Abstract
Introduction:
Numerous studies from high-income countries document the causal relationship between cigarette smoking during pregnancy and adverse maternal and child health (MCH) outcomes. Less research has been conducted in low and middle income countries, but a burgeoning literature can be found for Brazil.
Methods:
We review Brazilian studies of the prevalence of maternal smoking, the relative risk of smoking-attributable adverse MCH outcomes, and present new estimates for these outcomes, using the attributable fraction method.
Results:
We found that Brazilian studies of the relative risks of smoking-attributable adverse MCH outcomes were broadly consistent with previous reviews. Based on a comparison of maternal smoking over time, smoking during pregnancy has declined by about 50% over the last 20 years in Brazil. For 2008, we estimate that 5,352 cases of spontaneous abortion, 10,929 cases of preterm birth, 20,717 cases of low birth weight, and 29 cases of sudden infant death syndrome are attributable to maternal smoking. Between 1989 and 2008, the percent of smoking-attributable adverse MCH outcomes in Brazil was at least halved.
Conclusions:
The results show that over a 20-year period, during which Brazil implemented numerous effective tobacco control measures, the country experienced a dramatic decrease in both maternal smoking prevalence and smoking-attributable adverse MCH outcomes. Countries that implement effective tobacco control measures can expect to reduce both maternal smoking and adverse MCH outcomes, thereby improving the public health.
INTRODUCTION
Numerous studies from high-income countries document the causal relationship between cigarette smoking during pregnancy and adverse maternal and child health (MCH) outcomes, such as low birth weight (LBW), preterm birth, sudden infant death syndrome (SIDS), placenta previa, placental abruption, and spontaneous abortion (Cnattingius, 2004; Hackshaw, Rodeck, & Boniface, 2011; Tong et al., 2011; USDHHS, 2004, 2006). In addition, maternal cigarette smoking is likely to expose infants and children to secondhand smoke (USDHHS, 2006), which has been causally linked to an increased risk of SIDS, acute respiratory infections, middle ear disease, and more severe asthma.
Less research has been conducted in low and middle income countries (LMICs), where there is an increasing prevalence of smoking among women, limited access to maternity care, and substantially worse MCH outcomes. The United Nation’s Millennium Development Goals have focused on improving MCH as an important step for economic development and poverty reduction (United Nations, 2000). Therefore, there is an urgent need for more research assessing the effect of maternal smoking on MCH outcomes and how various tobacco control policies can influence these outcomes in LMICs.
With many country-specific studies available on maternal smoking and related MCH outcomes, and a relatively comprehensive surveillance network for health outcomes, Brazil is well suited for estimating the potential effects of reductions in smoking prevalence on smoking-attributable adverse MCH outcomes. Additionally, Brazil has successfully reduced its previously high rates of smoking. Between 1989 and 2008, overall adult smoking prevalence (aged 18 and above) dropped from 34.8% to 17.2%, and female smoking prevalence dropped from 27.0% to 13.1% (Almeida et al., 2012; Monteiro, Cavalcante, Moura, Claro, & Szwarcwald, 2007; Szklo et al., 2012). During the period from 1989 to 2008, Brazil implemented a set of strong tobacco control policies, including higher cigarettes taxes, antismoking media campaigns, cessation treatment policies, and strong advertising restrictions and health warnings on cigarette packages (Almeida et al., 2012; Monteiro et al., 2007; Szklo et al., 2012). These policies played a major role in the steep decline in smoking prevalence, accounting for a 46% relative reduction (Levy, de Almeida, & Szklo, 2012).
The purpose of this article is to develop estimates of smoking- attributable adverse MCH outcomes for 1989 and 2008—before and after implementation of many major tobacco control policies in Brazil. We first review existing studies on the prevalence of maternal smoking, the association of active smoking with adverse MCH outcomes, and the prevalence of adverse MCH outcomes for Brazil. With this information, we calculate the number of smoking-attributable adverse MCH outcomes for 2 years: 1989 and 2008.
METHODS
Smoking-Attributable MCH Outcomes Calculation
The number of smoking-attributable adverse MCH outcomes is the product of total number of cases for each adverse MCH outcome and the smoking attributable fraction (SAF) for that outcome. The total number of cases is the product of the prevalence of each MCH outcome and the total number of births. The other key component for the number of smoking-attributable adverse MCH outcomes, SAF, is defined as:
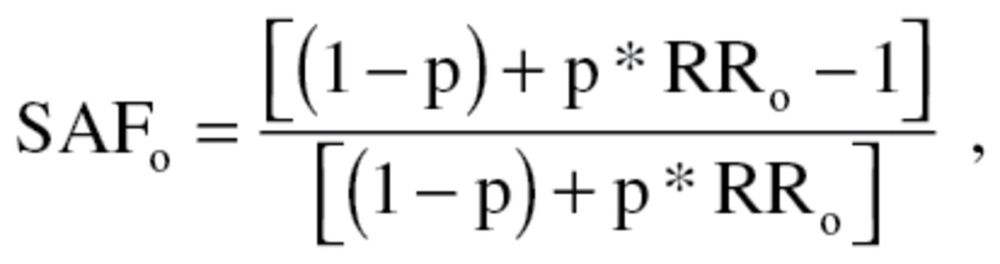 |
where p = maternal smoking prevalence and RRo = relative risk of MCH outcome for maternal smokers relative to maternal nonsmokers (Levin, 1953; Lilienfeld & Lilienfeld, 1980).
For each component of the model, we sought information from existing Brazilian studies and data. We conducted a literature search using PubMed with the keywords “Brazil & maternal & smoking” and “Brazil & pregnant & smoking.” We also added specific adverse MCH outcomes, such as LBW, preterm birth, and SIDS. We consulted the bibliography of each article to find any additional articles for Brazil that explicitly considered maternal smoking.
