Abstract
Cosmetics comprising either natural or synthetic components are used almost regularly and universally in different forms to enhance the beauty. The utmost disclosure of human membrane to sunlight and environmental pollution results in the exhibition of free radical, that react with deoxyribonucleic acid, proteins and fatty acids, causation oxidative destruction dysfunction of the antioxidant system. In skin, the formation of reactive oxygen species leads to skin diseases, predominantly cutaneous malignancies, immunosuppression, wrinkles, aging, etc., The human organism fosters a barrier practice against the destructive action of free radicals, comprising mostly of vitamins, carotenoids and enzymes. Cosmetic products are the best option to reduce skin disorders such as hyper pigmentation, skin aging, skin wrinkling and rough skin texture, etc., Hence in this review, we conferred various in vitro methods that are used for the development of novel cosmetic formulation. There is an expanding fascinate employing in vitro techniques because they are less time consuming, more cost-effective and lessen the participation of human volunteers.
Keywords: Aging, hyperpigmentation, in vitro methods, ultraviolet radiation, wrinkle
INTRODUCTION
In our body, skin is the biggest organ. The skin performs an excessively important role, providing a massive physical rampart against mechanical,[1] chemical, thermal, and microbial factors. Otherwise, all these factors may affect the physiological status of the body.[2,3,4] The ultraviolet (UV) radiation arrived to earth surface due to environmental pollution inceptions fade of the protective stratospheric ozone layer. Moreover, the consequent increases in UV radiation induced skin disorders, predominantly cutaneous malignancies, immunosuppression, wrinkles, aging, etc.[5] The UV radiation emitted by the sun is divided into Ultraviolet C (270-290 nm), Ultraviolet B (290-320 nm), and Ultraviolet A (320-400 nm), is subdivided into UVA2 (320-340 nm) and UVA1 (340-400 nm). The UVB and UVA radiation extent the earth surface in significant amounts and are able to incite biological effects. The ozone in the stratosphere captivated UVC and hence it does not spread to earth's surface.[6]
The UV radiation readily affected human skin, because it is the very perceptive organ. The UV radiation entered distinctive chromophore in the skin, such as melanin, deoxyribonucleic acid (DNA), Ribonucleic acid, proteins, lipids, water, aromatic amino acids, such as tyrosine and tryptophan, trans-urocanic acid, etc., The incorporation of UV radiation by these chromophore leads to distinct photochemical reactions and provokes reactive oxygen species (ROS), over exposure of UV radiation causes harmful effects.[7] The UV radiation generated ROS and this lead to histochemical changes of altering severity, thickening of the stratum spinosum, and flattening of dermoepidermal junction.[8] The destructive action of free radicals to the skin is controlled by vitamins, carotenoids, and enzymes present in the body. In human skin, approximately, 70% of carotenoids are β-carotene and lycopene, which can serve as markers for the whole antioxidative potential.[9,10] Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) comprise a cellular defense system against ROS toxicity. However, when skin is irradiation with solar UV rays provokes a reduction in the levels of antioxidants, deactivation of antioxidant enzymes and an upsurge in the markers of lipid peroxidation in skin, meanwhile skin suffers from skin disorders.[11,12]
The skin is the reflection of an individual's physical appearance and to take care of perfect is very decisive due to successively exposure of the skin to UV radiation. The exposure of the body to UV radiation, this lead to skin evoked to produce ROS and free radicals. These oxidative agents’ results to induce skin disorders such as hyper pigmentation, wrinkling, rough texture, and aging, etc., The imperfections of skin make it one of the most attractive subjects for skin research workers.[5,7]
The skins are protected from exogenous and endogenous harmful agents by applying cosmetic products and it reinforce the beauty and attractiveness of skin.[13] The cosmetic products not only improve the external appearance of skin; however, it also increases the longevity of decent health by discounting skin disorders. The skin care formulation nourish the health, texture and integrity of skin, moisturizing, maintaining elasticity of skin by reduction of type I collagen and photoprotection, etc., These characteristics of cosmetic are due to the presence of synthetic or natural ingredients in skin care formulation, because it helps to diminish the exhibition of free radicals in skin and manage the skin properties for a long time. The cosmetic products are the best choice to reduce skin disorders such as hyper pigmentation, skin aging, skin wrinkling and rough skin texture, etc., The development of novel skin care formulation constrained to assessment the potency of ingredients presents in formulations. The different in vivo models are used for computation of safety and efficacy of cosmetic formulation, but in maximum models human volunteers are used.[14,15,16] To perform in vivo assessment of cosmetic products on human volunteers has several inconveniences such as being overpriced, time-consuming, and is potentially perilous to human clinical subjects.[17] Moreover, to get human ethical clearance approval is a very difficult assignment. Consequently, there is an enlarging interest using in vitro methods because they are less time consuming, more cost-effective and lessen the participation of human volunteers. The assessment of effectiveness of skin care formulation by in vitro model may also help in reducing the cost of products.
This report precises how pigmentation, wrinkle and aging are produced in skin, and how the action of cosmetics can be evaluated to eradicate or minimize the skin ailments. Here, the recent advance experimental methods are discussed, which are used in the evaluation and to check the efficacy of skin care cosmetics formulations. This review will help the researcher working in the development of new skin care cosmetic formulations.
SKIN DISORDERS
Hyper pigmentation
The melanin plays a prominent role for color production over human skin.[18] The melanin is produced by melanocyte cells build in the stratum basale of the skin epidermis, and it is main defensive pigment in arresting of UV induced skin damage.[19,20] The melanocyte cells present in the human body produced two patterns of melanin namely Pheomelanin and Eumelanin.[21] The Pheomelanin originates the yellow to reddish-brown color in the body while eumelanin yield light brown to black color in the body.[22] Over disclosure of the body to UV radiation can persuade the generation of melanin in the skin. The hyper pigmentation in epidermal skin outcomes from enhanced activity of tyrosinase and effects in several dermatological disorders, such as melasma, post inflammatory melanoderma and solar lentigo. Tyrosinase oxidize the conversion of L-tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and of DOPA to DOPA quinone, both reactions being the first two rate-limiting steps in the melanin synthesis pathway.[23] UV radiation intensifies the formation ROS in the skin and these ROS boost melanin biosynthesis, damage DNA, and may induce proliferation of melanocytes.[24] In the human skin, the over formation and cumulation of melanin pigment produces a change in skin color, skin wrinkling and skin aging.[25] This hyper pigmentation could develop a serious problem in skin resultant in a great number of skin diseases, including chloasoma dermatitis, freckles, and geriatric pigment spots. Melanin biosynthesis can be inhibited by evading UV exposure, by inhibition of melanocyte metabolism and proliferation, by inhibition of tyrosinase.[20] Moreover, the hyperpigmentation can also be reduced by ROS scavengers or inhibitors such as antioxidants might suppress melanogenesis in the epidermal layer of skin. Hence, different whitening cosmetics and medicines are being developed to check melanogenesis.
