Abstract
This review represents the studies performed on some beneficial mangrove plants such as Ceriops decandra, Xylocarpus granatum, Xylocarpus moluccensis, Excoecaria agallocha, Sarcolobus globosus, Sonneratia caseolaris and Acanthus ilicifolius from the Sundarban estuary spanning India and Bangladesh with regard to their biological activities and chemical investigations till date. Sundarban is the largest single chunk of mangrove forest in the world. The forest is a source of livelihood to numerous people of the region. Several of its plant species have very large applications in the traditional folk medicine; various parts of these plants are used by the local people as cure for various ailments. Despite such enormous potential, remarkably few reports are available on these species regarding their biological activities and the active principles responsible for such activities. Though some chemical studies have been made on the mangrove plants of this estuary, reports pertaining to their activity-structure relationship are few in number. An attempt has been made in this review to increase the awareness for the medicinal significance as well as conservation and utilization of these mangrove species as natural rich sources of novel bioactive agents.
Keywords: Activity-structure relationship, bioactivities, folk-medicine, mangroves, phytochemicals, Sundarban estuary
INTRODUCTION
Mangroves are woody, specialized types of trees growing in brackish wetlands in the tropical and sub-tropical inter-tidal coastal zones and river deltas where other plants cannot grow. There are about 39.3 million acres of mangrove forests in the warm coastlines of tropical oceans all over the world. Among these the Sundarban (latitude 21° 31’ - 22° 30’ North and longitude 88° 10’ - 89° 51’ East) is the largest single block of tidal halophytic mangrove forest in the world.[1] The name Sundarban, literally meaning “beautiful forest” is believed to be derived from Sundari or Sundri (Heritiera fomes), one of the most abundant tree species found in this forest.[2] The forest lies in the Ganges-Brahmaputra Delta along the Bay of Bengal and is spread across areas of Bangladesh and West Bengal, India, forming the seaward fringe of the delta. The seasonally flooded Sundarban freshwater swamp forests lie inland from the mangrove forests. Of the total 10,000 km2 area, Indian Sundarban mangrove forest covers 4,266.66 km2 and the rest is covered with Bangladeshi Sundarban mangrove forest.[2] The biodiversity of Sundarban mangrove forest is rich and wide-ranging for which both of its Indian and Bangladeshi parts have been declared as World Heritage Site by UNESCO in 1987 and in 1997 respectively.[2,3] Mangroves thrive in extremely stressful and hostile environment of high salinity, high and low tides of water, high temperature and moisture, strong winds and muddy anaerobic soil. Besides these abiotic stress conditions, factors such as insects and microorganisms contribute largely in developing the biotic stress to the community. There is probably no other group of plants with such highly developed morphological and physiological adaptations to such extreme conditions. To thrive in such hostile environments, alterations in their physiological processes have occurred resulting in the synthesis of novel chemical compounds; these chemical compounds offer protection to these plants against various biotic and abiotic stresses mentioned above.[4,5,6] A number of these compounds or secondary metabolite molecules have significant biological and other medicinal properties that can be exploited in shaping better human health care needs. In fact, many mangrove plant species have their uses in folk or traditional medicine as cures for various ailments and for other commercial purposes.[7] These chemical compounds derived from the natural sources such as the mangroves can play very significant roles in the new drug discovery process.[8] However, intense scientific works need to be carried out to delve deeper into this scantily explored yet promising area to unfold the rich sources of valuable elixir for the health care of mankind.
The present review attempts discussion on the biological activities and chemical investigations carried out on mangrove plants Ceriops decandra, Xylocarpus granatum, Xylocarpus moluccensis, Excoecaria agallocha, Sarcolobus globosus, Sonneratia caseolaris and Acanthus ilicifolius from the Sundarban estuary spanning India and Bangladesh.
Ceriops decandra
C. decandra (Griff.) Ding Hou (Rhizophoraceae), locally called Jale Goran or Jhamti Goran in Bengali, is an evergreen shrub or small tree upto 5 m tall and are common in Indian Sundarbans.[2] The whole plant is used as an astringent and the plant parts are used to stop hemorrhage and treat ulcers, pain and hepatitis.[7,9] Though a few chemical investigations on C. decandra from other parts of India and the globe revealed the presence of diterpenoids, triterpenoids and lignins,[10,11,12,13,14] remarkably little studies on the chemistry and the biological activity of the species from Indian or Bangladeshi Sundarbans have been reported till date. Study by Ghosh et al.,[15] revealed significant contents of lipids, sterols and triterpenes from the leaves of C. decandra from Indian Sundarban region. The study also revealed the sterol and triterpene composition of the leaves of the species. Misra et al.,[16] reported the hydrocarbon and wax ester profile from the leaves of this species from Indian Sundarban along with six other species of mangroves. Uddin et al.,[9] reported the antinociceptive activity of ethanol extract of leaf and pneumatophore of the species from Bangladeshi Sundarban exhibiting significant inhibition of acetic acid-induced writhing in mice. A study on the leaf, bark and pneumatophore from the species of Bangladeshi origin showed potent broad spectrum activity against both Gram-positive and Gram-negative bacteria.[17] Another study by Banerjee et al.,[18] demonstrated strong antioxidant activity of the stem bark extract (reducing power as Ascorbic acid equivalent = 13.04 mg/g and diphenyl picryl hydrazyl [DPPH] radical scavenging ability as IC50 = 0.65 mg/ml) of C. decandra (Perr.) Robinson from Indian Sundarban. A recent study by Hossain et al.,[19] showed the antioxidative property of bark of the species from Bangladeshi Sundarbans which is in line with a previous study by Banerjee et al.[18] The study also demonstrated significant anti-inflammatory activity by the species. Chaudhuri and Guha[20] reported the presence of antifungal activity of the leaf and fruit extracts of the species against Fusarium oxysporum. Another recent study by Simlai and Roy[21] reported the phytochemical contents and the antimicrobial activity of C. decandra extracts and the stability of this activity against extreme thermal and pH treatment. The study also reported the partial identification of the nature of the active constituents exhibiting the antimicrobial activity using thin layer chromatography (TLC)-fingerprinting technique.
