Abstract
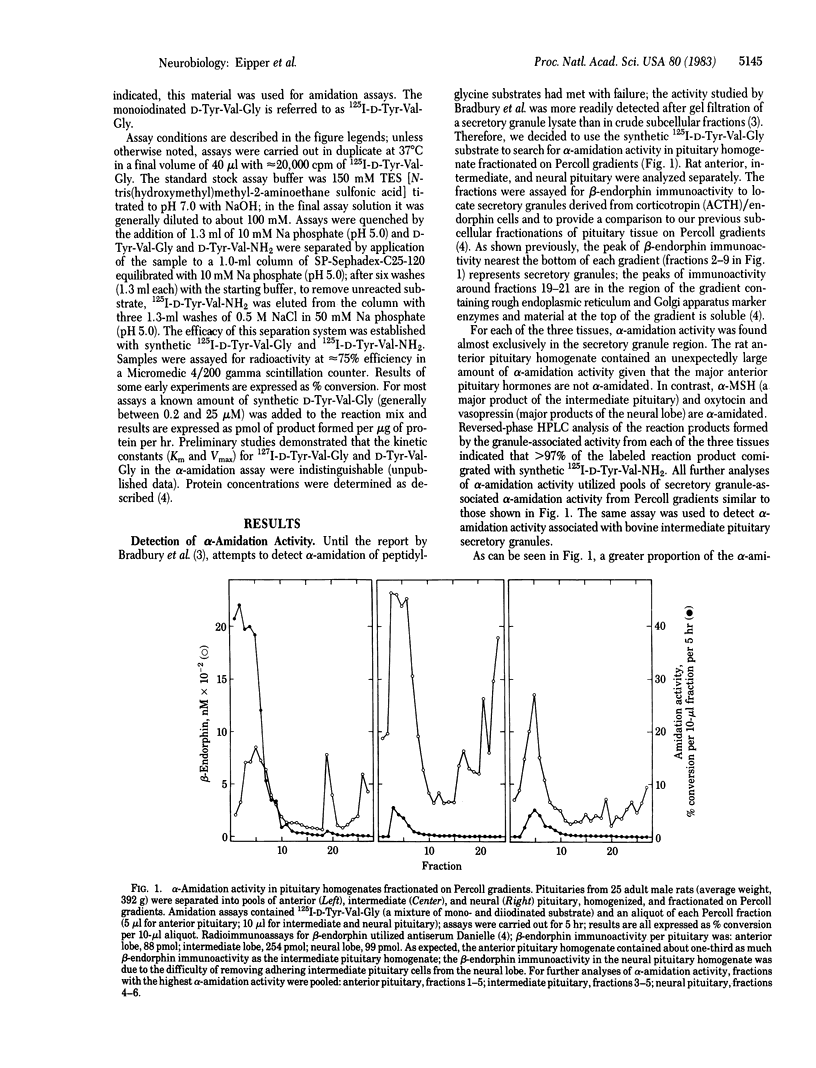
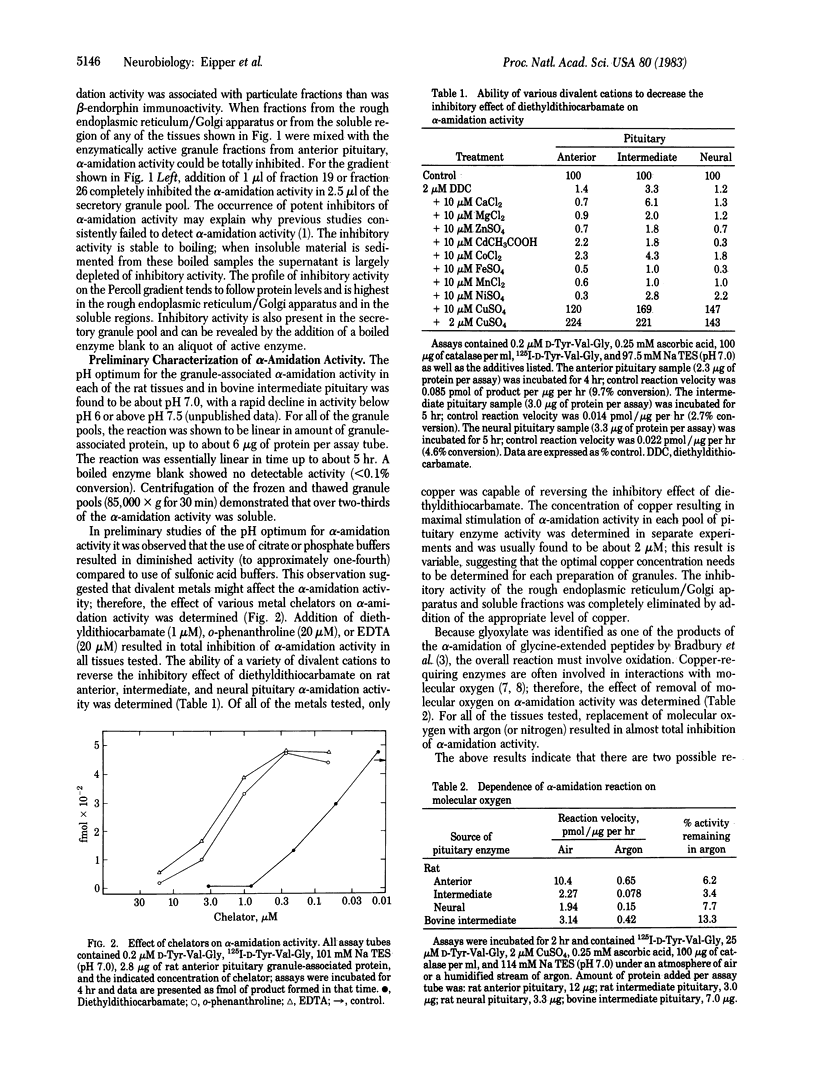
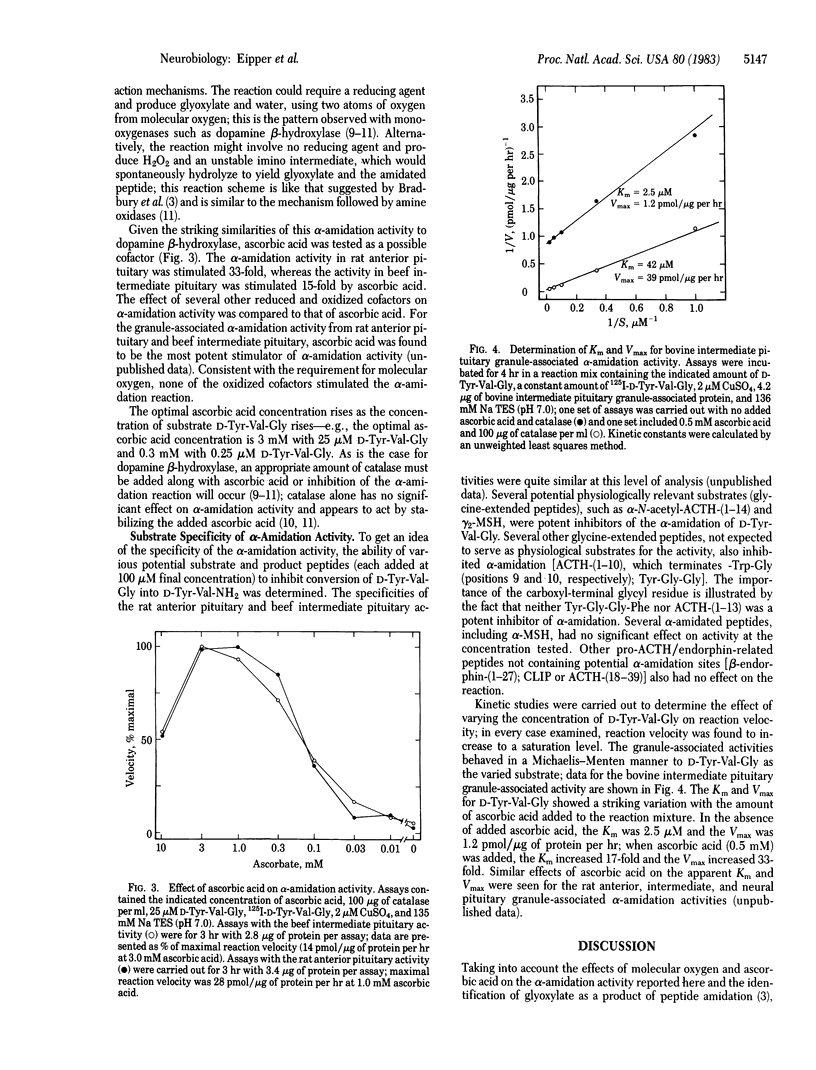
An enzymatic activity capable of producing an alpha-amidated peptide product from its glycine-extended precursor has been identified in secretory granules of rat anterior, intermediate, and neural pituitary and bovine intermediate pituitary. High levels of endogenous inhibitors of this alpha-amidation activity have also been found in tissue homogenates. The alpha-amidation activity is totally inhibited by addition of divalent metal ion chelators such as diethyldithiocarbamate, o-phenanthroline, and EDTA; alpha-amidation activity is restored to above control levels upon addition of copper. The alpha-amidation reaction requires the presence of molecular oxygen. Of the various cofactors tested, ascorbic acid was the most potent stimulator of alpha-amidation. The alpha-amidation activity has a neutral pH optimum and is primarily soluble following several cycles of freezing and thawing. Kinetic studies with the bovine intermediate pituitary granule-associated activity demonstrated a linear Lineweaver-Burk plot when D-Tyr-Val-Gly was the varied substrate; the apparent Km and Vmax varied with the concentration of ascorbic acid. The substrate specificity of the alpha-amidation activity appears to be quite broad; the conversion of D-Tyr-Val-Gly into D-Tyr-Val-NH2 is inhibited by the addition of a variety of