Maternal Smoking Prevalence
We used two nationally representative surveys to directly calculate maternal smoking prevalence for 2008: the Pesquisa de Orçamentos Familiares (POF) (Instituto Brasileiro de Geografia e Estatistica) and the Global Adult Tobacco Survey (GATS) (Almeida et al., 2012; Instituto Nacional de Cancer (Brasil), 2010). Both surveys were conducted in 2008 by the Instituto Brasileiro de Geografia e Estatistica using similar sampling strategies and data collection methods. Weighted comparisons of the sociodemographic characteristics from the two surveys are almost identical. POF provides information on pregnancy status but uses a proxy measure of maternal smoking prevalence by asking “have you spent some money on tobacco products for yourself in the last 7 days?” This may underestimate smoking prevalence because smokers may not have purchased cigarettes themselves in the previous 7 days. The GATS, on the other hand, provides reliable estimates of smoking prevalence but does not determine pregnancy status. Assuming that the relative difference between pregnant and nonpregnant women by age group observed by POF could be applied to the GATS prevalence, we multiplied the relative difference by the female smoking prevalence for each age group.
Due to the lack of a nationally representative estimate for maternal smoking in 1989, we used a different approach. First, we estimated overall female smoking rate using the 1989 National Survey of Health and Nutrition (Monteiro et al., 2007). We assumed that a fixed percentage of female smokers quit during pregnancy (40%) and applied that percent to the smoking prevalence. Detail about how we obtained the percentage is provided in the Results section. To consider the consistency of this estimate, we also examined Brazilian studies of trends in maternal smoking prevalence over time.
A review found that the extent of underreporting of smoking by pregnant women was between 5% and 20% (Gorber, Schofield-Hurwitz, Hardt, Levasseur, & Tremblay, 2009). Based on a study in Spain (Aranda Regules, Mateos Vilchez, González Villalba, Sanchez, & Luna del Castillo, 2008), we corrected both prevalence estimates upward by 15% to account for underreporting of smoking status.
Relative Risks of Adverse MCH Outcomes
We examined the relative risk (RR) from existing Brazilian studies for four adverse MCH outcomes for which the literature provides strong support for a causal association with maternal smoking (USDHHS, 2010): preterm birth, LBW, spontaneous abortion, and SIDS. Because the number of studies for Brazil was limited, we compared the results to reviews based on studies conducted mostly in the United States and other high-income countries.
Prevalence of Adverse MCH Outcomes in Brazil
Births were obtained from the Instituto Brasileiro de Geografia e Estatística (Instituto Brasileiro de Geografia e Estatistica). SIDS deaths, stratified by maternal age, were obtained from the System on Mortality Information of the Ministry of Health, created in 1975/76. Spontaneous abortions were obtained from the Hospital-Based Information System of the Ministry of Health created in 1990/91.
The number of LBW and preterm births was obtained from the Information System on Live Births (SINASC) (Jorge, Gotlieb, Soboll, Almeida, & Latorre, 1993; Jorge, Laurenti, & Gotlieb, 2007), which was implemented in 1990 to collect information from birth certificates (for hospital births) and registries (for home births). In 2008, home births represented 1.1% of all births. SINASC provides vital information on live births for epidemiological, statistical, and demographic analyses, with information on prenatal care, birthplace, method of delivery, and gender and birth weight of the newborn. The prevalence of LBW was defined as (birth weight < 2500g)/live births, and the prevalence of preterm birth was defined as (gestational age < or equal 36 weeks)/live births. Because many LBW infants are also of early gestational age, we provide estimates of LBW that subtract out those that are defined as preterm births.
In the past, gestational age was not widely and accurately reported in SINASC, resulting in an underestimate of the prevalence of preterm births due to classification errors (Silva, Ribeiro, Borba Júnior, Coimbra, & Silva, 2001). In recent years, the quality of SINASC data has improved, with only 0.4% unreported gestational age, compared with 2.5% in 1994 (Silveira, Santos, Matijasevich, Malta, & Duarte, 2009).
RESULTS
Maternal Smoking Prevalence in Brazil
For 2008, we obtained a national smoking prevalence of 7.7% (95% confidence interval [CI]: 5.2%–11.0%) for Brazilian pregnant women aged 15–49 . By age, the prevalence of smoking among pregnant women was 7.2% (3.6%, 14.4%) for women aged 15–19, 7.5% (4.2%, 13.0%) for women aged 20–29, 5.2% (5.4%, 19.8%) for women aged 30–39, and 5.2% (0.5%, 38.0%) for women aged 40–49.
The 2008 GATS survey indicates that 12.7% of women smoked (Instituto Nacional de Cancer [Brasil], 2010). Combined with our nationally representative estimate of maternal smoking prevalence (7.7%), this suggests that 40% (1 − [7.7/12.7]) of pregnant smokers quit smoking during the course of their pregnancy. Halal, Victora, and Barros (1993) found that 36% of pregnant smokers in Brazil quit during pregnancy. A study reported that, in 2003–4, 21.1% of pregnant women at the beginning of the first trimester smoked, of which 36% stopped by the end of the first trimester (Reis, da Silva, Trindade, Abrahão, & da Silva, 2008). Therefore, we conclude that the quit rate during pregnancy has remained stable over time. Based on the National Survey of Health and Nutrition (Monteiro et al., 2007), the overall female smoking rate was 26.9% in 1989. Assuming that 40% of female smokers quit during pregnancy, we estimated that maternal smoking prevalence was 16.1% in 1989.