Aging
The aging development is the cumulation of oxidative destruction to cells and tissues, integrated with an imperfect multiply in the chance of morbidity and mortality.[26,27,28] The long-term exposure of skin to solar UV radiation is a serious environmental threat that creates to acute and chronic effects in the human skin. The UV radiation causes comprehensive generation of ROS and reactive nitrogen species can outcome in the structural and functional alteration of cutaneous proteins, for example collagen, elastin and glycosaminoglycans,[29] which may contribute to photoaging. The increased ROS causes oxidative stress and oxidative photodamage of macromolecules and plasma membrane components in the skin. This further lead to immature aging of the skin that is marked by the rough skin textures, wrinkles, laxity, atrophy, pigmentary changes or blotchy dyspigmentation, elastosis, telangiectasia, and pre-cancerous lesions such as actinic keratosis and malignant tumors.[30,31,32,33] These all are the fundamental causative agent for the evolvement of a wrinkle on the face and parts of the body. The early sign of skin aging is due to periorbital wrinkle formation. The type I collagen is the main component of the skin dermis, and its reduction result in aging of the skin. The collagen is the chief structural unit of the extracellular matrix (ECM). The type I collagen help in the maintenance of the skin dermis structure.[32] The enzyme such as SOD, CAT, and GPX as well as the peptides with thiol groups as glutathione, present in skin cells help in formation of antioxidants. Whereas, additional antioxidants are acquired from nature through nutrition, as vitamin C, vitamin E, and carotenoids, afforded protection against UV-induced photoaging.[33,26]
Wrinkle
The formation of a wrinkle in human skin has been integrated with marked decreases in skin elasticity and is identified by dry and flaky rough skin, both fine and coarse wrinkles, poor elastic recoil, solar lentigines, sallow color, and impaired wound healing.[34,35] The prolonged disclosure of human skin to sun or UV irradiation excels to variations in the constitution of skin.[29] Hence, this persuades to be diminished of skin flexibility as an outcome of the degeneration of the three-dimensional structure of resilient fibers, that is closely connected with a consequent lowering in the resilient properties of the skin. In human skin, two types of elastases are present, neutrophil elastase and skin fibroblast elastase both are mainly responsible for the deterioration of springy fibers associated with wrinkle formation. Neutrophil elastase is a serine proteinase, whereas skin fibroblast elastase is in the family of metalloproteinases and thus they differ significantly in substrate specificity. Neutrophil elastase can debase all types of elastic fibers with a high susceptibility to elaunin fibers and mature elastic fibers. Although fibroblast elastase acts on oxytalan fibers and elaunin fibers, but has limited effects on mature elastic fibers.[36,37,38,39] The exposure of UV radiation in skin surges the origination of ROS; it overwhelmed antioxidant defence mechanisms, resulting in oxidative stress and oxidative photodamage of proteins and other macromolecules in the skin. These ROS leads to overproduction of elastases, a reduction and degeneration of collagen and deposition of glycosaminoglycans, induced by UV irradiation affect the elastic-fiber network.[40,41,42] Such devastation to the skin is conceived to create to perceivable alterations such as wrinkling and sagging.
IN VITRO DETERMINATIONS OF SKIN CARE FORMULATIONS
Determination of sun protection factor (SPF) determination
The SPF is characterized as the UV energy required to produce a minimal erythema dose (MED) on protected skin, divided by the UV energy required producing a MED on unprotected skin. The SPF indicates the effectiveness of a sunscreen cream:

The lowest time interval or dosage of UV light irradiation sufficient to produce a smallest, perceptible erythema on unprotected skin is known as the MED. The higher value of SPF indicates the product is more effective in preventing sunburn.
The in vitro screening method was examined by Kaur and Saraf 2011 and Ashawat and Saraf 2006. Prepare 200 μg ml−1 of the sample and determine absorbance values of the aliquot from 290 nm to 320 nm, at 5 nm intervals, using UV-Visible spectrophotometer.[56,57,58]
The observed absorbance values at 5 nm intervals (290-320 nm) were calculated by using the formula:
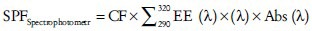
Where, CF is a correction Factor, EE (λ) is erythmogenic effect of radiation with wavelength λ and Abs (λ) is spectrophotometric absorbance values at wavelength λ. The values of EE (λ) × I (λ) are constants.
Discussion on SPF action
The exposure of UV solar radiation to Human body fabricates harmful effects on the skin, including sunburn, photoaging,[46] cutaneous malignancies,[47] local immunosuppression[48] and damage DNA.[49,50] Although there are few methods are available by which persons protected themselves against the sun, evidence from paintings counsels that clothes to cover the body; veils and large brim hats were used by ancient Greeks, and umbrellas were used in aged Egypt, Mesopotamia, China and India.[51] The sun rays continuously reaches to earth with approximately 50% visible light, 40% infrared radiation, and 10% UV radiation.[52] UV A (320-400 nm) wavelengths is mainly responsible for photoaging because it pierce deeper into the skin. The shorter wavelengths (UV B) are 30-40 times more energetic, and penetrate mostly into the epidermis. Further this may lead to skin photocarcinogenesis and immunosuppression.[53,54,55] The sunscreen products are applied to prevent sunburn and other skin damage. The sunscreen products available in the market contain UV absorbers that have been chiefly controlled at protecting against UV induced sunburn and DNA damage. Since, the biological endpoint for the determination of the SPF is the UV erythema. The SPF label is the indicator only for a protection against erythemally effective solar UV, largely confined to the UVB and partially short-wavelength UVA radiation.[52,56] Hence, there is a continuous need of quantitative determination of different parameters, such as SPF, protection against UV radiations, to support the efficacy and safety of the products.[57] For economical, practical and ethical reasons, there is an increasing curiosity employing in vitro methods because they are less time consuming, more cost-effective and give the additional information, for example critical wavelength and photostability.[58] The in vitro method for SPF determination is arrogant to in vivo methods because this can minimize risks related to UV exposure of human subjects during a sunscreen product development.
