Abstract
This review highlights the potential role that post-copulatory sexual selection plays in elasmobranch reproductive systems and the utility of this group to further understanding of evolutionary responses to the post-copulatory processes of sperm competition and cryptic female choice. The growing genetic evidence for female multiple mating (polyandry) in elasmobranchs is summarized. While polyandry appears to be common in this group, rates of multiple paternity are highly variable between species suggesting that there is large variance in the strength of post-copulatory sexual selection among elasmobranchs. Possible adaptations of traits important for post-copulatory sexual selection are then considered. Particular emphasis is devoted to explore the potential for sperm competition and cryptic female choice to influence the evolution of testes size, sperm morphology, genital morphology and sperm storage organs. Finally, it is argued that future work should take advantage of the wealth of information on these reproductive traits already available in elasmobranchs to gain a better understanding of how post-copulatory sexual selection operates in this group.
Keywords: cryptic female choice, genitals, multiple paternity, oviducal gland, sperm competition, testis
INTRODUCTION
Darwin (1871) realized that survival alone is not enough to secure the transmission of genes to future generations and suggested that another form of selection, which he called sexual selection, influences the evolution of traits that determine mating success. Sexual selection can promote the evolution of costly pre-copulatory (before mating) traits, such as weapons and extravagant ornaments, that enhance mating success during male-male competition and mate choice (Andersson, 1994). In most animals, however, females mate with multiple males within a single reproductive episode and sperm from different males can overlap both temporally and spatially within the female’s reproductive tract. Consequently, sexual selection can continue after mating through the post-copulatory processes of sperm competition, the competition among ejaculates from rival males for fertilization of the female’s ova (Parker, 1970), and cryptic female choice, when females influencing the outcome of sperm competition through variation in their behaviour, physiology or morphology (Thornhill, 1983; Eberhard, 1985, 1996). Evidence from numerous taxa reveals that these post-copulatory processes influence the evolution of sexual behaviours, ejaculate traits (e.g. ejaculate volume and sperm morphology, swimming speed and viability), genital and reproductive tract morphology, and reproductive physiology (Birkhead, 1998; Hosken & Stockley, 2004; Snook, 2005; Birkhead et al., 2009; Evans & Meisner, 2009; Montgomerie & Fitzpatrick, 2009). Indeed, due to the prevalence and importance of post-copulatory sexual selection in influencing fitness, sperm competition and cryptic female choice are now recognized as a potent selective force influencing the evolution of myriad sexual traits (Birkhead & Møller, 1998; Birkhead et al., 2009).
Over the past four decades, studies of post-copulatory sexual selection have grown almost exponentially to include most major groups of animals (Birkhead & Møller, 1998; Birkhead et al., 2009). Studies of post-copulatory sexual selection in fishes have notably lagged behind that of other taxa (Montgomerie & Fitzpatrick, 2009), however, and this is particularly obvious for elasmobranchs. In this review, the available evidence for post-copulatory sexual selection in elasmobranchs is reviewed and hypotheses are presented on the role that it plays in shaping male reproductive traits in this group. As will be clear from this review, although post-copulatory sexual selection is an inevitable consequence of their reproductive biology, studies of sperm competition and cryptic female choice are a very underdeveloped field in elasmobranchs. Therefore, the many features that make this group ideal for gaining novel insights into post-copulatory sexual selection are highlighted in order to identify fruitful avenues for future research. The evidence that females mate with multiple males in elasmobranchs is reviewed first, as this is a prerequisite for any putative model system of post-copulatory sexual selection. Then, a range of possible adaptations for post-copulatory sexual selection displayed by elasmobranchs are considered, focusing on traits that are known to play important roles in sperm competition, cryptic female choice and sexual conflict in other taxa.
MULTIPLE PATERNITY IN ELASMOBRANCHS
Direct evidence that females mate with multiple males over the course of a single breeding season (polyandry) comes either from infrequent behavioural observations of multiple matings in the field (Chapman et al., 2003) or, more commonly, from genetic studies where advances in molecular techniques have led to an increase in the number of studies assessing multiple paternity (the siring of a single brood of offspring by multiple fathers) in elasmobranchs (Feldheim et al., 2001; Chapman et al., 2004; Daly-Engel et al., 2006). Genetic studies have supported the conclusion that polyandry occurs in elasmobranchs, thereby enabling researchers to confidently infer patterns of reproductive behaviour without direct behavioural observations. While the application of molecular methods to assign paternity is relatively recent in elasmobranchs, the available evidence demonstrates that polyandry is both prevalent and highly variable in this group. Furthermore, among sharks, it is likely that multiple paternity evolved relatively early, prior to the division of the two major shark superorders (Squalomorphii and Galeomorphii) c. 350 million years ago (Heinecke et al., 2009). Of the eight extant orders of shark, five have been shown to exhibit multiple paternity: Carcharhiniformes, Orectolobiformes, Hexanchiformes, Lamniformes and Squaliformes.
Multiple paternity has been documented in 12 of the 15 species (80%) of elasmobranchs summarized in this review (including 14 species of sharks and one skate; Table I). The frequency of multiple paternity, however, varies widely among elasmobranch species. For example, Daly-Engel et al. (2010) and Chapman et al. (2004) reported a predominance of genetic monogamy in the shortspine spurdog Squalus cf. mitsukurii Jordan & Snyder 1903 and the bonnethead shark Sphyrna tiburo (L. 1758), respectively, while a predominance of genetic polyandry has been reported in the lemon shark Negaprion brevirostris (Poey 1868) and the small spotted catshark Scyliorhinus canicula (L. 1758) (Feldheim et al., 2001; DiBattista et al., 2008; Griffiths et al., in press; see Table I). Rates of multiple paternity can also vary among populations of a single species. In the sandbar shark Carcharhinus plumbeus (Nardo 1827), rates of multiple paternity range from 40% in a tropical population in the central Pacific Ocean to 85% in a temperate population in the north-west Atlantic Ocean (Daly-Engel et al., 2007; Portnoy et al., 2007). Similarly, in the spiny dogfish Squalus acanthias L. 1758 populations sampled along the east coast of North America, rates of multiple paternity vary almost two-fold, ranging from 17 to 30% (Lage et al., 2008; Veríssimo et al., 2010).
Table I.