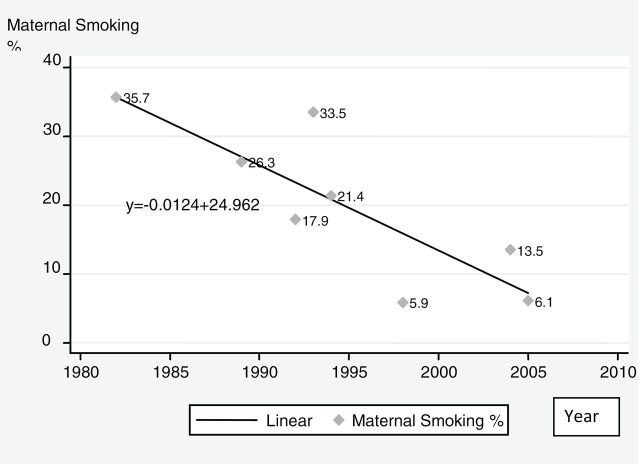
Between 1989 and 2003, the prevalence of smoking among females aged 18–44 dropped from 26.9% to 19.0% (Monteiro et al., 2007), about 0.56 absolute percentage points per year. Meanwhile, maternal smoking prevalence also fell, albeit with substantial variation by geographic location and socioeconomic status (SES). For example, studies conducted in Pelotas, a mid-size, relatively affluent southern Brazilian city, report that 35.7% of pregnant women smoked in 1982 and 33.5% in 1993, which further decreased to 25.1% in 2004 (Santos et al., 2008). However, the decline between 1982 and 2004 was far greater for high (24.9%–8.7%) than for low-income mothers (43.7%–33.6%). Additionally, a study of 5,539 pregnant women from six Brazilian cities between 1991 and 1995 found an overall maternal smoking rate of 17.9%, but prevalence ranged from 7.2% (Manaus) to 31.9% (Porto Alegre) (Kroeff et al., 2004). These variations were well correlated with the overall female smoking rates in those cities (Moura et al., 2008). Figure 1 shows the trend in maternal smoking prevalence from selected studies over the past three decades. The slope of the trend line indicates that maternal smoking fell at a rate of 1% per year. Our estimate for 2008 falls on the trend line as does an estimate of maternal smoking prevalence from a 2003 survey of 16 state capital cities with 11,419 reproductive age women aged 15–49 (Instituto Nacional de Câncer, 2004).
Figure 1.
Prevalence of maternal smoking: estimates from selected studies.
In summary, estimates from different sources indicate that, in Brazil, maternal smoking prevalence has fallen at the same or greater rate than overall female smoking prevalence.
Relative Risk of Adverse MCH Outcomes
Preterm Births
One study of preterm birth in Brazil for a 2004 birth cohort was located in a prosperous southern Brazilian state (Silveira et al., 2010) and the other a 2009 case-control study in northeastern Brazil (Silva, de Almeida, Matsuo, & Soares, 2009). They reported unadjusted odds ratios (ORs) of 1.31 and 1.61, respectively; however, after adjusting for other factors, the remaining effect was marginally significant (adjusted OR [AOR] = 1.19; p = .08) and not significant (AOR = 1.25; p = .31). A review by Cnattingius (2004) reported RRs for preterm birth among pregnant smokers between 1.2 and 1.6. Thus, Brazilian estimates are at the lower end of those reported in high-income countries, when adjusted for other factors.
Low Birth Weight
Using a historical cohort of 5,166 live births occurring in the city of Pelotas in 1993, researchers found that the AOR for LBW among children of smokers was 1.59 (95% CI 1.30–1.95), and smoking was associated with an AOR of 2.07 (95% CI 1.69–2.53) for growth retardation but was not associated with preterm delivery (Horta, Victora, Menezes, Halpern, & Barros, 1997). On the other hand, using the two population-based cohorts of singleton live births in southeast Brazil in 1978–79 and 1994, one study found that after adjusting for the yearly effect of preterm birth, the risk for LBW was smaller and no longer statistically significant (Barbieri, Silva, Bettiol, & Gomes, 2000). Using the same 1978–79 and 1994 cohorts, two other studies reported significant AORs, ranging from 1.31 to 1.80 for the 1978–79 cohort and from 1.39 to 1.98 for the 1994 cohort (Goldani, Barbieri, Silva, & Bettiol, 2004; Silva, Barbieri, Gomes, & Bettiol, 1998). Finally, a large-scale, case-control study of LBW infants delivered between 1986 and 2004 in Campinas obtained an AOR of 1.51 for smoking beyond the fourth month of pregnancy (Coutinho, Cecatti, Surita, Souza, & Morais, 2009).
A review of studies of LBW and maternal smoking in mostly high-income countries by DiFranza and Lew (1995) reported a pooled RR of 1.82, whereas the U.S. Surgeon General (USDHHS, 2004) estimated RR’s ranging from 1.5 to 2.5, similar to Cnattingius (2004). Therefore, estimates from Brazil are generally at the middle to lower end of estimates from high-income countries. However, because of difficulties in determining gestational age in many of the early Brazilian studies, few were able to accurately exclude preterm births, potentially masking the effects of smoking.
Spontaneous Abortion
One Brazilian study found no effect of smoking on spontaneous abortions in the current pregnancy, possibly due to the small sample, but found that active smoking was associated with the number of past spontaneous abortions (Nakamura et al., 2004). Among reviews, DiFranza and Lew (1995) reported a pooled RR of 1.2 for cohort studies and of 1.3 for case-control studies; Cnattingius (2004) estimated RR’s between 1.0 and 1.8; and the U.S. Surgeon General (USDHHS, 2004) obtained a pooled RR of 1.2 for cohort studies and an overall OR of 1.3.
Sudden Infant Death Syndrome
One Brazilian study obtained an AOR of 3.0 for SIDS among women in Porto Alegre City who smoked more than 10 cigarettes per day during pregnancy (Pinho, Aerts, & Nunes, 2008). These results are consistent with several reviews: a pooled OR of 2.98 (DiFranza & Lew, 1995), ORs ranging from 2.0 to 3.0 (Cnattingius, 2004), and ORs ranging from 1.4 to 3.0 (USDHHS, 2004).