Xylocarpus granatum
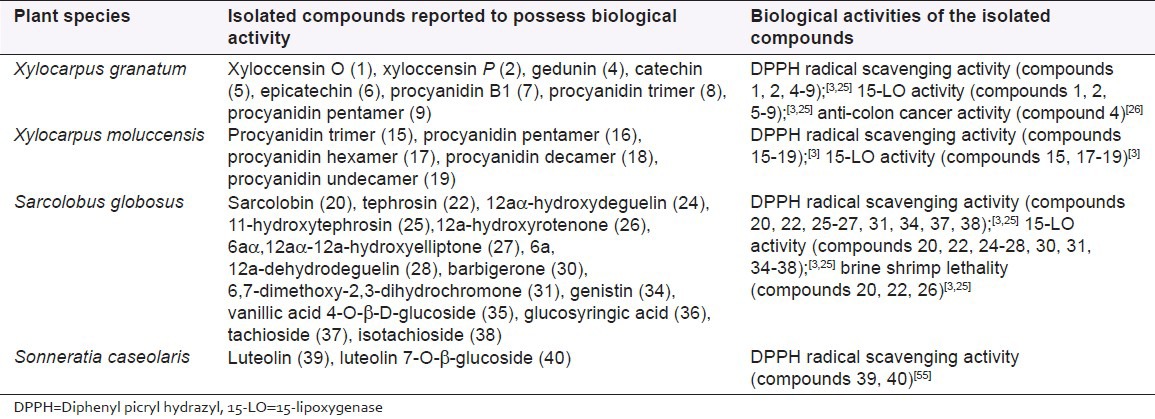
X. granatum Koeing (Meliaceae), locally called Dhundul in Bengali, is an evergreen tree with gray bark and grows in the inter-tidal ridge forest and river bank.[2] Extracts of different parts of the plant are reported to be used traditionally as relief for fever including one caused by malaria, inflammation, dysentery, diarrhea, cholera and other abdominal problems in certain parts of the globe.[3,7] Due to its quality reddish black colored timber, the wood is exploited for carpentry works, resulting in the scarcity of the species in the Indian part of the Sundarban.[2] The bark of the plant is used for tanning and for the preparation of amber dyes as well.[22] Investigation on the extracts of Bangladeshi X. granatum revealed potential for central nervous system (CNS) depressant activity and DPPH radical scavenging activity.[23,24] Chemical investigation by Wangensteen et al.[25] from the bark of X. granatum of Bangladeshi Sundarban revealed the presence of four previously reported limonoids [Figure 1], i.e., xyloccensin O (1), xyloccensin P (2), xyloccensin Q (3) and gedunin (4). The study also revealed the presence of two flavonoids, catechin (5) and epicatechin (6) and procyanidins of the B1 (7), trimer (8) and pentamer (9) type with catechin as the starter and epicatechin as the extender. Evaluation of the antioxidant activity (DPPH radical scavenging and 15-lipoxygenase [15-LO]) demonstrated high activity in case of catechin and procyanidins, of which procyanidin of the pentamer type was found to possess the maximum activity (IC50: DPPH: 3.3 ± 0.3 μM, 15-LO: 9 ± 1 μM) [Table 1]. This is in line with another study carried out by Wangensteen et al.[3] Uddin et al.[26] isolated and identified two limonoids [Figure 1], gedunin (4) and 1α-hydroxy-1,2-dihydrogedunin (10) from the bark of X. granatum of Bangladeshi Sundarban and reported the anticolon cancer activity of gedunin (4) [Table 1]. The IC50 value for cytotoxic potential of gedunin (4) against CaCo-2 colon cancer cell line was found to be 16.83 μM. This exhibits the significant anticancerous property of the compound. Antidiarrheal property of its bark of Bangladeshi Sundarban origin has also been reported thus justifying its use in the traditional herbal medicine.[22] The methanol extract of the bark at oral doses of 250 mg/kg and 500 mg/kg showed significant antidiarrheal activity in the castor oil and magnesium sulphate induced murine models respectively. Daula and Basher[27] demonstrated the plant rootlet and shoot growth inhibitory activity as well as antimicrobial activity of the species from Bangladeshi Sundarban. The antimicrobial potential of the plant is in line with the studies reported by Alam et al.,[28] and Wangensteen et al.[3] Potent broad spectrum antibacterial activity against both Gram-positive and Gram-negative bacteria was also reported by Uddin et al.[17] from this species of Bangladeshi origin.
Figure 1.