A compilation of all known studies on elasmobranch multiple paternity (MP) to date (N, number of broods sampled). For both N and % MP, a range is presented if multiple studies are cited under the same species or if the author gave a range as a finding. As 10 is considered the minimal number of broods able to generate a frequency estimate, studies with N <10 are listed as multiple paternity ‘Found’ or ‘Not found’
| Common name | Latin name | N | % MP | Methodology | Reference |
|---|---|---|---|---|---|
| Lemon shark | Negaprion brevirostris | 45–46 | 81–86 | Kinship reconstruction with microsatellites |
Feldheim et al. (2001), DiBattista et al. (2008) |
| Spiny dogfish | Squalus acanthias | 10–29 | 17–30 | Brood sampling, microsatellites |
Lage et al. (2008), Veríssimo et al. (2010) |
| Shortspine spurdog | Squalus cf. mitsukurii | 27 | 11 | Brood sampling, microsatellites | Daly-Engel et al. (2010) |
| Bonnethead shark | Sphyrna tiburo | 22 | 19 | Brood sampling, microsatellites | Chapman et al. (2004) |
| Sandbar shark | Carcharhinus plumbeus | 20 | 40–85 | Brood sampling, microsatellites |
Daly-Engel et al. (2007), Portnoy et al. (2007) |
| Small-spotted catshark | Scyliorhinus canicula | 13 | 85–92 | Brood sampling, microsatellites | Griffiths et al. (in press) |
| Thornback ray | Raja clavata | 4 | Found in all | Brood sampling, microsatellites | Chevolot et al. (2007) |
| Shortfin mako shark | Isurus oxyrinchus | 4 | Found in all | Brood sampling, microsatellites | Gubili et al. (2012) |
| Nurse shark | Ginglymostoma cirratum | 1–2 | Found in all | Brood sampling with MHC genes and RFLP |
Ohta et al. (2000), Saville et al. (2002) |
| Bignose shark | Carcharhinus altimus | 1 | Found | Brood sampling, microsatellites | Daly-Engel et al. (2006) |
| Bluntnose sixgill shark | Hexanchus griseus | 1 | Found | Brood sampling, microsatellites | Larson et al. (2011) |
| White shark | Carcharadon carcharias | 1 | Found | Brood sampling, microsatellites | Gubili et al. (2012) |
| Banded houndshark | Triakis scyllium | 1 | Not found | Brood sampling with MHC genes and RFLP |
Ohta et al. (2000) |
| Galapagos shark | Carcharhinus galapagensis | 1 | Not found | Brood sampling, microsatellites | Daly-Engel et al. (2006) |
| Whale shark | Rhincodon typus | 1 | Not found | Brood sampling, microsatellites | Schmidt et al. (2010) |
The occurrence of polyandrous mating among elamobranchs may actually be underestimated in the studies reviewed in Table I for a number of reasons. First, the molecular techniques used to assess rates of multiple paternity rely on adequate sample size to give authentic frequency estimates. Many studies of multiple paternity in elasmobranchs, however, particularly for rare or endangered species, utilize opportunistic analyses on a single brood, thereby precluding an accurate frequency estimate. Indeed, of the 15 species summarized in Table I, multiple paternity has been found in every case where more than one litter was examined. Further, species whose life history includes litter sizes smaller than three pups, a common trait in a number of elasmobranchs, cannot be easily examined for multiple paternity. Finally, factors such as fertilization bias due to sperm competition and cryptic female choice will lead to the underestimation of polyandry in elasmobranchs, as these processes inevitably exclude genetic representation by some mated males (García-González, 2008). Regardless of these limitations, genetic techniques are still useful for detecting the occurrence of multiple paternity in elasmobranchs, and the number of studies documenting this phenomenon continues to grow.
EVOLUTIONARY RESPONSES TO POST-COPULATORY SEXUAL SELECTION IN ELASMOBRANCHS
The preceding evidence that multiple mating occurs in numerous species of elasmobranchs (Table I) illustrates the potential role for post-copulatory sexual selection to shape reproductive trait evolution in this group. Little attention, however, has been paid to understanding such evolutionary processes in elasmobranchs, much less the underlying mechanisms responsible. This section explores a range of putative reproductive traits that may be influenced by post-copulatory sexual selection in elasmobranchs, including testes size, sperm morphology, genital morphology and sperm storage organs in females. These traits are known to be important determinants of competitive fertilization success and influence the outcome of post-copulatory sexual selection in other taxa and, as this review demonstrates, there is increasing evidence that these traits are shaped by similar evolutionary forces in elasmobranchs.
TESTES SIZE
Investment in testicular tissue is highly variable among species (Harcourt et al., 1981; Møller, 1991; Todd, 2008; Montgomerie & Fitzpatrick, 2009) and is influenced by a variety of factors including body size, seasonal effects and clutch size (Møller, 1991; Stockley et al., 1996; Montgomerie & Fitzpatrick, 2009). After controlling for these factors, however, relative (i.e. body size corrected) investment in testicular tissue often reflects the extent of multiple mating by females in a population, and thus the strength of post-copulatory sexual selection (Parker et al., 1997; Birkhead & Møller, 1998; Montgomerie & Fitzpatrick, 2009). Larger testes produce greater numbers of sperm, as revealed by the positive relationship between testes size (i.e. combined testes mass) and the number of sperm ejaculated (Parker, 1982; Møller, 1989; Marconato & Shapiro, 1996; Schärer et al., 2004). Thus, males with larger testes are able to ejaculate a greater number of sperm, and consequently enjoy a competitive advantage over rival males in contests to fertilize a female’s ova (Parker, 1982). Highly polyandrous species are, therefore, expected to have larger testes relative to closely related monogamous species, a prediction that has been validated across a wide range of taxa (Parker et al., 1997).
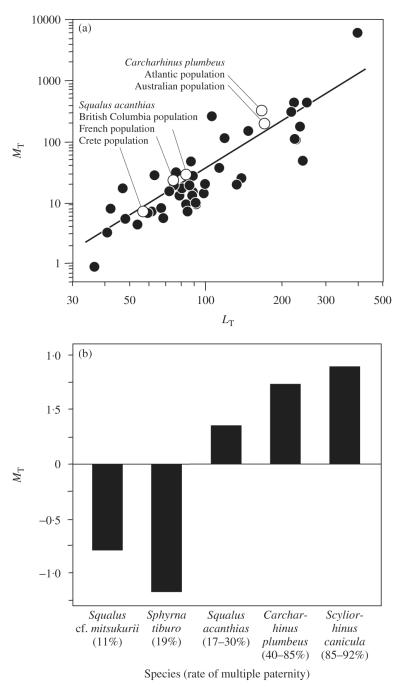
Given the clear link between mating behaviours and relative testes size, the use of size corrected testes mass is commonly used as a proxy measure for the risk of sperm competition (Parker et al., 1997). Therefore, to provide possible insights into how post-copulatory sexual selection influences testes size in elasmobranchs, the relationship between testes mass (MT) and body size (total length, LT) is explored in an effort to identify species whose relatively large or small testes may provide insight into sexual selection. Using information from the literature and unpublished work, data were compiled on MT (whenever possible using peak values to account for seasonal effects) and adult male LT for 43 species of sharks (data available in Table SI, Supporting Information). As with other fishes (Montgomerie & Fitzpatrick, 2009), there was a strong positive correlation between MT and LT in sharks [r = 0·87, P < 0·001, Fig. 1(a)]. It is clear, however, that there are several species that invest either relatively more or less in testes size than would be expected when only considering LT as an explanatory variable [Fig. 1(a)]. Interspecific variation in investment in MT is known to be a hallmark of the effect of post-copulatory sexual selection in shaping testes size in other taxa (Harcourt et al., 1981). Of the species of elasmobranchs examined [Fig. 1(a)], several may offer valuable insights into how selection acts on testes size and some of these are discussed in greater detail.
