Abstract
Fibroblast growth factor 2 (FGF2) consists of multiple protein isoforms (low [LMW] and high molecular weight [HMW]), which are localized to different cellular compartments, indicating unique biological activity. We previously showed that the LMW isoform is important in protecting the heart from myocardial dysfunction associated with ischemia-reperfusion (I/R) injury, but the roles of the HMW isoforms remain unknown. To elucidate the role of HMW isoforms in I/R and cardioprotection, hearts from novel mouse models,in which the murine FGF2 HMWs are knocked out (HMWKO) or the human FGF2 24 kDa HMW isoform is overexpressed (HMW Tg) and their wildtype (Wt) or non-transgenic (NTg) cohorts were subjected to an ex vivo work-performing heart model of I/R. There was a significant improvement in post-ischemic recovery of cardiac function in HMWKO hearts (76±5%, p<0.05) compared to Wt hearts (55±5%), with a corresponding decrease in HMW Tg function (line 20: 38±6% and line 28: 33±4%, p<0.05) compared to non-transgenic hearts (68±9%). FGF2 LMW isoform was secreted from Wt and HMWKO hearts during I/R, and a FGF receptor (FGFR) inhibitor, PD173074 caused a decrease in cardiac function when administered in I/R in Wt and FGF2 HMWKO hearts (p<0.05), indicating that FGFR is involved in FGF2 LMW isoform's biological effect in ischemia-reperfusion injury. Moreover, overexpression of HMW isoform reduced FGFR1 phosphorylation/activation with no further decrease in the phosphorylation state in the presence of the FGFR inhibitor. Overall, our data indicate that HMW isoforms have a detrimental role in the development of post-ischemic myocardial dysfunction.
Keywords: fibroblast growth factor, human or mouse FGF, ischemia-reperfusion injury, cardioprotection, low or high molecular weight isoforms, fibroblast growth factor receptor
Introduction
Fibroblast growth factor-2 (FGF2) is a heparin-binding protein involved in cell growth, differentiation, and death/survival [1]. FGF2 consists of multiple protein isoforms resulting from different translational start sites from a single Fgf2 gene [1]. One 18 kDa FGF2 (low molecular weight isoform [LMW]), is translated from a conventional Kozak AUG start codon [1]. Several high molecular weight (HMW) isoforms of FGF2 are identified in many species, including human, rat, bovine, guinea pig, and chicken [2–5]. In mice, there are two HMW isoforms (21 and 22 kDa) [1], and in humans, there are four HMW isoforms (21, 22.5, 24 and 34 kDa) [1] initiated at CUG start codons. HMW isoforms contain a nuclear localization signal that targets the HMW isoforms to the nucleus, while the LMW FGF2 isoform is predominantly cytoplasmic [6]. Recent evidence, however, indicates that the LMW and HMW isoforms are not always localized only to the cytoplasm or nucleus, respectively [7–9]. In addition to the complexity of their localization, FGF2 isoforms also display distinct biological activities. In vitro studies using neonatal cardiomyocytes show that both LMW and HMW FGF2 isoforms increase cell proliferation, but only the HMW FGF2 isoforms cause binucleation independent of FGFR pathways, possibly by directly affecting chromatin structure [7, 10]. FGF2 LMW isoform can induce cardioprotection in an angiogenic-dependent and -independent manner [11–13]. Our previous data indicate that inhibition of JNK signaling and the apoptotic process is essential to FGF2 LMW isoform-mediated cardioprotection [13]. However, the function of FGF2 HMW isoforms in ischemiareperfusion (I/R) injury is currently unknown. Also, unclear is the role that FGF receptor signaling plays in the effects of each isoform on the post-ischemic heart.
There are two classes of FGF receptor identified on the cell surface. Heparan sulfate proteoglycans (HSPGs), the low affinity-high capacity receptor class, and FGF receptor (FGFR), the high affinity-low capacity receptor [14]. In human hearts, FGFR1 and FGFR4 are the predominant FGF receptors [15]. In mouse cardiomyocytes, FGFR1 is present, with no FGFR4 expression [16]. FGFR1 is highly expressed during cardiac development [17] and gradually declines, but still is expressed in adult rat hearts [18]. FGFR1 signaling in the heart leads to developmental cardiomyocyte growth [17], acute cardioprotection after I/R injury [19], angiogenesis-induced cardioprotection [20], tumorogenesis in myxoma [21], and proliferation [22]. Both FGF2 isoforms are contain the amino acid sequence that binds to and activates FGFR (14), but which, if any, endogenous FGF2 isoforms interact with FGFR to modulate the outcome following cardiac I/R injury remain to be elucidated.
This present study investigates the role of FGF2 HMW isoforms in I/R injury and the involvement of FGFR in FGF2 LMW isoform's action in cardioprotection. This study demonstrates that FGF2 HMW isoforms, unlike the LMW isoform, does not protect against post-ischemic contractile dysfunction. Furthermore, this study establishes that FGFR is necessary for LMW isoform-mediated cardioprotection during I/R injury.
Materials and Methods
Animals and exclusion criteria
Mice were housed in a pathogen-free facility and handled in accordance with standard use protocols, animal welfare regulations, and the NIH Guide for the Care and Use of Laboratory Animals. All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee. Wildtype (Wt) and FGF2 HMWKO (HMW isoforms absent) mice were bred on a mixed Black Swiss (50%)/129 (50%) background. Non-transgenic (NTg) and two lines (20 and 28) of mice overexpressing the human HMW 24 kDa isoform (24 kDa HMW Tg) were bred on a FVB/N background. Wildtype, FGF2 HMWKO, non-transgenic mice and 24 kDa HMW Tg mice were randomly assigned to the studies. A total of 10 mice were excluded from I/R injury study. Exclusion from the ischemia-reperfusion study was based on the signs of aortic or pulmonary vein leak in the working heart preparation. Aortic leak was represented as an aortic pressure <60 mmHg on Langendorff, retrograde perfusion mode. Pulmonary vein leak was demonstrated as an aortic flow <2.0 mL/min, low (<4 mmHg) atrial pressure, and a blood gas pO2 >380 mmHg or a visible leak (i.e., hole in ventricle or atrium) in the heart.
Generation of FGF2 HMW knockout (HMWKO) mice
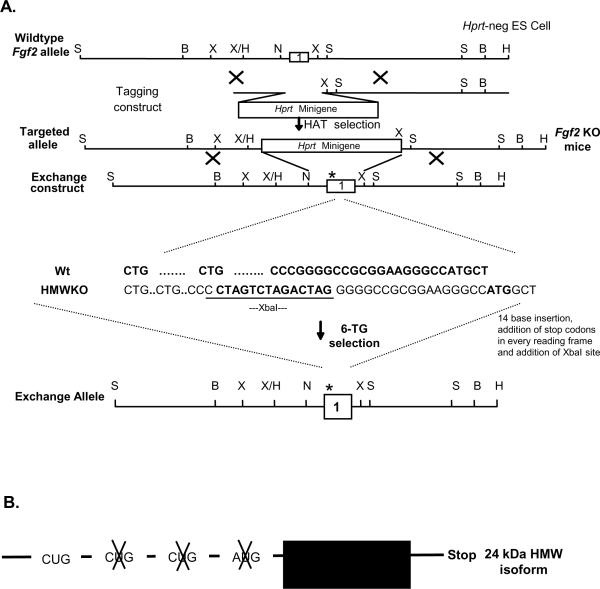
The FGF2 HMWKO mice were generated on by Drs. Ming Zhou and Mohamad Azhar in the Doetschman laboratory [23, 24] and utilized the “Tag & Exchange” procedure [13] as shown in Figure 1A. Briefly, a 14-bp oligo DNA (5'-CTA GTC TAG ACT AG-3'), which contained stop codons (TAG) in all 3 reading frames and which would cause a frame-shift between the ATG and the two upstream CTG start sites, was ligated to the Sma I site. The insertion caused a frame-shift of the HMW isoforms reading frame and kept only the LMW isoform reading frame. This manipulation resulted in an ablation of the FGF2 HMW isoforms.
Figure 1.
Schematic for the generation of FGF2 mice. (A) The Tag and Exchange procedure was used to generate Fgf2 KO and FGF2 HMWKO mice. (B) cDNA construct engineered to overexpress the human FGF2 24 kDa HMW isoform when driven by the phosphoglycerate kinase promoter. (C) Representative Western blot of FGF2 isoform expression in Wt, Fgf2 KO and FGF2 LMWKO hearts. (D) Quantitative analysis showing significant increase in the protein expression of the 18 kDa FGF2 LMW isoform in FGF2 HMWKO (dark gray) and Wt (black) hearts. (E) Representative Western blot of FGF2 isoform expression in NTg, FGF2 Tg and FGF2 HMW Tg hearts. (F) Quantitative analysis demonstrating no differences in the protein expression in endogenous 18 kDa, 21 kDa and 22 kDa mouse FGF2 isoform in 24 kDa Tg (white) and NTg (black) hearts. n=6 for Wt, FGF2 HMWKO hearts, n=6 for NTg, n=5 for 24 kDa HMW Tg. *p<0.05 vs. Wt hearts.
