Abstract
Sickle cell disease (SCD) is a debilitating hemolytic genetic disorder with high morbidity and mortality affecting millions of individuals worldwide. Although SCD was discovered more than a century ago, no effective mechanism-based prevention and treatment are available due to poorly understood molecular basis of sickling, the fundamental pathogenic process of the disease. SCD patients constantly face hypoxia. One of the best-known signaling molecules to be induced under hypoxic conditions is adenosine. Recent studies demonstrate that hypoxia-mediated elevated adenosine signaling plays an important role in normal erythrocyte physiology. In contrast, elevated adenosine signaling contributes to sickling and multiple life threatening complications including tissue damage, pulmonary dysfunction and priapism. Here, we summarize recent research on the role of adenosine signaling in normal and sickle erythrocytes, progression of the disease and therapeutic implications.
In normal erythrocytes, both genetic and pharmacological studies demonstrate that adenosine can enhance 2,3-bisphosphoglycerate (2,3-BPG) production via A2B receptor (ADORA2B) activation, suggesting that elevated adenosine has an unrecognized role in normal erythrocytes to promote O2 release and prevent acute ischemic tissue injury. However, in sickle erythrocytes, the beneficial role of excessive adenosine-mediated 2,3-BPG induction becomes detrimental by promoting deoxygenation, polymerization of sickle hemoglobin and subsequent sickling. Additionally, adenosine signaling via the A2A receptor (ADORA2A) on invariant natural killer T (iNKT) cells inhibits iNKT cell activation and attenuates pulmonary dysfunction in SCD mice. Finally, elevated adenosine coupled with ADORA2BR activation is responsible for priapism, a dangerous complication seen in SCD.
Overall, the research reviewed here reveals a differential role of elevated adenosine in normal erythrocytes, sickle erythrocytes, iNK cells and progression of disease. Thus, adenosine signaling represents a potentially important therapeutic target for the treatment and prevention of disease.
Keywords: sickle cell disease; malaria; adenosine; adenosine A2B receptor; 2,3-diphosphoglycerate; adenosine deaminase
I. Introduction
1. Definition of SCD
SCD is a devastating genetic hemolytic disorder associated with a high morbidity and mortality. It is the most prevalent autosomal recessive disorder affecting millions worldwide with approximately 300,000 infants born each year with SCD [1, 2]. In 2004, there were about 113,000 hospitalizations for SCD in the United States and the total hospital costs were approximately $488 million [3]. Therefore, SCD accounts for considerable human suffering and medical costs. The pathophysiology of SCD has long been speculated to be due to microvascular occlusion in the setting of erythrocyte sickling and adhesion due to constant hypoxia [4–6]. However, the specific factors and signaling pathways involved in the initiation of sickling and its progression remain unidentified. As a result we lack effective preventative approaches, or mechanism-specific treatment options for the disease.
2. Multiple Milestones Established by the Discoveries of SCD in Modern Medicine
More than a century ago Dr. James B. Herrick, an attending physician at Chicago Presbyterian Hospital and professor of medicine at Rush Medical College in Chicago, noted peculiar elongated and sickle shaped RBCs of a patient who was admitted to Chicago Presbyterian Hospital with anemia. The patient, Walter Noel, was a dental student from Grenada. These observations disclosed the cellular abnormality responsible for the anemia and provided the condition its name and a convenient diagnostic criterion [7]. Approximately 40 years later the noted chemist, Dr. Linus Pauling of Caltech, reported experiments showing that β-globin from patients with SCD had an altered electrophoretic mobility in comparison with normal β-globin[8]. Pauling concluding that SCD resulted from a molecular abnormality in β-globin and coined the term “molecular disease”. A few years later Vernon Ingram of Cambridge University identified the specific amino acid substitution that accounted for the altered electrophoretic mobility originally observed by Pauling [9]. Many years later Yuet W. Khan of the University of California San Francisco discovered a restriction fragment length polymorphism that resulted from the single nucleotide change in the genome of individuals harboring the gene encoding the mutant β-globin [10]. The findings of Khan provided a convenient genetic test for carrier detection and disease prevention through genetic counseling. Taken together, using electrophoresis, sequencing and enzyme digestion, SCD is the first disease identified at molecular level and the specific mutation has been identified.
3. HbS polymerization-mediated erythrocyte sickling is the primary central cause for the pathophysiology SCD
The substitution of valine for glutamic acid at position six in β-globin creats a hydrophobic patch in the HbS tetramer that results in a propensity to polymerize and form long polymers that distort the shape of the RBC [1, 11]. The tendency to polymerize is more pronounced for the deoxygenated form of HbS. HbS polymerization results in the distortion of the red blood cells (RBCs) into a sickle shape [1, 11]. Sickle cells are abnormally rigid, have reduced deformability and are easily destroyed. Due to membrane damage the life span of the RBCs of SCD patients is shortened and this leads to chronic intravascular and extravascular hemolysis and chronic hemolytic anemia [12]. In addition, damaged RBCs have abnormal surfaces that result in increased adherence to and damage of vascular endothelium, a process that enhances acute vaso-occlusion. The persistent and recurring ischemic-hypoxic mediated sickling and increased erythrocyte damage result in chronic injury to multiple organs and leads to multiple key pathophysiological features in SCD patients [1, 5, 13–16]. These include intermittent painful vaso-occlusive crises, painful episodes of priapism (an erectile disorder discussed below), life-threatening splenic autoinfarction, acute chest syndrome, stroke, pulmonary hypertension, and accumulative multi-organ damage and dysfunction and ultimately, shortened lifespan. Thus, patients with SCD suffer from tissue damage and life-threatening complications primarily caused by the polymerization of HbS and the resulting erythrocyte sickling. Despite significant advances in our knowledge of the molecular defect associated with HbS there are still no preventative approaches or mechanism-specific treatment options for the erythrocyte sickling and other key complications seen in the disease [17]. Thus, new approaches to the prevention and treatment of SCD are desperately needed.
II. Adenosine metabolism and signaling
1. Adenosine production and degradation
Adenosine is generated intracellularly and extracellularly by degradation of adenine nucleotides. Under normal physiological conditions, intracellular and extracellular levels of adenosine are in the nanomolar range, but they rise into millimolar concentrations under stressful conditions like hypoxia, ischemia and cellular damage. Intracellularly, adenosine is formed predominantly by dephosphorylation of adenosine monophosphate (AMP), catalyzed by intracellular 5'-nucleotidase [18, 19]. Hydrolysis of s-adenosyl-homocysteine also contributes to intracellular adenosine formation [20]. Inside the cell, adenosine is metabolized by two enzymes, adenosine kinase (ADK) and adenosine deaminase (ADA). ADK phosphorylates adenosine to AMP, and is critical for regulating intracellular levels of adenosine and maintaining intracellular levels of adenine nucleotides. ADA catalyses the irreversible conversion of adenosine to inosine. Intracellular adenosine homeostasis is also maintained by bi-directional equilibrative nucleoside transporters (ENTs) in the plasma membrane, through facilitated diffusion of adenosine in the direction of the concentration gradient [20].