Fig. 1.
Testes mass (MT) and post-copulatory sexual selection in elasmobranchs. (a) Relationship between mean MT and adult male total length (LT) in 43 species of sharks. Data are available in Table SI, Supporting Information. (b) Relationship between rates of multiple paternity for five shark species and relative investment in testes as measured using residual MT values. Rates of multiple paternity are presented in Table I. Residual MT values were calculated from a log10–log10 plot of MT and LT for Squalus acanthias (F. Hazin, unpubl. data), Squalus cf. mitsukurii (M. Broadhurst, unpubl. data), Sphyrna tiburo (G. Parsons, unpubl. data), Carcharhinus plumbeus (I. Baremore & L. Hale, unpubl. data) and Scyliorhinus canicula (Kousteni et al., 2010). For all species, the geographic location where testes and multiple paternity data were collected was matched as closely as possible.
Data on LT and MT were available for five species where rates of multiple paternity have been established (Table I): Squalus cf. mitsukurii, S. tiburo, S. acanthias, C. plumbeus and S. canicula. To explore the possible link between rates of multiple paternity and investment in MT, residual MT was calculated from a regression of log10 LT and log10 MT. These residual MT values were then compared to observed rates of multiple paternity. Residual values were used rather than the more traditional gonado-somatic index [IG = 100 MT (M – MT)−1, where M body mass] as the latter has been criticized on statistical grounds (Tomkins & Simmons, 2002). Because of the small sample size, residual MT values were used here for illustrative purposes only. This preliminary examination of the data reveals that species with higher rates of multiple paternity also invest relatively more in testicular tissue [Fig. 1(b)], suggesting that elasmobranch species experiencing a greater risk of sperm competition have relatively larger testes than those with lower risk of sperm competition.
Evidence of population-specific investment in MT was found in two elasmobranch species, which may indicate population-specific responses to the risk of sperm competition. For C. plumbeus, male investment in testes differs between populations, with males from Atlantic Ocean populations investing relatively more in testes compared with males from Australian populations [Fig. 1(a)]. This difference in testes size may result from geographically variable rates of multiple paternity observed in C. plumbeus populations. Although the degree of multiple paternity is currently unknown in Australian populations, in the north-west Atlantic Ocean population 85% of litters have multiple sires (Portnoy et al., 2007), suggesting that sperm competition may be prevalent in this population. The rate of multiple paternity in north-west Atlantic Ocean C. plumbeus is among the highest observed in elasmobranchs (Table I) and more than double that of the Hawaiian population of C. plumbeus (Daly-Engel et al., 2007). If rates of multiple paternity in the Australian population are similar to those in the Hawaiian population, the observed difference in testicular investment could represent a local response to differences in mating behaviours. Similarly, MT varies among three populations of S. acanthias [Fig. 1(a)], which may be another species where rates of multiple paternity vary among populations (Table I). Such intraspecific variance in testes size in response to differential rates of multiple paternity is not without precedent (Firman & Simmons, 2008) and probably represents a population-specific response to the level of sperm competition.
SPERM MORPHOLOGY AND QUALITY
The considerable variation in sperm morphology and components of sperm ‘quality’ (e.g. sperm swimming speed, viability or the amount of variation in sperm morphology) observed in numerous taxa is thought to reflect variance in the strength of post-copulatory sexual selection (Hunter & Birkhead, 2002; Montgomerie & Fitzpatrick, 2009; Pitnick et al., 2009; Fitzpatrick & Baer, 2011). Thus, sperm traits that make a male’s ejaculate more competitive than sperm from rival males are expected to provide a selective advantage in post-copulatory sexual selection. Many studies have upheld this prediction by showing that males with faster swimming sperm (Gage et al., 2004; Casselman et al., 2006; Liljedal et al., 2008; Gasparini et al., 2010) or sperm that are better suited to the characteristics of a females’ reproductive tract (García-González & Simmons, 2007) fertilize more eggs than sperm from rival males. Furthermore, several recent studies have demonstrated a link between the length of various sperm components (e.g. head, mid-piece and flagellum) and sperm swimming speed ( Gomendio & Roldan, 1991, 2008; Fitzpatrick et al., 2009, 2010; Lüpold et al., 2009; Mossman et al., 2009; Firman & Simmons, 2010; Helfenstein et al., 2010), leading researchers to speculate on the role of sperm competition in favouring relatively long, and therefore fast-swimming, sperm (Gomendio & Roldan, 1991, 2008; Fitzpatrick et al., 2009). Characteristics of the female’s reproductive tract can also influence the evolution of sperm morphology, as some females can exert cryptic choice for relatively long sperm (Pitnick et al., 2009). In many taxa, variance in sperm morphology is explained not only by the level of sperm competition but also by the length of the female’s reproductive tract (Pitnick et al., 2009). These latter studies reveal the importance of female traits in influencing selection on sperm length and suggest that the relationships observed between sperm length and the level of sperm competition may reflect more complex evolutionary patterns of selection on ejaculate traits.
Among elasmobranch species, researchers have been aware of the extensive inter-specific variation in sperm morphological traits for almost a century (Fig. 2; Leigh-Sharpe, 1920). Recently, Jamieson (2005) offered a more in-depth examination of the level of variation in sperm morphology, summarizing the extensive interspecific variation in sperm morphology across elasmobranchs. Of the 30 elasmobranch species considered in Jamieson’s (2005) analysis, all sperm components varied approximately three-fold in length, with sperm head length ranging from 33 μm in C. plumbeus to 93 μm in the roughskin dogfish Centroscymnus owstoni Garman 1906, sperm mid-piece length ranging from 7 μm in the dusky smooth hound Mustelus canis (Mitchill 1815) to 21 μm in the Japanese swellshark Cephaloscyllium umbratile Jordan & Fowler 1903 and sperm flagellum length ranging from 49 μm in the tiger shark Galeocerdo cuvier (Péron & LeSueur 1822) to 143 μm in the Japanese angelshark Squatina japonica Bleeker 1858 (Jamieson, 2005). This pattern of inter-specific variation in sperm length components could be characteristic of an evolutionary response in sperm size to the selective pressures associated with post-copulatory sexual selection.
Fig. 2.
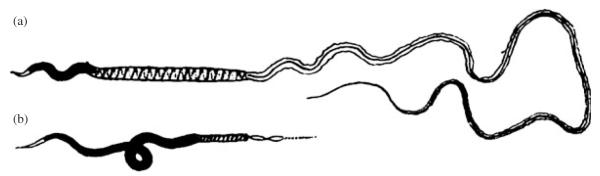
Variation in sperm morphology in elasmobranchs. A drawing of the sperm morphology of (a) Scyllium canicula and (b) Raia circularis (Modified from Leigh-Sharpe (1920) and reprinted with permission from the Journal of Morphology).
