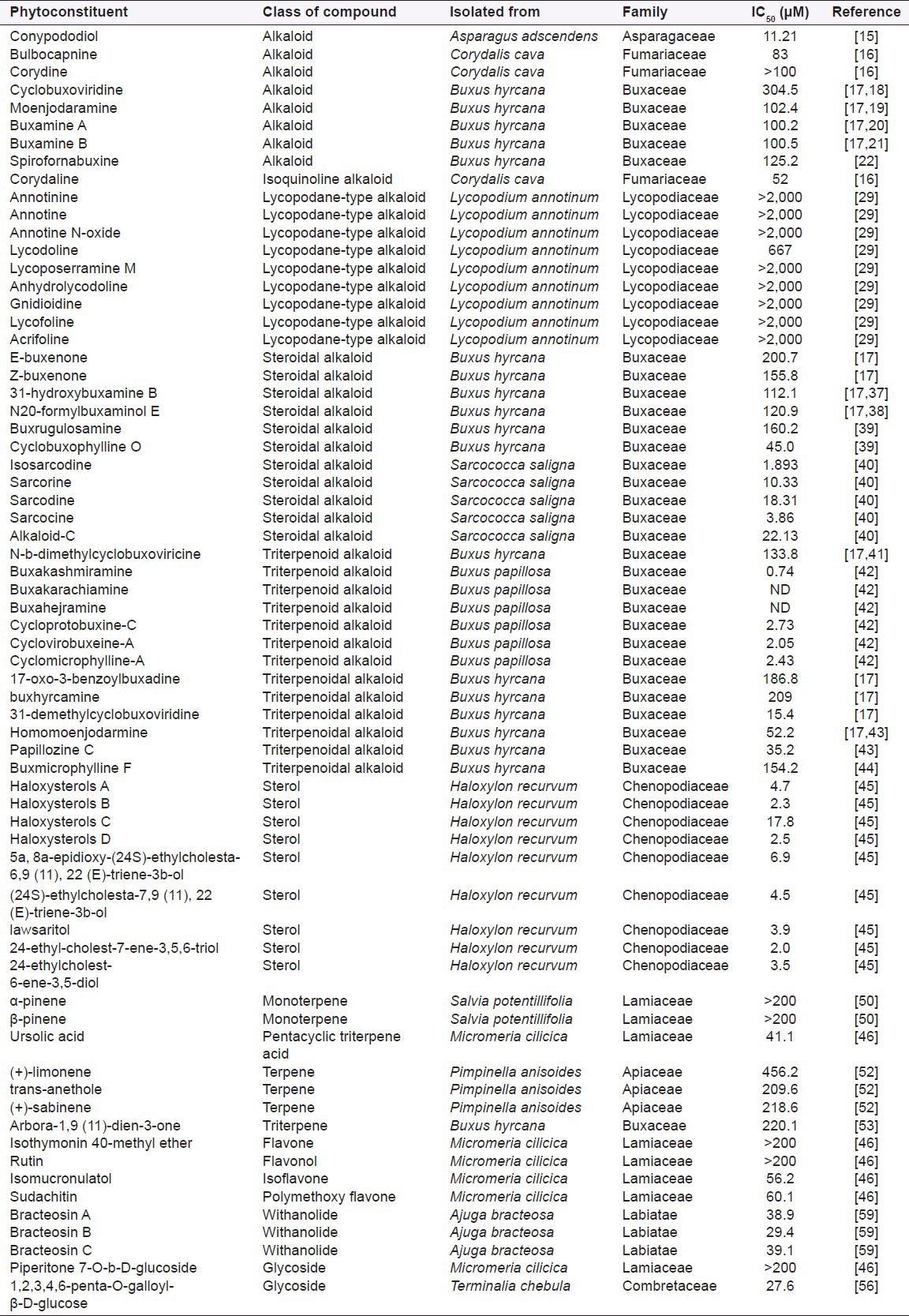
Figure 1a.
Structures of some important anticholinesterase compounds
Figure 1c.