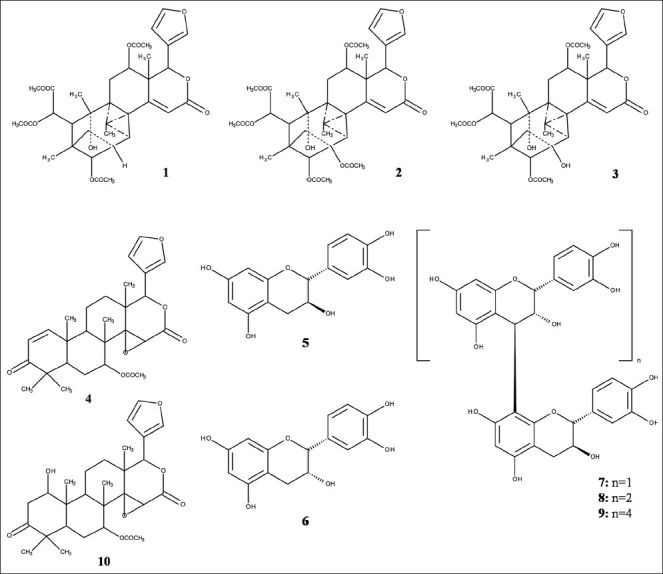
Structures of compounds isolated from Xylocarpus granatum (1-10)
Table 1.
Biological activities of the compounds reported to be isolated from the mangrove plants of Sundarban estuary
Xylocarpus moluccensis
X. moluccensis (Lamk.) Roem. (Meliaceae), a medium sized tree, generally grow away from the frequent tidal inundation.[2] Traditionally the bark of the plant is used to treat gastrointestinal disorders such as dysentery, diarrhea, fever including that from malaria and possesses astringent properties; the fruit is used as an aphrodisiac and used as a cure for elephantiasis and swelling of the breast.[3,7,29] Uddin et al.[30] showed the antidiarrheal activity of the methanolic extract of the bark of the plant from Bangladeshi Sundarban in the castor oil and magnesium sulphate induced mice and antibacterial property thus validating the plant's use in gastrointestinal disorders in traditional medicine. Alamgir et al.[23] and Sarker et al.[31] demonstrated the neuropharmacological property of X. moluccensis from Bangladeshi Sundarban exhibiting the CNS depressant activity in mice. Though no antimicrobial activity from leaf was observed by Uddin et al.[17], but Haque et al.[32] reported the presence of strong antimicrobial activities of crude extracts from stem bark and three isolated pure compounds (structures not elucidated) called XM-1, XM-2 and XM-3. The study also suggested the presence of cytotoxic activity towards brine shrimp nauplii, exhibited by the crude extract of Bangladeshi X. moluccensis. Mondal et al.[33] reported antimicrobial activity of pneumatophores of the species and suggested the species as an important source for antimicrobial compounds. The pneumatophore extracts of the plant of Bangladeshi (Sundarban) origin demonstrated moderate cytotoxic activity against human breast ductal carcinoma cells (MDA-MB-453S) and human gastric adenocarcinoma cells (AGS cell line).[29] In a recent study, a number of procyanidins have been reported from this plant by Wangensteen et al.[3] while chemically investigating the bark of the plant from Bangladeshi Sundarban. The study revealed the presence of flavonoids [Figure 2] catechin (11) and epicatechin (12) and few procyanidins, i.e. procyanidin B1 (13), procyanidin B3 (14), procyanidin trimer (15), procyanidin pentamer (16), procyanidin hexamer (17), procyanidin decamer (18) and procyanidin undecamer (19). Their studies revealed the presence of DPPH radical scavenging activity and 15-LO inhibiting activities in these isolated procyanidins and antimicrobial activities from the tissue extracts of this plant [Table 1].
Figure 2.

Structures of compounds isolated from Xylocarpus moluccensis (11-19)
Excoecaria agallocha
E. agallocha L. (Euphorbiaceae), locally called Geoa or Gneoa in Bengali, is a medium dioecious tree upto 15-20 m tall.[2] Parts of the plant possess medicinal properties and are used to treat epilepsy, conjunctivitis, dermatitis, hematuria, leprosy and toothache.[7] The species from Bangladeshi Sundarban is reported to possess neuropharmacological activity when tested on mice at higher dosage.[23] Subhan et al.[34] reported potent antinociceptive and gastroprotective effect of the crude ethanolic extracts of bark from E. agallocha of Bangladeshi Sundarbans. Another study by Subhan et al.[35] demonstrated a few biological activities such as neuropharmacological, antimicrobial and cytotoxicity effect of the ethanolic extract from the plant bark of Bangladeshi origin. In this study, the extract was found to possess potential effect on the CNS, exhibited significant antimicrobial activity and considerable cytotoxic effect on brine shrimps; however, the extract showed low level of toxicity in mice. The study by Subhan et al.[36] also demonstrated the presence of antioxidant activities in the tissue extracts of this plant from Bangaladeshi Sundarban. In another study, Hossain et al.[37] also reported the antioxidative property, along with the antiallergic property of different solvent extracts from the bark of the species of Bangladeshi origin. It was observed that the water and the ethanol fraction exhibited the maximum antioxidant and histamine release inhibitory activity compared to the other fractions. Kumar et al.[38] performed preliminary gas chromatography – mass spectrometry (GC-MS) analysis of the compounds present in the root exudates of the species from Indian Sundarbans. It is the first report on the presence of aminopyrine and palmitic acid in the root exudates of this species. A recent study by Rahman et al.[39] on the methanolic extract of stem from the species of Bangladeshi origin demonstrated more potent antihyperglycemic activity when compared to a standard antihyperglycemic drug, glibenclamide thus indicating the species as a potential source for antidiabetic drugs. Another study by Chaudhuri and Guha[20] showed that the water extract of bark of this species had antifungal activity against a pathogenic fungus, F. oxysporum. Despite these potential bioactivities, relatively little studies on the chemical content or compounds responsible for these activities of the species of Sundarban origin have been carried out. Some studies on the chemistry revealing the presence of phorbol ester, flavanone glycoside, various di- and triterpenoids in the E. agallocha from other parts of India and abroad have already been reported.[40,41,42,43,44,45,46,47,48,49,50] A very recent study by Mun et al.[51] revealed the chemical characteristics of E. agallocha along with few other mangrove species from Bangladeshi Sundarban demonstrating the dichloromethane, lignin, pentosan, α-cellulose etc., content in them.