Summary
In general, Brazilian studies of the relationship between maternal smoking and adverse MCH outcomes find RRs consistent with those for high-income countries, albeit mostly at the lower end. For the adjusted estimates, both the inverse relationship between SES and smoking prevalence and the high correlation between low SES status and adverse MCH outcomes may explain the inability to distinguish smoking effects in multivariate analyses. The lower RR estimates may also reflect different smoking patterns because studies find a dose-response relationship between amount smoked and MCH outcomes (Dietz et al., 2010; USDHHS, 2004). As shown in Table 1, we used RRs based on reviews from high-income countries for our analysis; Brazilian estimates of RRs were broadly consistent with these findings, and they allow us to obtain more reliable estimates of adverse MCH outcomes.
Table 1.
Relative Risks for Adverse Maternal and Child Health Outcomes Among Maternal Smokers in Brazil
| Relative risks | Best estimate | Lower bound | Upper bound |
|---|---|---|---|
| Preterm births | 1.4 | 1.2 | 1.6 |
| Low birth weight infants | 2 | 1.5 | 2.5 |
| Spontaneous abortion | 1.25 | 1.1 | 1.4 |
| Sudden infant death syndrome | 2.2 | 1.4 | 3.0 |
Source: Cnattingius (2004); USDHHS (2004).
Prevalence of Adverse MCH Outcomes in Brazil
Table 2 provides the prevalence of adverse MCH outcomes from 2000 to 2008, as estimated by SINASC. Between 2000 and 2008, rates of preterm birth have remained fairly constant (6.3%–6.9%). However, data from sources other than SINASC indicate much higher rates. For example, Silveira and coworkers (2008) reviewed 12 population-based studies of preterm birth in Brazil and extrapolated rates of about 13% in 2005. As noted previously, the large discrepancy may be because SINASC used less standardized measures for the gestation period and contains more unknown values than the reviewed studies. With improved reporting in more recent years, the increases of preterm birth may be explained mostly by higher rates of induced labor and caesarean births. On the other hand, the U.S. preterm birth rate has increased more than 20% since 1990, to 12.8% in 2006, with a small decrease to 12.3% in 2008 (Martin, Osterman, & Sutton, 2010). Thus, current rates of preterm birth from population-based studies in Brazil are close to those of the United States and are consistent with an upward trend, albeit at a higher rate.
Table 2.
Prevalence of Adverse Maternal and Child Health Outcomes in Brazil, 2000–2008
| Maternal and child health outcome/year | 2000 | 2002 | 2004 | 2005 | 2006 | 2007 | 2008 |
|---|---|---|---|---|---|---|---|
| Preterm births (%) | 6.8 | 6.3 | 6.5 | 6.6 | 6.7 | 6.6 | 6.9 |
| Low birth weight babies (%) | 7.7 | 8.1 | 8.2 | 8.1 | 8.2 | 8.2 | 8.2 |
| Low birth weight infants excluding preterm births (%) | 4.0 | 4.2 | 4.1 | 3.9 | 4.1 | 4.1 | 4.0 |
| Spontaneous abortions (%) | 3.1 | 3.6 | 4.2 | 4.2 | 4.0 | 4.0 | 3.9 |
| Sudden Infant Death Syndrome (rate per 100,000 live births) | 10.2 | 8.1 | 5.7 | 5.8 | 5 | 5.8 | 4.8 |
Data Source: SINASC.
After increasing from 2000 to 2002, rates of LBW in Brazil, as estimated by SINASC, have remained stable. Trends from other studies indicate a doubling of LBW since 1990, with two of the more rigorous studies having found more than a doubling in the southeast from 1978 to 2004 and in the northeast from 1978–9 to 1994 (Barros et al., 2005; Silva et al., 1998). Other studies of LBW report similar findings. One study found a 10% increase between 1994 and 2004 in Rio Grande do Sul State (Moraes, Zanini, Giugliani, & Riboldi, 2011). In a study of 15,142 hospital live births in five cities in 1992, as part of SINASC, researchers found that the percentage of LBW was 8.5% (Jorge et al., 1993), similar to a 2005 estimate of 8.6% in communities with ≥ 50,000 population (Andrade, Szwarcwald, & Castilho, 2008). Also using SINASC data, with corrections for underreporting, researchers did not detect a change between 1995 and 2007 (Silva et al., 2010).
Preterm births accounted for 48% of LBW cases in 1978–79 and 55% of LBW cases in 1994 (Silva et al., 1998). Using data from SINASC, we found that preterm birth accounted for about 50% of LBW cases in each of the years 2000–2008. Table 2 indicates that, after excluding preterm births, the prevalence of LBW was about 4.0.
The percentage of spontaneous abortion has increased between 2000 and 2008. These figures are much lower than a 13.3% reported lifetime occurrence of spontaneous abortion estimate reported in Brazil (Camargo et al., 2011) and the estimated 20% in the United States (Eskenazi et al., 1995; Savitz, Hertz-Picciotto, Poole, & Olshan, 2002).
The rate of SIDS in Brazil has declined to 4.8 per 100,000 live births in 2008. This rate is much lower than that found in the United States (52.9 per 100,000 live births) (Pinho & Nunes, 2011). Underdiagnosis may help explain this discrepancy because an earlier study found comparable rates of SIDS in Brazil and the United States, after adjusting for underdiagnosis (Nunes et al., 2001). On the other hand, a recent study using death certificates found much lower rates of SIDS in Brazil compared with the United States, suggesting that underdiagnosis may still contribute to Brazil’s lower rates (Woida, Saggioro, Ferro, & Peres, 2008).