Unfortunately, the functional significance and selective forces responsible for generating the observed variation in elasmobranch sperm morphology have yet to be investigated. Consequently, there remains extensive scope for examinations of how selection is acting on sperm morphology. At the intraspecific level, competitive fertilization studies which take advantage of recent artificial insemination techniques in elasmobranchs (Luer et al., 2007) would help to determine if variation in sperm morphology influences competitive fertilization success. At the interspecific level, phylogenetically controlled studies assessing whether sperm size increases in response to post-copulatory sexual selection in elasmobranchs, as is often the case in other taxa (Gomendio & Roldan, 1991, 2008; Fitzpatrick et al., 2009), would offer further insights into the evolution of sperm traits in this group.
GENITAL MORPHOLOGY
The primary function of male genitalia is to transfer sperm to females during copulation, while the role of the female genitalia is to receive the male intromittent organ. In many species, however, genitalia also serve a sexually selected role. In competitive matings, males with certain (species-specific) genital morphologies are more successful at inseminating females and fertilizing ova than rival males (Arnqvist & Danielsson, 1999; Danielsson & Askenmo, 1999; House & Simmons, 2003; Simmons et al., 2009; Evans et al., 2011). The strength of selection on male genitalia is therefore expected to co-vary with mating systems, and previous studies in insects have demonstrated that male genitalia exhibit dramatic divergence in morphology in polyandrous species compared to monandrous species (Arnqvist, 1998). Comparative studies in mammals also provided some evidence of a positive correlation between genital length and sperm competition risk at the species level (Ramm, 2007). Post-copulatory sexual selection may also influence the morphology of male genitalia to act as a mechanism to remove sperm from rival males from the female’s reproductive tract, thereby reducing the likelihood of sperm competition (Waage, 1979).
Female genital morphology and reproductive tracts, which can also be highly variable across closely related species, may co-evolve with male genitals as a result of post-copulatory sexual selection and sexual conflict (Arnqvist & Rowe, 2002; Hosken & Stockley, 2004; Evans et al., 2011). For example, female genital and reproductive tract morphologies may serve as an avenue for extending cryptic female choice for males with particular reproductive traits (Eberhard, 1985). In many species, however, the underlying evolutionary interest of males and females differs, leading to sexual conflict over control of inseminations and fertilizations (Eberhard, 1985; Hosken & Stockley, 2004; Arnqvist & Rowe, 2005). Sexual conflict can have a particularly important influence on genital evolution if genital traits provide advantages to one sex at the expense of the other during matings (Arnqvist & Rowe, 2005). Clear evidence for sexual conflict influencing genital evolution comes from studies of insects, where in many species male genitalia are armed with spines that can physically injury females during copulation and reduce a female’s likelihood of re-mating (Hosken & Stockley, 2004). Thus, the harmful effects of male genital armaments can benefit males at the expense of females, as males benefit by reducing the intensity of sperm competition experienced by their ejaculate, while induced female chastity prevents females from securing genetic benefits associated with multiple mating (Jennions & Petrie, 2000). In cases such as these, females often exhibit counter-adaptations (e.g. protective ‘pads’ or behaviours) that minimize the harmful effects of male appendages (Rönn et al., 2007).
In elasmobranchs, males have external, paired intromittent appendages, claspers, which extend from the posterior base of the pectoral fins (Fig. 3). Male elasmobranchs use either one or both of their claspers during copulation to transfer sperm to the female. Claspers have been used extensively as an indicator of male maturity because, as a male matures the claspers become calcified and rigid and the base of the clasper is able to rotate so that it can be directed anteriorly (Clark & von Schmidt, 1965). Given the importance of assessing reproductive maturity in fisheries science (Walker, 2005) and the relative ease with which male maturity may be estimated, there are extensive historical and contemporary data available on clasper length for a wide variety of elasmobranch species. In a pioneering series of papers, the external clasper morphology of 87 species of elasmobranchs has been described (Leigh-Sharpe, 1920, 1921, 1922a, b, c, 1924a, b, 1926a, b, c, d), and a review of the literature over the last 30 years uncovered clasper length data for an additional 70 species. Despite the abundance of data available and the known role of sexual selection in shaping genital morphology in other taxa, no study to date has attempted to assess whether clasper morphology is influenced by sexual selection and mating strategy. Several possible directions for future research on this topic in elasmobranchs are therefore presented here.
Fig. 3.
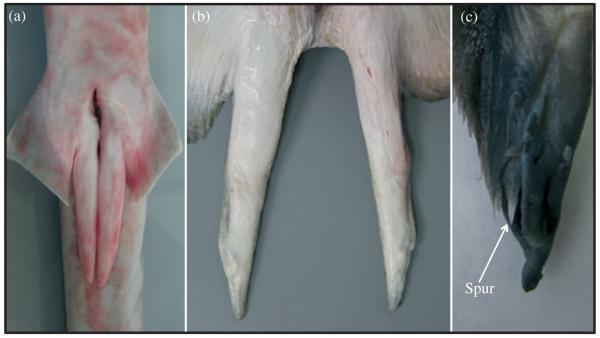
Variation in clasper morphology in elasmobranchs: (a) Mustelus stevensi and (b) Neotrygon kuhlii. For clarity, the tail has been removed in the pictures. (c) The clasper of Etmopterus baxteri showing the clasper spur.
The first of these takes advantage of the fact that male genital (clasper) size and morphology can vary dramatically (Fig. 3), even among closely related elasmobranchs. For example, after comparing the reproductive biology of three species of catsharks in the family Scyliorhinidae, Flammang et al. (2008) reported that clasper lengths for mature brown catsharks Apristurus brunneus (Gilbert 1892) and long-nose catsharks Apristurus kampae Taylor 1972 were 8·3 and 8 6% of the male LT, respectively. In contrast, claspers of the filetail catshark Parmaturus· xaniurus (Gilbert 1892) were 13·4% of the adult LT (Flammang et al., 2008). It remains unclear if this variation in clasper length is functionally important or influenced by the selective force of post-copulatory sexual selection. Tentative evidence suggests, however, that sperm competition may influence the relative investment in clasper length in cat-sharks as IG values, a proxy measure for sperm competition risk, were greater in P. xaniurus than A. brunneus (Flammang et al., 2008). Due to the relative ease in maintaining catshark populations in captivity (Griffiths et al., in press), this family therefore represents an intriguing avenue for research.