Generation of transgenic (Tg) mice overexpressing human FGF2 24 kDa HMW isoform
The human FGF2 24 kDa HMW (line 20 and 28) transgenic mice were generated by Dr. Douglas Coffin in the Doetschman laboratory [23] as shown in Figure 1B. Briefly, the AUG and first 2 CUG codons of the human Fgf2 cDNA (provided by R. Florkiewicz), were point-mutated, leaving only the human FGF2 24 kDa HMW isoform. The mutated vector was ligated to the 3' end of the phosphoglycerate kinase (PGK) promoter with an SV40 intron and poly A sequence at the downstream end of the cDNA. The chimeric gene was injected into the pronuclei of FVB/N strain fertilized mouse oocytes by the Transgenic Mouse Service Facility of the University of Cincinnati. Founder mice harboring the transgene were identified by PCR.
Isolated work-performing heart model of global low-flow ischemia (see online supplement)
Age- (10–12 weeks) and sex-matched Wt, FGF2 HMWKO, NTg and human FGF2 24 kDa HMW Tg mice were anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and heparinized (5000U/kg, i.p.) to protect the heart against microthrombi. The isolated work-performing heart preparation and global low-flow ischemia protocol were performed as previously described [13]. The low-flow ischemia resulted in a 90% reduction in coronary flow, with a constant infusion of 95% O2 and 5% CO2 into the modified Krebs-Henseleit perfusate.
Pharmacological agents
1-t-Butyl-3-(6-(3,5-dimethoxyphenyl)-2-(4-diethylaminobutylamino)-pyrido[2,3-d] pyrimidin-7-yl)urea (PD173074), a FGF receptor (FGFR) inhibitor, which prevents the autophosphorylation of FGFR [25], was a gift generously donated from Pfizer, New York, NY. This inhibitor has been shown to be extremely selective for FGFR and VEGFR2, with IC50 over 1000-fold higher for the receptors for insulin (IGF), epidermal growth factor (EGF) and platelet-derived growth factor (PDGF), and several serine/threonine kinases and other kinases including Src [25]. PD173074 was dissolved in DMSO and diluted in Kreb's solution to obtain a final concentration of 25 nmol/L. The 25 nM concentration was chosen as it has been shown to inhibit FGFR without inhibiting VEGFR2 receptors [25] and also inhibited FGFR without causing any adverse cardiac effect (i.e, cardiac dysrhythm). PD 173074 was administered 15 minutes prior to and for the first 15 minutes of ischemia and the last 15 minutes of ischemia and first 15 minutes of reperfusion. DMSO as vehicle treatment was administrated at the same length and time points as PD173074.
Myocardial infarction
Infarct size was determined by the histochemical stain, 2, 3, 5-triphenyltetrazolium chloride (TTC) which delineates viable versus necrotic tissue [26], as previously described by our lab [12, 13] (see online supplement).
Creatine kinase release in coronary effluent
The coronary effluent was collected at designated time points of baseline, ischemia and reperfusion (Figure S1, see online supplement) and protease inhibitor tablets were added to the collected coronary effluent. The amount of creatine kinase (CK) was determined using a CK Reagent Set. Creatine kinase release was normalized to coronary flow (mL/min) and heart weight (g) and represented as U/min*g.
Detection of FGF2 release in coronary effluent (see online supplement)
Quantitative determination of FGF2 in coronary effluent at various time points of baseline/equilibration, ischemia, and reperfusion (Figure S1, see online supplement) was performed by ELISA as previous described by our lab [12, 13] and according to the Quantikine human FGF2 immunoassay. FGF2 concentration (pg/mL) in coronary effluent was normalized for coronary flow rate (mL/min) and heart weight (g) and depicted as pg/min/g.
Nuclear and cytosolic preparation for detection of FGF2 isoforms (see online supplement)
Nuclear and cytosolic preparation of non-ischemic Wt, LMWKO and HMWKO hearts were prepared as described by Fryer and colleagues [27], and protein concentration was determined via a Bio-Rad Lowry protein assay.
FGF2 extraction and immunoblotting (see online supplement)
Snap-frozen, non-ischemic hearts were homogenized in homogenization buffer. FGF2 was extracted as previously described [12, 13]. Expression of FGF2 was determined by Western immunoblotting against FGF2 antibody. The purity of the cytosolic and nuclear fractions was determined by the enrichment of β-actin (cytosolic fraction) and histone-1 (nuclear fraction).
Immunohistochemistry for FGF2 localization
Immunohistochemistry on paraffin-embedded tissue sections was done as described previously [24]. Briefly, non-ischemic Wt, LMWKO and HMWKO adult hearts were fixed overnight at 4°C in 4% paraformaldehyde. Freshly fixed tissue was processed and embedded in paraffin, and sectioned at 7 μm in a microtome. Immunohistochemistry was carried out using LSAB+ System-HRP Kit (DakoCytomation, CA), following the recommended standard protocol of the manufacturer. Antigen retrieval occurred by boiling tissue sections in a microwave oven in Target Retrieval Solution (DakoCytomation, CA) for 40 minutes. Tissue sections were incubated rabbit polyclonal FGF2 antibody (Santa Cruz Biotechnology Inc, CA) (1:50 dilution) overnight at 4°C. This FGF2 antibody had been shown to cross-react to both the LMW and HMW isoforms of FGF2 [24]. For control staining, IgG isotype control (Pierce, IL) was used instead of the primary antibody. All tissue sections were counterstained by hematoxylin, and analyzed using bright-field optics with a Zeiss Axio Imager M1 microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY). Images were captured using similar magnification and light intensity and by using an AxioVision 4.6.3 imaging software (Carl Zeiss Microimaging, Inc., Thornwood, NY).
Whole heart preparation to detect FGFR activation (see online supplement)
Snap-frozen DMSO- and PD173074-treated ischemic-reperfused Wt and FGF2 HMWKO, as well as NTg and FGF2 24 kDa HMW Tg hearts were homogenized as previously described by our laboratory [13]. The homogenate was centrifuged at 13,000g for 15 minutes and the supernatant collected. Protein concentration was determined via Lowry protein assay.
Western immunoblotting for FGFR1 and FGFR4 expression and phosphorylation
Activation of FGFR1 and FGFR4 were determined via Western blot analysis. The blots were incubated with primary antibody (1:500) against phospho-FGFR. Total expression levels of FGFR1 and FGFR4 were performed by stripping the blot and incubating with primary antibody (1:500) against total FGFR1 or FGFR4. The activation (i.e., phosphorylation) of FGFR expression was visualized by ECL and densitometry of protein bands were quantitated using a Fluorchem 8800 gel imager.
Statistical analysis
All values in the text and figures were represented as mean±SEM of n independent experiments. Percent recovery of cardiac function, infarct size, vascular density, immunoblotting, and CK release were subjected to one-way analysis of variance (ANOVA) followed by Students' t-test. Pharmacological treatment studies were subjected to a two-way ANOVA following by Students't-test. Probabilities of 0.05 or less (p<0.05) were considered statistically significant.
Results
Cardiac characterization of mice deficient in or overexpressing the FGF2 HMW isoforms
A gross characterization of hearts was performed to determine whether ablation of only the FGF2 HMW isoforms (FGF2 HMWKO) or overexpression of the human FGF2 24 kDa HMW isoform (FGF2 24 kDa HMW Tg) affected cardiac growth, cardiac vasculogenesis and angiogenesis or the expression of the endogenous protein isoforms of FGF2, thereby influencing the outcomes following I/R injury. There was no significant difference in heart weight-to-body weight ratio (mg/g) between Wt (6.1±0.4) and HMWKO (6.0±0.4) mice or between NTg (5.0±0.1) and 24 kDa Tg (line 20: 5.2±0.1 and line 28: 4.9±0.1) animals, indicating that neither ablation of the HMW nor overexpression of the human FGF2 24 kDa HMW isoform altered cardiac growth or induced spontaneous cardiac hypertrophy.
No significant alteration in vasculogenesis or angiogenesis was detected in any of the groups. There was no significant difference in the number of smooth muscle-containing blood vessels per square millimeter (mm2) between Wt (8.6±0.2) and FGF2 HMWKO (8.8±0.4) hearts or between NTg (10.3±0.7) and 24 kDa HMW Tg hearts (line 20: 9.6±0.4 and line 28: 9.2±0.6). Also, the cardiac capillary density was similar in FGF2 HMWKO (54.0 ±10.6) and Wt (57.3±8.6) groups as well as in NTg (49.8±13.7) and 24 kDa Tg (line 20: 53.7 ±8.79 and line 28: 51.5±11.0) hearts.
