Abstract
Introduction:
Tobacco use is a leading cause of cancer, and continued use after cancer diagnosis puts patients at greater risk for adverse health outcomes, including increased risk for cancer recurrence. This study surveyed National Cancer Institute (NCI)–designated Cancer Centers to assess the availability of tobacco use treatment (TUT) services.
Methods:
Directors and oncology providers of 58 NCI-designated Cancer Centers received invitations to participate in an online survey. The questionnaire asked about attitudes, awareness, policies, and practices related to TUT; barriers to treatment provision; and factors likely to increase services.
Results:
All 58 Cancer Centers participated. Twelve (20.7%) Centers reported no TUT services for their patients. Of the remainder, 34 (58.6%) reported a TUT program within their Center and 12 (20.7%) reported external TUT services in their health care system or affiliated university. Only 62% of Centers reported routinely providing tobacco education materials to patients, just over half reported effective identification of patient tobacco use, and less than half reported an employee dedicated to providing TUT services or a clear commitment to providing TUT services from Center leadership. The 34 centers with internal TUT programs reported significantly greater services and administration support for TUT Services.
Conclusions:
These data demonstrate a national need for Cancer Centers to embrace and incorporate recommended standards for TUT. Tying TUT services to NCI recognition and providing stable funding for TUT services in Cancer Centers could lead to better health outcomes, treatment efficacy, and satisfaction for all U.S. Cancer Centers and their patients.
Introduction
Patients who continue to smoke after a cancer diagnosis are at greater risk for a range of adverse health outcomes, including shorter recurrence-free survival (Fleshner et al., 1999) and greater risk for a second primary tumor (Do et al., 2004; Johnson, 1998; Tucker et al., 1997). Smoking is also associated with worse outcomes and increased costs of care following cancer surgery. Continued smoking impairs quality of life across physical, psychological, and social domains (Garces et al., 2004).
Cancer centers and the clinicians and staff who work there should help cancer patients who use tobacco quit and help them eliminate exposure to secondhand smoke. Even though many cancer patients continue to smoke, a majority express interest in getting help to stop (Cooley et al., 2011) and significant numbers feel ready to quit (Sanderson Cox et al., 2002; Schnoll et al., 2003, 2004). The 2008 Clinical Practice Guideline emphasizes combining medication and counseling to significantly increase quit rates for all patients who want to stop smoking (Fiore et al., 2008). Key recommendations from the guideline include consistent identification and documentation of tobacco use, initiation of treatment for every tobacco user seen in a health care setting, and broad dissemination of these treatment guidelines into all health care clinical organizations.
The National Cancer Institute (NCI) awards substantive grants to support designated Cancer Centers across the United States that model transdisciplinary translational cancer research. It views these Cancer Centers as the centerpiece of efforts to reduce morbidity and mortality from cancer. Some combination of three principal focus areas—laboratory science, clinical research, and population science—is required for NCI designation (National Cancer Institute, 2010). In 2009, the NCI website listed 65 designated Cancer Centers (National Cancer Institute, 2009). Forty of these, exhibiting work in all three focus areas, were labeled Comprehensive Cancer Centers. Of the remaining 25 Cancer Centers, 7 Centers did not engage in clinical research and therefore provided no direct patient care services.
Given their visibility and status as champions in cancer control efforts, these designated Centers and their providers should be leading by example in all areas of cancer care and prevention, including implementing recommended evidence-based tobacco use treatment (TUT) for patients under their care. To date, no studies have assessed the kinds and extent of tobacco treatment services offered by NCI-supported Cancer Centers.
This study sought to learn what NCI Cancer Centers are currently doing to address tobacco use in their patients and staff. It identifies the characteristics of Centers that provide active TUT programs and the barriers that may prevent Cancer Centers from offering more tobacco treatment services.
Methods
The study utilized a cross-sectional design, gathering web-based survey data in October 2009 from key staff of 58 NCI-designated Cancer Centers. Unless otherwise noted, the term “Cancer Centers” includes the 40 Comprehensive Cancer Centers and the additional 18 Cancer Centers, all of which offered direct patient care services.