In terms of intraspecific variation in male genital size and shape and concomitant adaptations in female genital traits, there are striking similarities between elasmobranchs and other taxa including teleosts (Evans & Meisner, 2009; Evans et al., 2011), insects (Arnqvist & Rowe, 2005; Röonn et al., 2007) and mammals (Dixson, 1987; Baryshnikov et al., 2003; Ramm, 2007). In some shark species, the terminal cartilages of claspers are armed with sharp ridges, spurs or hooks [Fig. 3(c)] that act as holdfasts during copulations but can subsequently cause damage to the females’ reproductive tract (Pratt & Carrier, 2005). Vaginal scars caused by claspers are commonly observed in sharks and can be used as an indicator of recent mating activity (Pratt, 1979). Together with male behaviours of aggressively biting the fins and bodies of females during matings (Carrier et al., 1994; Kajiura et al., 2000), these vaginal scars suggest that mating can be costly for female elasmobranchs. In at least one species of shark, the blue shark Prionace glauca (L. 1758), females have thick-walled vaginas that may represent a co-evolutionary adaptation to the evolution of clasper spurs and hooks (Pratt, 1979; Pratt & Carrier, 2005). Thus, the presence of both male and female genital modifications suggests that post-copulatory sexual selection may be an important selective force driving genital evolution in elasmobranchs.
CRYPTIC FEMALE CHOICE AND SPERM STORAGE ORGANS
The most direct method for biasing fertilization success available to females is to exert pre-copulatory mate choice for preferred males (Andersson, 1994). Even in the absence of pre-copulatory processes, however, females can exert cryptic choice for preferred males, and thereby control male reproductive success, through a variety of post-copulatory processes. During copulation, or immediately thereafter, females can bias paternity by either accepting fewer sperm from undesirable males during matings (Pilastro et al., 2004) or by ejecting sperm from undesirable males directly after mating has occurred (Pizzari & Birkhead, 2000). After mating, biochemical interactions between ejaculates and the ovarian fluid in the female’s reproductive tract can influence male fertilization success by differentially influencing sperm swimming speed among competing males to disadvantage sperm from undesirable males (Gasparini & Pilastro, 2011). In addition, in species where females mate multiply and store sperm in specialized sperm storage organs, females may be able to differentially store and utilize sperm to fertilize their eggs with sperm from preferred males (Eberhard, 1996). In some insect species, such differential use of sperm from competing males is facilitated by the presence of multiple sperm storage organs that allows sperm from different males to be partitioned and increases female control of patterns of paternity (Eberhard, 1996; Hellriegel & Bernasconi, 2000; Snow & Andrade, 2005).
Evaluating the evolutionary significance of cryptic female choice in elasmobranchs can be challenging, particularly due to the inherent difficulties in documenting reproductive behaviours in this group (Pratt & Carrier, 2005). Studies of the oviducal gland a paired organ in the female reproductive tract where sperm are stored that is also the site of ooctye fertilization and egg case manufacture (Pratt, 1993; Hamlett et al., 1998; Carrier et al., 2004), represent an interesting avenue for future investigation. Sperm storage in the oviducal gland, which has been documented in numerous elasmobranch species (Parsons et al., 2008), can last for days to years and allows females to decouple mating with fertilizations (Pratt, 1993; Parsons et al., 2008). Following insemination, sperm are stored in the main cavity of the oviducal gland’s terminal zone or packaged into discrete seminiferous tubules embedded in the epithelium (Hamlett et al., 1998). Intriguingly, the prevalence of female multiple mating (see Table I) coupled with the evidence that sperm storage is common in elasmobranchs, suggests that sperm from multiple males may be stored separately and that this may facilitate cryptic female choice. Additionally, the complex anatomy of the oviducal gland in elasmobranchs and the various ways that sperm may be stored and partitioned suggest that the oviducal gland has the potential to enhance post-copulatory sexual selection by alternately facilitating or inhibiting sperm mixing prior to fertilization (Hamlett et al., 1998; Carrier et al., 2004). While the function of the oviducal gland in facilitating cryptic female choice remains speculative at this point, future investigations of the role that this organ plays in influencing patterns of paternity, and comparative investigations of whether oviducal gland morphology co-varies with the strength of post-copulatory sexual selection would greatly enhance understanding of how cryptic female choice might proceed in elasmobranchs.
CONCLUSIONS
This review has argued that post-copulatory sexual selection is a powerful selective force operating in elasmobranchs, and highlighted several reproductive traits that are likely to be shaped by sperm competition, cryptic female choice and sexual conflict. From the available evidence, it is clear that there is extensive interspecific variation in patterns of polyandry, testes size, sperm morphology and genital morphology in elasmobranchs. In some cases, species exhibit intraspecific variation in these reproductive traits, suggesting that this variation may also be shaped by population-specific responses to the level of post-copulatory sexual selection. The major challenge now is to explain the adaptive significance of this variation and the evolutionary processes that have shaped it in elasmobranchs and to greatly enhance understanding of the importance of female traits during post-copulatory episodes of sexual selection. The latter would necessitate a thorough investigation of whether the oviducal gland facilitates cryptic female choice and would hopefully lead to a better understanding of the physiological processes that might allow females to exert such cryptic choice.
Although an understanding of how post-copulatory sexual selection operates in elasmobranchs is limited, there is a wealth of information on reproductive traits in elasmobranchs that are available to address these questions. With recent advances in understanding of the phylogenetic relationships among elasmobranchs (Vélez-Zuazo & Agnarsson, 2011), future comparative studies promise to offer robust phylogenetically controlled analyses of how post-copulatory sexual selection influences reproductive traits. Furthermore, recent successes in the development of artificial insemination protocols for elasmobranchs (Luer et al., 2007) will be instrumental in future studies that aim to determine how various sperm traits influence competitive mating success. Additionally, examining how genital morphology influences reproductive success in elasmobranch species where mating can be observed in the field and in captive environments promises to greatly enhance understanding of how selection acts on both genital morphology and reproductive strategies. While often challenging, incorporating post-copulatory sexual selection into studies of elasmobranch reproductive biology is key to understanding how male and female reproductive traits evolved in this ancient group of fishes.
Supplementary Material
Acknowledgments
We thank E. Garza Gisholt for sharing clasper photographs, I. Baremore, L. Hale, F. Hazin, M. Broadhurst and G. Parsons for sharing raw data, A. Griffiths and C. Gubiliand for giving permission to cite their unpublished work and the Australian Research Council Discovery Program for financial support.