Ablation of the HMW FGF2 isoform resulted in a significantly increased amount of FGF2 LMW isoform present (Figure 1C and D). In hearts overexpressing the human 24 kDa HMW isoform, the endogenous murine 18, 21 and 22 kDa FGF2 isoforms were not different compared to NTg hearts, demonstrating that overexpression of the FGF2 HMW isoforms did not affect the expression of endogenous FGF2 isoforms (Figure 1E and F). No FGF2 HMW isoforms were detected in FGF2 HMWKO mice (Figure 1C).
Concerns that the human 24 kDa isoform of FGF2 may have different effects from endogenous murine HMW isoforms are addressed by previously work of Gualandris and colleagues [28] in which this group demonstrated that human 24, 22.5 and 22 kDa HMW isoforms, expressed in murine fibroblasts, localized to the nucleus and induced growth in fibroblasts similar to those normally expressing endogenous FGF2 HMW isoforms.
Effect of ablation or overexpression of the FGF2 HMW isoforms in post-ischemic myocardial function
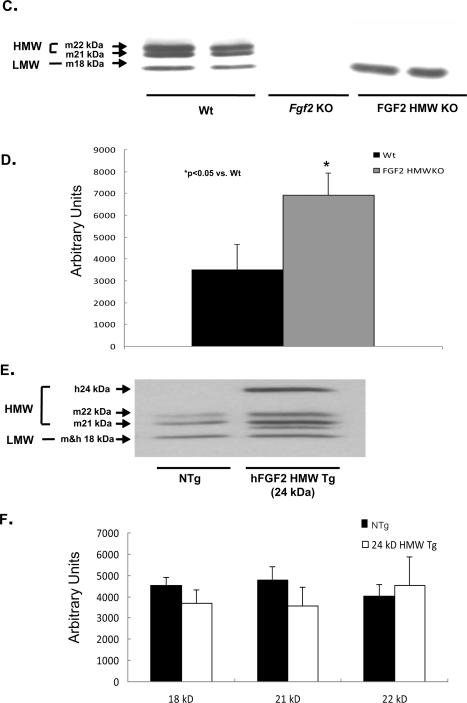
Hearts from wildtype (Wt), FGF2 HMWKO, non-transgenic (NTg), and two lines of FGF2 24 kDa HMW Tg mice were subjected to 60 minutes global low-flow ischemia and 120 minutes reperfusion. There was a significant increase in post-ischemic recovery of contractile function in FGF2 HMWKO hearts (76±5%) compared to Wt hearts (55±5%, p<0.05, Figure 2A) and in post-ischemic recovery of diastolic function (HMWKO: 60±4% vs. Wt: 46±6%). Following I/R injury, there was a significant improvement in systolic and diastolic function in FGF2 HMWKO hearts compared to Wt hearts (p<0.05, Table 1, Series 1). On the other hand, there was a significant decrease in post-ischemic recovery of contractile function in 24 kDa HMW Tg (line 20: 38±6% and line 28: 33±4%) compared to NTg hearts (68±9%, p<0.05, Figure 2B) and in post-ischemic recovery of relaxation function in 24 kDa HMW Tg (line 20: 36±1% and line 28: 36±2%) compared to NTg hearts (52±6%). This result demonstrating that the HMW isoform of FGF2 is not cardioprotective is supported by our previous work in which only the murine HMW isoforms (i.e., LMWKO hearts) lead to a poorer recovery of post-ischemic cardiac function compared to wildtype hearts [13]. Other cardiac function parameters were also significantly decreased in ischemic-reperfused 24 kDa HMW Tg hearts compared to ischemic-reperfused NTg hearts (Table 1, Series 2). Since transgenic and knockout mice were generated on different genetic backgrounds (FVBN and 129/Black Swiss, respectively), care was taken to compare each group to its wildtype littermates, as strain differences in mice have been shown to impact susceptibility to ischemic injury [29].
Figure 2.
Percent recovery of post-ischemic contractile function in Wt (black bar), Fgf2 KO (gray bar), FGF2 HMWKO (dark gray bar), NTg (black bar) and 24 kDa Tg (line 20: light gray bar, line 28: white bar) hearts following I/R injury. Recovery of cardiac function was calculated as the percent +dP/dt at 120 minutes reperfusion to baseline measure. (A) There was a significant decrease in Fgf2 KO and an increase in FGF2 HMWKO hearts in recovery of post-ischemic contractile function compared to Wt hearts. (B) There was a significant decrease in recovery of post-ischemic contractility in 24 kDa HMW Tg (line 20 and line 28) hearts compared to NTg hearts. n= 8 for Wt, FGF2 HMWKO hearts, n= 7 for NTg, n=5 for 24 kDa HMW Tg line 20, and n=6 for 24 kDa HMW Tg line 28. *p<0.05 vs. Wt hearts.
Table 1.
Cardiac function (systolic and diastolic parameters) at baseline and following ischemia-reperfusion injury.
| BASELINE | 60' ISCHEMIA/120' REPERFUSION | |||||||
|---|---|---|---|---|---|---|---|---|
| LVSP | LVEDP | +dP/dt | −dP/dt | LVSP | LVEDP | +dP/dt | −dP/dt | |
| Series 1 | ||||||||
| Wildtype | 99±3 | 8±1 | 4206±159 | −3347±162 | 62±8 † | 23±2 † | 2361±204 † | −1544±215 † |
| Fgf2 KO | 98±2 | 10±l | 4111±124 | −3048±126 | 55±8 † | 31±5 † | 1609±184 * † | −1078±103 * † |
| FGF2 HMWKO | 94±3 | 7±1 | 3906±139 | −3300±196 | 73±5 † | 19±3 † # | 2977±204 * † # | −1963±177 † # |
| Series 2 | ||||||||
| NTg | 100±l | 5±1 | 4079±24 | −3319±137 | 76±5 † | 22±6 † | 2773±358 † | −1734±199 † |
| 24 kDa HMW Tg line 20 | 97±2 | 4±1 | 4265±108 | −3180±162 | 56±5 * † | 27±10 † | 1625±270 * † | −1148±49 * † |
| 24 kDa HMW Tg line 28 | 99±1 | 6±1 | 4331±88 * | −3270±142 | 55±6 * † | 31±7 † | 1452±165 * † | −1180±70 * † |
| Series 3 | ||||||||
| Wt (DMSO) | 98±2 | 6±1 | 4411±124 | −3319±125 | 39±9 † | 24±3 † | 2045±202 † | −1301±154 † |
| FGF2 HMWKO (DMSO) | 98±1 | 5±1 | 4285±162 | −3602±89 * | 88±4 * † | 14±2 * † | 3837±159 * † | −2482±212 * † |
| Wt (PD173074) | 97±2 | 5±1 | 4102±87 | −3228±88 | 48±5 † | 27±5 † | 1397±140 * † # | −1060±58 † # |
| FGF2 HMWKO (PD173074) | 97±3 | 8±2 | 4068±133 | −3060±189 | 50±7 † # | 31±6 * † | 1268±104 * † # | −1051±113 † # |
All values were presented as mean ± SEM of n independent experiments. LVSP: left ventricle systolic pressure (mmHg). LVEDP: left ventricle end diastolic pressure (mmHg). ±dP/dt: derivative of change in contractile and relaxation pressure over time (mmHg/sec)
Series 1: n=7–8 per group.
p<0.05 vs. Wt.
p<0.05 vs. baseline cohort.
p<0.05 vs. Fgf2 KO.
Series 2: n=6 per group.
p<0.05 vs. NTg.
p<0.05 vs. baseline cohort.
Series 3: n=12 per group.
p<0.05 vs. vehicle (DMSO)-treated Wt.
p<0.05 vs. vehicle (DMSO)-treated cohort.
<0.05 vs. baseline cohort.
These data suggest that the increased amounts of LMW FGF2 in the absence of HMW FGF2 most likely play a protective role in preventing myocardial dysfunction during ischemiareperfusion injury. Conversely, overexpressing HMW FGF2, while keeping LMW FGF2 at levels normally expressed in the myocardium, results in lowered recovery.
Effect of FGF2 HMW isoforms on myocardial cell injury after ischemia-reperfusion injury
Myocardial infarct size was measured following I/R. There was no difference between Wt (39±2%) and FGF2 HMWKO (34±2%) or NTg (31± 3%) and 24 kDa HMW Tg (line 20: 33±1% and line 28: 35±3%) (see Figure S2, online supplement).