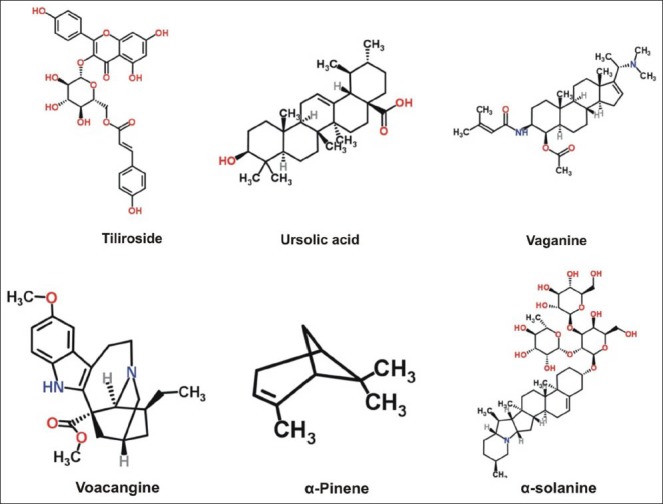
Structures of some important anticholinesterase compounds
Figure 1b.
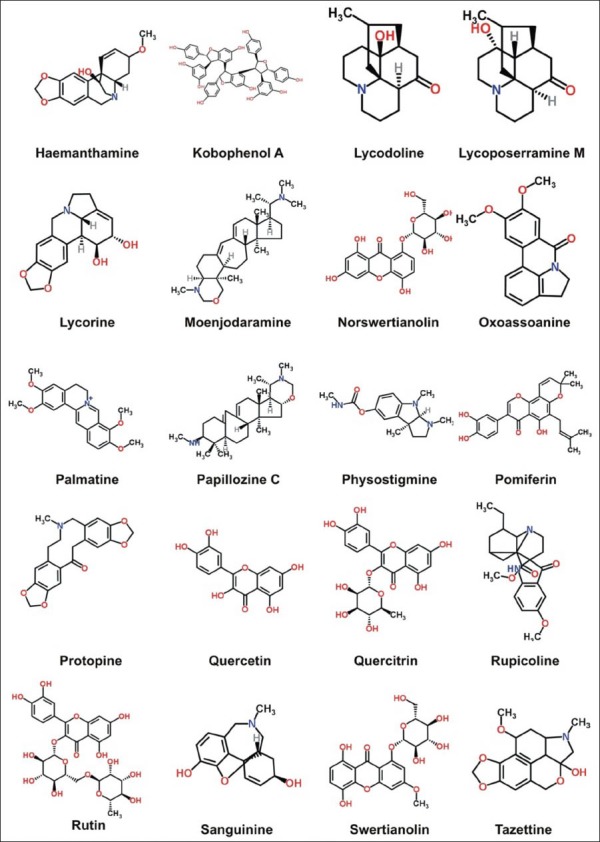
Structures of some important anticholinesterase compounds
Buxaceae
Buxaceae is a small family of 4-5 genera consisted of about 90-120 species of flowering plants which are usually shrubs or small trees with a cosmopolitan distribution.[60] The plants of this family find extensive uses in the folkloric medicine particularly for memory-related disorders. Furthermore, studies have evidenced that terpenoidal alkaloids are the major chemical constituents responsible for the biological activities of the plants of this family.[61]
Amaryllidaceae
The plants of Amaryllidaceae family are well-known for their ornamental value and medicinal properties. The family has attracted considerable attention due to the content of alkaloids of its species, which showed interesting biological properties.[62] The chemical structures of these alkaloids are very variable as well as their pharmacological properties. Some species of this family contain galanthamine, an acetylcholinesterase inhibitor approved for the treatment of AD, as well as other alkaloids with interesting pharmacological activities: Antimalarial, antiviral, and antiproliferative.[63,64,65,66] Galanthamine is an important reversible, long-lasting, selective, and competitive inhibitor of AChE isolated from Amaryllidaceae plant species such as Amaryllis, Galanthus, Leucojum, Pancratium, and Zephyranthes.[67] It is a good example of a natural product substituting synthetic drugs in the treatment of AD.