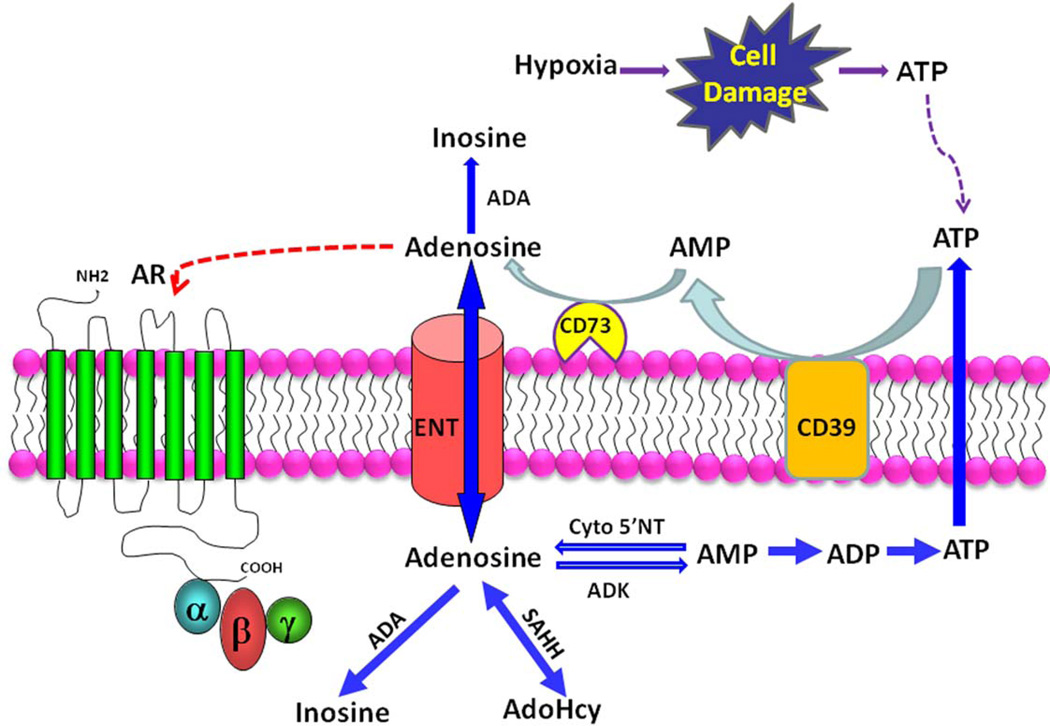
Adenosine is also generated extracellularly by degradation of adenine nucleotides. ATP is released from neurons as neurotransmitters, and also released from cells through connexin hemichannels, and when the cell membrane is subjected to mechanical stress [21]. ATP is also released from cells in response to hypoxia [18, 19]. Of relevance to this review, ATP release from erythrocytes is increased in response to malarial infection and contributes to parasite invasiveness [22–24]. Intracellular ATP levels are in millimolar range, hence when the cell membrane is damaged or lysed, extracellular ATP levels increase substantially. Extracellular adenine nucleotides are dephosphorylated by ectonucleotidases. Ectonucleotidases such as CD39, hydrolyse ATP and ADP to AMP. Ecto-5’-nucleotidase (CD73) catalyses dephosphorylation of AMP to adenosine [25]. Extracellular adenosine is transported into the cells through ENTs, depending upon concentration gradient, and is also degraded to inosine by intracellular ADA [20]. Adenosine metabolism is depicted in Figure 1.
Figure 1. Adenosine metabolism and signaling.
Cells release ATP in response to hypoxia and cell damage. The extracellular ATP is converted to adenosine (A) by the consecutive action of the ecto-nucleotidases, CD39 and CD73. The resulting adenosine can activate adenosine receptors (AR), be a substrate for extracellular adenosine deaminase (ADA), or reenter cells via equilibrative nucleoside transporters (ENTs). Within cells adenosine has multiple fates: 1) conversion to inosine via deamination, 2) conversion to adenosylhomocysteine (AdoHcy) via S-adenosylhomocytesine hydrolase (SAHH), or conversion to AMP by adenosine kinase (ADK). Adenosine can also be derived from AMP by the action of cytosolic 5’-nucleotidase (Cyto 5’NT).
2. Adenosine signaling via four adenosine receptors
Extracellular adenosine affects physiological and pathological processes on target cells by signaling through four different receptors including ADORA1, ADORA2A, ADORA2B and ADORA3. All four receptors are G-protein coupled and each has a distinct affinity for adenosine and a distinct cellular and tissue distribution. ADORA1 and ADORA3 couple to inhibitory G-protein, Gi, to inhibit adenylyl cyclase that results in decreased cyclic AMP [26] levels. A2A and A2B couple to stimulatory G-protein, Gs, to activate adenylyl cyclase that results in increased cAMP levels [20]. Thus, adenosine is a signaling nucleoside that elicits many physiological and pathological effects by engaging membrane receptors on multiple cell types [18].
III. Novel role of adenosine signaling in normal and sickle erythrocytes
Recent studies have revealed a previously unrecognized role of adenosine signaling in normal and sickle erythrocytes. The expression of adenosine receptors on erythrocytes and the role of adenosine signaling via its receptor in normal erythrocyte physiology and sickle pathology will be thoroughly reviewed in this section.
1. Role of adenosine signaling normal erythrocyte physiology
1a) Role of adenosine in 2,3-BPG induction in normal mouse erythrocytes via ADORA2B Activation
Adenosine is a signaling nucleoside that elicits many physiological effects by engaging membrane receptors [18]. However, nothing is known about the functions of adenosine signaling on normal erythrocyte physiology until recent studies revealing functional role of adenosine signaling in regulation of production of 2,3-BPG, an erythroid specific metabolite that induces O2 release from Hb. Zhang et al. [27] demonstrated that treatment of normal mature primary mouse erythrocytes with 5′-N-ethylcarboxamidoadenosine (NECA), a potent, nonmetabolizable adenosine analog, stimulated an increase in 2,3-BPG concentrations, indicating that adenosine can directly induce 2,3-BPG levels in mature mouse RBCs. NECA-mediated induction of 2,3-BPG in normal mouse RBCs was prevented by theophylline, a broad-spectrum adenosine receptor antagonist, indicating that induction of 2,3-BPG in erythrocytes by adenosine is mediated through adenosine receptors. Additional experiments were conducted using RBCs from mice deficient in each of the four different adenosine receptors. RBCs isolated from mice genetically deficient in Adora1, Adora2a and Adora3, or from wild type (WT) mice showed similar increases in 2,3-BPG concentration after treatment with NECA. By contrast, NECA did not induce 2,3-DPG in RBCs from mice genetically deficient in Adora2b. Finally, hypoxia-mediated induction of 2,3-BPG was significantly decreased in RBCs from ADORA2B-deficient mice. These results provide strong genetic evidence that ADORA2B signaling is required for hypoxia-mediated induction of 2,3-BPG in normal mouse RBCs. Overall, functional studies using both pharmacologic and genetic approaches revealed an unrecognized role of adenosine signaling via ADORA2B activation in induction of 2,3-DPG production.
Ib) ADORA2B is expressed in normal mouse erythrocytes
There are limited studies available to directly characterize adenosine receptor expression profiles in red blood cells. Extending functional studies, immunostaining with A2BR-specific antibodies confirmed that A2BR was expressed on isolated wild type (WT) mouse erythrocytes but not in those from A2BR-deficient mice [27]. This is the only attempt to characterize the adenosine receptors in RBCs using this method.
Ic) Protein kinase A functions downstream of ADORA2B responsible for 2,3-DPG induction in normal mouse erythrocytes
ADORA2B is commonly coupled to adenylyl cyclase by the stimulatory G-protein subunit (Gαs) and increases intracellular cAMP, which activates PKA. Zhang et al. [27] demonstrated that NECA induced cAMP production in RBCs from WT mice, but not in those from ADORA2B-deficient mice. Moreover, treatment of normal mouse RBCs with H-89, a specific, potent PKA inhibitor, significantly inhibited NECA-mediated induction of 2,3-BPG. These findings suggest that ADORA2B-mediated cAMP-dependent activation of PKA is responsible for adenosine-mediated induction of 2,3-BPG in mouse RBCs.