Determination of tyrosinase inhibitory activity
The mushroom tyrosinase and L-DOPA used for the tyrosinase inhibitory bioassay as discussed by Sharma et al. 2004 and Leu et al. 2008. Dissolve test substances in 0.1 ml of 10% dimethyl sulfoxide (DMSO) in an aqueous solution and incubated with 0.1 ml of mushroom tyrosinase (135 U/ml phosphate buffered solution [PBS], pH 6.8) at 25°C for 10 min, and then add 0.1 ml of 0.5 mM L-DOPA in a (PBS, pH 6.8). Incubate the reaction mixture for 5 min. The amount of dopachrome in the mixture is determined by the optical density (OD) at 475 nm and Kojic acid use as the standard agent. The inhibitory percentage of tyrosinase is calculated as follows:
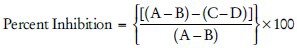
Where A is the OD at 475 nm without test substance, B is the OD at 475 nm without the test substance but with tyrosinase, C is the OD at 475 nm with the test substance and D is the OD at 475 nm with the test substance, but without tyrosinase.[59,60,61]
Determination of melanin content
Culture melanoma cell lines (B16 cells) in Dulbecco's modified Eagle's medium (DMEM) (13.4 mg/ml DMEM, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 143 U/ml benzylpenicillin potassium, 100 μg/ml streptomycin sulfate, 24 mM NaHCO3, pH 7.1) with 10% fetal bovine serum (and penicillin/streptomycin (100 IU/50 mg/ml) in a humidified atmosphere containing 5% CO2 in the air at 37°C. Culture the B16 cells in 24-well plates for the melanin quantification and determination of enzyme activity. This assay was previously conversed by Tada et al. 1986, Ha et al. 2007, Kim et al. 2006 and Hoshino et al. 2010.[62,63,64,65]
Discussion on melanin action
The extent and distribution of melanin pigmentation in human skin play a vital role for production of color in the skin.[66] The melanocytes cell producing pigment are present in the outermost layer of the skin to originate melanin. The human skin is protected from the destructive effects of UV sun radiation by melanin. Further, melanin participate in scavenging toxic drugs and chemicals.[67] The synthesis of melanin is mostly monitored by tyrosinase, a copper-containing enzyme and is found exclusively in melanocytes.[59,68] Tyrosinase catalyses three distinctive reactions in the biosynthetic pathway of melanin in melanocytes: The hydroxylation of tyrosine to L-DOPA and the oxidation of L-DOPA to dopaquinone; furthermore, in humans, dopaquinone is altered by a series of complex reactions to melanin.[69,70,71] The control of melanin synthesis is a prominent strategy in the treatment of abnormal skin pigmentation for cosmetic purposes. The above mentioned bioassay can be used to ascertain the inhibition property of melanin of sample.
Determination of elastase inhibition
Porcine pancreatic elastase was assayed spectrophotometrically using N-Succ-(Ala) 3-nitroanilide (SANA) as the substrate by Sahasrabudhe and Deodhar, 2010. The release of p-nitroaniline for 15 min at 25°C is monitored by measuring the absorbance at 410 nm. The reaction mixture contained 800 μl of 0.2 M Tris buffer (pH 8.0), 100 μl of enzyme elastase and 100 μl of 0.8 mM SANA as substrate and different concentrations of the test sample in Tris-HCl buffer. Preincubated the test sample with the enzyme for 20 min at 25°C and the reaction is started with the addition of substrate. The buffer is used as control.[72] The change in absorbance is monitored at 410 nm using UV spectrophotometer. Inhibitory effect of the samples on the Elastase activity calculated as:
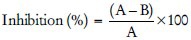
Where A, is the absorbance at 410 nm without test sample, and B is the change in absorbance at 410 nm with the test sample.
Discussion on elastase action
Elastin is an ECM protein and most abundant in organs providing elasticity to the connective tissues. It forms elastic fiber in the skin dermis and this may increase the skin elasticity. Damage to the elastin fibers leads to the declined skin resilience. The proteinase enzyme produced elastase, and this enzyme able to erode elastin.[73,74] Therefore, inhibition of the elastase activity ingredients could be used in cosmetic formulation to protect against skin aging and wrinkles.
Determination of collagenase assay
The assay employed was based on spectrophotometric methods reported by Thring et al. 2009, with some modifications for use in a microplate reader. The assay was performed in 50 mM Tricine buffer (pH 7.5 with 400 mM NaCl and 10 mM CaCl2). Dissolved collagenase from Clostridium histolyticum in the buffer for use at an initial concentration of 0.8 units/mL according to the supplier's activity data. The synthetic substrate N-[3-(2-furyl) acryloyl]-Leu-Gly-Pro-Ala (FALGPA) was dissolved in Tricine buffer to 2 mM. Incubate the sample with the enzyme in the buffer for 15 min before adding substrate to start the reaction. The final reaction mixture (150 μl total volumes) contained Tricine buffer, 0.8 mM FALGPA, 0.1 units Clostridium histolyticum and 25 μg test samples. Absorbance at 335 nm is measured immediately after adding substrate and then continuously for 20 min using a Cary 50 Microplate Reader in Nunc 96 well microtitre plates. Epigallocatechin-3-gallate, 250 μM (0.114 mg/ml) used as a positive control.[75]
Discussion on collagenase action
The collagen present in eighty percent of human skin, that is responsible for the tensile strength of the skin.[75] ROS leads to distinct changes in skin collagenous tissues by the breakdown of collagen, a major in the ECM. These modifications in the ECM most probable mediated by matrix metalloproteinases (MMP) are known to be an origin of the skin wrinkling ascertained in premature skin aging as well as in aged skin.[76,77,78,79] Collagen fibrils and elastin are responsible for strength and resiliency of skin. Aging and irradiation accelerate the degradation of the ECM, resulting in a decrease in dermal collagen and an increase in the level of the matrix MMP-1, which cleaves interstitial collagen leads the skin appear to be aged.[80,81,82,83] Furthermore, collagenase activity has been required to inhibit for retention of skin elasticity and tensile strength of the skin.