Lycopodiaceae
Lycopodiaceae family is comprised of four genera: Huperzia Bernh., Phylloglossum Kunze, Lycopodium L., and Lycopodiella Holub and has a wide distribution throughout the world.[68] Lycopodium species are used widely in Argentinian traditional medicine for memory improvement and Huperzine A is an alkaloid having potent, specific, and reversible acetylcholinesterase inhibiton isolated from Huperzia serrata.[69] Extensive studies on Huperzine A as a lead compound for the development of more effective anti-AChE drugs for the treatment of AD relative to those approved by the Food and Drug Association (FDA), such as donepezil, (–)-galanthamine, and rivastigmine, have been attributed to its better penetration through the blood brain barrier, its higher oral bioavailability and its longer duration of AChE inhibitory action.[70,71,72,73,74,75,76]
CONCLUSIONS
AD has great impact on the personal and social life of human beings and no doubt, cholinesterase inhibitors offer great help in the effective management and treatment of AD. It is clearly evidenced that alkaloids are the major phytoconstituents responsible for the anticholiesterase activity of plant extracts and this information could be exploited for the synthesis of novel anticholinesterase drugs using alkaloids as intermediate compounds. Although, large number of natural plants extracts has been found to effective inhibitors of AChE and BChE, very few plants have been studied in-depth. Thus, detailed studies involving β-amyloid and receptor binding studies are warranted for optimum therapeutic utilization of these phytoconstituents. Further, limited data is available on the safety aspects of both the plant extracts and the isolated phytoconstituents. Since, very few animal studies and clinical trials are available; scope exists to undertake extensive research in these areas. It was also noted that, the alkaloids are the major compounds responsible for the anticholinesterase activity of plant extracts and these alkaloids can be used as starting materials for new classes of synthetic drugs for the treatment of AD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Bartolucci C, Perola E, Pilger C, Fels G, Lamba D. Three-dimensional structure of a complex of galanthamine (Nivalin) with acetylcholinesterase from Torpedo californica: Implications for the design of new anti-Alzheimer drugs. Proteins. 2001;42:182–91. doi: 10.1002/1097-0134(20010201)42:2<182::aid-prot50>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Lahiri DK, Farlow MR, Greig NH, Sambamurti K. Current drug targets for Alzheimer's disease treatment. Drug Dev Res. 2002;56:267–81. [Google Scholar]
- 3.Perry EK. The cholinergic hypothesis: Ten years on. Br Med Bull. 1986;42:63–9. doi: 10.1093/oxfordjournals.bmb.a072100. [DOI] [PubMed] [Google Scholar]
- 4.Bartus BT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–17. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 5.Greig NH, Lahiri DK, Sambamurti K. Butyrylcholinesterase: An important new target in Alzheimer's disease therapy. Int Psychogeriatr. 2002;14:77–91. doi: 10.1017/s1041610203008676. [DOI] [PubMed] [Google Scholar]
- 6.Shetty HG, Woodhouse K. Geriatrics. In: Walker R, Edwards C, editors. Clinical Pharmacy and Therapeutics. 2nd ed. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- 7.Lopez S, Bastida J, Viladomat F, Codina C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002;71:2521–9. doi: 10.1016/s0024-3205(02)02034-9. [DOI] [PubMed] [Google Scholar]
- 8.Das A, Shanker G, Nath C, Pal R, Singh S, Singh HK. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba anticholinesterase and cognitive enhancing activities. Pharmacol Biochem Behav. 2002;73:893–900. doi: 10.1016/s0091-3057(02)00940-1. [DOI] [PubMed] [Google Scholar]