1d) ADORA2B signaling-mediated PKA activation underlies 2,3-BPG induction in human RBCs
Similar to mouse studies, a role for adenosine signaling in elevated 2,3-BPG was supported by experiments showing that NECA stimulates an increase in 2,3-BPG levels in a dose- and time-dependent manner in primary cultured human RBCs from healthy individuals. NECA-stimulated induction of 2,3-BPG was inhibited by theophylline and by an ADORA2B antagonist (MRS1754), but not by other adenosine receptor antagonists tested (PSB36, SCH442416 and MRS3777, antagonists of ADORA1, ADORA2A, ADORA3 respectively). The ADORA2B agonist BAY 60-6583, but not the ADORA2A agonist CGS21680, induced 2,3-DPG levels in a dose-dependent manner in RBCs [27]. Finally, H-89, PKA specific inhibitor, significantly reduced NECA-induced 2,3-DPG induction in normal human erythrocytes [27]. As in mice, ADORA2B is specifically expressed on normal human erythrocytes and the ADORA2B-mediated PKA activation is required for adenosine-mediated induction of 2,3-DPG in normal human RBCs [27].
2. Detrimental role of excessive adenosine signaling in erythrocyte sickling
Recent studies demonstrate the adenosine levels are elevated in the circulation of SCD Berkley mice and humans with SCD and increased adenosine plays an important role in the sickling process. We will review these findings in this section.
2a) Metabolomic profiling revealed that both adenosine and 2,3-BPG levels are elevated in the circulation of SCD Berkeley mice
It is well-know that hypoxic conditions promote deoxygenation and subsequent polymerization of HbS resulting in RBC sickling, hemolysis, vasocclusion and eventual end organ damage [11, 28]. Multiple factors and metabolites are altered in response to hypoxia and may contribute to the pathogenesis of SCD. Using high throughput metabolomic profiling, recent studies identified several metabolites, including adenosine and 2,3-BPG, that are highly elevated in the blood of SCD Berkley mice. These mice are a well-accepted animal model of SCD in which the endogenous mouse globin genes have been replaced with human α and βS globin genes [29, 30]. These mice have a relatively severe form of SCD resembling that seen in humans.
2b) Elevated adenosine contributes to sickling in SCD Berkley mice
Metabolomic profiling revealed that adenosine was among the metabolites most highly elevated in the whole blood of SCD transgenic mice compared to controls [27]. Subsequent HPLC measurement confirmed the finding of metabolomic profiling and further showed that the adenosine concentration was also significantly elevated in the plasma of SCD Berkley mice. To determine whether increased adenosine levels contribute to sickling in vivo, SCD Berkley mice were treated with polyethylene glycol–modified adenosine deaminase (PEG-ADA), a well tolerated drug that has been successfully used to lower adenosine levels in ADA-deficient humans and mice. Following 8 weeks of PEG-ADA treatment, blood smear analysis and flow cytometry showed that the percentages of sickled RBCs and the relative abundance of reticulocytes were significantly reduced. Intravascular hemolysis was also signficantly reduced by PEG-ADA treatment as demonstrated by decreased plasma hemoglobin, increased plasma haptoglobin and decreased total billirubin concentrations. Consistent with improved RBC survival, the half-life of RBCs in SCD transgenic mice was increased from 2 d to 4 d following chronic PEG-ADA treatment. Complete blood count analysis showed that chronic PEG-ADA treatment significantly increased the total number of RBCs, hemoglobin concentration and hematocrit and lowered the total number of white blood cells. The increase in hematocrit presumably reflects the corresponding increase in RBC numbers. Red cell distribution width (RDW) was also significantly reduced by PEG-ADA treatment, suggesting that the sizes of RBCs were more uniform and regular in treated than untreated mice. These results show that decreased sickling, reduced hemolysis and prolonged lifespan of RBCs in SCD transgenic mice treated with PEG-ADA resulted in significantly increased erythrocyte number and total hemoglobin content and decreased inflammatory response.
2c) The role of elevated adenosine in the acute sickle crisis
Hypoxia followed by reoxygenation triggers an acute sickle crisis in the Berkeley mouse model of SCD. Because hypoxia is characterized by elevated adenosine, we were not surprised to find that plasma adenosine concentrations in SCD transgenic mice increased significantly following 2 h hypoxia and 4 h reoxygenation. Treatment with PEG-ADA before hypoxia-reoxygenation prevented the increase in adenosine concentrations, reduced sickling, and attenuated hemolysis, as evidenced by decreased plasma hemoglobin and billirubin concentrations [27]. Pretreatment with PEG-ADA also significantly prevented the hypoxia-reoxygenation–induced decrease in RBC numbers, and hematocrit and also attenuated the hypoxia-reoxygenation–induced increase in RDW and in WBC numbers. These findings provide strong evidence that elevated adenosine levels contribute to increased erythrocyte sickling and hemolysis during an acute sickle crisis event triggered by hypoxia-reoxygenation.
In addition to sickling and hemolysis, vaso-occlusion is a key endpoint of an acute crisis event [28]. For this purpose we assessed the effect of PEG-ADA therapy on hypoxia-reoxygenation-induced lung inflammation, a well-accepted measure of vaso-occlusion. Immunostaining with neutrophil-specific antibodies showed that hypoxia-reoxygenation increased neutrophil infiltration in the lungs of SCD mice compared to normoxic conditions [27]. PEG-ADA treatment reduced neutrophil infiltration in the lungs after hypoxia-reoxygenation. PEG-ADA treatment also significantly decreased the abundance of proinflammatory cytokines in the lungs of SCD transgenic mice, including interferon (IFN)-γ, interleukin (IL)-6, IL-1β and granulocyte-macrophage colony-stimulating factor (GM-CSF) [27]. These findings indicate that elevated adenosine levels not only underlie sickling induced by hypoxia-reoxygenation but also contribute to hypoxia-reoxygenation-induced lung inflammation, a major outcome of vaso-occlusion in acute sickle crisis.
2d) ADORA2B mediated 2,3-BPG induction underlies adenosine-mediated sickling in SCD Berkley mice
2,3-BPG is an erythrocyte-specific metabolite that binds to hemoglobin and decreases its oxygen-binding affinity. Previous studies have shown that 2,3-BPG concentrations are increased in the RBCs of individuals with SCD and contribute to erythrocyte sickling [31–33]. Thus, it is possible that elevated erythrocyte 2,3-BPG in SCD mice contributes to adenosine-induced sickling. In support of this hypothesis, Zhang et al. [27] demonstrated that lowering adenosine concentrations in SCD transgenic mice by chronic PEG-ADA treatment resulted in a decrease in 2,3-BPG concentrations in RBCs, increased hemoglobin oxygen-binding affinity measured by an increased percentage of saturated hemoglobin and attenuated chronic sickling. We also found that 2,3-BPG levels increased in response to hypoxia-reoxygenation and that pretreatment with PEG-ADA inhibited this elevation. These results indicate that adenosine is responsible for increased 2,3-BPG concentrations in erythrocytes from SCD transgenic mice and suggest that elevated 2,3-DPG concentrations contribute to both chronic sickling and acute sickle crisis in these mice.
2e) In vivo effects of ADORA2B antagonism in SCD Berkley mice
To determine the in vivo effect of ADORA2B-mediated induction of 2,3-BPG on sickling in SCD Berkley mice, we treated these mice for 8 weeks with the A2BR-specific antagonist PSB1115. Like PEG-ADA treatment, PSB1115 treatment reduced both 2,3-BPG concentrations in RBCs and the percentage of cells that were sickled. Moreover, chronic treatment with PSB1115 increased the half-life of RBCs in SCD transgenic mice from 2 d to 5.5 d, a slightly greater improvement than that seen in mice treated with PEG-ADA [27]. In addition, complete blood count analysis showed significant improvement with PSB1115 treatment, including increased total RBC numbers and hemoglobin concentration as well as decreased total WBC numbers, percentage of reticulocytes and RDW. Overall, these studies provide in vivo evidence that A2BR-mediated induction of 2,3-BPG contributes to erythrocyte sickling.