Questionnaire Development
The questionnaire assessed recognized components of effective TUT and the facilitators and barriers to implementing the interventions recommended by the Clinical Practice Guidelines. An NCI working group on treating tobacco use within Cancer Centers helped draft a pilot instrument. The final 44-item questionnaire included demographic questions, questions about respondents’ awareness, opinions, and involvement in TUT in their Cancer Center, and questions about TUT programs within Centers to support patients in tobacco cessation. Those reporting TUT services within the Cancer Center were asked about sources of funding and the administrative home for such services. Respondents answering “no” or “unsure” when asked about the availability of in-house services were asked if TUT services were available through their affiliated university or health care system. They were also asked about the likelihood of their Cancer Center instituting a TUT program in the next year.
Respondents identified the specific practices related to TUT currently offered by their Centers. These included routinely identifying tobacco use as a vital sign in medical records, having designated individual(s) to provide TUT, offering employee programs for TUT, supporting quality improvement measures related to TUT, having environmental policies in place to support tobacco use cessation (e.g., 100% tobacco-free grounds policy), having local champions to promote TUT treatment efforts, and offering research programs in tobacco control.
Fielding the Survey
The survey goal was to ensure that we obtained a respondent who demonstrated knowledge about the availability or lack thereof for TUT services in every Cancer Center. After pilot testing, the questionnaire was sent by email from the director of the University of North Carolina Lineberger Cancer Center to the directors of the other 57 NCI Cancer Centers. We anticipated that some directors or their designees might not respond or have little awareness of the TUT services in their Center. To increase the likelihood of accurate information from every Cancer Center, questionnaires were also emailed to one radiation oncologist and one medical oncologist in either the head and neck or thoracic departments at each Cancer Center. These providers were identified through information provided by directors on their returned questionnaires or on Cancer Centers’ websites. Those who did not respond to the initial email contact received up to two reminder emails. The Public Health-Nursing Institutional Review Board of the University of North Carolina at Chapel Hill determined that this project did not constitute human subjects research.
Survey Respondents
From the distributed surveys, we received 48 responses from either the director or the director’s designee and 62 responses from oncology treatment providers. We received at least one response from every Cancer Center and received more than one response from 60% of Centers. For analysis, we used data from only one respondent from each Cancer Center, choosing the director or director’s designee in all cases unless compelling information from returned questionnaires recommended another, better informed respondent. Our protocol defined compelling as satisfying one of the following conditions: either the oncology treatment respondent’s combined awareness and involvement were greater than that of the director or the director indicated a high number of unsure responses. Final analyses are based on responses from 43 directors or their designees and 15 oncology providers with highest awareness of TUT services at their center. Additional data for each Cancer Center was assembled from the NCI’s website, specifically about Cancer Center designation and the amount of NCI funding received in 2008.
Analysis
All quantitative data were entered into SPSS 17.0 and initially presented through frequency distributions. Bivariate associations were examined with chi-square analysis for categorical variables.
Results
Demographics
Respondents (the 43 directors/designees and 15 oncology providers) were predominantly male (65%) and had served in their Cancer Center for more than ten years (43%). Almost a third (31%) of respondents indicated their primary Cancer Center role to be director, with an additional 17% indicating other significant administrative roles (e.g., medical director, research program director, tobacco treatment program director). Twenty-six percent reported their primary role as physicians, 17% as researchers, and 5% as TUT clinicians.
Attitudes and Knowledge About TUT Services
Virtually all (97%) respondents indicated they felt that providing TUT services to cancer patients was important or very important. Eighty-eight percent thought that they were very or highly aware of their Cancer Center policies and services related to tobacco use, and 59% reported that they were personally “very” or “highly” involved in ensuring these tobacco treatment services and policies were in place. Only 48% of respondents indicated that they had substantive knowledge about the U.S. Health and Human Services clinical practice guidelines related to TUT.
TUT Programs
When asked if their Cancer Center had a TUT program for patients, 34 (58.6%) responded yes. Of the remaining Centers, 12 (20.7%) reported a TUT program within the health care system or affiliated university to which patients could be referred, and 12 (20.7%) reported no program or being unsure about affiliated TUT programs. Respondents stated that their Cancer Center–based TUT programs were administratively housed in a variety of clinics and departments, the most common being patient support services (24%), free standing tobacco research programs (15%), and prevention and control research programs (15%). Other bases for housing TUT services included clinics within psychiatry, family medicine, pulmonary medicine, and multidisciplinary clinics.