Myocardial cell injury, indicated as CK release, was measured at designated time points of baseline (equilibration), ischemia, and reperfusion (Figure S1, see online supplement). CK release was significantly increased during early reperfusion in all groups compared to their baseline cohorts (Table S1 and S2, see online supplement). Yet, there was no difference in CK release at early reperfusion between Wt and FGF2 HMWKO or NTg and human FGF2 24 kDa HMW Tg groups. Furthermore, our previous published results showed that myocardial infarction and creatine kinase release were significantly decreased in FGF2 Tg hearts overexpressing all FGF2 isoforms (LMW and HMW) [12] compared to the hearts expressing only the high molecular isoforms of FGF2 (LMWKO) [13] and Wt hearts. Therefore, these data suggest that all FGF2 isoforms are necessary to protect the heart from myocardial infarction and cell injury.
The localization and secretion of FGF2 LMW and HMW isoforms in non-ischemic and ischemic-reperfused hearts
The localization of the FGF2 LMW isoform is not well-defined; most studies demonstrate a cytosolic location, yet more recent studies also show a nuclear location [7–9]. FGF2 HMW isoforms are localized to the nucleus [30], but recent evidence indicates that the HMW isoforms can be released out of the cell through vesicle shedding [9]. With the compartmentalization of the FGF2 isoforms to multiple sites in the cell, elucidating the localization of the FGF2 LMW and HMW isoform will allow a greater understanding of the subcellular targets of FGF2 isoform signaling. Furthermore, which of these FGF2 isoforms act in a paracrine/autocrine manner (i.e., activate FGFRs) or act in an intracrine fashion during I/R is also unknown.
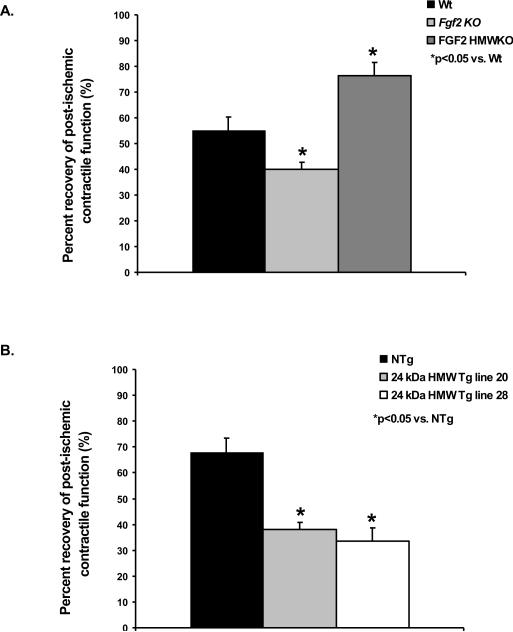
The localization of the LMW and HMW isoforms of FGF2 in non-ischemic Wt, FGF2 LMWKO (only HMW isoforms present) and FGF2 HMWKO (only LMW isoform present) hearts was identified. Western immunoblot data showed that the HMW isoforms were localized only to the nucleus in Wt and LMWKO hearts; whereas, the LMW isoform was found in the cytoplasm as well as in the nucleus in Wt and FGF2 HMWKO hearts (Figure 3A). Confirmation of the localization of the LMW and HMW protein isoforms of FGF2 occurred via immunohistochemistry on non-ischemic Wt, LMWKO, and HMWKO hearts (Figure 3B). The LMW isoform, using the HMWKO heart, was detected in the cytosol and nucleus (Figure 3B, last panel); whereas, the HMW isoform, using the LMWKO heart, was identified in the nucleus (Figure 3B, middle panel). These results are consistent with those of other investigators who identified the LMW isoform in both cytoplasm and nucleus and HMW isoforms were localized only to the nucleus [1]. This localization may indicate as well as dictate the unique activity of FGF2 LMW and HMW isoforms in I/R injury.
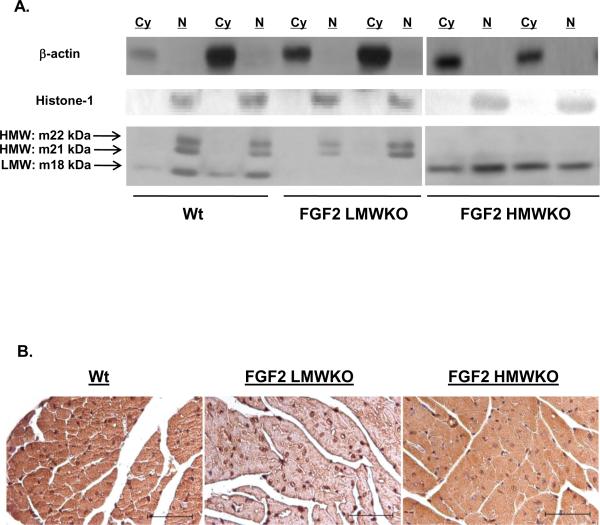
Figure 3.
(A) Representative Western blot of FGF2 isoform localization in non-ischemic wildtype (Wt), FGF2 LMWKO and FGF2 HMWKO mouse hearts. In Wt hearts, the LMW, 18 kDa isoform was localized to the cytosolic (Cy) and nuclear (N) fractions of the heart; whereas, the HMW, 21 and 22 kDa, isoforms were nuclear localized. In the absence of the LMW isoform (FGF2 LMWKO), the HMW isoforms were only expressed in the nucleus. In the absence of the HMW isoforms (FGF2 HMWKO), the LMW isoform was localized in the cytoplasm and nucleus of the heart. β-actin, a cytosolic protein, used as a measure of cytosolic fraction enrichment. Histone-1, a nuclear protein, used as a measure of nuclear fraction enrichment. (B) Localization of FGF2 isoforms via immunohistochemistry in adult mouse ventricular myocardium. Wildtype (Wt) heart (first panel), FGF2 LMW KO heart (middle panel), and FGF2 HMWKO heart (last panel). Brown color indicates FGF2, and blue color marks nuclei that are counterstained by hematoxylin. In wildtype and FGF2 HMWKO (expression of only LMW isoform) hearts, FGF2 is found in both cytoplasm and nucleus of the cardiomyocytes. FGF2 is detected in FGF2 LMW KO (expression of only HMW isoforms) hearts. Scale bar in A–C, 50 μm. n=3/group.
Coronary effluent samples, collected at various time points during baseline and reperfusion from Wt, LMWKO, and HMWKO hearts, demonstrated that secretion of the HMW isoforms did not occur in the FGF2 LMWKO hearts (Table 2), which suggests that the HMW effects in the heart are most likely due to intracrine signaling. In the FGF2 HMWKO hearts, the LMW isoform was secreted out of the cell during ischemia-reperfusion injury (Table 2), suggesting that it may act through FGFR to elicit its activity.
Table 2.
Level of FGF2 release detected in coronary effluent during baseline, ischemia and reperfusion.
| Wildtyoe | LMWKO | HMWKO | |
|---|---|---|---|
| Baseline (Equilibration) | 8.3±3.3 | not detected (ND) | 14.5±4.3 |
| Ischemia (0–30 min) | ND | ND | ND |
| Early Rep (0–14 min) | 10.6±4.0 | ND | 15.7±3.6 |
| Late Rep (110–120 min) | 12.3±4.3 | ND | 16.8±7.1 |
Concentration (in pg/min/g heart weight) of FGF2 secreted in coronary effluent from wildtype, hearts deficient of the LMW isoform (LMWKO) and deficient of HMW isoforms (HMWKO) collected at baseline, ischemia and reperfusion (Rep). ND: not detected. n=4 per group.
The involvement of FGFR in protection against myocardial dysfunction
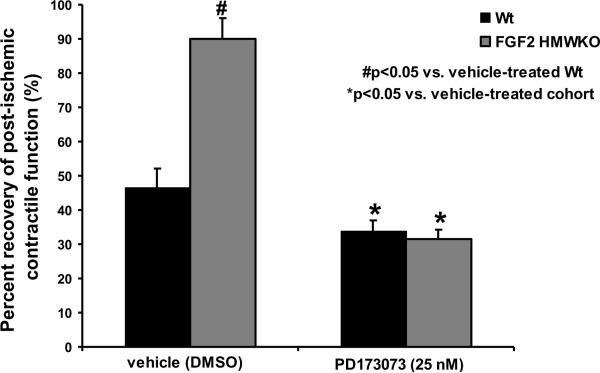
To determine whether the secreted FGF2 LMW isoform acts through FGFR to elicit cardioprotection, Wt and FGF2 HMWKO hearts were subjected to 60 minutes of global, low-flow ischemia and 120 minutes of reperfusion, and treated with vehicle (DMSO) or PD173074 (25 nM), a FGFR inhibitor that binds the tyrosine kinase motif on the FGFR and inhibits the tyrosine kinase activity of FGFR [25]. Following I/R injury, there was significantly less systolic and diastolic dysfunction in ischemic-reperfused, DMSO-treated FGF2 HMWKO vs. DMSO-treated Wt hearts (p<0.05, Table 1, Series 3). Furthermore, there was a significant increase in post-ischemic recovery of contractile function in DMSO-treated FGF2 HMWKO hearts (90±6%) vs. DMSO-treated Wt (47±5%) hearts (p<0.05, Figure 4). With similar FGF2 LMW isoform release in Wt and FGF2 HMWKO hearts observed (Table 2), the cardioprotective effect in FGF2 HMWKO hearts was not only due to the presence of FGF2 LMW isoform, but also due to the absence of FGF2 HMW isoforms. After PD173074 treatment, percent recovery of post-ischemic contractile function was significantly attenuated in Wt (34±3%), and FGF2 HMWKO (31±3%) hearts (p<0.05, Figure 4), indicating that FGFR is involved in I/R injury and the cardioprotective effect of FGF2. Most likely, it is the released FGF2 LMW isoform in FGF2 HMWKO hearts that acts on FGFR and elicits cardioprotection.