Footnotes
SUPPORTING INFORMATION Supporting Information may be found in the online version of this paper:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Andersson M. Sexual Selection. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Arnqvist G. Comparative evidence for the evolution of genitalia by sexual selection. Nature. 1998;393:784–786. [Google Scholar]
- Arnqvist G, Danielsson I. Copulatory behavior, genital morphology, and male fertilization success in water striders. Evolution. 1999;53:147–156. doi: 10.1111/j.1558-5646.1999.tb05340.x. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Antagonistic coevolution between the sexes in a group of insects. Nature. 2002;415:787–789. doi: 10.1038/415787a. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Sexual Conflict. Princeton University Press; Princeton, NJ: 2005. [Google Scholar]
- Baryshnikov GF, Bininda-Emonds ORP, Abramov AV. Morphological variability and evolution of the baculum (os penis) in Mustelidae (Carnivora) Journal of Mammalogy. 2003;84:673–690. [Google Scholar]
- Birkhead TR. Cryptic female choice: criteria for establishing female sperm choice. Evolution. 1998;52:1212–1218. doi: 10.1111/j.1558-5646.1998.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Birkhead TR, Møller AP. Sperm Competition and Sexual Selection. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Birkhead TR, Hosken DJ, Pitnick S. Sperm Biology: An Evolutionary Perspective. Academic Press; Burlington, MA: 2009. [Google Scholar]
- Carrier JC, Pratt HL, Martin LK. Group reproductive behaviors in free-living nurse sharks, Ginglymostoma cirratum. Copeia. 1994;1994:646–656. [Google Scholar]
- Carrier JC, Pratt HL, Castro JI. Reproductive biology of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of Sharks and Their Relatives. CRC Press; Boca Raton, FL: 2004. pp. 269–286. [Google Scholar]
- Casselman SJ, Schulte-Hostedde AI, Montgomerie R. Sperm quality influences male fertilization success in walleye (Sander vitreus) Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:2119–2125. [Google Scholar]
- Chapman DD, Corcoran MJ, Harvey GM, Malan S, Shivji MS. Mating behavior of southern stingrays, Dasyatis americana (Dasyatidae) Environmental Biology of Fishes. 2003;68:241–245. [Google Scholar]
- Chapman DD, Prodohl PA, Gelsleicher J, Manire CA, Mahmood SS. Predominance of genetic monogamy by females in a hammerhead shark, Sphyrna tiburo: implications for shark conservation. Molecular Ecology. 2004;13:1965–1974. doi: 10.1111/j.1365-294X.2004.02178.x. [DOI] [PubMed] [Google Scholar]
- Chevolot M, Ellis JR, Rijnsdorp AD, Stam WT, Olsen JL. Multiple paternity analysis in the thornback ray Raja clavata L. Journal of Heredity. 2007;98:712–715. doi: 10.1093/jhered/esm077. [DOI] [PubMed] [Google Scholar]
- Clark E, von Schmidt K. Sharks of the central gulf coast of Florida. Bulletin of Marine Science. 1965;15:13–83. [Google Scholar]
- Daly-Engel TS, Grubbs RD, Holland KN, Toonen RJ, Bowen BW. Assessment of multiple paternity in single litters from three species of carcharhinid sharks in Hawaii. Environmental Biology of Fishes. 2006;76:419–424. [Google Scholar]
- Daly-Engel TS, Grubbs RD, Bowen BW, Toonen RJ. Frequency of multiple paternity in an unexploited tropical population of sandbar sharks (Carcharhinus plumbeus) Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:198–204. [Google Scholar]
- Daly-Engel TS, Grubbs RD, Feldheim KA, Bowen BW, Toonen RJ. Is multiple mating beneficial or unavoidable? Low multiple paternity and genetic diversity in the shortspine spurdog (Squalus mitsukurii) Marine Ecology Progress Series. 2010;403:255–267. [Google Scholar]
- Danielsson I, Askenmo C. Male genital traits and mating interval affect male fertilization success in the water strider Gerris lacustris. Behavioral Ecology and Sociobiology. 1999;46:149–156. [Google Scholar]
- Darwin C. The Descent of Man, and Selection in Relation to Sex. John Murray; London: 1871. [Google Scholar]
- DiBattista JD, Feldheim KA, Thibert-Plainte X, Gruber SH, Hendry AP. A genetic assessment of polyandry and breeding site fidelity in lemon sharks. Molecular Ecology. 2008;17:3337–3351. doi: 10.1111/j.1365-294X.2008.03833.x. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Observations on the evolution of the genitalia and copulatory behaviour in male primates. Journal of Zoology. 1987;213:423–443. [Google Scholar]
- Eberhard WG. Sexual Selection and Animal Genitalia. Harvard University Press; Cambridge, MA: 1985. [Google Scholar]
- Eberhard WG. Female Control: Sexual Selection by Cryptic Female Choice. Princeton University Press; Princeton, NJ: 1996. [Google Scholar]
- Evans JP, Meisner AD. Copulatory structures: taxonomic overview and the potential for sexual selection. In: Jamieson BGM, editor. Reproductive Biology and Phylogeny of Fishes. Science Publishers, Inc.; Enfield, NH: 2009. pp. 138–180. [Google Scholar]
- Evans JP, Gasparini C, Holwell GI, Ramnarine IW, Pitcher TE, Pilastro A. Intraspecific evidence from guppies for correlated patterns of male and female genital trait diversification. Proceedings of the Royal Society B. 2011;278:2611–2620. doi: 10.1098/rspb.2010.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim KA, Gruber SH, Ashley MV. Multiple paternity of a lemon shark litter (Chondrichthyes: Carcharhinidae) Copeia. 2001;2001:781–786. [Google Scholar]
- Firman RC, Simmons LW. Polyandry, sperm competition, and reproductive success in mice. Behavioral Ecology. 2008;19:695–702. [Google Scholar]
- Firman RC, Simmons LW. Sperm midpiece length predicts sperm swimming velocity in house mice. Biology Letters. 2010;6:513–516. doi: 10.1098/rsbl.2009.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick JL, Baer B. Polyandry reduces sperm length variation in social insects. Evolution. 2011;65:3006–3012. doi: 10.1111/j.1558-5646.2011.01343.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JL, Montgomerie R, Desjardins JK, Stiver KA, Kolm N, Balshine S. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1128–1132. doi: 10.1073/pnas.0809990106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick JL, Garcia-Gonzalez F, Evans JP. Linking sperm length and velocity: the importance of intramale variation. Biology Letters. 2010;6:797–799. doi: 10.1098/rsbl.2010.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammang BE, Ebert DA, Cailliet GM. Reproductive biology of deep-sea catsharks (Chondrichthyes: Scyliorhinidae) in the eastern North Pacific. Environmental Biology of Fishes. 2008;81:35–49. [Google Scholar]
- Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA. Spermatozoal traits and sperm competition in Atlantic salmon: relative velocity is the primary determinant of fertilization success. Current Biology. 2004;14:44–47. [PubMed] [Google Scholar]