Most respondents for the 34 Cancer Centers that offered TUT programs reported that internal funding sources supported their programs. The second most often cited source of support was through federal grants (50%). Eighty-five percent of TUT programs reported multiple sources of funding.
Of the 24 programs reporting no cancer center TUT program, seven (29%) reported that their cancer center would likely or very likely institute a TUT program within the next year.
Current Cancer Center Policy and Practice
Figure 1 displays the percentage of respondents reporting specific system policies and programs that support TUT. Having smoke-free campus policies and a tobacco use research program were each reported by over 75% of all respondents. Slightly more than half reported an employee treatment program or a physician champion to promote TUT. Less than half reported a person(s) whose designated role involved TUT or coordination, tobacco use as a vital sign, or the use of any tobacco measure in quality improvement. For comparison, 78% of responding Cancer Centers reported having a designated individual who provides nutritional counseling to their patients.
Figure 1.
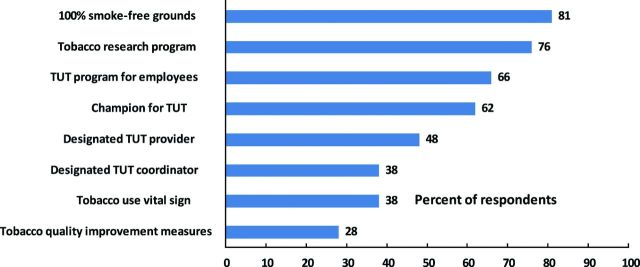
Percent of Centers reporting policies and programs to support tobacco use treatment (n = 58).
Components of adopting and implementing recommended TUT (Bradley et al., 2001, Fiore et al., 2008, Partnership for Prevention, 2008) and the percent of responding centers reporting these can be found in Figure 2. Other than providing tobacco education materials, no recommended TUT practice was found in more than 60% of Cancer Centers. Less than half of respondents believed that there was clear commitment to TUT services from Center leadership or that providers received adequate training in TUT. Less than a third believed that their Center actively promoted either TUT to family members or utilization of quitline fax referrals. Few (9%) reported that clinicians were offered regular feedback on how they identify, refer, or counsel patients who use tobacco.
Figure 2.
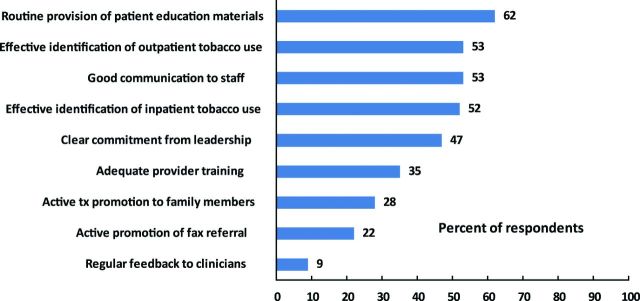
Percent of Centers reporting components of adopting and implementing recommended TUT (n = 58).
Table 1 shows that Cancer Centers with a TUT program within their Center (n = 34), compared with those without such a program (n = 24), were more likely to report a broad array of many meaningful TUT services, such as on-site coordination and provision of TUT, and systems for identification of inpatient and outpatient tobacco use among cancer patients. Centers with a TUT program were also significantly more likely to have strong administrative support for TUT, such as communication to staff, a clear commitment from leadership, and champions who made TUT a major part of their professional role.
Table 1.