glycine-extended peptides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett H. P., Browne C. A., Solomon S. Purification of the two major forms of rat pituitary corticotropin using only reversed-phase liquid chromatography. Biochemistry. 1981 Aug 4;20(16):4530–4538. doi: 10.1021/bi00519a004. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Glembotski C. C., Mains R. E. Selective loss of alpha-melanotropin-amidating activity in primary cultures of rat intermediate pituitary cells. J Biol Chem. 1983 Jun 25;258(12):7292–7298. [PubMed] [Google Scholar]
- Glembotski C. C. Characterization of the peptide acetyltransferase activity in bovine and rat intermediate pituitaries responsible for the acetylation of beta-endorphin and alpha-melanotropin. J Biol Chem. 1982 Sep 10;257(17):10501–10509. [PubMed] [Google Scholar]
- Glembotski C. C., Eipper B. A., Mains R. E. Adrenocorticotropin(1-14)OH-related molecules in primary cultures of rat intermediate pituitary cells. Identification and role in the biosynthesis of alpha-melanotropin. J Biol Chem. 1983 Jun 25;258(12):7299–7304. [PubMed] [Google Scholar]
- Harris E. D., Rayton J. K., Balthrop J. E., DiSilvestro R. A., Garcia-de-Quevedo M. Copper and the synthesis of elastin and collagen. Ciba Found Symp. 1980;79:163–182. doi: 10.1002/9780470720622.ch9. [DOI] [PubMed] [Google Scholar]