Smoking-Attributable Adverse MCH Outcomes
Table 3 presents the estimates for SAFs and the number of smoking-attributable adverse MCH outcomes for 1989 and 2008, as well as upper and lower bounds for the 2008 estimates. The upper and lower bound estimates for RRs are shown in Table 1 and for maternal smoking prevalence are from the confidence intervals of the estimates. In addition, the lower bound estimate is not adjusted upward for underreporting of smoking. To account for underreporting of MCH outcomes, we adjusted the preterm birth estimates by 50% for the best estimate and by 300% for upper bound; the spontaneous abortion and SIDS estimates by 100% for mid range and by 300% for upper bound. Spontaneous abortions were also increased by 2% to reflect the additional stillbirths based on data from SINASC.
Table 3.
Smoking-Attributable Adverse Maternal and Child Health (MCH) Outcomes in Brazil, All Ages, 1989 and 2008
| Year and measure/MCH outcome | Live births | Preterm birth | Low birth weight | Low birth weight (excluding preterm births) | Spontaneous abortion | Sudden Infant Death Syndrome | |
|---|---|---|---|---|---|---|---|
| 2008 | Smoking-attributable fraction | 3.4% | 8.1% | 2.2% | 8.1% | 9.6% | |
| Best estimate | 3,105,800 | 10,929 | 20,717 | 5,352 | 10,106 | 29 | |
| Lower bound | 2,423 | 7,394 | 734 | 7,394 | 3 | ||
| Upper bound | 57,843 | 40,618 | 23,802 | 19,814 | 120 | ||
| 1989 | Smoking-attributable fraction | 6.9% | 15.6% | 4.4% | 15.6% | 18.2% | |
| Best estimate | 3,679,935 | 25,882 | 44,267 | 9,963 | 22,996 | 138 | |
The best estimate for total number of preterm birth cases attributable to smoking is obtained by multiplying total births in 2008 (3,105,800) by the prevalence of preterm birth from Table 2 (6.9%) and by the SAF (3.4%), adjusted upward by 50% for the best estimate, which results in 10,929 preterm births attributable to smoking for that year. Because we do not have the upper and lower bound for maternal smoking prevalence for 1989, Table 3 presents only point estimates. For the 1989 estimates, we use the estimates of MCH prevalence from the year 2000. The SAFs for each outcome fell by at least 50% between 1989 and 2008 due to reduced maternal smoking prevalence. As a consequence, the number of smoking-attributable spontaneous abortions fell from 9,963 to 5,352, the number of preterm births fell from 25,882 to 10,518, the number of LBW cases fell from 44,267 to 20,717, the number of LBW cases (minus the overlap with preterm births) fell from 22.966 to 10,106, and the number of SIDS deaths fell from 138 to 29.
DISCUSSION
Our data show that smoking during pregnancy has declined substantially in Brazil over the last 20 years, during which time the country introduced many strong tobacco control policies. The high quality of data and the large number of studies allowed us to estimate the number of smoking-attributable adverse MCH outcomes for Brazil, now an upper middle income Latin American country. The methods employed here can be applied to other countries if data on maternal smoking prevalence and adverse MCH outcomes are available.
We found that Brazilian studies of the RR for adverse MCH outcomes among maternal smokers were broadly consistent with those from the high-income countries. In some cases, Brazilian studies had small sample sizes, were not nationally representative, or failed to report smoking during pregnancy, differences in maternal smoking behaviors or the gestation period studied. Nevertheless, the lower RRs observed in Brazilian studies may reflect different measures of smoking or different maternal smoking behaviors. In particular, Brazilian women may smoke fewer cigarettes and be less nicotine dependent than women in high-income countries. The difference in RR may also reflect differences in other risk factors for adverse MCH outcomes, including SES, parity, the presence or absence of chronic disease in the mother, maternal nutrition status, and the use of alcohol. These and other factors will need to be considered in cross-country analysis.
Although it is difficult to distinguish the role of maternal smoking on trends in adverse MCH outcomes, the recent leveling off or decrease of all major adverse MCH outcomes in Brazil is likely related to reduced maternal smoking. Because the extent of underreporting of adverse MCH outcomes has been reduced over time, improvements due to reduced smoking may have been less apparent. Although we corrected for underreporting and apparent trends, a limitation of our analysis is that we used data for 2000 to obtain estimates of adverse MCH outcomes for 1989, and our estimates of maternal smoking prevalence for 1989 were indirect.
We considered only those adverse MCH outcomes for which the literature demonstrates a strong and consistent causal link to maternal smoking, which have been studied in Brazil. However, strong causal associations with maternal smoking have also been identified for placenta previa, placental abruption, infertility, delayed conception, ectopic pregnancy, congenital malformations, and respiratory distress (Hackshaw et al., 2011; USDHHS, 2004), and the adverse effects of exposure to secondhand smoke on pregnant women, infants, and children are also well documented (Hackshaw et al., 2011; USDHHS, 2006). Furthermore, maternal cigarette smoking also provides role models for older children, increasing the risk that these children will themselves become tobacco users. For these reasons, our results may well underestimate the health burden of maternal smoking in Brazil.
Between 1989 and 2008, Brazil enacted numerous strong tobacco control policy measures, including higher cigarettes taxes, antismoking media campaigns, cessation treatment policies, and strong advertising restrictions and health warnings on cigarette packages. Brazil’s experience can be compared with that of other Latin American nations, such as Uruguay, which has recently enacted strong tobacco control policies, and Argentina, which still lacks strong tobacco control policies. Uruguay and Argentina had an overall smoking prevalence for women aged 18–44 of more than 30% (WHO, 2011); similarly, the maternal smoking prevalence in both countries is much higher than in Brazil, with rates of 18.0% in Uruguay and 10.3% in Argentina for select cities (Bloch et al., 2008).