Percent of Cancer Centers Reporting TUT Components, by Centers With and Without a TUT Program (n = 58)
| Components related to tobacco use treatment (TUT) programs with % of centers reporting | TUT program | No TUT program |
| n = 34 | n = 24 | |
| Yes (%) | No (%) | |
| Routine provision of patient education materials | 79* | 38 |
| Designated person who provides TUT | 76* | 8 |
| Cancer center has tobacco research program | 76 | 75 |
| Offers employee program for staff/faculty | 74 | 54 |
| Has a champion for TUT | 74** | 46 |
| Clear commitment from leadership | 71* | 13 |
| Good communication to staff | 68** | 33 |
| Effective identification of outpatient tobacco use | 62 | 42 |
| Effective identification of inpatient tobacco use | 62 | 37 |
| Designated person who coordinates TUT | 76* | 8 |
| Adequate provider training | 41 | 25 |
| Identifies tobacco use as vital sign in medical record | 38 | 38 |
| Active TUT promotion to family members | 38** | 13 |
| Tracks quality improvement measures TUT | 35 | 17 |
| Active promotion of fax referral | 29 | 13 |
| Regular feedback to clinicians | 9 | 8 |
Note. TUT = tobacco use treatment.
*p < .001. **p < .05.
Improving TUT Services
Factors that respondents indicated would improve TUT services in their Cancer Center are reported in Figure 3, with the top three factors being provision of stable funding, qualified tobacco treatment specialists (TTS) on staff, and adequate space. These suggested improvements were recommended by the majority of respondents from Centers with and without a TUT program in their Center.
Figure 3.
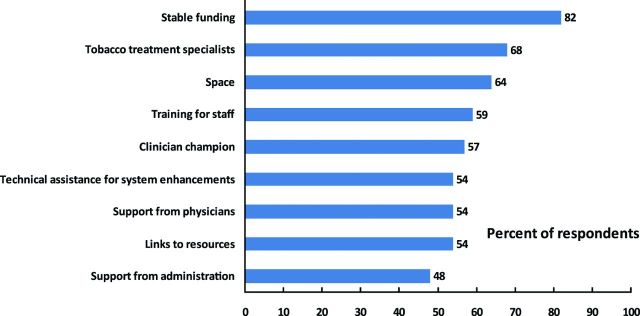
Factors perceived “likely” or “very likely” to improve tobacco use treatment services at Cancer Centers (n = 58).
Discussion
This study provides the first comprehensive look at TUT practices at NCI Cancer Centers. While this study shows that the majority of directors and key leadership of NCI Cancer Centers believe that TUT services are important and have TUT research programs, many do not provide recommended evidenced-based tobacco cessation services to their cancer patients. A sizable minority offer their patients no TUT services.
Based on our results, the following recommendations should be considered:
1. All Cancer Centers who treat patients should have a TUT program within their center.
Cancer Centers exist to effectively treat cancer patients, to prevent future cancer recurrences, and to conduct research on cancer treatment and prevention. Given that tobacco use is a leading cause of cancer and a significant cause of morbidity and mortality following a cancer diagnosis, TUT program availability to Center patients should be central to the mission of all Cancer Centers. Literature also indicates that cancer patients benefit from specialized services to help them quit smoking (McBride & Ostroff, 2003). Given the positive impacts on cancer patients, family members, and staff, it is surprising that comprehensive TUT is not uniformly integrated into all Centers. Since Centers that have a TUT program appear to provide significantly more meaningful TUT services and enjoy stronger administrative support, all Cancer Centers without a TUT program should have one within their Center.
The 2008 U. S. Department of Health and Human Services’ Treating Tobacco Use and Dependence Clinical Practice Guideline promotes dissemination of effective TUT medications and counseling strategies, as well as care for specific populations, including cancer patients (Fiore et al., 2008). Having a TUT program within a cancer center will increase Cancer Center providers’ familiarity with best practices in TUT, a foundational step in helping the Centers incorporate evidence-based tools to support cancer patients in becoming tobacco free.
2. The NCI should facilitate the incorporation of TUT services into Cancer Center care.
The NCI can play a pivotal role in helping their designated Cancer Centers offer TUT programs. Since tobacco research programs in and of themselves were not related to the provision of TUT services, research on tobacco addiction, while desirable, is not sufficient to ensure service provision. NCI Cancer Centers, recognized as leaders in cancer treatment and research, cannot be seen as offering substandard care when it comes to treating tobacco use, a highly addictive behavior that causes cancer, compromises cancer treatment, and increases risk of cancer recurrence. To begin changing this paradigm, the NCI sponsored a conference in 2009, attended by representatives from over three dozen Cancer Centers, to discuss collaboration and research for TUT (Morgan et al., 2011). This conference highlighted model programs to serve as best practices in providing guidance to Cancer Centers as they develop or expand TUT services. Further promotion of TUT services may occur if quality indicators for NCI Center designation and funding include published, evidenced-based TUT guidelines. Providing recommended care in this vital area would further set these Cancer Centers apart, improve cancer prevention and treatment efforts in these Centers, and accelerate diffusion of such services to other cancer programs across the United States.