Figure 4.
Percent recovery of post-ischemic cardiac function in Wt (black bar) and FGF2 HMWKO (dark gray bar) hearts following DMSO (vehicle) or PD173074 (25 nM) treatment. Inhibition of FGFR with PD173074 (25 nM) completely abolished the FGF2 HMWKO post-ischemic recovery of contractile function. Recovery of cardiac function was calculated as the percent +dP/dt at 120 minutes reperfusion to baseline measure. n=6 for vehicle (DMSO)- and PD173074-treated Wt and FGF2 HMWKO hearts. *p<0.05 vs. vehicle (DMSO)-treated cohort. #p<0.05 vs. vehicle (DMSO)-treated Wt hearts.
Effect of FGFR inhibition on myocardial cell injury
Myocardial infarct size was measured after 60 minutes global, low-flow ischemia and 120 minutes reperfusion. There was no difference in DMSO-treated groups: Wt (26±6%) and FGF2 HMWKO (28±3%) (see Figure S3, online supplement). Following FGFR inhibition, there was a significant increase in myocardial infarct size in both Wt (36±2%) and FGF2 HMWKO (42±1%, p<0.05) compared to DMSO-treated cohorts (see Figure S3, online supplement). Creatine kinase release was measured from coronary effluent at designated time points of baseline, ischemia and reperfusion (Figure S1, see online supplement). There was no significant difference in CK release between vehicle-treated Wt and FGF2 HMWKO hearts (Table S3, see online supplement). Following PD173074 treatment, creatine kinase release was slightly, but significantly, increased in Wt vs. its vehicle cohort at baseline level (p<0.05, Table S3, see online supplement), but no difference in early reperfusion. There was no difference in creatine kinase release between PD173074-treated FGF2 HMWKO and vehicle-treated FGF2 HMWKO hearts. This evidence suggests that inhibition of FGFR resulted in increased myocardial cell damage independent of FGF2 isoforms.
Effect of FGFR inhibition on FGFR activation
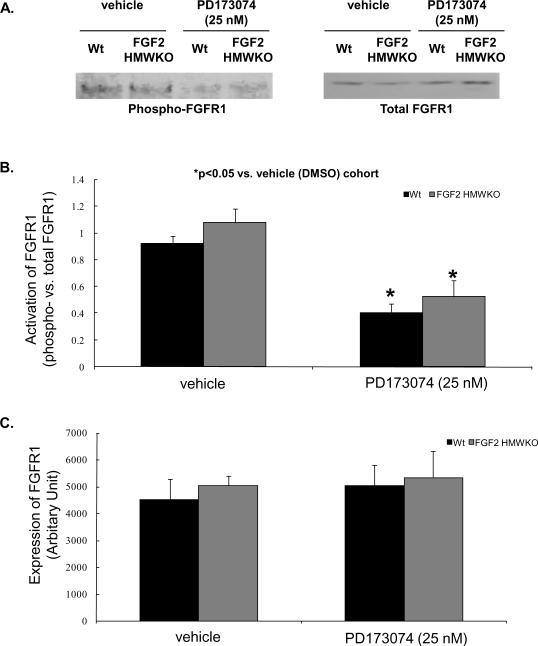
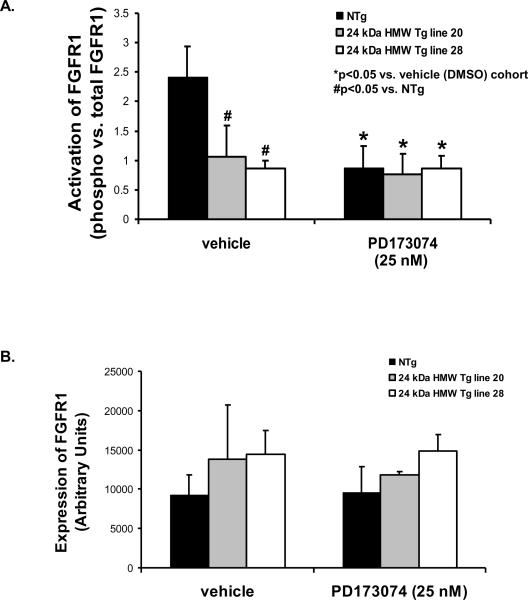
PD173074 abolishes FGFR activation by binding the tyrosine kinase cleft to inhibit FGFR autophosphorylation [25]. To determine whether this concentration of PD173074 blocked FGFR phosphorylation in Wt and FGF2 HMWKO and NTg and HMW Tg mouse hearts subjected to I/R injury, Western immunoblotting was performed. Phosphorylation of FGFR1 was significantly decreased in PD173074-treated Wt, FGF2 HMWKO and NTg hearts compared to their vehicle (DMSO)-treated cohorts (p<0.05, Figures 5A, 5B, and Figure 6A). Overexpression of the human 24 kDa HMW Tg hearts resulted in reduced FGFR1 phosphorylation and treatment with the FGFR inhibitor to HMW Tg hearts had no further decrease in receptor phosphorylation (Figure 6A). In addition, there was no difference in FGFR1 expression between DMSO-treated Wt and FGF2 HMWKO hearts and NTg and HMW Tg hearts (Figures 5A, 5C, and Figure 6B). Cardiac expression of FGFR4 was not observed (data not shown). These findings suggest that the activation of FGFR1, the subtype of FGFR expressed on rodent hearts [16], and ultimately the cardiac actions of FGF2 were reduced by overexpression of the human 24 kDa HMW isoform or by 25 nM PD173074, the selective FGFR inhibitor.
Figure 5.
FGFR1 activation, as measured by phosphorylation state, in DMSO- and PD173074-treated mouse hearts. (A) Representative Western blot depicting activation of FGFR1 in vehicle (DMSO)- and PD173074-treated Wt and FGF2 HMWKO hearts. (B) There was a significant decrease in FGFR1 phosphorylation in PD173074-treated Wt and HMWKO groups compared to their vehicle (DMSO)-treated cohorts. (C) FGFR1 expression was not different in vehicle (DMSO)- and PD173074-treated Wt and FGF2 HMWKO hearts following I/R injury. n=4/group for Wt and HMWKO. *p<0.05 vs. vehicle (DMSO)-treated cohort.
Figure 6.
FGFR1 activation, as measured by phosphorylation state, in DMSO- and PD173074-treated NTg and human 24 kDa HMW Tg mouse hearts. (A) There was a significant decrease in FGFR1 phosphorylation in the PD173074-treated NTg group compared to its vehicle (DMSO)-treated cohorts; whereas, there was no difference in the FGFR1 phosphorylation state in vehicle (DMSO)-treated and PD173074-treated HMW Tg groups. (B) FGFR1 expression was not different in vehicle (DMSO)- and PD173074-treated NTg and FGF2 24 kDa HMW Tg hearts following I/R injury. n= 6 for NTg, n=9 for 24 kDa HMW Tg line 20, and n=6 for 24 kDa HMW Tg line 28. *p<0.05 vs. vehicle (DMSO)-treated cohort. #p<0.05 vs. NTg.