Sarcolobus globosus
S. globosus Wall. (Asclepiadaceae), known as Caw Phal in Bengali, is a prostrate or climbing shrub growing in the mangrove forest of Sundarban estuary.[2] Traditionally, the plant is used as a relief for rheumatism, dengue and fever.[52] In a study carried out on the species from Bangladeshi Sundarban, Wangensteen et al.[52] for the first time reported the presence of a new rotenoid [Figure 3] sarcolobin (20) and a new isoflavone sarcolobone (21). The study also reported a few known rotenoids [Figure 3] such as tephrosin (22), 12aα-hydroxyrotenone (23), 12aα-hydroxydeguelin (24), 11-hydroxytephrosin (25), 12a-hydroxyrotenone (26), 6aα, 12aα-12a-hydroxyelliptone (27), 6a, 12a-dehydrodeguelin (28), 13-homo-13-oxa-6a, 12a-dehydrodeguelin (29), the isoflavone barbigerone (30) and a chromone 6,7-dimethoxy-2,3-dihydrochromone (31). The group for the first time reported 6,7-dimethoxy-2,3-dihydrochromone (31) as a natural product. Later on, Wangensteen et al.[53] identified two rotenoids, villosinol (32) and 6-oxo-6a, 12a-dehydrodeguelin (33), one isoflavone called genistin (34) and four phenolic glycosides named vanillic acid 4-O-β-D-glucoside (35), glucosyringic acid (36), tachioside (37) and isotachioside (38) for the first time from the species [Figure 3]. In this study, the rotenoids were found to inhibit 15-LO but lacked DPPH scavenging activity thus suggesting that the observed 15-LO inhibitory effect might be due to other mechanism than antioxidative activity [Table 1]. The results were found to be in line with a separate study by Wangensteen et al.[3] The latter study also revealed the potent cytotoxic effect and brine shrimp lethality of the lipophilic extracts, which could be attributed to the high amount of tephrosin and other rotenoids [Table 1]. No activity was observed by Alamgir et al.[23] when S. globosus was tested for neuropharmacological effect on the mice. In a recent study by Kuddus et al.[54] the bark extract of the species of Bangladeshi origin exhibited significant cytotoxic effect in the brine shrimp lethality bioassay, along with the membrane stabilizing activity using the murine erythrocyte in the hypotonic solution and significant thrombolytic activity in human blood specimen.
Figure 3.
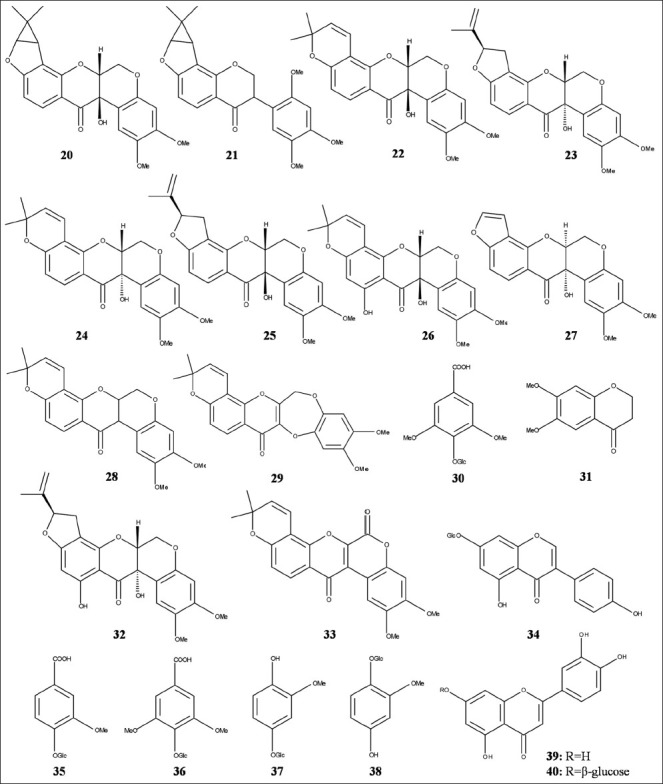
Structures of compounds isolated from Sarcolobus globosus (20-38) and Sonneratia caseolaris (39,40)
Sonneratia caseolaris
S. caseolaris (L.) Engl. (Sonneratiaceae), locally known as Chak Keora in Bengali, is an evergreen, medium to tall tree growing upto a height of 10 m.[2] Fruits from the species are used to treat bleeding, hemorrhages, piles, sprain poultices.[7] Study by Sadhu et al.[55] revealed the presence of two flavonoids [Figure 3], luteolin (39) and luteolin 7-O-β-glucoside (40) possessing antioxidant activity (DPPH radical scavenging activity on TLC) [Table 1] from the dried powdered leaf of the species from Sundarban mangroves of Bangladesh. Ahmed et al.[56] observed significant dose dependent effect on the serum glucose and lipid profiles in rats when administered with the dried leaf powder from this species to their diet. The administration had led to the significant decrease in serum glucose, serum triglyceride, serum total cholesterol and serum low density lipoprotein cholesterol levels when compared to controls. The use of leaf powder in the diets had also resulted in significant increases in the serum high density lipoprotein cholesterol levels. The study suggests S. caseolaris leaf as a potential source for antidiabetic agents and cure for coronary diseases. A study by Chaudhuri and Guha[20] revealed the ethanolic extract of the leaf of S. caseolaris to possess antifungal activity against F. oxysporum. In a recent study on the species from Bangladeshi Sundarban, Mubassara et al.,[57] showed the species to possess strong antioxidative activity and reducing power. The study also exhibited inhibition of both histamine and leukotriene B4 suggesting the species to be a good source for the development of anti-allergic agents.