Determination of antiglycation activity
Prepared reaction mixtures containing 5 mg bovine serum albumin, 25 mM threose and 1 mM diethylenetriaminepentaacetic acid in 1.0 ml of 0.1 M phosphate buffer (pH 7.4) containing 0.02% sodium azide, and incubated at 37°C. Each reaction mixture is placed into a 2 ml cryogenic vial and sealed and following the incubation for 7 days in an incubator and remove 100 μl aliquots. Measure the fluorescence intensity at an excitation of 370 nm and an emission of 440 nm with a spectrofluorometer. The activity of each blank (without threose) is subtracted from the activity of each sample.[84]

Discussion on antiglycation action
One of the causes of aging is the appearance of the Advanced Glycosylation End Products (AGE) during life. In our skin, oxidative stress causes damage to skin cell membrane by acceleration of protein glycation and AGE formation. Protein glycation occurs when blood sugar reacts with protein such as collagen, an essential component of skin, to form AGE, which leads to the degradation of collagen.[85,86] Damage to and loss of collagen (which provides skin firmness), elastin (which supplies skin elasticity and rebound) and glycosaminoglycans (which keep the skin hydrated), result in the appearance of roughness, uneven tone, brown patches, thin skin and deep wrinkles. Accelerated skin aging is expressly apparent in diabetic patients, where glycation is increased because of high serum glucose levels. This inspection has developed as a screening tool for searching of glycation inhibitors from a natural product samples that often contain interfering substances such as fluorescence and quencher materials.[87] Therefore, by incorporating substances containing glycation inhibitory effect in cosmetic preparation is a strategy for the prevention of aging and wrinkle.
Determination of hyaluronidase inhibition
Hyaluronidase inhibition was determined by measuring the amount of N-acetylglucosamine splited from sodium hyaluronate, this method was described by Sahasrabudhe and Deodhar, 2010. Fifty microliter of bovine hyaluronidase (7900 units ml−1) dissolved in 0.1 M acetate buffer (pH 3.6) and mixed with 50 μl of designated concentrations of sample dissolved in 5% DMSO. The control group is treated with 50 μl of 5% DMSO instead of samples and is incubated for 20 min at 37°C. After 20 min add 50 μl of calcium chloride (12.5 mM) to the reaction mixture and again incubated for 20 min at 37°C. This Ca2+ activated hyaluronidase is treated with 250 μl sodium hyaluronate (1.2 mg ml−1) and incubated at 37°C for 40 min. After incubation 50 μl of 0.4 M sodium hydroxide and 100 μl of 0.2 M sodium borate are added to the reaction mixture and then incubated in the boiling water bath for 3 min. After cooling to room temperature add 1.5 ml of p-Dimethylaminobenzaldehyde solution (4 g PDMAB dissolved in 50 mL of 10 N HCL and 350 ml of glacial acetic acid) to the reaction mixture and incubated in a water bath at 37°C for 20 min till color developed. The absorbance is measured at 585 nm on UV spectrophotometer.[72] Inhibitory effect is expressed as follows:
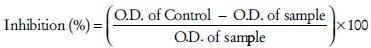
Discussion on hyaluronidase action
The almost every tissue of all vertebrates containing hyaluronic acid, but is most abundant in the ECM of soft connective tissues.[19] Hyaluronic acid play vital role in keeping the body smooth, moist and lubricated due to its water holding property.[1] Enzyme hyaluronidase lowers hyaluronic acid by decreases the viscosity of body fluids and increases the permeability of connective tissues lowering its viscosity and increasing the permeability and is also involved in bacterial pathogenesis.[88,89,90] Hyaluronidase inhibitors are potent, ubiquitous regulating agents who are involved in maintaining the balance between the anabolism and catabolism of Hyaluronic acid, and this keep skin moist as well as smooth.[91]
DETERMINATION OF IN VITRO ANTIOXIDANT USED FOR SKIN CARE FORMULATION
Determination of total polyphenol content
The total polyphenol content is determined using Folin-Ciocalteu reagent. The reaction mixture contained: 2.0 ml of the sample, 400 μl of freshly prepared diluted Folin-Ciocalteu reagent and then sodium carbonate solution (75 g/l) added to the reaction mixture to reach a 10.0 ml volume. The above solution is incubated at room temperature for 10 min, and measure absorption at 760 nm wavelength. The amount of total polyphenol is calculated using the gallic acid calibration curve. The results are expressed as gallic acid equivalent mg/100 ml of the sample.[92,93,94]
Determination of total flavone and flavonol content
The total flavones and flavonols contents are determined using aluminum chloride (AlCl3). Mix 9.8 ml of the prepared sample with a 10% solution of AlCl3 (200 μl). After 30 min, absorption is measured at a 425 nm wavelength. The amount of total flavone is calculated using quercetin calibration curve. The results are expressed as the quercetin equivalent (QE) mg/100 ml of the sample.[92,93,94]
Determination of 2,2-diphenyl-1-picrylhydrazyl phosphate reduction
The free radical scavenging ability is investigated using stable DPPH radical. The prepared sample (400 μl) is replenished up to 2.0 ml with 0.1 mM of DPPH methanol solution. Prepared control without test compound in an identical manner. The reaction is allowed to be completed in the dark for about 30 min. Take absorption of sample at a 517 nm wavelength. DPPH reduction is calculated when taking into account the absorption of the control investigation, and the observed activity is compared to quercetin calibration curve. The results are expressed as quercetin antioxidant activity equivalent (QE) μmol/100 ml of the solution.[95,96,97]

Where, Asample and Acontrol are absorbance of sample and control.