Discussion
Our previous data suggest that the FGF2 LMW isoform is important for protecting the heart from post-ischemic cardiac dysfunction and this protection involved the MKK7/JNK pathway [13]. The roles of FGF HMW isoforms in I/R injury are currently unknown. The present study characterizes for the first time the results of ablation or overexpression of the HMW isoforms of FGF2 in a mouse model, and demonstrates that FGF2 HMW isoforms play an opposite role to the LMW isoform in cardiac ischemia-reperfusion injury. Ablation of FGF2 HMW isoforms resulted in a protective effect against myocardial dysfunction, and overexpression of the human HMW 24 kDa isoform lead to a poorer recovery of cardiac function following I/R injury (Figure 2, Table 1, Series 1 and 2), demonstrating that the HMW isoforms are deleterious in I/R injury. This effect observed in FGF2 HMWKO hearts may not only be due to the increased presence of the cardioprotective isoform (the LMW isoform), but also due to the absence of the injurious isoforms (the HMW isoforms). The data suggest that both explanations may contribute to the observed phenotype, as the percent recovery of post-ischemic cardiac function in FGF2 HMWKO hearts (with the presence of only the LMW isoform) was greater than in wildtype hearts, indicating that the LMW isoform is protective. Alternately, an opposing role for HMW FGF2 in the development of cardiac dysfunction is established in the ischemicreperfused hearts overexpressing the human FGF2 24 kDa HMW isoform, which had a significant decrease in percent recovery of post-ischemic cardiac function (Figure 2B, Table 1, Series 2) compared to NTg hearts, indicating that that HMW isoforms are injurious. The HMW Tg findings presented here support previously published data from our lab, which demonstrated that mice only expressing the HMW isoforms (LMWKO) recovered to a lesser degree than their wildtype cohorts [13] and suggest that the nuclear-targeted FGF2 HMW isoforms have a deleterious role in myocardial I/R injury. These studies suggest that the LMW and the HMW isoforms might work in opposition during I/R injury to modulate cardiac function. It has been speculated that HMW and LMW FGF2 may serve different functions by controlling each other's biological activity [30, 31], a supposition supported by our work here showing an increase in total LMW FGF2 protein in the absence of HMW FGF2, as well as the finding that overexpression of HMW FGF2 reduces FGFR1 activation, through which LMW FGF2 cardioprotection from I/R injury is mediated.
Most studies of FGF2 in I/R injury focus on the LMW isoform, which has been shown to be a cardioprotective molecule both in vivo and in vitro [32]. Until recently, there was no evidence implicating a role of FGF2 HMW isoforms in I/R injury. Kardami and colleagues [33, 34] showed that exogenous administration of the recombinant rat FGF2 23 kDa HMW isoform protected the heart from myocardial dysfunction and reduced myocardial infarction in the short-term (24 hours post-MI) but not long-term (1–8 weeks post-MI), most likely due to the post-ischemic hypertrophic response triggered by the FGF2 HMW isoform. Our study reveals that endogenous HMW isoforms or overexpression of the human 24 kDa HMW isoform are/is detrimental to the recovery of post-ischemic cardiac function and infarct size following acute I/R injury. Our findings are opposite of what Kardami and colleagues observed with the recombinant rat HMW isoform. These differences can be explained as follows: 1) since FGF2 HMW isoforms and the FGF2 LMW isoform bind to heparan sulfate proteoglycans with similar affinity, it is likely that the exogenously administered FGF2 HMW isoform can interact with FGFR [35], eliciting a similar cardioprotective effect as the FGF2 LMW isoform, 2) the FGF2 HMW isoform effects in the myocardium could be dependent on duration such that in the short term, FGF2 HMW isoforms may elicit a cardioprotective phenomenon; however, if the expression of FGF2 HMW protein isoforms are manipulated for a long period of time, the activation status of proteins involved in cardioprotection or cardiotoxicity might change, causing an opposite effect on post-ischemic cardiac function, and 3) since the HMW isoform induces a hypertrophic response [33], cardiac function may decrease because of the cardiac pathology initiated by the hypertrophic response.
In vitro studies by Kardami and colleagues [35, 36] show that myocytes exogenously treated with murine FGF2 HMW isoforms or hearts overexpressing the rat FGF2 HMW isoforms (21–22 kDa) display an increase in cardiomyocyte size. Yet, in our FGF2 mice overexpressing the human 24 kDa HMW isoform, there was no spontaneous cardiac growth. This inconsistency in FGF2 HMW isoforms in cardiac hypertrophy may be due to the models that were used to determine cardiac hypertrophy. In those studies [35, 36], the FGF2 HMW isoforms are given exogenously to cardiomyocytes for two days. In our mouse models, the human FGF2 24 kDa HMW isoform is overexpressed chronically from birth which may modulate the activity of other molecules and/or signals. For example, our unpublished data demonstrated a 2-fold decrease in Fgf1 mRNA level in non-ischemic FGF2 LMWKO hearts, a significant increase in Fgf6 mRNA level in non-ischemic Fgf2 KO hearts, and a significant decrease in Fgf13 mRNA level in non-ischemic Fgf2 KO and FGF2 LMWKO hearts. Therefore, in FGF2 HMWKO or 24 kDa HMW Tg hearts, there might be alteration in some similar intrinsic mitogenic or angiogenic molecules and that could be the reason for the inconsistent cardiac anatomy and morphology between our mouse model and Kardami's observation.
Similar to what our laboratory previously demonstrated [13], there was no significant difference in myocardial cell injury, measured by TTC staining and CK release, in FGF2 HMWKO hearts compared to Wt hearts (Figure S2 and Table S1, see online supplement). Also, overexpressing the human FGF2 24 kDa HMW isoform did not alter myocardial cell injury compared to non-transgenic hearts (Figure S3 and Table S2, see online supplement). However, our lab has previously shown that ablating both LMW and HMW isoforms of FGF2 at the same time results in a greater infarct size after I/R [12]. Based on these findings, neither the LMW nor the HMW isoforms alone protect the heart from cellular injury, but both are necessary to reduce infarct size in the post-ischemic heart. Evidence indicates that the effect of the FGF2 HMW isoforms on cell death is related to its concentration given, such that a high concentration of FGF2 HMW (100 ng/mL) promotes cell growth and inhibits cell death, while a low concentration of FGF2 HMW (1 ng/mL) promotes cell death [37]. This dual effect of FGF2 HMW isoforms in cell death may contribute to the absence of change in myocardial infarction between groups. Furthermore, and consistent with other studies, the level of myocardial infarction is not always a predictor of post-ischemic improvement in left ventricular function after I/R injury [38].
FGF2 activity is, in part, mediated through its interaction with FGFR [14]. FGFR has been linked to protection against I/R-induced myocardial dysfunction [19]. The cardioprotective effect with administration of FGF2 requires an interaction with FGFR as binding to the low affinity HSPG sites alone is not sufficient for cardioprotection [19]. The present study found that following FGFR inhibition (PD173074), the improvement observed in post-ischemic cardiac function was not only inhibited in Wt hearts, but also in FGF2 HMWKO hearts (Figure 4, Table 1, Series 3). Our current study also demonstrated that upon FGFR inhibition, there was a significant increase in myocardial infarction and creatine kinase release in PD-treated Wt and FGF2 HMWKO hearts compared to DMSO-treated cohort (Figure S4 and Table S3, see online supplement). These findings indicate that FGFR activation is involved in protecting the heart from myocardial cell death. This evidence reveals two important conclusions: 1) FGFR is involved in I/R injury in Wt hearts, since the recovery of post-ischemic cardiac function was significantly decreased and myocardial infarct size was greatly increased upon FGFR receptor inhibition and 2) the cardioprotective effect in FGF2 HMWKO hearts is receptor-mediated, since FGFR inhibition completely abolished the protection. However, whether it is due to the FGF2 LMW isoform's interaction with FGFR and/or due to the ablation of FGF2 HMW isoforms affecting downstream targets of FGFR signaling remains to be elucidated. FGF2 HMW isoform activity has been linked to several signaling pathways [39, 40], including PKC, ERK, and c-JUN, a downstream substrate of JNK. Preliminary unpublished data from our laboratory suggests that PKC isoforms and JNK are downregulated in HMW Tg mice, and further studies will determine if these kinases are responsible for the deleterious effects of the HMW FGF2 isoforms. Moreover, unpublished preliminary data from our laboratory suggests that in HMWKO hearts, there may be some level of regulation of sarco(endo)plasmic reticulum calcium cycling and the contractile apparatus to influence post-ischemic recovery of cardiac function following I/R injury. Ongoing studies subsequent to the data presented here should further elucidate this area of FGF2 isoform and cardioprotection research.