Despite being the seventh largest economy in the world, disparities in health and SES remain a major challenge for Brazil. Approximately 14.2 million people (10% of Brazilians) are illiterate, of whom 4 million are smokers; about 12 million people live on less than US$2.0 per day, of whom 3 million are smokers. Moreover, according to the National Household Survey (IBGE 2011), women with the lowest level of education (less than 1 year of education) and those with the lowest purchasing power (less than ¼ minimum wage a month) had a higher number of pregnancies than other women. Although the studies we reviewed indicated higher smoking rates among pregnant women of low SES, further research is needed on how maternal smoking prevalence varies by SES and other demographic factors.
National tobacco control measures are not necessarily followed by “the same opportunity of individual access to health services for the same needs” (Almeida et al., 2001). Similar to other LMICs, Brazil faces the challenge of ensuring that all populations, especially low SES populations who typically smoke at higher rates, benefit from its strong national tobacco control policies (Szklo et al., 2012). In spite of the availability of smoking cessation services, the country’s higher mean nominal income, and considerable reduction in poverty since the 1980s, the delivery of and access to health care in Brazil still needs to be improved, as shown by the United Nations’ Human Development Index. Almeida and colleagues (2012) have called attention to the need for health professionals employed by the Brazilian Health System to document the smoking status of their patients during routine visits and to offer evidence-based support for smoking cessation. Our study also highlights the importance of establishing a national system for the epidemiological surveillance of tobacco consumption and related social, economic, and health indicators, as stated in article 20 of the FCTC, in order to better understand the evolution of the tobacco epidemic, including its impact on adverse MCH outcomes.
The adverse impact of smoking during pregnancy on birth outcomes has been well documented for high-income countries, including the United States, where, despite recent declines, maternal smoking continues to cause a substantial number of infant deaths (Dietz et al., 2010). In contrast, few studies have assessed the impact of maternal smoking in LMICs or the potential for tobacco control measures to improve MCH. The experience of Brazil strongly suggests that effective tobacco control policies will reduce maternal smoking and smoking-attributable adverse MCH outcomes. These important benefits are often overlooked and merit greater consideration in decisions regarding tobacco control policy.
FUNDING
The funding was in the form of a Scientific and Technical Support Contract (#HHSN261201000043C) from the Tobacco Control Research Branch of the National Cancer Institute.
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
We would like to thank Martha Waller for her work on earlier drafts.
REFERENCES
- Almeida C., Braveman P., Gold M. R., Szwarcwald C. L., Ribeiro J. M., Miglionico A, … Viacava F. (2001). Methodological concerns and recommendations on policy consequences of the World Health Report 2000. Lancet, 357, 1692–1697.10.1016/S0140-6736(00)04825-X [DOI] [PubMed] [Google Scholar]
- Almeida L., Szklo A., Sampaio M., Souza M., Martins L. F., Szklo M, … Caixeta R. (2012). Global Adult Tobacco Survey data as a tool to monitor the WHO Framework Convention on Tobacco Control (WHO FCTC) implementation: The Brazilian case. International Journal of Environmental Research and Public Health, 9, 2520–2536.10.3390/ijerph9072520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C. L., Szwarcwald C. L., Castilho E. A. (2008). Low birth weight in Brazil according to live birth data from the Ministry of Health, 2005. Cadernos de Saúde Pública, 24, 2564–2572 [DOI] [PubMed] [Google Scholar]
- Aranda Regules J. M., Mateos Vilchez P., González Villalba A., Sanchez F., Luna del Castillo Jde. D. (2008). Validity of smoking measurements during pregnancy: Specificity, sensitivity and cut-off points. Revista Española de Salud Pública, 82, 535–545 [DOI] [PubMed] [Google Scholar]
- Barbieri M. A., Silva A. A., Bettiol H., Gomes U. A. (2000). Risk factors for the increasing trend in low birth weight among live births born by vaginal delivery, Brazil. Revista de Saúde Pública, 34, 596–602 [DOI] [PubMed] [Google Scholar]
- Barros F. C., Victora C. G., Barros A. J. D., Santos I. S., Albernaz E., Matijasevich A, … Vaughan J. P. (2005). The challenge of reducing neonatal mortality in middle- income countries: Findings from three Brazilian birth cohorts in 1982, 1993, and 2004. Lancet, 365, 847–854.10.1016/S0140-6736(05)71042–4 [DOI] [PubMed] [Google Scholar]
- Bloch M., Althabe F., Onyamboko M., Kaseba-Sata C., Castilla E. E., Freire S, … Goldenberg R. (2008). Tobacco use and secondhand smoke exposure during pregnancy: An investigative survey of women in 9 developing nations. American Journal of Public Health, 98, 1833–1840.10.2105/AJPH.2007.117887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo R. S., Santana D. S., Cecatti J. G., Pacagnella R. C., Tedesco R. P., Melo E. F., Jr., Sousa M. H. (2011). Severe maternal morbidity and factors associated with the occurrence of abortion in Brazil. International Journal of Gynaecology and Obstetrics, 112, 88–92.10.1016/j.ijgo.2010.08.013 [DOI] [PubMed] [Google Scholar]