2f) Adenosine is elevated in the circulation of SCD patients and contributes to sickling by induction of 2,3-BPG in human sickle erythrocytes via ADORA2B
Similar to the mouse in vivo findings, both adenosine and 2,3-BPG levels are elevated in patients with SCD. Functional studies demonstrated that hypoxic conditions resulted in an induction of 2,3-BPG, which was attenuated by PEG-ADA, MRS1754 (an ADORA2B inhibitor), H-89 or glycolate (an inhibitor of 2,3-BPG production) treatment to a similar degree, indicating that adenosine induces 2,3-BPG through ADORA2B signaling. By contrast, NECA stimulated a further increase in 2,3-BPG under hypoxic conditions. Finally, treatment with PEG-ADA, MRS1754, H-89 or glycolate significantly reduced the percentage of sickled cells, whereas NECA treatment significantly enhanced the percentage of sickled cells under hypoxic conditions [27]. Overall, these findings show that the induction of 2,3-BPG by adenosine-mediated ADORA2B activation followed by downstream signaling through PKA is a major underlying mechanism in hypoxia-mediated erythrocyte sickling in RBCs from individuals with SCD.
2g) Elevated adenosine-mediated ADORA2B activation contributes to multi-tissue damage in SCD Berkley mice
The elongated and abnormally shaped RBCs associated with SCD result in obstruction of blood flow in capillary beds of many tissues, resulting in end organ damage [11]. Histological analysis showed that vascular congestion, vascular damage and necrosis in lung, liver and spleen of SCD transgenic mice were significantly improved following chronic treatment with PEG-ADA. Consistent with these histological improvements, PEG-ADA treatment of SCD Berkley mice significantly decreased the elevated heme content in all tissues examined including lung, liver and spleen. The ratio of spleen weight to body weight was significantly reduced after chronic PEG-ADA treatment. Because SCD transgenic mice show marked pathological changes in their kidneys [30] and because approximately 25% of people with SCD develop renal dysfunction with proteinuria [34], chronic PEG-ADA treatment reduced kidney injury and decreased proteinuria and increased urine osmolality of SCD Berkley mice. Much like chronic PEG-ADA enzyme therapy, chronic treatment with PSB1115 reduced vascular congestion, vascular damage and necrosis in multiple tissues [27]. These studies provide in vivo evidence for the detrimental role of excess adenosine coupled with ADORA2B signaling in the pathophysiology of SCD and for the beneficial effects of chronic PEG-ADA enzyme therapy and ADORA2B antagonism.
3. Working model of adenosine signaling in normal and sickle erythrocytes
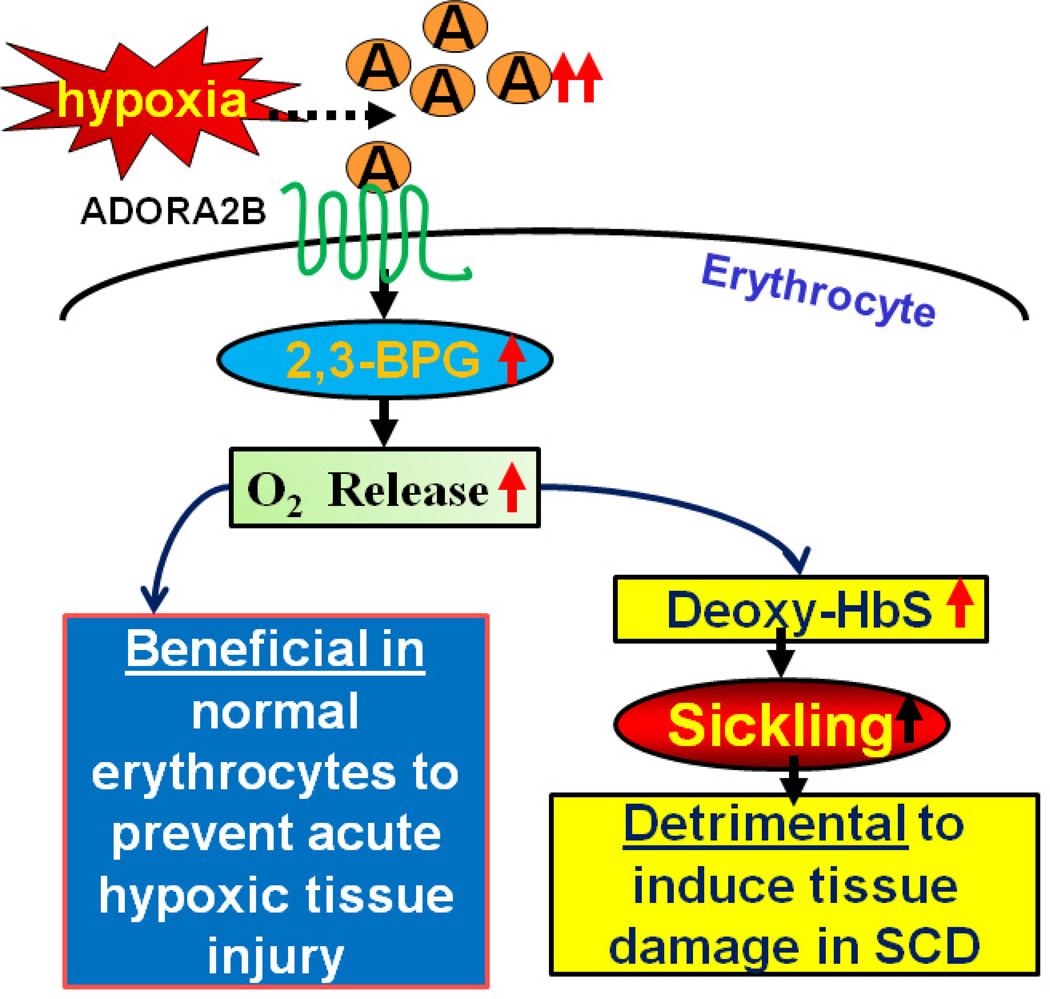
Numerous studies indicate that elevated adenosine signaling via ADORA2B protects against tissue injury in multiple well-accepted models of tissue hypoxia [18, 35, 36]. Thus, our studies raised a novel, but compelling working model that for normal healthy individuals increased adenosine is physiologically a beneficial process that promotes O2 release from Hb to hypoxic tissues to maintain normal tissue function. From this perspective, adenosine stimulated oxygen release from RBCs represents a previously unrecognized beneficial consequence of the hypoxic adenosine response. However, for the individual with SCD this process is detrimental because the increased adenosine promotes O2 release, increased formation of deoxyHbS and its subsequent polymerization leading to sickled RBCs, hemolysis and multiple tissue injury (Figure 2).
Figure 2. Novel role of adenosine signaling in normal and sickle erythrocytes.
Extracellular levels of adenosine increase in response to hypoxia (see Figure 1). Elevated adenosine activates ADORA2B adenosine receptors on erythrocytes, thereby activating downstream signaling pathways resulting in increased intracellular 2,3-bisphosphoglycerate (2,3-BPG), an allosteric regulator of hemoglobin (Hb) that reduces oxygen-binding affinity. This signaling pathway is beneficial to for normal individuals leading to increased oxygen release to hypoxic tissues. However, this process is detrimental for individuals with sickle cell disease by promoting the release of oxygen from sickle hemoglobin (HbS), resulting in increased concentrations of deoxy-HbS, increased deoxy-HbS polymerization and sickling.
IV. Excessive adenosine signaling via ADORA2B contributes to priapism and penile fibrosis-a serious complication of SCD
1. Definition of priapism
Priapism is defined as abnormal prolonged and painful penile erection occurring unassociated with sexual interest [37]. About 40% of men with SCD experience priapism [15, 38, 39]. The disorder is dangerous and urgent given its association with erectile tissue damage and erectile dysfunction. Current strategies to manage the disorder are poor due to lack of fundamental understanding of the etiology and pathophysiology of priapism.