Determination of total carotenoid content
Mix 6.0 g of the sample and 20 ml of petroleum ether, and the resulting mixture is agitated for 5 min. Filter the solution into a 25 ml measuring flask and diluted up to the mark. The extinction value of the resulting yellow-colored solution is measured spectrophotometrically at a 440 nm. Measure the extinction value of standard potassium bichromate solution contained 0.0900 g of potassium bichromate in 250 ml of purified water.[98,99] Total carotenoid content is expressed as ß-carotene (mg/100 g of cream), and calculated using the following formula:
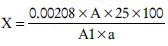
X = Total carotenoid
A = Extinction value of the studied solution
A1 = Extinction value of the standard solution
a = Amount of the cream, g
Discussion on antioxidant activity
Antioxidants are compounds competent of either interruption or inhibit the oxidation progressions which occur under the impact of atmospheric oxygen or ROS. Antioxidant entities can rummage ROS, inhibit the generation of liberated radicals, chain breaking activity and metal chelation. Antioxidants are implicated in the defense mechanism of the organism against the pathologies associated with the attack of released radicals.[92,93] When endogenous factors cannot ensure a stringent control and a complete protection of the organism against the ROS, the need for exogenous antioxidants arises, as nutritional additives or pharmaceutical/cosmetics products, which contain as the active principle an antioxidant compound. The antioxidant assays calculated the oxidative outputs at the primitive and concluding stages of oxidation. Phenolic compounds possess anti-inflammatory, anti-carcinogenic, anti-atherosclerotic, and other properties that may be related to skin disorders.[17] Polyphenolic compositions might have a repressive effect on mutagenesis and carcinogenesis in humans. Flavonoids and flavonols are two polyphenolic supplements that perform a significant part in stabilizing lipid oxidation and are accompanying with antioxidant activity.[93,94,95,99]
ß-carotene is the most copious and mainly efficient precursor of vitamin A. ß-carotene is a radical scavenger, quenching singlet oxygen and free radicals without damage to cells and tissue. This property of ß-carotene makes it applicable for UV protection of skin. The increase cell turn-over and regeneration in the outer layers of the skin are regulated by ß-carotene, making it effective for diseases and skin conditions related to epithelium damage. It also intensifies the appearance of dry or damaged skin by reducing, flaking and restoring suppleness. In skin-care products, beta-carotene is used for its antioxidant properties, its capacity to screen the skin from sun damage, and its endowment to help even the skin tone, deeming it an active anti-aging ingredient. Furthermore it is used in anti-aging products for its sun damage protection capabilities.[94,99,100]
CONCLUSION
The increasing fascinate acquired by in vitro approach for evaluation of skin care formulation is due to the less time consuming, more cost-effective and reduce the participation of human volunteers. In this review, plenty of in vitro methods are notified they differ from each other in terms of skin disorders. Exposure to UV radiation results in skin ailments like hyperpigmentation, wrinkle, aging, photodamage, etc., The antioxidant activity and SPF assay can be used to curb skin from photodamage. The tyrosinase inhibitory activity and melanin assay used to reduce the hyperpigmentation and marks from skin. The elastase inhibition assay can be used for determination of skin elasticity; furthermore, collagenase has been required to inhibit for retention of skin elasticity and tensile strength of the skin. Moreover, antiglycation assay checks the devastation and loss of collagen, and hence it reduces wrinkle and aging from skin. Hyaluronic acid holds the water together and keeps the body smooth, watery and lubricated, so that hyaluronidase inhibition assay can check the moisture present in the body. This article would be helpful for the development of safety and efficacy skin care cosmetic formulations. In vitro, screening techniques may represent a fast and rational tool reducing the number of in vivo experiments and risks related to human subjects; hence this can reduce the Research and Development expenses. Furthermore, it substantiates to slash the cost of cosmetic products [Table 1].
Table 1.
Comparative data of in vitro and in vivo model
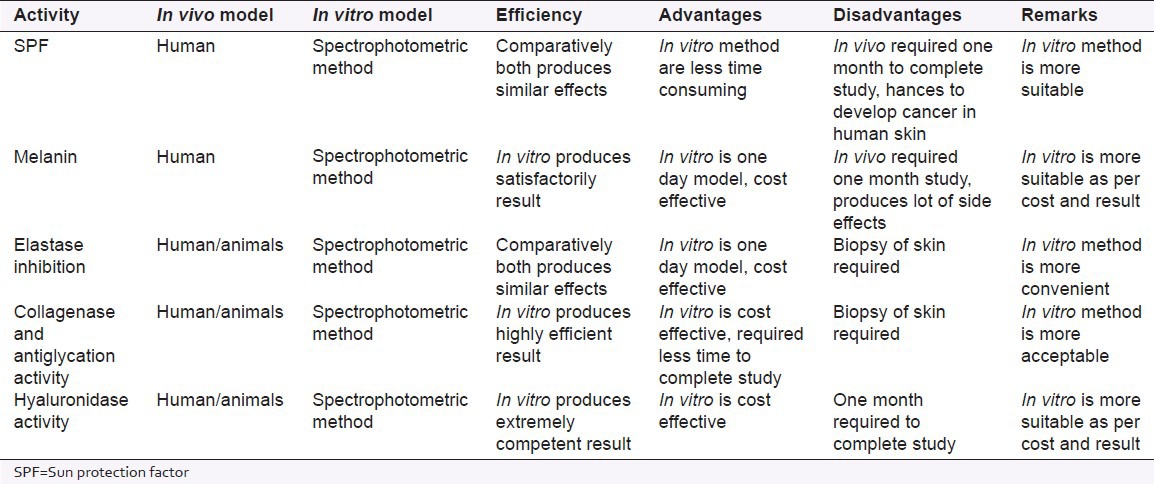
The in vitro method mentioned in this review are very useful for the scientist those who are working in the cosmetic discipline. This is the incomparable and initial article which produces utter in vitro techniques used for determination of efficacy of novel products of cosmetic in a unique platform. Moreover, until the date, no individual article produces all these in vitro techniques in a single step. The researchers expend their lot of time during the literature survey of research work; we hope this review will produce utmost methodology in unshared steps and economize their expensive time.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Costin GE, Hearing VJ. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–94. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 2.Svobodova A, Walterova D, Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 3.Hussein MR. Ultraviolet radiation and skin cancer: Molecular mechanisms. J Cutan Pathol. 2005;32:191–205. doi: 10.1111/j.0303-6987.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 4.Palm MD, O’Donoghue MN. Update on photoprotection. Dermatol Ther. 2007;20:360–76. doi: 10.1111/j.1529-8019.2007.00150.x. [DOI] [PubMed] [Google Scholar]
- 5.Sgarbi FC, Carmo ED, Rosa LE. Radiation ultraviolet carcinogens. Revista de Ciencias Medicas. 2007;16:245–50. [Google Scholar]
- 6.Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol. 2005;52:937–58. doi: 10.1016/j.jaad.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 7.González S, Fernández-Lorente M, Gilaberte-Calzada Y. The latest on skin photoprotection. Clin Dermatol. 2008;26:614–26. doi: 10.1016/j.clindermatol.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Balogh TS, Velasco MV, Pedriali CA, Kaneko TM, Baby AR. Ultraviolet radiation protection: Current available resources in photoprotection. An Bras Dermatol. 2011;86:732–42. doi: 10.1590/s0365-05962011000400016. [DOI] [PubMed] [Google Scholar]