- Cnattingius S. (2004). The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research, 6(Suppl. 2)S125–S140.10.1080/14622200410001669187 [DOI] [PubMed] [Google Scholar]
- Coutinho P. R., Cecatti J. G., Surita F. G., Souza J. P., Morais S. S. (2009). Factors associated with low birth weight in a historical series of deliveries in Campinas, Brazil. Revista da Associação Médica Brasileira (1992), 55, 692–699 [DOI] [PubMed] [Google Scholar]
- Dietz P. M., England L. J., Shapiro-Mendoza C. K., Tong V. T., Farr S. L., Callaghan W. M. (2010). Infant morbidity and mortality attributable to prenatal smoking in the U.S. American Journal of Preventive Medicine, 39, 45–52.10.1016/j.amepre.2010.03.009 [DOI] [PubMed] [Google Scholar]
- DiFranza J. R., Lew R. A. (1995). Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. The Journal of Family Practice, 40, 385–394 [PubMed] [Google Scholar]
- Eskenazi B., Gold E. B., Lasley B. L., Samuels S. J., Hammond S. K., Wight S, … Schenker M. B. (1995). Prospective monitoring of early fetal loss and clinical spontaneous abortion among female semiconductor workers. American Journal of Industrial Medicine, 28, 833–846 [DOI] [PubMed] [Google Scholar]
- Goldani M. Z., Barbieri M. A., Silva A. A., Bettiol H. (2004). Trends in prenatal care use and low birthweight in southeast Brazil. American Journal of Public Health, 94, 1366–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorber S. C., Schofield-Hurwitz S., Hardt J., Levasseur G., Tremblay M. (2009). The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine & Tobacco Research, 11, 12–24.10.1093/ntr/ntn010 [DOI] [PubMed] [Google Scholar]
- Hackshaw A., Rodeck C., Boniface S. (2011). Maternal smoking in pregnancy and birth defects: A systematic review based on 173 687 malformed cases and 11.7 million controls. Human Reproduction Update, 17, 589–604.10.1093/humupd/dmr022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halal I. S., Victora C. G., Barros F. C. (1993). Determining factors of smoking habit and its cessation during pregnancy in a urban locality in the southern region of Brazil. Revista de Saúde Pública, 27, 105–112.10.1590/S0034-89101993000200005 [DOI] [PubMed] [Google Scholar]
- Horta B. L., Victora C. G., Menezes A. M., Halpern R., Barros F. C. (1997). Low birthweight, preterm births and intrauterine growth retardation in relation to maternal smoking. Paediatric and Perinatal Epidemiology, 11, 140–151 [DOI] [PubMed] [Google Scholar]
- Instituto Brasileiro de Geografia e Estatistica (2010). Consumer Expenditure Study, 2008–2009 Retrieved November 3, 2010, from www.ibge.gov.br/english/estatistica/populacao/condicaodevida/pof/2008_2009/default.shtm
- Instituto Nacional de Câncer (2004). Inquérito domiciliar sobre comportamentos de risco e morbidade referida de doenças e agravos não transmissíveis: Brasil, 15 capitais e Distrito Federal, 2002–2003. Rio de Janeiro, Brasil: Instituto Nacional de Câncer, Ministério da Saúde; [Google Scholar]
- Instituto Nacional de Cancer (Brasil) (2010). Global Adult Tobacco Survey Report. Rio de Janeiro, Brazil: INCA, Pan-American Health Organization; [Google Scholar]
- Jorge M. H., Gotlieb S. L., Soboll M. L., de Almeida M. F., Latorre Mdo. R. (1993). An information system on live births and the use of its data in epidemiology and health statistics. Revista de Saúde Pública, 27(Suppl)1–44 [PubMed] [Google Scholar]
- Jorge M. H., Laurenti R., Gotlieb S. L. (2007). Quality analysis of Brazilian vital statistics: The experience of implementing the SIM and SINASC systems. Ciência & Saúde Coletiva, 12, 643–654.S1413-81232007000300014 [DOI] [PubMed] [Google Scholar]
- Kroeff L. R., Mengue S. S., Schmidt M. I., Duncan B. B., Favaretto A. L., Nucci L. B. (2004). Correlates of smoking in pregnant women in six Brazilian cities. Revista de Saúde Pública, 38, 261–267.10.1590/S0034-89102004000200016 [DOI] [PubMed] [Google Scholar]
- Levin M. L. (1953). The occurrence of lung cancer in man. Acta-Unio Internationalis Contra Cancrum, 9, 531–541 [PubMed] [Google Scholar]
- Levy D., de Almeida L. M., Szklo A. (2012). The Brazil SimSmoke policy simulation model: The effect of strong tobacco control policies on smoking prevalence and smoking-attributable deaths in a middle income nation. PLoS Medicine, 9, e1001336.10.1371/journal.pmed.1001336PMEDICINE-D-12-00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld A. M., Lilienfeld D. E. (1980). Foundations of epidemiology. New York: Oxford University Press; [Google Scholar]
- Martin J. A., Osterman M. J. K., Sutton P. D. (2010). Are preterm births on the decline in the United States? Recent data from the National Vital Statistics System NCHS data brief. Hyattsville, MD: National Center for Health Statistics; [PubMed] [Google Scholar]
- Monteiro C. A., Cavalcante T. M., Moura E. C., Claro R. M., Szwarcwald C. L. (2007). Population-based evidence of a strong decline in the prevalence of smokers in Brazil (1989-2003). Bulletin of the World Health Organization, 85, 527–534.10.2471/BLT.06.039073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes A. B., Zanini R. R., Giugliani E. R., Riboldi J. (2011). Trends in the proportion of low birth weight from 1994 to 2004 in Rio Grande do Sul State, Brazil: A multilevel analysis. Cadernos de Saúde Pública, 27, 229–240.10.1590/S0102-311X2011000200004 [DOI] [PubMed] [Google Scholar]