- 9.Orhan I, Sener B, Choudhary MI, Khalid A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J Ethnopharmacol. 2004;91:57–60. doi: 10.1016/j.jep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Perry NS, Houghton PG, Theolad AE, Jenner P, Perry EK. In vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol. 2000;52:895–902. doi: 10.1211/0022357001774598. [DOI] [PubMed] [Google Scholar]
- 11.Perry NS, Houghton PG, Sampson J, Theolad AE, Hart S, Lis-balchin M, et al. In vitro activities of Salvia lavandulaefolia (Spanish Sage) relevant to treatment of Alzheimer's disease. J Pharm Pharmacol. 2001;53:1347–56. doi: 10.1211/0022357011777846. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee PK, Kumar V, Mal M, Houghtona PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Orhan I, Sener B. Franz C, Máthé A, Craker LE, Gardner ZE, editors. Sustainable use of various Amaryllidaceae plants against Alzheimer's disease. Proc. WOCMAP III. Targeted Screening of MAPs, Economics and Law. 2005;4 Acta Hort 678, ISHS. [Google Scholar]
- 14.Kim SR, Hwang SY, Jang YP, Park MJ, Markelonis GJ, Oh TH, et al. Protopine from Corydalis ternata has anticholinesterase and antiamnesic activities. Planta Med. 1999;65:218–21. doi: 10.1055/s-1999-13983. [DOI] [PubMed] [Google Scholar]
- 15.Khan I, Nisar M, Khan N, Saeed M, Nadeem S, Fazal-ur-Rehman, et al. Structural insights to investigate Conypododiol as a dual cholinesterase inhibitor from Asparagus adscendens. Fitoterapia. 2010;81:1020–5. doi: 10.1016/j.fitote.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Adsersen A, Kjolbye A, Dall O, Jager AK. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Corydalis cava Schweigg and Kort. J Ethnopharmacol. 2007;113:179–82. doi: 10.1016/j.jep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Ata A, Iverson CD, Kalhari KS, Akhter S, Betteridge J, Meshkatalsadat MH, et al. Triterpenoidal alkaloids from Buxus hyrcana and their enzyme inhibitory, anti-fungal and anti-leishmanial activities. Phytochemistry. 2010;71:1780–6. doi: 10.1016/j.phytochem.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Choudhary MI, Atta-ur-Rahman, Freyer AJ, Shamma M. New alkaloids from Buxus papilosa. J Nat Prod. 1987;50:84–8. [Google Scholar]
- 19.Atta-ur-Rahman, Nisa M, Farhi S. The isolation and structure of “moenjodaramine” and “harappamine”-two new alkaloids from Buxus papilosa. Z Naturforsch C. 1984;39B:524–7. [Google Scholar]
- 20.Mokry P, Voticky Z. Buxus alkaloids. X X. Alkaloids of Buxus arborescens Mill. Chemicke Zvesti. 1984;38:101–9. [Google Scholar]
- 21.Vassova A, Voticky Z, Cernik J, Tomko J. Buxus alkaloids. XVIII. Alkaloids of Buxus harlandi Hance. Chemicke Zvesti. 1980;34:706–11. [Google Scholar]
- 22.Fourneau C, Hocquemiller R, Guedon D, Cave A. Spirofornabuxine, a novel type of Buxus alkaloid. Tetrahedron Lett. 1997;38:2965–8. [Google Scholar]
- 23.Roddick JG. The acetylcholinesterase inhibitory activity of steroidal glycoalkaloids and their aglycones. Phytochemistry. 1989;28:2631–4. [Google Scholar]
- 24.McGehee DS, Krasowski MD, Fung DL, Wilson B, Gronert GA, Moss J. Cholinesterase inhibition by potato glycoalkaloids slows mivacurium metabolism. Anesthesiology. 2000;93:510–9. doi: 10.1097/00000542-200008000-00031. [DOI] [PubMed] [Google Scholar]
- 25.Andrade MT, Lima JA, Pinto AC, Rezende CM, Carvalho MP, Epifanio RA. Indole alkaloids from Tabernaemontana australis (Muell. Arg) Miers that inhibit acetylcholinesterase enzyme. Bioorg Med Chem. 2005;13:4092–5. doi: 10.1016/j.bmc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 26.Karczmar A. Invited review: Anticholinesterases: Dramatic aspects of their use and misuse. Neurochem Int. 1998;32:401–11. doi: 10.1016/s0197-0186(97)00123-x. [DOI] [PubMed] [Google Scholar]