This present study has demonstrated that the FGF2 HMW isoforms are localized to the nucleus, and the LMW isoform is localized both to the cytosol and nucleus of the heart (Figure 3). Studies show that internalized FGF2 LMW isoform, occurring through a putative nuclear localization signal or heparan sulfate proteoglycans [41, 42], can stimulate cell proliferation and down-regulation of FGFR [43]. Yet, there is evidence of FGF2 HMW isoform release facilitated by HSP27 [44] or the shedding of membrane vesicles [9] in endothelial cells, particularly during stress (i.e., tumorogenesis, hypoxia) [45]. Less is known about whether or which FGF2 isoforms are released during I/R injury to facilitate the cardioprotective effect. The present study evaluates whether any of the HMW or LMW effects during ischemia-reperfusion injury are due to secretion of these isoforms, leading to activation of receptor (paracrine/autocrine model), or interaction directly with intracellular signaling (intracrine mechanism). Evidence obtained from Wt, FGF2 LMWKO and FGF2 HMWKO coronary effluent samples, collected at various time-points of baseline equilibration, early reperfusion and late reperfusion, show release of FGF2 LMW isoform from the heart, but no FGF2 HMW isoforms were detected in coronary effluent during I/R injury (Table 2). Therefore, the cardioprotective effect in FGF2 HMWKO hearts is most likely due to the release of the LMW isoform and its interaction with the cell surface receptor, FGFR. The amount of FGF2 LMW isoform release in FGF2 HMWKO shows no significant difference to that in Wt hearts; however, the percent recovery of post-ischemic cardiac function in FGF2 HMWKO hearts is significantly higher compared to Wt hearts, indicating that beside the interaction between LMW isoform and FGFR, absence of the FGF2 HMW isoforms elicits cardioprotection through a yet to be determined mechanism. Davis and colleagues [23] demonstrated that in vascular smooth muscle, the LMW isoform mediated DNA synthesis in an autocrine fashion and the HMW isoforms induced DNA synthesis through an intracrine manner. This observation is similar to our current findings which showed that the LMW and HMW isoforms may act on the heart through different modes of action. The HMW and LMW FGF2 isoforms are speculated to associate, either directly or indirectly, in a reciprocal mechanism, controlling each other's biological activity in a concentration- and/or localization-dependent manner [31, 32]. Therefore, the presence or absence of the HMW FGF2 isoforms may affect the biological function of the LMW isoform in the heart as demonstrated in the current study by the alteration in FGF receptor activation (Figure 6A) or post-ischemic recovery of cardiac function (Figure 2).
In summary, these studies have shown that adjusting the balance of HMW and LMW FGF2, by either ablating or increasing HMW FGF2 expression, results in altered pos-ischemic function, with HMW FGF2 playing a detrimental role in recovery. At the same time, we have confirmed that both LMW and HMW FGF2 isoforms are necessary to protect the heart from myocardial cell injury/death, as seen in our previous work [12, 13]. The HMW effects in the heart are most likely due to intracrine signaling, while LMW activity in the heart is due to its interaction with FGFR. This evidence reveals key roles of FGF2 LMW and HMW isoforms in I/R injury which may aid in the development of novel strategies for the reduction of morbidity and mortality from ischemic heart disease. Furthermore, the mouse models, HMWKO and human 24 kDa HMW Tg, are novel resources that now can be employed to delineate the roles of the low and high molecular weight protein isoforms of FGF2 not only in cardiac biology and disease, but in other organ system physiology and pathophysiology. Unlike other models used to study HMW FGF2, these mice have altered endogenous expression of these isoforms, allowing a substantially more physiologically relevant examination of their effects in vivo. This is of particular importance as it becomes clear that HMW FGF2 has a distinct role to play intracellularly, which is not addressed by the application of exogenous HMW FGF2.
Supplementary Material
Acknowledgments
This work was supported by grants from the American Heart Association (SDG 23004N), the Pharmaceutical Research and Manufacturers of America (Research Starter Grant), NIH/NHLBI R01 (HL075633) to Jo El J. Schultz and (HL070174) to Doetschman. The authors are grateful to Dr. Ming Zhou for his contribution of generating the initial construct for the FGF2 HMWKO mouse model. The authors would like to acknowledge M. Bender, A. Whitaker and S. Pawlowski for their excellent animal husbandry. Also, the authors would like to acknowledge Pfizer, Inc (Groton, CT) for the generous gift of the FGFR inhibitor, PD173074.
Non-standard abbreviations
- LMW
low molecular weight
- HMW
high molecular weight
- MKK7
MAPK kinase kinase 7
- FGFR
fibroblast growth factor receptor
- I/R
ischemia/reperfusion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures None
References
- [1].Okada-Ban M, Thiery JP, Jouanneau J. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32(3):263–7. doi: 10.1016/s1357-2725(99)00133-8. [DOI] [PubMed] [Google Scholar]
- [2].Doble BW, Fandrich RR, Liu L, Padua RR, Kardami E. Calcium protects pituitary basic fibroblast growth factors from limited proteolysis by co-purifying proteases. Biochem Biophys Res Commun. 1990;173(3):1116–22. doi: 10.1016/s0006-291x(05)80901-5. [DOI] [PubMed] [Google Scholar]
- [3].Dono R, Zeller R. Cell-type-specific nuclear translocation of fibroblast growth factor-2 isoforms during chicken kidney and limb morphogenesis. Dev Biol. 1994;163(2):316–30. doi: 10.1006/dbio.1994.1151. [DOI] [PubMed] [Google Scholar]
- [4].Powell PP, Klagsbrun M. Three forms of rat basic fibroblast growth factor are made from a single mRNA and localize to the nucleus. J Cell Physiol. 1991;148(2):202–10. doi: 10.1002/jcp.1041480204. [DOI] [PubMed] [Google Scholar]
- [5].Sommer A, Brewer MT, Thompson RC, Moscatelli D, Presta M, Rifkin DB. A form of human basic fibroblast growth factor with an extended amino terminus. Biochem Biophys Res Commun. 1987;144(2):543–50. doi: 10.1016/s0006-291x(87)80001-3. [DOI] [PubMed] [Google Scholar]
- [6].Okada-Ban M, Moens G, Thiery JP, Jouanneau J. Nuclear 24 kD fibroblast growth factor (FGF)-2 confers metastatic properties on rat bladder carcinoma cells. Oncogene. 1999;18(48):6719–24. doi: 10.1038/sj.onc.1203092. [DOI] [PubMed] [Google Scholar]
- [7].Sun G, Doble BW, Sun JM, Fandrich RR, Florkiewicz R, Kirshenbaum L, et al. CUG-initiated FGF-2 induces chromatin compaction in cultured cardiac myocytes and in vitro. J Cell Physiol. 2001;186(3):457–67. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1044>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [8].Claus P, Doring F, Gringel S, Muller-Ostermeyer F, Fuhlrott J, Kraft T, et al. Differential intranuclear localization of fibroblast growth factor-2 isoforms and specific interaction with the survival of motoneuron protein. J Biol Chem. 2003;278(1):479–85. doi: 10.1074/jbc.M206056200. [DOI] [PubMed] [Google Scholar]
- [9].Taverna S, Ghersi G, Ginestra A, Rigogliuso S, Pecorella S, Alaimo G, et al. Shedding of membrane vesicles mediates fibroblast growth factor-2 release from cells. J Biol Chem. 2003;278(51):51911–9. doi: 10.1074/jbc.M304192200. [DOI] [PubMed] [Google Scholar]
- [10].Pasumarthi KB, Kardami E, Cattini PA. High and low molecular weight fibroblast growth factor-2 increase proliferation of neonatal rat cardiac myocytes but have differential effects on binucleation and nuclear morphology. Evidence for both paracrine and intracrine actions of fibroblast growth factor-2. Circ Res. 1996;78(1):126–36. doi: 10.1161/01.res.78.1.126. [DOI] [PubMed] [Google Scholar]
- [11].Detillieux KA, Sheikh F, Kardami E, Cattini PA. Biological activities of fibroblast growth factor-2 in the adult myocardium. Cardiovasc Res. 2003;57(1):8–19. doi: 10.1016/s0008-6363(02)00708-3. [DOI] [PubMed] [Google Scholar]
- [12].House SL, Bolte C, Zhou M, Doetschman T, Klevitsky R, Newman G, et al. Cardiac-specific overexpression of fibroblast growth factor-2 protects against myocardial dysfunction and infarction in a murine model of low-flow ischemia. Circulation. 2003;108(25):3140–8. doi: 10.1161/01.CIR.0000105723.91637.1C. [DOI] [PubMed] [Google Scholar]