- 9.Darvin ME, Haag SF, Lademann J, Zastrow L, Sterry W, Meinke MC. Formation of free radicals in human skin during irradiation with infrared light. J Invest Dermatol. 2010;130:629–31. doi: 10.1038/jid.2009.283. [DOI] [PubMed] [Google Scholar]
- 10.Hata TR, Scholz TA, Ermakov IV, McClane RW, Khachik F, Gellermann W, et al. Non-invasive raman spectroscopic detection of carotenoids in human skin. J Invest Dermatol. 2000;115:441–8. doi: 10.1046/j.1523-1747.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 11.Yasui H, Sakurai H. Age-dependent generation of reactive oxygen species in the skin of live hairless rats exposed to UVA light. Exp Dermatol. 2003;12:655–61. doi: 10.1034/j.1600-0625.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 12.Sohal RS, Allen RG. Oxidative stress as a causal factor in differentiation and aging: A unifying hypothesis. Exp Gerontol. 1990;25:499–522. doi: 10.1016/0531-5565(90)90017-v. [DOI] [PubMed] [Google Scholar]
- 13.Saraf S, Kaur CD. Phytoconstituents as photoprotective novel cosmetic formulations. Pharmacogn Rev. 2010;4:1–11. doi: 10.4103/0973-7847.65319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhtar N, Khan BA, Mahmood T, Parveen R, Qayum M, Anwar M, et al. Formulation and evaluation of antisebum secretion effects of sea buckthorn w/o emulsion. J Pharm Bioallied Sci. 2010;2:13–7. doi: 10.4103/0975-7406.62698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashawat MS, Banchhor M, Saraf S. Herbal cosmetics: Trends in skin care formulation. Pharmacogn Rev. 2009;3:82–9. [Google Scholar]
- 16.Datta HS, Paramesh R. Trends in aging and skin care: Ayurvedic concepts. J Ayurveda Integr Med. 2010;1:110–3. doi: 10.4103/0975-9476.65081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kale S, Kavade E, Yadav AV. Formulation and in-vitro evaluation for sun protection factor of Crinum asiaticum Linn flower (Family-Amaryllidaceae) extract sunscreen creams. Indian Journal of Pharmaceutical Education and Research. 2010;46:112–9. [Google Scholar]
- 18.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–50. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 19.Hanamura T, Uchida E, Aoki H. Skin-lightening effect of a polyphenol extract from Acerola (Malpighia emarginata DC.) fruit on UV-induced pigmentation. Biosci Biotechnol Biochem. 2008;72:3211–8. doi: 10.1271/bbb.80421. [DOI] [PubMed] [Google Scholar]
- 20.Wang KH, Lin RD, Hsu FL, Huang YH, Chang HC, Huang CY, et al. Cosmetic applications of selected traditional Chinese herbal medicines. J Ethnopharmacol. 2006;106:353–9. doi: 10.1016/j.jep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Pilawa B, Buszman E, Latocha M, Wilczok T. Free radicals in DOPA-melanin-chloroquine complexes. Pol J Med Phys Eng. 2007;10:35–42. [Google Scholar]
- 22.Sugumaran M. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res. 2002;15:2–9. doi: 10.1034/j.1600-0749.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 23.Song TY, Chen CH, Yang NC, Fu CS, Chang YT, Chen CL. The correlation of in vitro mushroom tyrosinase activity with cellular tyrosinase activity and melanin formation in melanoma cells A2058. J Food Drug Anal. 2009;17:156–62. [Google Scholar]
- 24.Yamakoshi J, Otsuka F, Sano A, Tokutake S, Saito M, Kikuchi M, et al. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003;16:629–38. doi: 10.1046/j.1600-0749.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 25.Herrling T, Jung K, Fuchs J. The important role of melaninas protector against free radicals in skin. SOFW Journal. 2007;133:26–32. [Google Scholar]
- 26.Junqueira VB, Barros SB, Chan SS, Rodrigues L, Giavarotti L, Abud RL, et al. Aging and oxidative stress. Mol Aspects Med. 2004;25:5–16. doi: 10.1016/j.mam.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Udompataikul M, Sripiroj P, Palungwachira P. An oral nutraceutical containing antioxidants, minerals and glycosaminoglycans improves skin roughness and fine wrinkles. Int J Cosmet Sci. 2009;31:427–35. doi: 10.1111/j.1468-2494.2009.00513.x. [DOI] [PubMed] [Google Scholar]
- 28.Suwannalert P, Povichit N, Puchadapirom P, Junking M. Anti-aging activity and non-toxic dose of phytooxyresveratrol from Artocarpus lakoocha Roxb. Tropical Journal of Pharmaceutical Research. 2012;11:69–74. [Google Scholar]
- 29.Kambayashi H, Yamashita M, Odake Y, Takada K, Funasaka Y, Ichihashi M. Epidermal changes caused by chronic low-dose UV irradiation induce wrinkle formation in hairless mouse. J Dermatol Sci. 2001;27:S19–25. doi: 10.1016/s0923-1811(01)00113-x. [DOI] [PubMed] [Google Scholar]
- 30.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 31.Fisher GJ, Datta S, Wang Z, Li XY, Quan T, Chung JH, et al. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106:663–70. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho HS, Lee MH, Lee JW, No KO, Park SK, Lee HS, et al. Anti-wrinkling effects of the mixture of vitamin C, vitamin E, pycnogenol and evening primrose oil, and molecular mechanisms on hairless mouse skin caused by chronic ultraviolet B irradiation. Photodermatol Photoimmunol Photomed. 2007;23:155–62. doi: 10.1111/j.1600-0781.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 33.Kang TH, Park HM, Kim YB, Kim H, Kim N, Do JH, et al. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol. 2009;123:446–51. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Vayalil PK, Mittal A, Hara Y, Elmets CA, Katiyar SK. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J Invest Dermatol. 2004;122:1480–7. doi: 10.1111/j.0022-202X.2004.22622.x. [DOI] [PubMed] [Google Scholar]
- 35.Brenneisen P, Sies H, Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann N Y Acad Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji N, Moriwaki S, Suzuki Y, Takema Y, Imokawa G. The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective inhibition of elastase activity. Photochem Photobiol. 2001;74:283–90. doi: 10.1562/0031-8655(2001)074<0283:troesb>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Sander CS, Chang H, Salzmann S, Müller CS, Ekanayake-Mudiyanselage S, Elsner P, et al. Photoaging is associated with protein oxidation in human skin in vivo. J Invest Dermatol. 2002;118:618–25. doi: 10.1046/j.1523-1747.2002.01708.x. [DOI] [PubMed] [Google Scholar]