- 27.Kim DK. Inhibitory effect of corynoline isolated from the aerial parts of Corydalis incisa on the acetylcholinesterase. Arch Pharm Res. 2002;25:817–9. doi: 10.1007/BF02976997. [DOI] [PubMed] [Google Scholar]
- 28.Kim DK, Lee KT, Baek NI, Kim SH, Park HW, Lim JP, et al. Acetylcholinesterase inhibitors from the aerial parts of Corydalis speciosa. Arch Pharm Res. 2004;27:1127–31. doi: 10.1007/BF02975117. [DOI] [PubMed] [Google Scholar]
- 29.Halldorsdottir ES, Jaroszewski JW, Olafsdottir ES. Acetylcholinesterase inhibitory activity of lycopodane-type alkaloids from the Icelandic Lycopodium annotinum ssp. alpestre. Phytochemistry. 2010;71:149–57. doi: 10.1016/j.phytochem.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Park CH, Kim SH, Choi W, Lee YJ, Kim JS, Kang SS, et al. Novel anticholinesterase and antiamnesic activities of dehydroevodiamine, a constituent of Evodia rutaecarpa. Planta Med. 1996;62:405–9. doi: 10.1055/s-2006-957926. [DOI] [PubMed] [Google Scholar]
- 31.Tang XC, Kindel GH, Kozikowski AP, Hanin I. Comparison of the effects of natural and synthetic huperzine: A on rat brain cholinergic function in vitro and in vivo. J Ethnopharmacol. 1994;44:147–55. doi: 10.1016/0378-8741(94)01182-6. [DOI] [PubMed] [Google Scholar]
- 32.Ashani Y, Grunwald J, Kronman C, Velan B, Shafferman A. Role of tyrosine 337 in the binding of huperzine A to the active site of human acetylcholinesterase. Mol Pharmacol. 1994;45:555–60. [PubMed] [Google Scholar]
- 33.Rahman AU, Choudhary MI. Bioactive natural products as a potential source of new pharmacophores-a theory of memory. Pure Appl Chem. 2001;73:555–60. [Google Scholar]
- 34.Rhee IK, Meent MV, Ingkaninan K, Verpoorte R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J Chromatogr A. 2001;915:217–23. doi: 10.1016/s0021-9673(01)00624-0. [DOI] [PubMed] [Google Scholar]
- 35.Rizzi A, Schuh R, Bruckner A, Cvitkovich B, Kremser L, Jordis U, et al. Enantiomeric resolution of galantamine and related drugs used in anti-Alzheimer therapy by means of capillary zone electrophoresis employing derivatized cyclodextrin selectors. J Chromatogr B. 1999;730:167–75. doi: 10.1016/s0378-4347(99)00186-3. [DOI] [PubMed] [Google Scholar]
- 36.Ingkaninan K, Temkitthawon P, Chuenchom K, Yuyaem T, Thongnoi W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol. 2003;89:261–4. doi: 10.1016/j.jep.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Atta-ur-Rahman, Alam M, Nasir H, Dagne E, Yenesew A. Three steroidal alkaloids from Buxus hildebrandtii. Phytochemistry. 1990;29:1293–6. [Google Scholar]
- 38.Loru F, Duval D, Aumelas A, Akeb F, Guedon D, Guedj R. Four steroidal alkaloids from the leaves of Buxus sempervirens. Phytochemistry. 2000;54:951–7. doi: 10.1016/s0031-9422(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 39.Guo H, Cai XH. Triterpenoid alkaloids from Buxus rugulosa. Chem Nat Comp. 2008;44:206–7. [Google Scholar]
- 40.Khalid A, Zaheer-ul-Haq, Ghayur MN, Fareeda Feroz F, Atta-ur-Rahman, Gilanib AH, et al. Cholinesterase inhibitory and spasmolytic potential of steroidal alkaloids. J Steroid Biochem Mol Biol. 2004;92:477–84. doi: 10.1016/j.jsbmb.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Choudhary MI, Shahnaz S, Parveen S, Khalid A, Mesaik MA, Ayatollahi SAM, et al. New cholinesterase-inhibiting triterpenoid alkaloids from Buxus hyrcana. Chem Biodivers. 2006;3:1039–52. doi: 10.1002/cbdv.200690102. [DOI] [PubMed] [Google Scholar]