- García-González F. Male genetic quality and the inequality between paternity success and fertilization success: consequences for studies of sperm competition and the evolution of polyandry. Evolution. 2008;62:1653–1665. doi: 10.1111/j.1558-5646.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- García-González F, Simmons LW. Shorter sperm confer higher competitive fertilization success. Evolution. 2007;61:816–824. doi: 10.1111/j.1558-5646.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Gasparini C, Pilastro A. Cryptic female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proceedings of the Royal Society B. 2011;278:2495–2501. doi: 10.1098/rspb.2010.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini C, Simmons LW, Beveridge M, Evans JP. Sperm swimming velocity predicts competitive fertilization success in the green swordtail Xiphophorus helleri. PLoS One. 2010;5:e12146. doi: 10.1371/journal.pone.0012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomendio M, Roldan ERS. Sperm competition influences sperm size in mammals. Proceedings of the Royal Society B. 1991;243:181–185. doi: 10.1098/rspb.1991.0029. [DOI] [PubMed] [Google Scholar]
- Gomendio M, Roldan RS. Implications of diversity in sperm size and function for sperm competition and fertility. International Journal of Developmental Biology. 2008;52:439–447. doi: 10.1387/ijdb.082595mg. [DOI] [PubMed] [Google Scholar]
- Griffiths A, Jacoby D, Casane D, McHugh M, Croft D, Genner MJ, Sims DW. First analysis of multiple paternity in an oviparous shark, the small-spotted catshark (Scyliorhinus canicula L.) Journal of Heredity. doi: 10.1093/jhered/esr112. (in press) doi: 10.1093/jhered/esr1112. [DOI] [PubMed] [Google Scholar]
- Gubili C, Duffy CAJ, Cliff G, Wintner S, Shivji MS, Chapman DD, Bruce BD, Martin AP, Sims DW, Jones CS, Noble LR. Application of molecular genetics for conservation of the great white shark, Carcharodon carcharias, L. 1758. In: Domeier ML, editor. Global Perspectives on the Biology and Life History of the Great White Shark. CRC Press; Boca Raton, FL: 2012. pp. 357–380. [Google Scholar]
- Hamlett WC, Knight DP, Koob TJ, Jezior M, Luong T, Rozycki T, Brunette N, Hysell MK. Survey of oviducal gland structure and function in elasmobranchs. Journal of Experimental Biology. 1998;282:399–420. [Google Scholar]
- Harcourt AH, Harvey PH, Larson SG, Short RV. Testis weight, body weight and breeding system in primates. Nature. 1981;293:55–57. doi: 10.1038/293055a0. [DOI] [PubMed] [Google Scholar]
- Heinecke MP, Naylor GJP, Hedges SB. Cartilaginous fishes (Chondrichthyes) In: Hedges SB, Kumar S, editors. The Timetree of Life. Oxford University Press; New York, NY: 2009. pp. 320–327. [Google Scholar]
- Helfenstein F, Podevin M, Richner H. Sperm morphology, swimming velocity, and longevity in the house sparrow Passer domesticus. Behavioral Ecology and Sociobiology. 2010;64:557–565. [Google Scholar]
- Hellriegel B, Bernasconi G. Female-mediated differential sperm storage in a fly with complex spermathecae, Scatophaga stercoraria. Animal Behaviour. 2000;59:311–317. doi: 10.1006/anbe.1999.1308. [DOI] [PubMed] [Google Scholar]
- Hosken DJ, Stockley P. Sexual selection and genital evolution. Trends in Ecology and Evolution. 2004;19:87–93. doi: 10.1016/j.tree.2003.11.012. [DOI] [PubMed] [Google Scholar]
- House CM, Simmons LW. Genital morphology and fertilization success in the dung beetle Onthophagus taurus: an example of sexually selected male genitalia. Proceedings of the Royal Society B. 2003;270:447–455. doi: 10.1098/rspb.2002.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter FM, Birkhead TR. Sperm viability and sperm competition in insects. Current Biology. 2002;12:121–123. doi: 10.1016/s0960-9822(01)00647-9. [DOI] [PubMed] [Google Scholar]
- Jamieson BGM. Chondrichthyan spermatozoa and phylogeny. In: Hamlett WC, editor. Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras. Science Publishers, Inc.; Enfield, NH: 2005. pp. 201–236. [Google Scholar]
- Jennions MD, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biological Review. 2000;75:21–64. doi: 10.1017/s0006323199005423. [DOI] [PubMed] [Google Scholar]
- Kajiura SM, Sebastian AP, Tricas TC. Dermal bite wounds as indicators of reproductive seasonality and behaviour in the Atlantic stingray, Dasyatis sabina. Environmental Biology of Fishes. 2000;58:23–31. [Google Scholar]
- Kousteni V, Kontopoulou M, Megalofonou P. Sexual maturity and fecundity of Scyliorhinus canicula (Linnaeus, 1758) in the Aegean Sea. Marine Biology Research. 2010;6:390–398. [Google Scholar]
- Lage CR, Petersen CW, Forest D, Barnes D, Kornfield I, Wray C. Evidence of multiple paternity in spiny dogfish (Squalus acanthias) broods based on microsatellite analysis. Journal of Fish Biology. 2008;73:2068–2074. [Google Scholar]
- Larson S, Christiansen J, Griffling D, Ashe J, Lowry D, Andrews K. Relatedness and polyandry of sixgill sharks, Hexanchus griseus, in an urban estuary. Conservation Genetics. 2011;12:679–690. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands. Memoir I. Journal of Morphology. 1920;34:245–265. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands. Memoir II. Journal of Morphology. 1921;35:359–380. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands. Memoir III. Journal of Morphology. 1922a;36:191–198. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands. Memoir IV. Journal of Morphology. 1922b;36:199–220. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands. Memoir V. Journal of Morphology. 1922c;36:221–243. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands. Memoir VI. Journal of Morphology. 1924a;39:553–566. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands. Memoir VII. Journal of Morphology. 1924b;39:567–577. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands. Memoir VIII. Journal of Morphology. 1926a;42:307–320. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands. Memoir IX. Journal of Morphology. 1926b;42:321–334. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands. Memoir X. Journal of Morphology. 1926c;42:335–348. [Google Scholar]
- Leigh-Sharpe WH. The comparative morphology of the secondary sexual characters of elasmobranch fishes, the claspers, clasper siphons, and clasper glands together with a dissertation on Cowper’s glands of humans. Memoir XI. Journal of Morphology. 1926d;42:349–358. [Google Scholar]
- Liljedal S, Rudolfsen G, Folstad I. Factors predicting male fertilization success in an external fertilizer. Behavioral Ecology and Sociobiology. 2008;62:1805–1811. [Google Scholar]
- Luer CA, Walsh CJ, Bodine AB, Wyffels JT. Normal embryonic development in the clearnose skate, Raja eglanteria, with experimental observations on artificial insemination. Environmental Biology of Fishes. 2007;80:239–255. [Google Scholar]