- 38.Homsy R, Pelletier-Lebon P, Tixier JM, Godeau G, Robert L, Hornebeck W. Characterization of human skin fibroblasts elastase activity. J Invest Dermatol. 1988;91:472–7. doi: 10.1111/1523-1747.ep12476608. [DOI] [PubMed] [Google Scholar]
- 39.Benelli R, Venè R, Bisacchi D, Garbisa S, Albini A. Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo and serine proteases. Biol Chem. 2002;383:101–5. doi: 10.1515/BC.2002.010. [DOI] [PubMed] [Google Scholar]
- 40.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–70. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 41.Moon JY, Yim EY, Song G, Lee NH, Hyun CG. Screening of elastase and tyrosinase inhibitory activity from Jeju Island plants. Eur Asia J Biosci. 2010;4:41–53. [Google Scholar]
- 42.Inomata S, Matsunaga Y, Amano S, Takada K, Kobayashi K, Tsunenaga M, et al. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Invest Dermatol. 2003;120:128–34. doi: 10.1046/j.1523-1747.2003.12021.x. [DOI] [PubMed] [Google Scholar]
- 43.Venditti E, Spadoni T, Tiano L, Astolfi P, Greci L, Littarru GP, et al. In vitro photostability and photoprotection studies of a novel ‘multi-active’ UV-absorber. Free Radic Biol Med. 2008;45:345–54. doi: 10.1016/j.freeradbiomed.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Khazaeli P, Mehrabani M. Screening of sun protective activity of the ethyl acetate extracts of some medicinal plants. Iran J Pharmaceut Res. 2008;7:5–9. [Google Scholar]
- 45.Touitou E, Godin B. Skin nonpenetrating sunscreens for cosmetic and pharmaceutical formulations. Clin Dermatol. 2008;26:375–9. doi: 10.1016/j.clindermatol.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Afaq F, Katiyar SK. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini Rev Med Chem. 2011;11:1200–15. doi: 10.2174/13895575111091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katiyar SK. UV-induced immune suppression and photocarcinogenesis: Chemoprevention by dietary botanical agents. Cancer Lett. 2007;255:1–11. doi: 10.1016/j.canlet.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pissavini M, Alard V, Heinrich U, Jenni K, Perier V, Tournier V, et al. In vitro assessment of water resistance of sun care products: A reproducible and optimized in vitro test method. Int J Cosmet Sci. 2007;29:451–60. doi: 10.1111/j.1468-2494.2007.00407.x. [DOI] [PubMed] [Google Scholar]
- 49.Bendová H, Akrman J, Krejcí A, Kubác L, Jírová D, Kejlová K, et al. In vitro approaches to evaluation of sun protection factor. Toxicol in vitro. 2007;21:1268–75. doi: 10.1016/j.tiv.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 50.Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol. 2008;58:S149–54. doi: 10.1016/j.jaad.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 51.Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48:1–19. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 52.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 53.Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet. 2007;370:528–37. doi: 10.1016/S0140-6736(07)60638-2. [DOI] [PubMed] [Google Scholar]
- 54.Hojerová J, Medovcíková A, Mikula M. Photoprotective efficacy and photostability of fifteen sunscreen products having the same label SPF subjected to natural sunlight. Int J Pharm. 2011;408:27–38. doi: 10.1016/j.ijpharm.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 55.Stanfield J, Osterwalder U, Herzog B. In vitro measurements of sunscreen protection. Photochem Photobiol Sci. 2010;9:489–94. doi: 10.1039/b9pp00181f. [DOI] [PubMed] [Google Scholar]
- 56.Kaur CD, Saraf S. Photochemoprotective activity of alcoholic extract of Camellia sinensis. Int J Pharmacol. 2011;7:400–4. [Google Scholar]
- 57.Kaur CD, Saraf S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacognosy Res. 2010;2:22–5. doi: 10.4103/0974-8490.60586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashawat MS, Saraf S. Photo protective properties of Boerhavia diffusa. Bioscience Biotechnology Research Asia. 2006;3:257–60. [Google Scholar]
- 59.Sharma VK, Choi J, Sharma N, Choi M, Seo SY. In vitro anti-tyrosinase activity of 5-(hydroxymethyl)-2-furfural isolated from Dictyophora indusiata. Phytother Res. 2004;18:841–4. doi: 10.1002/ptr.1428. [DOI] [PubMed] [Google Scholar]
- 60.Leu YL, Hwang TL, Hu JW, Fang JY. Anthraquinones from Polygonum cuspidatum as tyrosinase inhibitors for dermal use. Phytother Res. 2008;22:552–6. doi: 10.1002/ptr.2324. [DOI] [PubMed] [Google Scholar]
- 61.Boissy RE, Visscher M, DeLong MA. DeoxyArbutin: A novel reversible tyrosinase inhibitor with effective in vivo skin lightening potency. Exp Dermatol. 2005;14:601–8. doi: 10.1111/j.0906-6705.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 62.Tada H, Shiho O, Kuroshima K, Koyama M, Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986;93:157–65. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- 63.Ha YM, Chung SW, Song S, Lee H, Suh H, Chung HY. 4-(6-Hydroxy-2-naphthyl)-1,3-bezendiol: A potent, new tyrosinase inhibitor. Biol Pharm Bull. 2007;30:1711–5. doi: 10.1248/bpb.30.1711. [DOI] [PubMed] [Google Scholar]
- 64.Kim KS, Kim JA, Eom SY, Lee SH, Min KR, Kim Y. Inhibitory effect of piperlonguminine on melanin production in melanoma B16 cell line by downregulation of tyrosinase expression. Pigment Cell Res. 2006;19:90–8. doi: 10.1111/j.1600-0749.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 65.Hoshino T, Matsuda M, Yamashita Y, Takehara M, Fukuya M, Mineda K, et al. Suppression of melanin production by expression of HSP70. J Biol Chem. 2010;285:13254–63. doi: 10.1074/jbc.M110.103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar KJ, Yang JC, Chu FH, Chang ST, Wang SY. Lucidone, a novel melanin inhibitor from the fruit of Lindera erythrocarpa Makino. Phytother Res. 2010;24:1158–65. doi: 10.1002/ptr.3018. [DOI] [PubMed] [Google Scholar]