- 42.Atta-ur-Rahman, Parveen S, Khalid A, Farooq A, Choudhary MI. Acetyl and butyrylcholinesterase-inhibiting triterpenoid alkaloids from Buxus papillosa. Phytochemistry. 2001;58:963–8. doi: 10.1016/s0031-9422(01)00332-6. [DOI] [PubMed] [Google Scholar]
- 43.Atta-ur-Rahman, Parveen S, Khalid A, Farooq A, Ayattollahi SA, Choudhary MI. Acetylcholinesterase inhibiting triterpenoidal alkaloids from Buxus hyrcana. Heterocycles. 1998;49:481–8. [Google Scholar]
- 44.Yan YX, Hu XD, Chen JC, Sun Y, Zhang XM, Qing C, et al. Cytotoxic triterpenoid alkaloids from Buxus microphylla. J Nat Prod. 2009;72:308–11. doi: 10.1021/np800719h. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed E, Nawaz SA, Malik A, Choudhary MI. Isolation and cholinesterase-inhibition studies of sterols from Haloxylon recurvum. Bioorg Med Chem Lett. 2006;16:573–80. doi: 10.1016/j.bmcl.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 46.Ozturk M, Kolak U, Topcu G, Oksuz S, Choudhary MI. Antioxidant and anticholinesterase active constituents from Micromeria cilicica by radical-scavenging activity-guided fractionation. Food Chem. 2011;126:31–8. [Google Scholar]
- 47.Jung M, Park M. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules. 2007;12:2130–9. doi: 10.3390/12092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orhan I, Senol FS, Kartal M, Dvorska M, Zemlicka M, Smejkal K, et al. Cholinesterase inhibitory effects of the extracts and compounds of Maclura pomifera (Rafin.) Schneider. Food Chem Toxicol. 2009;47:1747–51. doi: 10.1016/j.fct.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 49.Shahwar D, Shafiq-Ur-Rehman, Raza MA. Acetyl cholinesterase inhibition potential and antioxidant activities of ferulic acid isolated from Impatiens bicolor Linn. J Med Plant Res. 2010;4:260–6. [Google Scholar]
- 50.Kivrak I, Mehmet Emin Duru ME, Ozturk M, Mercan N, Harmandar M, Topcu G. Antioxidant, anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia potentillifolia. Food Chem. 2009;116:470–9. [Google Scholar]
- 51.Chung YK, Heo HJ, Kim EK, Kim HK, Huh TL, Lim Y, et al. Inhibitory effect of ursolic acid purified from Origanum majorana L on the acetylcholinesterase. Mol Cells. 2001;11:137–43. [PubMed] [Google Scholar]
- 52.Menichini F, Tundis R, Loizzo MR, Bonesi M, Marrelli M, Statti GA, et al. Acetylcholinesterase and butyrylcholinesterase inhibition of ethanolic extract and monoterpenes from Pimpinella anisoides V Brig. (Apiaceae) Fitoterapia. 2009;80:297–300. doi: 10.1016/j.fitote.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Vorbrueggen H, Pakrashi SC, Djerassi C. Terpenoids. LIV. Studies on Indian medicinal plants. Arborinol, a new triterpene type. Justus Liebigs Annalen der Chemie. 1963;668:57–76. [Google Scholar]
- 54.Orhan I, Terzioglu S, Sener B. α-onocerin: An acetylcholinesterase inhibitor from Lycopodium clavatum. Planta Med. 2003;69:1–3. doi: 10.1055/s-2003-38489. [DOI] [PubMed] [Google Scholar]
- 55.Urbain A, Marston A, Queiroz EF, Ndjoko K, Hostettmann K. Xanthones from Gentiana campestris as new acetylcholinesterase inhibitors. Planta Med. 2004;70:1011–4. doi: 10.1055/s-2004-832632. [DOI] [PubMed] [Google Scholar]
- 56.Sancheti S, Sanchet S, Um BH, Seo SY. 1,2,3,4,6-penta-O-galloyl-β-d-glucose: A cholinesterase inhibitor from Terminalia chebula. S Afr J Bot. 2010;76:285–8. [Google Scholar]
- 57.Lee KY, Sung SH, Kim YC. New acetylcholinesterase inhibitory pregnane glycosides of Cynanchum atratum roots. Helv Chim Acta. 2003;86:474–83. [Google Scholar]
- 58.Sung SH, Kang SY, Lee KY, Park MJ, Kim JH, Park JH, et al. (+)-α-Viniferin, a stilbene trimer from Caragana chamlague, inhibits acetylcholinesterase. Biol Pharm Bull. 2002;25:125–7. doi: 10.1248/bpb.25.125. [DOI] [PubMed] [Google Scholar]