Acanthus ilicifolius
A. ilicifolius L.(Acanthaceae), locally called Horkoch Kanta or Horgoja in Bengali, is a scarcely woody, bushy, dense vine shrub of height upto 2.5 m.[2] Parts of the species are used as aphrodisiac and relief for asthma, diabetes, diuretic, dyspepsia, hepatitis, leprosy, rheumatism and a number of ailments.[7] Chakraborty et al.[58] showed the chemopreventive potential of the aqueous leaf extract of the species in transplantable Ehrlich ascites carcinoma-bearing murine model manifested in limiting metallothionein protein expression and in preventing DNA alternations in the animal liver. The study demonstrated decreases in tumor cell count, increase in mean survival duration of the animals, restoration in hematological and hepatic histological profiles. Banerjee et al.[18] reported the antioxidant activity of the species from Indian Sundarbans. Senthil Kumar et al.[59] demonstrated the anti-inflammatory activity of the leaves of the species from Indian Sundarbans using the murine model. The study showed a considerable decrease of rat paw edema. The fraction of the extract was also found to be a free radical scavenger and is in line with another study by Banerjee et al.[18] Leaf and bark extract of the species have been reported to possess activity against a pathogenic fungus, F. oxysporum.[20] A recent study by Islam et al.[60] on the methanolic extract of A. ilicifolius leaves demonstrated dose dependent antinociceptive activity against acetic-acid induced writhing, formalin and hot plate induced murine models. In another report by Senthil Kumar et al.,[61] the gastroprotective potential of the methanolic extract of the leaves from the species was demonstrated using different models of gastric ulceration in rats. The extract exhibited protective activity against the aspirin, indomethacin, stress, ethanol and pylorus ligation induced gastric ulcerations.
The information presented in this review clearly indicate that the discussed mangrove species from the Sundarban estuary possess full potential for extraction of pharmacologically vital compounds. Various classes of phytochemicals such as flavonoids, limonoids, rotenoids, phenolic glycosides etc., have been isolated and characterized from these species, a number of which have been reported to possess different types of biological activities. These compounds and their derivatives might be useful in newer drug discovery process. Despite the noticeable biological activities, however, minimum initiatives have been taken for the ethnobotanical and ethnopharmacological studies, search of natural products and establishment of activity-structure relationship. In our literature survey it was noticed that though the species such as C. decandra, E. agallocha, A. ilicifolius exhibited a number of biological activities there's strong lacuna in the search for the active compounds responsible behind these activities. From the Sundarban estuary, among the species discussed, it was found that the majority of the works have been reported on X. granatum while S. caseolaris was found to be least reported. Attempt to establish activity-structure relationship may also reveal an array of compounds responsible for single or different activities, which might be of synergistic nature. For instance, in a study on C. decandra, two of the extracts, which showed significant antimicrobial activity during the disc-diffusion assay failed to exhibit any activity during bioautography after the separation of the compounds using TLC.[21] This might be due to the separation of the constituents, which were showing activity at the synergistic level. Activity guided isolation of bioactive compounds have potent application to establish the activity-structure relationship. The information available also clearly states the lack of intense research regarding the biological activity and the chemical investigation of the aforementioned mangrove species from Indian Sundarban mangrove forest. Majority of the studies on the plants have been reported from Bangladeshi Sundarban estuary. Intense search should be carried out to delve deeper into this sparsely explored promising area from where new bioactive compounds can be isolated eventually helping in the drug discovery process.