- Ingebretsen O. C., Terland O., Flatmark T. Subcellular distribution of ascorbate in bovine adrenal medulla. Evidence for accumulation in chromaffin granules against a concentration gradient. Biochim Biophys Acta. 1980 Mar 3;628(2):182–189. doi: 10.1016/0304-4165(80)90365-7. [DOI] [PubMed] [Google Scholar]
- LEVIN E. Y., LEVENBERG B., KAUFMAN S. The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J Biol Chem. 1960 Jul;235:2080–2086. [PubMed] [Google Scholar]
- May S. W., Phillips R. S., Mueller P. W., Herman H. H. Dopamine beta-hydroxylase. Demonstration of enzymatic ketonization of the product enantiomer S-octopamine. J Biol Chem. 1981 Mar 10;256(5):2258–2261. [PubMed] [Google Scholar]
- Milby K. H., Mefford I. N., Chey W., Adams R. N. In vitro and in vivo depolarization coupled efflux of ascorbic acid in rat brain preparations. Brain Res Bull. 1981 Sep;7(3):237–242. doi: 10.1016/0361-9230(81)90013-7. [DOI] [PubMed] [Google Scholar]
- Orcutt J. C., Molinoff P. B. Endogenous inhibitors of dopamine-beta- hydroxylase in rat organs. Biochem Pharmacol. 1976 May 15;25(10):1167–1174. doi: 10.1016/0006-2952(76)90364-6. [DOI] [PubMed] [Google Scholar]
- Paquette T. L., Gingerich R., Scharp D. Altered amidation of pancreatic polypeptide in cultured dog islet tissue. Biochemistry. 1981 Dec 22;20(26):7403–7408. doi: 10.1021/bi00529a013. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Carlquist M., Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982 Apr 15;296(5858):659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]