- Moura E. C., Morais Neto O. L., Malta D. C., Moura L., Silva N. N., Bernal R. (2008). Vigilância de Fatores de Risco para Doenças Crônicas por Inquérito Telefônico nas capitais dos 26 estados brasileiros e no Distrito Federal, 2006. Revista Brasileira de Epidemiologia, 11(Suppl. 1)20–37.10.1590/S1415-790X2008000500003 [Google Scholar]
- Nakamura M. U., Alexandre S. M., Kuhn dos Santos J. F., de Souza E., Sass N., Auritscher Beck A. P, … Kulay Júnior L. (2004). Obstetric and perinatal effects of active and/or passive smoking during pregnancy. São Paulo Medical Journal = Revista Paulista de Medicina, 122, 94–98.10.1590/S1516-31802004000300004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M. L., Pinho A. P., Aerts D., Sant’Anna A., Martins M. P., Costa J. C. (2001). Sudden infant death syndrome: Clinical aspects of an underdiagnosed disease. Jornal de Pediatria, 77, 29–34 [PubMed] [Google Scholar]
- Pinho A. P., Aerts D., Nunes M. L. (2008). Risk factors for sudden infant death syndrome in a developing country. Revista de Saúde Pública, 42, 396–401.10.1590/S0034-89102008000300002 [DOI] [PubMed] [Google Scholar]
- Pinho A. P., Nunes M. L. (2011). Epidemiological profile and strategies for diagnosing SIDS in a developing country. Jornal de Pediatria, 87, 115–122.10.1590/S0021-75572011000200006 [DOI] [PubMed] [Google Scholar]
- Reis L. G., da Silva C. J., Trindade A., Abrahão M., da Silva V. A. (2008). Women who smoke and stop during pregnancy: Who are they? Revista Brasileira de Saúde Materno Infantil, 8, 217–221.10.1590/S1519-38292008000200009 [Google Scholar]
- Santos I. S., Barros A. J., Matijasevich A., Tomasi E., Medeiros R. S., Domingues M. R, … Victora C. G. (2008). Mothers and their pregnancies: A comparison of three population-based cohorts in Southern Brazil. Cadernos de Saúde Pública, 24(Suppl. 3)S381–S389 [DOI] [PubMed] [Google Scholar]
- Savitz D. A., Hertz-Picciotto I., Poole C., Olshan A. F. (2002). Epidemiologic measures of the course and outcome of pregnancy. Epidemiologic Reviews, 24, 91–101.10.1093/epirev/mxf006 [DOI] [PubMed] [Google Scholar]
- Silva A. A., Barbieri M. A., Gomes U. A., Bettiol H. (1998). Trends in low birth weight: A comparison of two birth cohorts separated by a 15-year interval in Ribeirão Preto, Brazil. Bulletin of the World Health Organization, 76, 73–84 [PMC free article] [PubMed] [Google Scholar]
- Silva A. A., Silva L. M., Barbieri M. A., Bettiol H., Carvalho L. M., Ribeiro V. S., Goldani M. Z. (2010). The epidemiologic paradox of low birth weight in Brazil. Revista de Saúde Pública, 44, 767–775.10.1590/S0034-89102010005000033 [DOI] [PubMed] [Google Scholar]
- Silva A. M., de Almeida M. F., Matsuo T., Soares D. A. (2009). Risk factors for pre-term birth in Londrina, Paraná State, Brazil. Cadernos de Saúde Pública, 25, 2125–2138 [DOI] [PubMed] [Google Scholar]
- Silva A. A., Ribeiro V. S., Borba A. F., Jr., Coimbra L. C., Silva R. A. (2001). Evaluation of data quality from the Information System on Live Births in 1997–1998. Revista de Saúde Pública, 35, 508–514 Retrieved from www.scielosp.org/scielo.php?script=sci_arttext&pid= S0034-89102001000600003&nrm=iso [DOI] [PubMed] [Google Scholar]
- Silveira M. F., Santos I. S., Barros A. J., Matijasevich A., Barros F. C., Victora C. G. (2008). Increase in preterm births in Brazil: Review of population-based studies. Revista de Saúde Pública, 42, 957–964 [DOI] [PubMed] [Google Scholar]
- Silveira M. F., Santos I. S., Matijasevich A., Malta D. C., Duarte E. C. (2009). Preterm births in Brazil from 1994 to 2005 according to the Information System on Live Births (SINASC). Cadernos de Saúde Pública, 25, 1267–1275 [DOI] [PubMed] [Google Scholar]
- Silveira M. F., Victora C. G., Barros A. J., Santos I. S., Matijasevich A., Barros F. C. (2010). Determinants of preterm birth: Pelotas, Rio Grande do Sul State, Brazil, 2004 birth cohort. Cadernos de Saúde Pública, 26, 185–194 [DOI] [PubMed] [Google Scholar]
- Szklo A. S., de Almeida L. M., Figueiredo V. C., Autran M., Malta D., Caixeta R., Szklo M. (2012). A snapshot of the striking decrease in cigarette smoking prevalence in Brazil between 1989 and 2008. Preventive Medicine, 54, 162–167.10.1016/j.ypmed.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Tong V. T., Dietz P. M., England L. J., Farr S. L., Kim S. Y., D’Angelo D., Bombard J. M. (2011). Age and racial/ethnic disparities in prepregnancy smoking among women who delivered live births. Preventing Chronic Disease, 8, A121 [PMC free article] [PubMed] [Google Scholar]
- United Nations (2000). United Nations Millennium Declaration. In 55th Session Agenda Item 60(b). UN General Assembly. Geneva, Switzerland: United Nations; [Google Scholar]
- USDHHS (2004). The health consequences of smoking: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; [Google Scholar]
- USDHHS (2006). The health consequences of second-hand smoke: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; [Google Scholar]
- USDHHS (2010). How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; [PubMed] [Google Scholar]
- WHO (2011). World Health Organization Global Infobase: NCID Inidicators, Tobacco Use Retrieved August 4, 2011, from https://apps.who.int/infobase/Indicators.aspx
- Woida FM, Saggioro FP, Ferro MA, Peres LC. (2008). Sudden infant death syndrome in Brazil: fact or fancy? Sao Paulo Med J,. 126(1), 48–51 [DOI] [PMC free article] [PubMed] [Google Scholar]