Footnotes
Source of Support: This work has been supported in part by a grant from UGC to AR (F. No. 40-176/2011[SR]) and other departmental funds from UGC and DBT, Government of India
Conflict of Interest: None declared
REFERENCES
- 1.Ghosh A, Mukherjee S, Sen N, Dasgupta M, Naskar KR. Check-list of mangroves and mangrove associated species in the Indian Sundarbans. Seshaiyana. 2002;10:03–5. [Google Scholar]
- 2.Naskar KR. Delhi: Daya Publishing House; 2004. Manual of Indian Mangroves. [Google Scholar]
- 3.Wangensteen H, Alamgir M, Duong GM, Gronhaug TE, Samuelsen AB, Malterud KE. Chemical and biological studies of medicinal plants from the Sundarbans mangrove forest. In: Eddouks M, editor. Advances in Phytotherapy Research. India: Research Signpost; 2009. pp. 59–78. [Google Scholar]
- 4.Reyes LF, Cisneros-Zevallos L. Wounding stress increases the phenolic content and antioxidant capacity of purple-flesh potatoes (Solanum tuberosum L.) J Agric Food Chem. 2003;51:5296–300. doi: 10.1021/jf034213u. [DOI] [PubMed] [Google Scholar]
- 5.Edreva A, Velikova V, Tsonev T, Dagnon S, Gurel A, Aktas L, et al. Stress-protective role of secondary metabolites: diversity of functions and mechanisms. Gen Appl Plant Physiol. 2008;34:67–78. [Google Scholar]
- 6.Shulaev V, Cortes D, Miller G, Mittler R. Metabolomics for plant stress response. Physiol Plant. 2008;132:199–208. doi: 10.1111/j.1399-3054.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 7.Bandaranayake WM. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes. 1998;2:133–48. [Google Scholar]
- 8.Bandaranayake WM. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl Ecol Manag. 2002;10:421–52. [Google Scholar]
- 9.Uddin SJ, Shilpi JA, Barua J, Rouf R. Antinociceptive activity of Ceriops decandra leaf and pneumatophore. Fitoterapia. 2005;76:261–3. doi: 10.1016/j.fitote.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Sakagami H, Kashimata M, Toguchi M, Satoh K, Odanaka Y, Ida Y, et al. Radical modulation activity of lignins from a mangrove plant, Ceriops decandra (Griff.) Ding Hou. In Vivo. 1998;12:327–32. [PubMed] [Google Scholar]
- 11.Anjaneyulu AS, Rao VL. Ceriopsins A-D, diterpenoids from Ceriops decandra. Phytochemistry. 2002;60:777–82. doi: 10.1016/s0031-9422(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 12.Anjaneyulu AS, Rao VL, Lobkovsky E, Clardy J. Ceriopsin E, a new epoxy ent-kaurene diterpenoid from Ceriops decandra. J Nat Prod. 2002;65:592–4. doi: 10.1021/np010540p. [DOI] [PubMed] [Google Scholar]
- 13.Anjaneyulu AS, Rao VL. Ceriopsins F and G, diterpenoids from Ceriops decandra. Phytochemistry. 2003;62:1207–11. doi: 10.1016/s0031-9422(02)00627-1. [DOI] [PubMed] [Google Scholar]
- 14.Ponglimanont C, Thongdeeying P. Lupane-triterpene esters from the leaves of Ceriops decandra (Griff.) Ding Hou. Aus J Chem. 2005;58:615–8. [Google Scholar]
- 15.Ghosh A, Misra S, Dutta AK, Choudhury A. Pentacyclic triterpenoids and sterols from seven species of mangrove. Phytochemistry. 1985;24:1725–7. [Google Scholar]
- 16.Misra S, Datta AK, Chattopadhyay S, Choudhury A, Ghosh A. Hydrocarbons and wax esters from seven species of mangrove leaves. Phytochemistry. 1987;26:3265–8. [Google Scholar]
- 17.Uddin SJ, Rouf R, Shilpi JA, Alamgir M, Nahar L, Sarker SD. Screening of some Bangladeshi plants for in vitro antibacterial activity. Orient Pharm Exp Med. 2008;6:316–21. [Google Scholar]
- 18.Banerjee D, Chakrabarti S, Hazra AK, Banerjee S, Ray J, Mukherjee B. Antioxidant activity and total phenolics of some mangroves in Sunderbans. Afr J Biotechnol. 2008;7:805–10. [Google Scholar]
- 19.Hossain H, Moniruzzaman S, Nimmi I, Kawsar H, Hossain A, Islam A, et al. Anti-inflammatory and antioxidant activities of the ethanolic extract of Ceriops decandra (Griff.) Ding Hou bark. Orient Pharm Exp Med. 2011;11:215–20. [Google Scholar]
- 20.Chaudhuri P, Guha S. Potentiality of mangrove plant extracts for biocontrol of a pathogenic fungi, Fusarium oxysporum. Sci Cult. 2010;76:271–4. [Google Scholar]
- 21.Simlai A, Roy A. Analysis of and correlation between phytochemical and antimicrobial constituents of Ceriops decandra, a medicinal mangrove plant, from Indian Sundarban estuary. J Med Plants Res. 2012;6:4755–65. [Google Scholar]