2. An unexpected priapic phenotype in adenosine deaminase deficient mice leads to a novel discovery of novel role of excessive adenosine in priapism in SCD Berkley mice
A potential role for adenosine in priapism was revealed by an unexpected priapism phenotype in adenosine deaminase (ADA)-deficient mice. ADA is a purine metabolic enzyme that catalyzes the conversion of adenosine to inosine. As a result of ADA deficiency these mice exhibit a marked increase in adenosine concentrations, particularly in the penis, which has the highest level of adenosine among all the tissues examined. These mice display features of priapism seen in humans, including spontaneous prolonged penile erection, increased vascular relaxation in response to neurostimulation and penile fibrosis. The spontaneously occurring priapism observed with ADA-deficient mice is quickly relieved by intraperitoneal injection of PEG-ADA. This observation provided the initial clue that elevated adenosine in the penes of Ada−/− mice was responsible for the priapism phenotype presented by these mice. ADA-deficient mice represent a novel and important animal model to study the role of adenosine signaling in priapism. These findings are strongly supported by earlier studies in multiple animal models, including humans, showing that intracavernous injection of adenosine resulted in tumescence and penile erection. Adenosine was shown to increase intracavernous pressure by local injection in multiple animal models [40–45], including humans [46]. Finally, to determine the general pathological significance of excess adenosine in priapism, SCD Tg mice, a well-accepted animal model of SCD displaying priapism [29, 47], were used. These studies further demonstrated that increased adenosine also contributes to priapism in SCD Berkley mice [48–50].
3. ADORA2B on penile vascular smooth muscle cells is responsible for adenosine-induced relaxation of corpus cavernosum in both ADA-deficient mice and SCD Berkley mice
Using 4 adenosine receptor-deficient mice, the studies revealed that the ADORA2B is essential for adenosine-dependent cavernosal smooth muscle relaxation and penile erection. Supporting this finding, RT-PCR studies confirmed that ADORA2B is the major receptor expressed in cavernosal smooth muscle cells. More importantly, genetic and pharmacological studies demonstrated that ADORA2B-mediated cAMP and cGMP induction is required for excess adenosine induced priapism seen in both ADA-deficient mice and SCD Berkley mice. Thus, these studies reveal a general contributory role of adenosine and ADORA2B signaling in priapism.
4. The role of adenosine signaling in penile fibrosis associated with priapism
Penile fibrosis is dangerous and urgent complication of priapism that leads to erectile dysfunction [51]. Recent studies demonstrated that elevated adenosine levels in penile tissue contribute to vascular damage and penile fibrosis associated with priapism and further studies have shown that chronic reduction of adenosine by PEG-ADA enzyme therapy prevented and attenuated the progression of vascular damage [52] and the increased profibrotic gene expression associated with penile fibrosis in both ADA-deficient mice and SCD Tg mice [53]. Mechanistically, using both pharmacologic and genetic tools, the studies revealed that TGF-beta functions downstream of the ADORA2BR and is responsible for excess adenosine-mediated penile fibrosis seen in both lines of mice [53]. Overall, these studies have identified a previously unrecognized novel application of PEG-ADA as a safe, effective and mechanism-based drug to treat and prevent priapism and penile fibrosis in animals and provide a strong justification for clinical trials in men suffering from priapism (Figure 3).
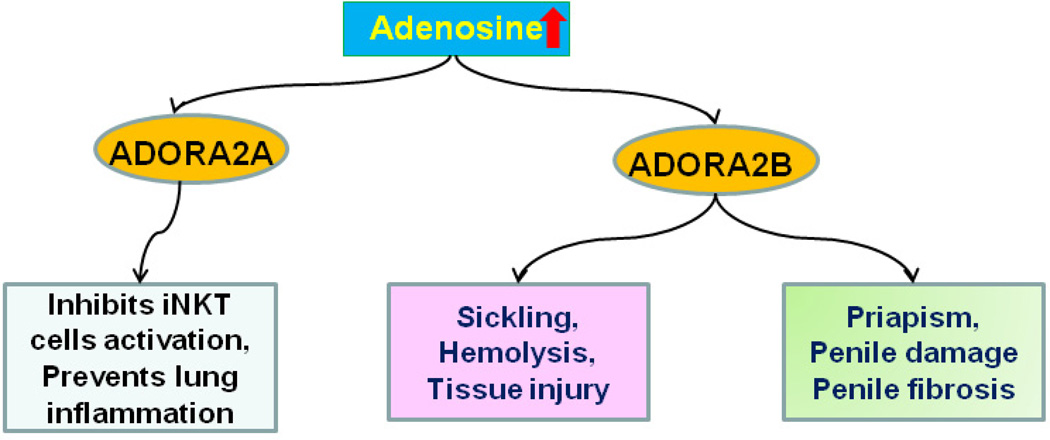
Figure 3. Beneficial and detrimental effects of adenosine signaling in sickle cell disease.
Elevated adenosine associated with SCD has beneficial effects by activating ADORA2A receptors on invariant natural killer T (iNKT) cells, a process that prevents iNKT cells activation and pulmonary inflammation. However, elevated adenosine activates ADORA2B receptors on erythrocytes thereby activating a signaling pathway leading to deoxy-HbS polymerization and erythrocyte sickling (see Fig. 2). The activation of ADORA2B receptors on penile endothelial cells and cavernosal smooth muscle cells results in priapism, a serious complication of SCD, leading to penile fibrosis and erectile dysfunction.
IV. Adenosien signaling via ADORA2A inhibits invariant natural killer T cell activation and prevents lung dysfunction in IN NY1DD SCD mice
1. Protective role of ADORA2A in NY1DD mouse model of SCD by inhibition of natural killer cell activation
In contrast to the detrimental effects of adenosine signaling via ADORA2B in sickling, multiple tissue damage and priapism, recent studies from Dr. Joel Linden’s group have demonstrated the beneficial effects of activation of ADORA2A is to inhibit invariant natural killer T (iNKT) cell activation and subsequent decreases pulmonary dysfunction in the NY1DD mouse model of SCD [54]. Briefly, these studies have shown that iNKT cells are activated in transgenic mice expressing human Hb S (NY1DD), a mild mouse model of SCD compared to SCD Berkley mice. Similarly, iNKT cells were also activated in the patients with SCD. Functional studies show that activated iNKT cells contributes to lung injury in NU1DD mice during acute sickle crisis induced by experimental hypoxia and reoxygenation. Pharmacological studies demonstrated that activation of the ADORA2A in iNKT cells with a specific receptor agonist inhibits iNKT cell function and reduces lung injury in these mice.
2. Novel adenosine-based therapies in SCD
Adenosine functions as a signaling molecule responsible for multiple pathophysiological roles in SCD by activating various receptors on different cell types. For example, on erythrocytes, activation of ADORA2B contributes to sickling by induction of 2,3-BPG; on penile vascular smooth muscle cells, activation of ADORA2B contributes to priapism by induction of cAMP and cGMP and subsequent relaxation of corpus cavernosum; on iNKT cells, activation of ADORA2A inhibits their activation and prevents pulmonary inflammation and dysfunction. These findings have shown that adenosine has differential roles in the pathogenesis of SCD and indicate that targeting on adenosine signaling in the treatment of SCD is complicated. PEG-ADA is a safe drug and has been used effectively for more than two decades to treat ADA-deficient individuals and mice to lower elevated adenosine levels [55, 56]. PEG-ADA is well-tolerated by ADA-deficient humans and mice and has resulted in no obvious side effects in SCD Berkeley mice. In view of the potentially beneficial effects of adenosine-mediated ADORA2A activation on iNKT cells, an ADORA2B antagonist may be a better choice than PEG-ADA, because it will specifically block only one of the four adenosine receptors without the loss of potentially beneficial effects resulting from the activation of other adenosine receptors on different cell types (Figure. 3). Because differential role of adenosine signaling in SCD, it will be interesting to conduct preclinical studies to assess the efficacy and safety of ADORA2A agonists and ADORA2B antagonists in combined treatment in these mouse models of SCD.