- Lüpold S, Calhim S, Immler S, Birkhead TR. Sperm morphology and sperm velocity in passerine birds. Proceedings of the Royal Society B. 2009;276:1175–1181. doi: 10.1098/rspb.2008.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconato A, Shapiro DY. Sperm allocation, sperm production and fertilization rates in the bucktooth parrotfish. Animal Behaviour. 1996;52:971–980. [Google Scholar]
- Møller AP. Ejaculate quality, testes size and sperm competition in mammals. Functional Ecology. 1989;3:91–96. [Google Scholar]
- Møller AP. Sperm competition, sperm deletion, paternal care, and relative testis size in birds. The American Naturalist. 1991;137:882–906. [Google Scholar]
- Montgomerie R, Fitzpatrick JL. Testis size, sperm size and sperm competition. In: Jamieson BGM, editor. Reproductive Biology and Phylogeny of Fishes. Science Publishers, Inc.; Enfield, NH: 2009. pp. 1–53. [Google Scholar]
- Mossman J, Slate J, Humphries S, Birkhead TR. Sperm morphology and velocity are genetically codetermined in the zebra finch. Evolution. 2009;63:2730–2737. doi: 10.1111/j.1558-5646.2009.00753.x. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Okamura K, McKinney EC, Bartl S, Hashimoto K, Flajnik MF. Primitive synteny of vertebrate major histocompatability complex class I and class II genes. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4712–4717. doi: 10.1073/pnas.97.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GA. Sperm competition and its evolutionary consequences in the insects. Biological Reviews. 1970;45:525–567. [Google Scholar]
- Parker GA. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. Journal of Theoretical Biology. 1982;96:281–294. doi: 10.1016/0022-5193(82)90225-9. [DOI] [PubMed] [Google Scholar]
- Parker GA, Ball MA, Stockley P, Gage MJG. Sperm competition games: a prospective analysis of risk assessment. Proceedings of the Royal Society B. 1997;264:1793–1802. doi: 10.1098/rspb.1997.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons GR, Hoffmayer ER, Hendon JM, Bet-Sayad WV. A review of shark reproductive ecology: life history and evolutionary implications. In: Rocha MA, Arukwe A, Kapoor BG, editors. Fish Reproduction. Science Publishers, Inc.; Enfield, NH: 2008. pp. 435–469. [Google Scholar]
- Pilastro A, Simonato M, Bisazza A, Evans JP. Cryptic female preferences for colorful males in guppies. Evolution. 2004;58:665–669. [PubMed] [Google Scholar]
- Pitnick S, Hosken DJ, Birkhead TR. Sperm morphological diversity. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology: An Evolutionary Perspective. Academic Press; Burlington, MA: 2009. pp. 69–149. [Google Scholar]
- Pizzari T, Birkhead TR. Female feral fowl eject sperm of subdominant males. Nature. 2000;405:787–789. doi: 10.1038/35015558. [DOI] [PubMed] [Google Scholar]
- Portnoy DS, Piercy AN, Musick JA, Burgess GH, Graves JE. Genetic polyandry and sexual conflict in the sandbar shark, Carcharhinus plumbeus, in the western North Atlantic and Gulf of Mexico. Molecular Ecology. 2007;16:187–197. doi: 10.1111/j.1365-294X.2006.03138.x. [DOI] [PubMed] [Google Scholar]
- Pratt HL. Reproduction in the blue shark, Prionace glauca. Fishery Bulletin. 1979;77:445–470. [Google Scholar]
- Pratt HL. The storage of spermatozoa in the oviducal glands of western North Atlantic sharks. Environmental Biology of Fishes. 1993;38:139–149. [Google Scholar]
- Pratt HL, Carrier JC. Elasmobranch courtship and mating behaviour. In: Hamlett WC, editor. Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras. Science Publishers, Inc.; Enfield, NH: 2005. pp. 129–169. [Google Scholar]
- Ramm SA. Sexual selection and genital evolution in mammals: a phylogenetic analysis of baculum length. American Naturalist. 2007;169:360–369. doi: 10.1086/510688. [DOI] [PubMed] [Google Scholar]
- Rönn J, Katvala A, Arnqvist G. Coevolution between harmful male genitalia and female resistance in seed beetles. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10921–10925. doi: 10.1073/pnas.0701170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville KJ, Lindley AM, Maries EG, Carrier JC, Pratt HL. Multiple paternity in the nurse shark, Ginglymostoma cirratum. Environmental Biology of Fishes. 2002;63:347–352. [Google Scholar]
- Schärer L, Ladurner P, Rieger R. Bigger testes do work more: experimental evidence that testis size reflects testicular cell proliferation activity in the marine invertebrate, the free-living flatworm Macrostomum sp. Behavioral Ecology and Socio-biology. 2004;56:420–425. [Google Scholar]
- Schmidt JV, Chen CC, Sheikh SI, Meekan MG, Norman BM, Joung SJ. Paternity analysis in a litter of whale shark embryos. Endangered Species Research. 2010;12:117–124. [Google Scholar]
- Simmons LW, House CM, Hunt J, García-González F. Evolutionary response to sexual selection in male genital morphology. Current Biology. 2009;19:1442–1446. doi: 10.1016/j.cub.2009.06.056. [DOI] [PubMed] [Google Scholar]
- Snook RR. Sperm in competition: not playing by the numbers. Trends in Ecology and Evolution. 2005;20:46–53. doi: 10.1016/j.tree.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Snow LSE, Andrade MCB. Multiple sperm storage organs facilitate female control of paternity. Proceedings of the Royal Society B. 2005;272:1139–1144. doi: 10.1098/rspb.2005.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P, Gage MJG, Parker GA, Moller AP. Female reproductive biology and the coevolution of ejaculate characteristics in fish. Proceedings of the Royal Society B. 1996;263:451–458. [Google Scholar]
- Thornhill R. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigricepts. American Naturalist. 1983;122:765–788. [Google Scholar]
- Todd AC. Using testis size to predict the mating systems of New Zealand geckos. New Zealand Journal of Zoology. 2008;35:103–114. [Google Scholar]
- Tomkins JL, Simmons LW. Measuring relative investment: a case study of testes investment in species with alternative male reproductive tactics. Animal Behaviour. 2002;63:1009–1016. [Google Scholar]
- Vélez-Zuazo X, Agnarsson I. Shark tales: a molecular species-level phylogeny of sharks (Selachimorpha, Chondrichthyes) Molecular Phylogenetics and Evolution. 2011;58:207–217. doi: 10.1016/j.ympev.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Veríssimo A, Grubbs D, McDowell J, Musick J, Portnoy D. Frequency of multiple paternity in the spiny dogfish Squalus acanthias in the western North Atlantic. Journal of Heredity. 2010;102:88–93. doi: 10.1093/jhered/esq084. [DOI] [PubMed] [Google Scholar]
- Waage JK. Dual function of the damselfly penis: sperm removal and transfer. Science. 1979;203:916–918. doi: 10.1126/science.203.4383.916. [DOI] [PubMed] [Google Scholar]
- Walker TI. Reproduction in fisheries science. In: Hamlett WC, editor. Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras. Science Publishers, Inc.; Enfield, NH: 2005. pp. 81–127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.