- 22.Rouf R, Uddin SJ, Shilpi JA, Alamgir M. Assessment of antidiarrhoeal activity of the methanol extract of Xylocarpus granatum bark in mice model. J Ethnopharmacol. 2007;109:539–42. doi: 10.1016/j.jep.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Alamgir M, Alam SM, Alaul M, Rashid M, Hasan M, Choudhuri MS. Preliminary evaluation of some medicinal plants of Sundarbans mangrove forest on central nervous system. Orient Pharm Exp Med. 2006;6:215–20. [Google Scholar]
- 24.Uddin SJ, Shilpi JA, Delazar A, Nahar L, Sarker SD. Free radical scavenging activity of some Bangladeshi plant extracts. Orient Pharm Exp Med. 2004;4:187–95. [Google Scholar]
- 25.Wangensteen H, Duong GM, Alamgir M, Sarder M, Samuelsen AB, Malterud KE. Biological activities of limonoids, catechins, procyanidins and extracts from Xylocarpus granatum. Nat Prod Commun. 2006;1:985–90. [Google Scholar]
- 26.Uddin SJ, Nahar L, Shilpi JA, Shoeb M, Borkowski T, Gibbons S, et al. Gedunin, a limonoid from Xylocarpus granatum, inhibits the growth of CaCo-2 colon cancer cell line in vitro. Phytother Res. 2007;21:757–61. doi: 10.1002/ptr.2159. [DOI] [PubMed] [Google Scholar]
- 27.Daula AF, Basher MA. Phytochemical screening, plant growth inhibition and antimicrobial activity studies of Xylocarpus granatum. Malays J Pharm Sci. 2009;7:09–21. [Google Scholar]
- 28.Alam MA, Sarder M, Awal MA, Sikder MMH, Daulla KA. Antibacterial activity of the crude ethanolic extract of Xylocarpus granatum stem barks. Bangladesh J Vet Med. 2006;4:69–72. [Google Scholar]
- 29.Uddin SJ, Grice ID, Tiralongo E. Cytotoxic effects of Bangladeshi medicinal plant extracts. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/nep111. 578092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uddin SJ, Shilpi JA, Alam SM, Alamgir M, Rahman MT, Sarker SD. Antidiarrhoeal activity of the methanol extract of the barks of Xylocarpus moluccensis in castor oil – and magnesium sulphate-induced diarrhoea models in mice. J Ethnopharmacol. 2005;101:139–43. doi: 10.1016/j.jep.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Sarker SD, Uddin SJ, Shilpi JA, Rouf R, Ferdous ME, Nahar L. Neuropharmacological properties of Xylocarpus moluccensis. Fitoterapia. 2007;78:107–11. doi: 10.1016/j.fitote.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 32.Haque ME, Islam MN, Rahman MH, Mohamad AU. Antimicrobial and cytotoxic activities of the crude extracts and isolated compounds of Xylocarpus mollucensis. Dhaka Univ J Pharm Sci. 2007;6:109–12. [Google Scholar]
- 33.Mondal S, Paul SK, Uddin SJ, Nahar L, Auzi AA, Sarker SD. A comparative study on the in vitro antibacterial activity of the pneumatophores of Heritiera fomes and Xylocarpus moluccensis. Ars Pharm. 2008;49:51–6. [Google Scholar]
- 34.Subhan N, Alam A, Ahmed F, Shahid IZ. Antinociceptive and gastroprotective effect of the crude ethanolic extracts of Excoecaria agallocha Linn. Turk J Pharm Sci. 2008;5:143–54. [Google Scholar]
- 35.Subhan N, Alam MA, Ahmed F, Shahid IJ, Nahar L, Sarker SD. Bioactivity of Excoecaria agallocha. Braz J Pharm. 2008;18:521–6. [Google Scholar]
- 36.Subhan N, Alam MA, Ahmed F, Awal MA, Nahar L, Sarker SD. In vitro antioxidant property of the extract of Excoecaria agallocha (Euphorbiaceae) DARU J Pharm Sci. 2008;16:149–54. [Google Scholar]
- 37.Hossain SJ, Aoshima H, El-Sayed M, Ahmed F. Antioxidative and anti-histamine-release activities of Excoecaria agallocha L. Pharmacologyonline. 2009;2:927–36. [Google Scholar]
- 38.Kumar T, Ray S, Brahmachary RL, Ghose M. Preliminary GC–MS analysis of compounds present in the root exudates of three mangrove species. Acta Chroma. 2009;21:117–25. [Google Scholar]
- 39.Rahman M, Siddika A, Bhadra B, Rahman S, Agarwala B, Chowdhury MH, et al. Antihyperglycemic activity studies on methanol extract of Petrea volubilis L. (Verbenaceae) leaves and Excoecaria agallocha L. (Euphorbiaceae) stems. Adv Nat Appl Sci. 2010;4:361–4. [Google Scholar]
- 40.Erickson KL, Beutler JA, Cardellina JH, 2nd, McMahon JB, Newman DJ, Boyd MR. A novel phorbol ester from Excoecaria agallocha. J Nat Prod. 1995;58:769–72. doi: 10.1021/np50119a020. [DOI] [PubMed] [Google Scholar]
- 41.Anjaneyulu AS, Rao VL. Five diterpenoids (agallochins A-E) from the mangrove plant Excoecaria agallocha Linn. Phytochemistry. 2000;55:891–901. doi: 10.1016/s0031-9422(00)00251-x. [DOI] [PubMed] [Google Scholar]
- 42.Anjaneyulu AS, Rao VL, Sreedhar K. ent-Kaurane and beyerane diterpenoids from Excoecaria agallocha. J Nat Prod. 2002;65:382–5. doi: 10.1021/np010262u. [DOI] [PubMed] [Google Scholar]