1. Sickle cell trait, purinergial signaling and malaria Individuals with sickle cell trait are resistant to malaria infection
The HbS mutation is not uniformly distributed among the world population. Instead the mutation is concentrated among inhabitants of regions where malaria infection is or was rampant [57]. For example the frequency of sickle cell trait (SCT) among members of African tribes in malarious regions can be as high as 40%. A high frequency of carriers is also present in portions of Italy, Greece and India where malaria was prevalent in the past. The genetic puzzle was originally stated by Allison in his seminal paper [57] “how can the sickle-cell gene be maintained at such a high frequency among so many people in spite of the constant elimination of these genes through deaths from the anemia?”
Data originally provided by Allison, confirmed and extended by many others, shows that retention of the HbS allele in the human gene pool can be explained by the survival benefit afforded to heterozygous individuals living in malarious regions [58–61]. A particularly vivid example comes from a detailed study of a cohort of 1022 Kenyon children living near Lake Victoria in a region where malaria is prevalent [62]. Childhood mortality between the ages of 2–16 months was determined for individuals homozygous for the HbS allele (SS), heterozygous for the HbS allele (AS, carriers) or wild type individuals (AA). As expected the highest mortality was observed among those with SCD. However, individuals with SCT (AS, carriers) had a significantly greater survival rate than wild type individuals (AA). If carrier status confers protection against malaria it would be predicted that the HbS allele would be present at increased frequency in regions where malaria transmission is, or has been, intense. This prediction is born out across much of central Africa where malaria transmission is rampant. Sickle cell heterozygote frequencies as high as 20% are also seen in regions of India and Greece that were formerly heavily malarious [63]. Results from genetic linkage studies indicate that the HbS mutation has occurred independently at least five times [64]. Thus, the high levels of HbS alleles occurring in Africa and India appear to represent independently occurring HbS mutations. Tens of thousands of individuals have been studied from around the world and high frequencies of the HbS allele have not been observed in any population that lacks a history of malaria infection. Although the relationship between HbS carrier status and resistance to malaria is now well established and well accepted, the biochemical basis for this relationship is unclear. In view of early studies of purinergic signaling in malaria invasiveness in normal RBCs [22–24] and our recent findings concerning the role of adenosine signaling in erythrocyte physiology [27] we were intrigued by the possibility that this new understanding of RBC regulation may provide clues to understanding the basis of malarial resistance provided by the presence of the βS allele[65]. This possibility will now be discussed below.
2. Purinergic remodeling may reduce efficiency of parasite invasion of RBC by reducing extracellular ATP
Recent evidence indicates that purinerigic signaling is involved in the malaria parasite P. falciparum invasion to normal RBCs. Erythrocytes infected by P. falciparum release large amounts of ATP that apparently binds to ATP receptors present on either schizont or trophozoite stage parasites and promote invasion of uninfected RBCs [24]. Activation of these receptors is important for RBC invasion because drugs that block these receptors impair invasion [22, 23]. Furthermore, the addition of apyrase, an enzyme that hydrolyzes ATP, to the medium during infection significantly reduces parasite invasion into RBCs. The purinergic receptors activated by ATP are of the P2Y category and activate intracellular Ca2+ signaling pathways required for efficient parasite invasion of the RBC. Independent lines of investigation suggest that the protection against P. falciparum infection accorded by pyruvate kinase deficiency also is due to a depletion of ATP resulting from the enzyme deficiency [66]. The pyruvate kinase reaction is critical for erythrocyte ATP production that is derived from phosphoenolpyruvate, a product of glycolysis. Thus, efficient parasite invasion and propagation in RBCs depends on the parasite-induced release of ATP from the infected host cell and the activation of P2Y purinergic receptors on the parasites prior to RBC invasion. Activation of P2Y receptors on cell free parasites is accompanied with activation of calcium signaling pathways within the plasmodium that promote RBC invasion.
3. The hypoxic adenosine response in SCT likely reduces parasite invasiveness to RBCs-possible explanation for resistance of SCT to malaria infection
The presence of the βS allele in those with sickle cell trait (SCT) is generally considered benign. However, numerous reports in recent years have presented convincing evidence that carrier status confers significantly increased risk for a number of mild to serious clinical conditions, including exercise related sudden death [58, 59]. It has long been known that SCT is associated with increased concentrations of free heme in the plasma, possibly due to accelerated autooxidation and heme loss due to instability of βS [67, 68]. This feature may contribute to a mild hemolysis and hypoxia that activates the “hypoxic adenosine response” that promotes changes in gene regulation that favor the breakdown of ATP and the accumulation of adenosine.
In response to hypoxic conditions cells release ATP and other adenine nucleotides that are then converted to extracellular adenosine by the ecto-nucleotidases, CD39 and CD73 (Figure 4). The genes encoding these ecto-enzymes of adenine nucleotide degradation are regulated by transcriptional mechanisms involving the hypoxia-dependent transcription factor, hypoxia inducible factor-1α (HIF-1α) [18, 69] (Figure 4). A critical pathway for terminating extracellular adenosine elevations is the cellular uptake of adenosine through facilitated equilibrative nucleoside transporters (ENTs). ENTs carry out the bidirectional facilitated transport of adenine nucleosides. ENTs are repressed under hypoxic conditions, and may thereby critically contribute to hypoxia-elicited elevations of extracellular adenosine [18]. Thus, inhibition or down regulation of ENTs promote extracellular adenosine elevations and enhance adenosine signaling. Thus, hypoxic conditions promote changes in gene regulation that favor the breakdown of ATP, and the accumulation of extracellular adenosine.
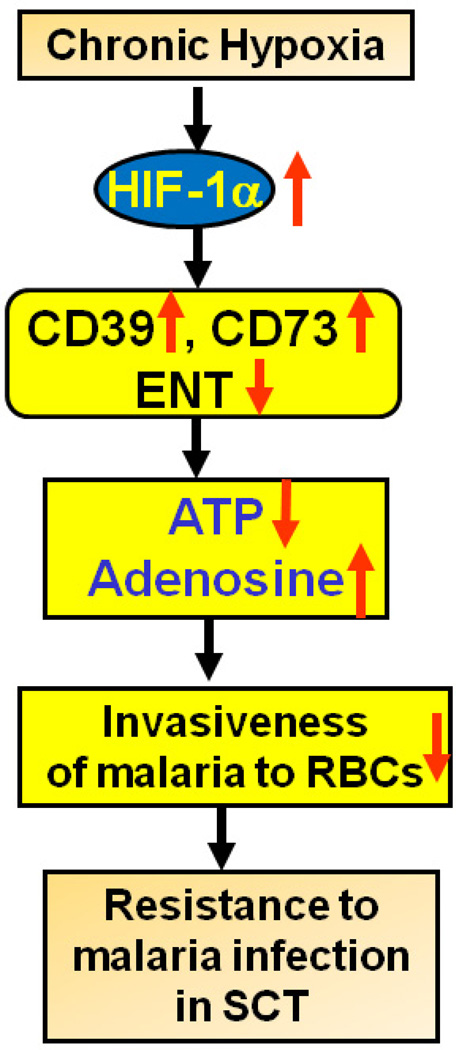
Figure 4. Sickle cell trait (SCT), purinergic signaling and malaria.
SCT is associated with increased concentrations of free heme in the plasma, possibly due to accelerated autooxidation and heme loss due to instability of βS. This feature may contribute to a mild hemolysis and hypoxia that activates purinergic remodeling in nucleated cells, a transcriptional process that promotes changes in gene expression that favor the breakdown of ATP and the accumulation of adenosine. The breakdown of extracellular ATP may result in reduced parasite P2Y signaling on and impaired parasitic invasion of the RBCs.