- 67.Solano F, Briganti S, Picardo M, Ghanem G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19:550–71. doi: 10.1111/j.1600-0749.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 68.Parvez S, Kang M, Chung HS, Cho C, Hong MC, Shin MK, et al. Survey and mechanism of skin depigmenting and lightening agents. Phytother Res. 2006;20:921–34. doi: 10.1002/ptr.1954. [DOI] [PubMed] [Google Scholar]
- 69.Song KK, Huang H, Han P, Zhang CL, Shi Y, Chen QX. Inhibitory effects of cis- and trans-isomers of 3,5-dihydroxystilbene on the activity of mushroom tyrosinase. Biochem Biophys Res Commun. 2006;342:1147–51. doi: 10.1016/j.bbrc.2005.12.229. [DOI] [PubMed] [Google Scholar]
- 70.Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003;16:101–10. doi: 10.1034/j.1600-0749.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 71.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 72.Sahasrabudhe A, Deodhar M. Anti-hyaluronidase, anti-elastase activity of Garcinia indica. Int J Bot. 2010;6:299–303. [Google Scholar]
- 73.Kim JH, Byun JC, Reddy AK, Hyun CG, Lee NH. Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus. J Med Plants Res. 2009;3:914–20. [Google Scholar]
- 74.Daamen WF, Veerkamp JH, van Hest JC, van Kuppevelt TH. Elastin as a biomaterial for tissue engineering. Biomaterials. 2007;28:4378–98. doi: 10.1016/j.biomaterials.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 75.Thring TS, Hili P, Naughton DP. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Altern Med. 2009;9:27. doi: 10.1186/1472-6882-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J, Jung E, Kim Y, Park J, Park J, Hong S, et al. Asiaticoside induces human collagen I synthesis through TGFbeta receptor I kinase (TbetaRI kinase)-independent Smad signaling. Planta Med. 2006;72:324–8. doi: 10.1055/s-2005-916227. [DOI] [PubMed] [Google Scholar]
- 77.Bae JY, Choi JS, Choi YJ, Shin SY, Kang SW, Han SJ, et al. (-) Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: Involvement of mitogen-activated protein kinase. Food Chem Toxicol. 2008;46:1298–307. doi: 10.1016/j.fct.2007.09.112. [DOI] [PubMed] [Google Scholar]
- 78.Masaki H. Role of antioxidants in the skin: Anti-aging effects. J Dermatol Sci. 2010;58:85–90. doi: 10.1016/j.jdermsci.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Papanagiotou VD. Skin aging and photoaging. Dermattikon. 2008;4:57–65. [Google Scholar]
- 80.Jung E, Lee J, Baek J, Jung K, Lee J, Huh S, et al. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. J Ethnopharmacol. 2007;112:127–31. doi: 10.1016/j.jep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 81.Bae JY, Choi JS, Kang SW, Lee YJ, Park J, Kang YH. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp Dermatol. 2010;19:e182–90. doi: 10.1111/j.1600-0625.2009.01044.x. [DOI] [PubMed] [Google Scholar]
- 82.Langton AK, Sherratt MJ, Griffiths CE, Watson RE. A new wrinkle on old skin: The role of elastic fibres in skin ageing. Int J Cosmet Sci. 2010;32:1–10. doi: 10.1111/j.1468-2494.2010.00574.x. [DOI] [PubMed] [Google Scholar]
- 83.Uitto J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7:s12–6. [PubMed] [Google Scholar]
- 84.Choi SY, Jung SH, Lee HS, Park KW, Yun BS, Lee KW. Glycation inhibitory activity and the identification of an active compound in Plantago asiatica extract. Phytother Res. 2008;22:323–9. doi: 10.1002/ptr.2316. [DOI] [PubMed] [Google Scholar]
- 85.Lee Y, Hong CO, Nam MH, Kim JH, Ma Y, Kim YB, et al. Antioxidant and glycation inhibitory activities of gold kiwifruit, Actinidia chinensis. J Korean Soc Appl Biol Chem. 2011;54:460–7. [Google Scholar]
- 86.Pageon H. Reaction of glycation and human skin: The effects on the skin and its components, reconstructed skin as a model. Pathol Biol (Paris) 2010;58:226–31. doi: 10.1016/j.patbio.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 87.Jedsadayanmata A. In vitro antiglycation activity of arbutin. Naresuan University Journal. 2005;13:35–41. [Google Scholar]
- 88.Ramamurthi A, Vesely I. Ultraviolet light-induced modification of crosslinked hyaluronan gels. J Biomed Mater Res A. 2003;66:317–29. doi: 10.1002/jbm.a.10588. [DOI] [PubMed] [Google Scholar]
- 89.Stern R, Jedrzejas MJ. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–39. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jedrzejas MJ, Stern R. Structures of vertebrate hyaluronidases and their unique enzymatic mechanism of hydrolysis. Proteins. 2005;61:227–38. doi: 10.1002/prot.20592. [DOI] [PubMed] [Google Scholar]
- 91.Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007;80:1921–43. doi: 10.1016/j.lfs.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 92.Lin CW, Yu CW, Wu SC, Yih KH. PPH free-radical scavenging activity, total phenolic contents and chemical composition analysis of forty-two kinds of essential oils. J Food Drug Anal. 2009;17:386–95. [Google Scholar]
- 93.Nakornriab M, Puangpronpitag D. Antioxidant activities and total phenolic contents of thai curry pastes. International Journal of Applied Chemistry. 2011;7:43–52. [Google Scholar]
- 94.Tibiri A, Sawadogo RW, Ouedraogo N. Evaluation of antioxidant activity, total phenolic and flavonoid contents of Entada africana Guill. et Perr. (Mimosaceae) organ extracts. Res J Med Sci. 2010;4:81–7. [Google Scholar]
- 95.Liu X, Zhao M, Wang J, Yang B, Jiang Y. Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. J Food Compost Anal. 2008;21:219–28. [Google Scholar]
- 96.Yang B, Wang J, Zhao M, Liu Y, Wang W, Jiang Y. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr Res. 2006;341:634–8. doi: 10.1016/j.carres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 1998;62:1201–4. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- 98.Ahmed S, Beigh SH. Ascorbic acid, carotenoids, total phenolic content and antioxidant activity of various genotypes of Brassica Oleracea encephala. J Med Biol Sci. 2009;3:1–8. [Google Scholar]
- 99.Bernatoniene J, Masteikova R, Davalgiene J, Peciura R, Gauryliene R, Bernatoniene R. Topical application of Calendula officinalis (L.): Formulation and evaluation of hydrophilic cream with antioxidant activity. Journal of Medicinal Plants Research. 2011;5:868–77. [Google Scholar]
- 100.Bayerl Ch. Beta-carotene in dermatology: Does it help? (164-6).Acta Dermatovenerol Alp Panonica Adriat. 2008;17:160–2. [PubMed] [Google Scholar]