- 43.Anjaneyulu AS, Rao VL. Seco diterpenoids from Excoecaria agallocha L. Phytochemistry. 2003;62:585–9. doi: 10.1016/s0031-9422(02)00269-8. [DOI] [PubMed] [Google Scholar]
- 44.Anjaneyulu AS, Rao VL, Sreedhar K. Agallochins J-L, new isopimarane diterpenoids from Excoecaria agallocha L. Nat Prod Res. 2003;17:27–32. doi: 10.1080/1057563021000027975. [DOI] [PubMed] [Google Scholar]
- 45.Konishi T, Konoshima T, Fujiwara Y, Kiyosawa S. Excoecarins D, E, and K, from Excoecaria agallocha. J Nat Prod. 2000;63:344–6. doi: 10.1021/np990366t. [DOI] [PubMed] [Google Scholar]
- 46.Konishi T, Konoshima T, Fujiwara Y, Maoka T. Novel diterpenes, excoecarins M and N from the resinous wood of Excoecaria agallocha. Tetrahedron Letters. 2000;41:3419–22. [Google Scholar]
- 47.Konishi T, Yamazoe K, Konoshima T, Fujiwara Y. Seco-labdane type diterpenes from Excoecaria agallocha. Phytochemistry. 2003;64:835–40. doi: 10.1016/j.phytochem.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Konishi T, Yamazoe K, Kanzato M, Konoshima T, Fujiwara Y. Three diterpenoids (excoecarins V1-V3) and a flavanone glycoside from the fresh stem of Excoecaria agallocha. Chem Pharm Bull. 2003;51:1142–6. doi: 10.1248/cpb.51.1142. [DOI] [PubMed] [Google Scholar]
- 49.Wang JD, Li ZY, Xiang WS, Guo YW. Further new secoatisane diterpenoids from the Chinese mangrove Excoecaria agallocha L. Helv Chim Acta. 2006;89:1367–72. [Google Scholar]
- 50.Zou JH, Dai J, Chen X, Yuan JQ. Pentacyclic triterpenoids from leaves of Excoecaria agallocha. Chem Pharm Bull. 2006;54:920–1. doi: 10.1248/cpb.54.920. [DOI] [PubMed] [Google Scholar]
- 51.Mun SP, Jahan MS, Al-Maruf A, Chowdhury DA. Chemical characterization of six mangrove species in Bangladesh. Wood Sci Technol. 2011;45:281–8. [Google Scholar]
- 52.Wangensteen H, Alamgir M, Rajia S, Samuelsen AB, Malterud KE. Rotenoids and isoflavones from Sarcolobus globosus. Planta Med. 2005;71:754–8. doi: 10.1055/s-2005-864182. [DOI] [PubMed] [Google Scholar]
- 53.Wangensteen H, Miron A, Alamgir M, Rajia S, Samuelsen AB, Malterud KE. Antioxidant and 15-lipoxygenase inhibitory activity of rotenoids, isoflavones and phenolic glycosides from Sarcolobus globosus. Fitoterapia. 2006;77:290–5. doi: 10.1016/j.fitote.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Kuddus MR, Aktar F, Miah MK, Baki MA, Rashid MA. Polyphenols content, cytotoxic, membrane stabilizing and thrombolytic activities of Sarcolobus globosus: A medicinal plant from Sundarban forest. Bol Latinoam Caribe Plant Med Aromat. 2011;10:363–8. [Google Scholar]
- 55.Sadhu SK, Ahmed F, Ohtsuki T, Ishibashi M. Flavonoids from Sonneratia caseolaris. J Nat Med. 2006;60:264–5. doi: 10.1007/s11418-006-0029-3. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed R, Moushumi SJ, Ahmed H, Ali M, Haq WM, Jahan R, et al. Serum glucose and lipid profiles in rats following administration of Sonneratia caseolaris (L.) Engl. (Sonneratiaceae) leaf powder in diet. Adv Nat Appl Sci. 2010;4:171–3. [Google Scholar]
- 57.Mubassara S, Takasugi M, Iga R, Hossain SJ, Aoshima H. Inhibition of the histamine and leukotriene B 4 release from rat peritoneal exudate cells by six Bangladeshi plants. Pharmacologyonline. 2011;2:76–85. [Google Scholar]
- 58.Chakraborty T, Bhuniya D, Chatterjee M, Rahaman M, Singha D, Chatterjee BN, et al. Acanthus ilicifolius plant extract prevents DNA alterations in a transplantable Ehrlich ascites carcinoma-bearing murine model. World J Gastroenterol. 2007;13:6538–48. doi: 10.3748/wjg.v13.i48.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Senthil Kumar KT, Gorain B, Roy DK, Zothanpuia, Samanta SK, Pal M, et al. Anti-inflammatory activity of Acanthus ilicifolius. J Ethnopharmacol. 2008;120:7–12. doi: 10.1016/j.jep.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 60.Islam MA, Saifuzzaman M, Ahmed F, Rahman MM, Sultana NA, Naher K. Antinociceptive activity of methanolic extract of Acanthus ilicifolius Linn leaves. Turk J Pharm Sci. 2012;9:51–60. [Google Scholar]
- 61.Senthil Kumar KT, Puia Z, Samanta SK, Barik R, Dutta A, Gorain B, et al. The Gastroprotective Role of Acanthus ilicifolius-A Study to Unravel the Underlying Mechanism of Anti-Ulcer Activity. Sci Pharm. 2012;80:701–17. doi: 10.3797/scipharm.1108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