In view of the study showing that extracellular ATP can increase invasiveness of P. falciparum to RBCs [66] and SCT individuals may face chronic hypoxic conditions [68, 70], we propose the following model. It is possible that mild chronic hypoxic conditions associated with SCT promote activation of Hif-1α and the hypoxic adenosine response. According to this scenario the ecto-enzymes, CD39 and CD73, will be up regulated. Increased CD39 and CD73 will result in the breakdown of extracellular ATP to adenosine. The outcome of these conditions will be a reduction of ATP available to activate P2Y receptors on the parasite that contributes to successful RBC invasion. Thus, one possible mechanism by which SCT confers resistance to P. falciparum is by leading to the reduction of extracellular ATP that is needed to promote parasite invasion of the RBC.
Concluding Remarks
Adenosine is a metabolic signaling molecule induced under energy depletion and ischemic/hypoxic conditions [20, 21]. Although adenosine signaling is involved in numerous cellular functions by engaging its membrane receptors, nothing was known about its role in RBCs until recent studies revealed a previously unrecognized beneficial role for adenosine stimulated-2,3-BPG induction in normal erythrocytes [27]. This study immediately suggests the beneficial effects of enhanced adenosine signaling in acute ischemic injury by stimulating O2 release to hypoxic tissue in normal individuals by induction of 2,3-BPG. In contrast to the beneficial role of adenosine signaling in normal RBCs, these studies have revealed that increased adenosine contributes to sickling by ADORA2B-mediated elevation of 2,3-BPG. Moreover, activation of ADORA2A on iNKT cells is capable of inhibiting their activation and prevents pulmonary inflammation and dysfunction seen in a mouse model of SCD [54]. Thus, the differential role of adenosine on different cells seen in SCD could have profound medical relevance and significant impact in the management of disease. Finally, our findings raised an intriguing hypothesis that hypoxic adenosine response in SCT individual may favor to increase circulating adenosine, decrease ATP and in turn provide resistance to malaria infection by reduction of invasiveness of malaria to RBCs. Taken together, recent new insight of adenosine signaling in erythrocyte physiology provide us better understanding of molecular basis of sickling, novel therapeutics and possible explanation for the survival benefit of SCT for those living in regions where malaria infection is common.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Madigan C, Malik P. Pathophysiology and therapy for haemoglobinopathies. Part I: sickle cell disease. Exp. Rev. Mol. Med. 2006;8:1–23. doi: 10.1017/S1462399406010659. [DOI] [PubMed] [Google Scholar]
- 2.Urbinati F, Madigan C, Malik P. Pathophysiology and therapy for haemoglobinopathies. Part II: thalassaemias. Exp. Rev. Mol. Med. 2006;8:1–26. doi: 10.1017/S1462399406010805. [DOI] [PubMed] [Google Scholar]
- 3.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2011;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 4.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N. Engl. J. Med. 2008;359:2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 6.Akinsheye I, Klings ES. Sickle cell anemia and vascular dysfunction: the nitric oxide connection. J. Cell. Physiol. 2010;224:620–625. doi: 10.1002/jcp.22195. [DOI] [PubMed] [Google Scholar]
- 7.Herrick JB. Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. Arch. Intern. Med. 1910;6:517–521. [PMC free article] [PubMed] [Google Scholar]
- 8.Pauling L, Itano HA, et al. Sickle cell anemia a molecular disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 9.Ingram VM. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956;178:792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- 10.Chang JC, Kan YW. Antenatal diagnosis of sickle cell anaemia by direct analysis of the sickle mutation. Lancet. 1981;2:1127–1129. doi: 10.1016/s0140-6736(81)90584-5. [DOI] [PubMed] [Google Scholar]
- 11.Hebbel RP. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991;77:214–237. [PubMed] [Google Scholar]
- 12.de Montalembert M. Advances in sickle cell disease. Bull. Acad. Nat. Med. 2008;192:1375–1381. discussion 1381. [PubMed] [Google Scholar]
- 13.Kato GJ, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, Brennan ML, Hazen SL, Gladwin MT. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br. J. Haematol. 2005;130:943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato GJ, Onyekwere OC, Gladwin MT. Pulmonary hypertension in sickle cell disease: relevance to children. Pediatr. Hematol. Oncol. 2007;24:159–170. doi: 10.1080/08880010601185892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebastiani P, Nolan VG, Baldwin CT, Abad-Grau MM, Wang L, Adewoye AH, McMahon LC, Farrer LA, Taylor JGt, Kato GJ, Gladwin MT, Steinberg MH. A network model to predict the risk of death in sickle cell disease. Blood. 2007;110:2727–2735. doi: 10.1182/blood-2007-04-084921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gladwin MT. Prevalence, risk factors and mortality of pulmonary hypertension defined by right heart catheterization in patients with sickle cell disease. Expert review of hematology. 2011;4:593–596. doi: 10.1586/ehm.11.66. [DOI] [PubMed] [Google Scholar]
- 17.Ballas SK. Current issues in sickle cell pain and its management, Hematology / the Education Program of the American Society of Hematology. Am. Soc. Hematol. 2007:97–105. doi: 10.1182/asheducation-2007.1.97. [DOI] [PubMed] [Google Scholar]
- 18.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 2011;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 21.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 22.Tanneur V, Duranton C, Brand VB, Sandu CD, Akkaya C, Kasinathan RS, Gachet C, Sluyter R, Barden JA, Wiley JS, Lang F, Huber SM. Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. Faseb J. 2006;20:133–135. doi: 10.1096/fj.04-3371fje. [DOI] [PubMed] [Google Scholar]
- 23.Levano-Garcia J, Dluzewski AR, Markus RP, Garcia CR. Purinergic signalling is involved in the malaria parasite Plasmodium falciparum invasion to red blood cells. Purinergic signalling. 2010;6:365–372. doi: 10.1007/s11302-010-9202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akkaya C, Shumilina E, Bobballa D, Brand VB, Mahmud H, Lang F, Huber SM. The Plasmodium falciparum-induced anion channel of human erythrocytes is an ATP-release pathway. Pflugers Arch. 2009;457:1035–1047. doi: 10.1007/s00424-008-0572-8. [DOI] [PubMed] [Google Scholar]
- 25.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5'-nucleotidase (CD73) Purinergic signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV, Carter-Dawson L, Lewis DE, Zhang W, Eltzschig HK, Kellems RE, Blackburn MR, Juneja HS, Xia Y. Detrimental effects of adenosine signaling in sickle cell disease. Nat. Med. 2011;17:79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129–151. [PubMed] [Google Scholar]
- 29.Paszty C. Transgenic and gene knock-out mouse models of sickle cell anemia and the thalassemias. Curr. Opin. Hematol. 1997;4:88–93. doi: 10.1097/00062752-199704020-00003. [DOI] [PubMed] [Google Scholar]
- 30.Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 31.Poillon WN, Kim BC. 2,3-Diphosphoglycerate and intracellular pH as interdependent determinants of the physiologic solubility of deoxyhemoglobin S. Blood. 1990;76:1028–1036. [PubMed] [Google Scholar]
- 32.Poillon WN, Kim BC, Labotka RJ, Hicks CU, Kark JA. Antisickling effects of 2,3-diphosphoglycerate depletion. Blood. 1995;85:3289–3296. [PubMed] [Google Scholar]