- 59.Riaz N, Malik A, Aziz-ur-Rehman, Muhammad P, Nawaz SA, Choudhary MI. Cholinesterase inhibiting withanolides from Ajuga brateosa. Chem Biodivers. 2004;1:1289–95. doi: 10.1002/cbdv.200490091. [DOI] [PubMed] [Google Scholar]
- 60.Von BM, Endress PK, Qiu YL. Phylogenetic relationships in Buxaceae based on nuclear internal transcribed spacers and plastid ndhF sequences. Int J Plant Sci. 2000;161:785–92. [Google Scholar]
- 61.Devkota KP, Lenta BN, Fokou PA, Sewald N. Terpenoid alkaloids of the Buxaceae family with potential biological importance. Nat Prod Rep. 2008;25:612–30. doi: 10.1039/b704958g. [DOI] [PubMed] [Google Scholar]
- 62.Vieira Pde B, Giordani RB, De Carli GA, Zuanazzi JA, Tasca T. Screening and bioguided fractionation of Amaryllidaceae species with anti-Trichomonas vaginalis activity. Planta Med. 2011;77:1054–9. doi: 10.1055/s-0030-1270740. [DOI] [PubMed] [Google Scholar]
- 63.Hostettmann K, Borloz A, Urbain A, Marston A. Natural product inhibitors of acetylcholinesterase. Curr Org Chem. 2006;10:825–47. [Google Scholar]
- 64.Campbell WE, Nair JJ, Gammon DW, Bastida J, Codina C, Viladomat F, et al. Cytotoxic and antimalarial alkaloids from Brunsvigia littoralis. Planta Med. 1988;64:91–3. doi: 10.1055/s-2006-957381. [DOI] [PubMed] [Google Scholar]
- 65.Hohmann J, Forgo P, Molnár J, Wolfard K, Molnár A, Thalhammer T, et al. Antiproliferative Amaryllidaceae alkaloids isolated from the bulbs of Sprekelia formosissima and Hymenocalisxfestalis. Planta Med. 2002;68:454–7. doi: 10.1055/s-2002-32068. [DOI] [PubMed] [Google Scholar]
- 66.Szlávik L, Gyuris Á, Minárovits J, Forgo P, Molnár J, Hohmann J. Alkaloids from Leucojum vernum and antiretroviral activity of Amaryllidaceae alkaloids. Planta Med. 2004;70:871–3. doi: 10.1055/s-2004-827239. [DOI] [PubMed] [Google Scholar]
- 67.Park SY. Potential therapeutic agents against Alzheimer's disease from natural sources. Arch Pharm Res. 2010;33:1589–609. doi: 10.1007/s12272-010-1010-y. [DOI] [PubMed] [Google Scholar]
- 68.Ortega MG, Agnese AM, Barboza GE, Cabrera JL. Seasonal study of the alkaloid pattern of Huperzia saururus with habitat in Córdoba province (Argentina) J Argent Chem Soc. 2007;95:1–9. [Google Scholar]
- 69.Ma X, Gang DR. The Lycopodium alkaloids. Nat Prod Rep. 2004;21:752–72. doi: 10.1039/b409720n. [DOI] [PubMed] [Google Scholar]
- 70.Kawakami Y, Inoue A, Kawai T, Wakita M, Sugimoto H, Hopfinger AJ. The rationale for E2020 as a potent acetylcholinesterase inhibitor. Bioorg Med Chem Lett. 1996;4:1429–46. doi: 10.1016/0968-0896(96)00137-x. [DOI] [PubMed] [Google Scholar]
- 71.Kryger G, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with E2020 (Aricept): Implications for the design of new anti-Alzheimer drugs. Structure. 1999;7:297–307. doi: 10.1016/s0969-2126(99)80040-9. [DOI] [PubMed] [Google Scholar]
- 72.Nightingale SL. Donepezil approved for treatment of Alzheimer's disease. JAMA. 1997;277:10. [Google Scholar]
- 73.Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3Å resolution. FEBS Lett. 1999;463:321–6. doi: 10.1016/s0014-5793(99)01637-3. [DOI] [PubMed] [Google Scholar]
- 74.Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, et al. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry. 2002;41:3555–64. doi: 10.1021/bi020016x. [DOI] [PubMed] [Google Scholar]
- 75.Bai DL, Tang XC, He XC, Huperzine A. A potential therapeutic agent for treatment of Alzheimer's disease. Curr Med Chem. 2000;7:355–74. doi: 10.2174/0929867003375281. [DOI] [PubMed] [Google Scholar]
- 76.Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin. 2006;27:1–26. doi: 10.1111/j.1745-7254.2006.00255.x. [DOI] [PubMed] [Google Scholar]