- [13].Liao S, Porter D, Scott A, Newman G, Doetschman T, Schultz Jel J. The cardioprotective effect of the low molecular weight isoform of fibroblast growth factor-2: the role of JNK signaling. J Mol Cell Cardiol. 2007;42(1):106–20. doi: 10.1016/j.yjmcc.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7(3):165–97. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- [15].Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem. 1997;45(7):1005–19. doi: 10.1177/002215549704500710. [DOI] [PubMed] [Google Scholar]
- [16].Cool SM, Sayer RE, van Heumen WR, Pickles JO, Nurcombe V. Temporal and spatial expression of fibroblast growth factor receptor 4 isoforms in murine tissues. Histochem J. 2002;34(6–7):291–7. doi: 10.1023/a:1023326524562. [DOI] [PubMed] [Google Scholar]
- [17].Mima T, Ueno H, Fischman DA, Williams LT, Mikawa T. Fibroblast growth factor receptor is required for in vivo cardiac myocyte proliferation at early embryonic stages of heart development. Proc Natl Acad Sci U S A. 1995;92(2):467–71. doi: 10.1073/pnas.92.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu L, Pasumarthi KB, Padua RR, Massaeli H, Fandrich RR, Pierce GN, et al. Adult cardiomyocytes express functional high-affinity receptors for basic fibroblast growth factor. Am J Physiol. 1995;268(5 Pt 2):H1927–38. doi: 10.1152/ajpheart.1995.268.5.H1927. [DOI] [PubMed] [Google Scholar]
- [19].Jiang ZS, Padua RR, Ju H, Doble BW, Jin Y, Hao J, et al. Acute protection of ischemic heart by FGF-2: involvement of FGF-2 receptors and protein kinase C. Am J Physiol Heart Circ Physiol. 2002;282(3):H1071–80. doi: 10.1152/ajpheart.00290.2001. [DOI] [PubMed] [Google Scholar]
- [20].Muinck ED, Nagy N, Tirziu D, Murakami M, Gurusamy N, Goswami SK, et al. Protection against myocardial ischemia-reperfusion injury by the angiogenic Masterswitch protein PR 39 gene therapy: the roles of HIF1alpha stabilization and FGFR1 signaling. Antioxid Redox Signal. 2007;9(4):437–45. doi: 10.1089/ars.2006.1501. [DOI] [PubMed] [Google Scholar]
- [21].Fujisawa H, Koide N, Kono T, Takayama K, Tsukioka K, Wada Y, et al. Expression of basic fibroblast growth factor and its receptor-1 in cardiac myxoma. J Cardiovasc Surg (Torino) 2002;43(5):589–94. [PubMed] [Google Scholar]
- [22].Pennisi DJ, Ballard VL, Mikawa T. Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR-1, but not for transmural myocardial patterning in the embryonic chick heart. Dev Dyn. 2003;228(2):161–72. doi: 10.1002/dvdy.10360. [DOI] [PubMed] [Google Scholar]
- [23].Davis MG, Zhou M, Ali S, Coffin JD, Doetschman T, Dorn GW., 2nd Intracrine and autocrine effects of basic fibroblast growth factor in vascular smooth muscle cells. J Mol Cell Cardiol. 1997;29(4):1061–72. doi: 10.1006/jmcc.1997.0383. [DOI] [PubMed] [Google Scholar]
- [24].Azhar M, Yin M, Zhou M, Li H, Mustafa M, Nusayr E, et al. Gene targeted ablation of high molecular weight fibroblast growth factor-2. Dev Dyn. 2009;238(2):351–7. doi: 10.1002/dvdy.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH, et al. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. Embo J. 1998;17(20):5896–904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, et al. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101(5):593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- [27].Fryer RM, Patel HH, Hsu AK, Gross GJ. Stress-activated protein kinase phosphorylation during cardioprotection in the ischemic myocardium. Am J Physiol Heart Circ Physiol. 2001;281(3):H1184–92. doi: 10.1152/ajpheart.2001.281.3.H1184. [DOI] [PubMed] [Google Scholar]
- [28].Gualandris A, Arese M, Shen B, Rifkin DB. Modulation of cell growth and transformation by doxycycline-regulated FGF-2 expression in NIH-3T3 cells. J Cell Physiol. 1999;181(2):273–84. doi: 10.1002/(SICI)1097-4652(199911)181:2<273::AID-JCP9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- [29].Bao WGY, Tang X, Wu W, Bolli R. Variability in susceptibility to ischemia among different strains of mice. J Mol Cell Cardiol. 2000;32(5):A21. [Google Scholar]
- [30].Delrieu I. The high molecular weight isoforms of basic fibroblast growth factor (FGF-2): an insight into an intracrine mechanism. FEBS Lett. 2000;468(1):6–10. doi: 10.1016/s0014-5793(00)01189-3. [DOI] [PubMed] [Google Scholar]
- [31].Pintucci G, Yu PJ, Saponara F, Kadian-Dodov DL, Galloway AC, Mignatti P. PDGF-BB induces vascular smooth muscle cell expression of high molecular weight FGF-2, which accumulates in the nucleus. J Cell Biochem. 2005;95(6):1292–300. doi: 10.1002/jcb.20505. [DOI] [PubMed] [Google Scholar]
- [32].Quarto N, Fong KD, Longaker MT. Gene profiling of cells expressing different FGF-2 forms. Gene. 2005;356:49–68. doi: 10.1016/j.gene.2005.05.014. [DOI] [PubMed] [Google Scholar]
- [33].Jiang ZS, Jeyaraman M, Wen GB, Fandrich RR, Dixon IM, Cattini PA, et al. High- but not low-molecular weight FGF-2 causes cardiac hypertrophy in vivo; possible involvement of cardiotrophin-1. J Mol Cell Cardiol. 2007;42(1):222–33. doi: 10.1016/j.yjmcc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- [34].Jiang ZS, Wen GB, Tang ZH, Srisakuldee W, Fandrich RR, Kardami E. High molecular weight FGF-2 promotes postconditioning-like cardioprotection linked to activation of the protein kinase C isoforms Akt and p70 S6 kinase. Can J Physiol Pharmacol. 2009;87(10):798–804. doi: 10.1139/Y09-049. [DOI] [PubMed] [Google Scholar]
- [35].Kardami E, Jiang ZS, Jimenez SK, Hirst CJ, Sheikh F, Zahradka P, et al. Fibroblast growth factor 2 isoforms and cardiac hypertrophy. Cardiovasc Res. 2004;63(3):458–66. doi: 10.1016/j.cardiores.2004.04.024. [DOI] [PubMed] [Google Scholar]
- [36].Sheikh F, Sontag DP, Fandrich RR, Kardami E, Cattini PA. Overexpression of FGF-2 increases cardiac myocyte viability after injury in isolated mouse hearts. Am J Physiol Heart Circ Physiol. 2001;280(3):H1039–50. doi: 10.1152/ajpheart.2001.280.3.H1039. [DOI] [PubMed] [Google Scholar]
- [37].Thomas-Mudge RJ, Okada-Ban M, Vandenbroucke F, Vincent-Salomon A, Girault JM, Thiery JP, et al. Nuclear FGF-2 facilitates cell survival in vitro and during establishment of metastases. Oncogene. 2004;23(27):4771–9. doi: 10.1038/sj.onc.1207638. [DOI] [PubMed] [Google Scholar]
- [38].Cohen MV, Yang XM, Downey JM. Smaller infarct after preconditioning does not predict extent of early functional improvement of reperfused heart. Am J Physiol. 1999;277(5 Pt 2):H1754–61. doi: 10.1152/ajpheart.1999.277.5.H1754. [DOI] [PubMed] [Google Scholar]
- [39].Gaubert F, Escaffit F, Bertrand C, Korc M, Pradayrol L, Clemente F, et al. Expression of the high molecular weight fibroblast growth factor-2 isoform of 210 amino acids is associated with modulation of protein kinases C delta and epsilon and ERK activation. J Biol Chem. 2001;276(2):1545–54. doi: 10.1074/jbc.M001184200. [DOI] [PubMed] [Google Scholar]
- [40].Hortala M, Estival A, Pradayrol L, Susini C, Clemente F. Identification of c-Jun as a critical mediator for the intracrine 24 kDa FGF-2 isoform-induced cell proliferation. Int J Cancer. 2005;114(6):863–9. doi: 10.1002/ijc.20744. [DOI] [PubMed] [Google Scholar]
- [41].Bouche G, Baldin V, Belenguer P, Prats H, Amalric F. Activation of rDNA transcription by FGF-2: key role of protein kinase CKII. Cell Mol Biol Res. 1994;40(5–6):547–54. [PubMed] [Google Scholar]
- [42].Florkiewicz RZ, Baird A, Gonzalez AM. Multiple forms of bFGF: differential nuclear and cell surface localization. Growth Factors. 1991;4(4):265–75. doi: 10.3109/08977199109043912. [DOI] [PubMed] [Google Scholar]
- [43].Bugler B, Amalric F, Prats H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol Cell Biol. 1991;11(1):573–7. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Piotrowicz RS, Martin JL, Dillman WH, Levin EG. The 27-kDa heat shock protein facilitates basic fibroblast growth factor release from endothelial cells. J Biol Chem. 1997;272(11):7042–7. doi: 10.1074/jbc.272.11.7042. [DOI] [PubMed] [Google Scholar]
- [45].Yu PJ, Ferrari G, Galloway AC, Mignatti P, Pintucci G. Basic fibroblast growth factor (FGF-2): the high molecular weight forms come of age. J Cell Biochem. 2007;100(5):1100–8. doi: 10.1002/jcb.21116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.