- 33.Poillon WN, Kim BC, Welty EV, Walder JA. The effect of 2,3-diphosphoglycerate on the solubility of deoxyhemoglobin S. Archives of biochemistry and biophysics. 1986;249:301–305. doi: 10.1016/0003-9861(86)90006-8. [DOI] [PubMed] [Google Scholar]
- 34.Scheinman JI. Sickle cell disease and the kidney. Nat. Clin. Pract. 2009;5:78–88. doi: 10.1038/ncpneph1008. [DOI] [PubMed] [Google Scholar]
- 35.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burnett AL. Therapy insight: Priapism associated with hematologic dyscrasias. Nat. Clin. Pract. Urol. 2005;2:449–456. doi: 10.1038/ncpuro0277. [DOI] [PubMed] [Google Scholar]
- 38.Bennett N, Mulhall J. Sickle cell disease status and outcomes of African-American men presenting with priapism. J. Sex. Med. 2008;5:1244–1250. doi: 10.1111/j.1743-6109.2008.00770.x. [DOI] [PubMed] [Google Scholar]
- 39.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood. 2005;106:3264–3267. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbasi S, Su B, Kellems RE, Yang J, Xia Y. The essential role of MEKK3 signaling in angiotensin II-induced calcineurin/nuclear factor of activated T-cells activation. J.Biol. Chem. 2005;280:36737–36746. doi: 10.1074/jbc.M506493200. [DOI] [PubMed] [Google Scholar]
- 41.Yonezawa A, Sakurada S, Furukawa K, Kimura Y. Adenosine and adenosine triphosphate. Nippon Rinsho. 2002;60(Suppl 6):52–56. [PubMed] [Google Scholar]
- 42.Noto T, Inoue H, Mochida H, Kikkawa K. Role of adenosine and P2 receptors in the penile tumescence in anesthetized dogs. Eur. J. Pharmacol. 2001;425:51–55. doi: 10.1016/s0014-2999(01)01167-0. [DOI] [PubMed] [Google Scholar]
- 43.Filippi S, Mancini M, Amerini S, Bartolini M, Natali A, Mancina R, Forti G, Ledda F, Maggi M. Functional adenosine receptors in human corpora cavernosa. Int. J. Androl. 2000;23:210–217. doi: 10.1046/j.1365-2605.2000.00232.x. [DOI] [PubMed] [Google Scholar]
- 44.Shalev M, Staerman F, Allain H, Lobel B, Saiag B. Stimulation of P2y purinoceptors induces, via nitric oxide production, endothelium-dependent relaxation of human isolated corpus cavernosum. J. Urol. 1999;161:955–959. [PubMed] [Google Scholar]
- 45.Sharifzadeh M, Zarrindast MR, Samini M. Effects of adenosine analogues on apomorphine-induced penile erection in rats. Gen. Pharmacol. 1995;26:1785–1790. doi: 10.1016/0306-3623(95)00114-x. [DOI] [PubMed] [Google Scholar]
- 46.Kilic S, Salih M, Anafarta K, Baltaci S, Kosar A. Adenosine: a new agent in the diagnosis of impotence. Int. J. Impot. Res. 1994;6:191–198. [PubMed] [Google Scholar]
- 47.Hsu L, Diwan B, Ward JM, Noguchi CT. Pathology of "Berkeley" sickle-cell mice includes gallstones and priapism. Blood. 2006;107:3414–3415. doi: 10.1182/blood-2005-11-4500. [DOI] [PubMed] [Google Scholar]
- 48.Mi T, Abbasi S, Zhang H, Uray K, Chunn JL, Xia LW, Molina JG, Weisbrodt NW, Kellems RE, Blackburn MR, Xia Y. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J. Clin. Invest. 2008;118:1491–1501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai Y, Zhang Y, Phatarpekar P, Mi T, Zhang H, Blackburn MR, Xia Y. Adenosine signaling, priapism and novel therapies. J. Sex. Med. 2009;6(Suppl 3):292–301. doi: 10.1111/j.1743-6109.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 50.Phatarpekar PV, Wen J, Xia Y. Role of Adenosine Signaling in Penile Erection and Erectile Disorders. J. Sex. Med. 2010;7:3553–3564. doi: 10.1111/j.1743-6109.2009.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol. Clin. North Am. 2007;34:631–642. doi: 10.1016/j.ucl.2007.08.006. viii. [DOI] [PubMed] [Google Scholar]
- 52.Wen J, Jiang X, Dai Y, Zhang Y, Tang Y, Sun H, Mi T, Kellems RE, Blackburn MR, Xia Y. Adenosine deaminase enzyme therapy prevents and reverses the heightened cavernosal relaxation in priapism. J. Sex. Med. 2010;7:3011–3022. doi: 10.1111/j.1743-6109.2009.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen J, Grenz A, Zhang Y, Dai Y, Kellems RE, Blackburn MR, Eltzschig HK, Xia Y. A2B adenosine receptor contributes to penile erection via PI3K/AKT signaling cascade-mediated eNOS activation. Faseb J. 2011;25:2823–2830. doi: 10.1096/fj.11-181057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010 doi: 10.1182/blood-2010-06-290643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hershfield MS. PEG-ADA replacement therapy for adenosine deaminase deficiency: an update after 8.5 years. Clin. Immunol. Immunopathol. 1995;76:S228–S232. doi: 10.1016/s0090-1229(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 56.Blackburn MR, Aldrich M, Volmer JB, Chen W, Zhong H, Kelly S, Hershfield MS, Datta SK, Kellems RE. The use of enzyme therapy to regulate the metabolic and phenotypic consequences of adenosine deaminase deficiency in mice. Differential impact on pulmonary and immunologic abnormalities. J. Biol. Chem. 2000;275:32114–32121. doi: 10.1074/jbc.M005153200. [DOI] [PubMed] [Google Scholar]
- 57.Allison AC. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans. R. Soc.Trop. Med. Hyg. 1954;48:312–318. doi: 10.1016/0035-9203(54)90101-7. [DOI] [PubMed] [Google Scholar]
- 58.Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am. J. Med. 2009;122:507–512. doi: 10.1016/j.amjmed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 59.Key NS, Derebail VK. Sickle-cell trait: novel clinical significance. Hematology / the Education Program of the American Society of Hematology. Am. Soc. Hematol. 2010:418–422. doi: 10.1182/asheducation-2010.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allison AC. Protection afforded by sickle-cell trait against subtertian malareal infection. Br. Med. J. 1954;1:290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allison AC. Genetic factors in resistance to malaria. Ann. New York Acad. Sci. 1961;91:710–729. doi: 10.1111/j.1749-6632.1961.tb31102.x. [DOI] [PubMed] [Google Scholar]
- 62.Aidoo M, Terlouw DJ, Kolczak MS, McElroy PD, ter Kuile FO, Kariuki S, Nahlen BL, Lal AA, Udhayakumar V. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–1312. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 63.Allison AC. Genetic control of resistance to human malaria. Curr. Opin. Immunol. 2009;21:499–505. doi: 10.1016/j.coi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Lopez C, Saravia C, Gomez A, Hoebeke J, Patarroyo MA. Mechanisms of genetically-based resistance to malaria. Gene. 2010;467:1–12. doi: 10.1016/j.gene.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Fairhurst RM, Bess CD, Krause MA. Abnormal PfEMP1/knob display on Plasmodium falciparum-infected erythrocytes containing hemoglobin variants: fresh insights into malaria pathogenesis and protection. Microbes Infect. 2012;14 doi: 10.1016/j.micinf.2012.05.006. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ayi K, Liles WC, Gros P, Kain KC. Adenosine triphosphate depletion of erythrocytes simulates the phenotype associated with pyruvate kinase deficiency and confers protection against Plasmodium falciparum in vitro. J. Infect. Dis. 2009;200:1289–1299. doi: 10.1086/605843. [DOI] [PubMed] [Google Scholar]
- 67.Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha-Rodrigues M, Portugal S, Soares MP, Mota MM. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 68.Hebbel RP, Morgan WT, Eaton JW, Hedlund BE. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc. Nat. Acad. Sci. U.S.A. 1988;85:237–241. doi: 10.1073/pnas.85.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 70.Rosenthal PJ. Lessons from sickle cell disease in the treatment and control of malaria. N. Engl. J. Med. 2011;364:2549–2551. doi: 10.1056/NEJMcibr1105118. [DOI] [PubMed] [Google Scholar]