3. Cancer centers should embrace new quality improvement measures and opportunities for TUT services.
Increasingly, the gold standard for TUT includes comprehensive on-site services for patients, training of providers, and quality improvement initiatives to improve outcomes. Quality improvements include system changes that ensure identification of all tobacco users as well as provision of evidence-based treatment. Consistent identification, such as making tobacco use a vital sign, results in more advice to quit and more counseling (McCullough, Fisher, Goldstein, Kramer, & Ripley-Moffitt, 2009). An added incentive for including regular identification and treatment for tobacco users comes from the current Meaningful Use of Data guidelines, which outline requirements for receipt of funds through the American Recovery and Reinvestment Act 2009 (U.S. Government, 2009). These guidelines include tobacco measures that track tobacco use identification and treatment for both eligible health care organizations and providers. The Joint Commission currently mandates TUT for inpatients with pneumonia, acute myocardial infarction, and heart failure. As of January 1, 2012, a new Joint Commission tobacco measure set expands TUT services to all tobacco users, regardless of diagnosis (Joint Commission, 2011). Canadian hospitals, adopting the Ottawa Model of Smoking Cessation, have shown impressive results from offering TUT, improving quality of care across multiple hospital settings (Reid et al., 2010).
Our data indicate that even among those Centers that have a TUT program, significant room exists for improvement in on-site counseling, as feedback systems to clinicians are particularly uncommon. Giving feedback to individual physicians or clinics on the care they provide has shown to be effective in changing physician behavior (Bradley et al., 2001). Cancer Center administrators should work with information technology staff to produce reports on tobacco use identification and treatment for feedback to providers and the institution.
Improving TUT outcomes in Cancer Centers will also involve training and support of providers and administrators, as provision of TUT to cancer patients poses some unique challenges not found when providing similar services to other outpatient populations. These unique challenges include: beliefs among some Center providers that TUT is the responsibility of the primary care provider, lack of training among oncologists for how to provide TUT, management of pharmacotherapy for patients with complex treatment regimens, and the physical, psychological, and spiritual issues related to facing cancer diagnosis and treatment (McBride & Ostroff, 2003).
4. Insititutional funding should support TUT services in Cancer Centers.
Perceived barriers to augmentation of TUT services in all Centers included shortages of funding, trained personnel, and space. Obtaining these resources, especially the funding required to sustain a program, is made more difficult because of the limited reimbursement currently available for such services. TTS offer more in-depth, individualized, and comprehensive treatment than that which physicians or nurses can incorporate into their already extensive clinic visit agendas. As members of the health care team, TTS can disseminate new treatment approaches and work on quality improvement around tobacco use (Hurt, Ebbert, Hays, & McFadden, 2009). Providing a mechanism that will allow TTS to bill for their services will greatly enhance the sustainability of their involvement with Centers and ensure continuity of care for patients.
Limitations
This study has several limitations. Because we used data from only one respondent per center, there is the possibility that others would have interpreted or responded to questions differently. It is possible that some respondents, perhaps directors or their designees, may have overstated their awareness and regard for TUT services because of the need to appear informed, thus potentially overstating program availability. Furthermore, because we measured self-reports of quality and effectiveness, variations in the nature and breadth of TUT programs (e.g., number of staff, reach of program) are not compared.
Conclusions
NCI cancer centers are increasingly interested in offering TUT programs as part of their core patient services. Nevertheless, TUT programs appear to lag behind other commonly accepted Cancer Center services, such as nutrition counseling. Having sustainable TUT programs at all Cancer Centers will improve Centers’ quality of cancer care and has the potential to reduce morbidity and mortality related to tobacco use among cancer patients.
Funding
This work was support by the University of North Carolina Lineberger Comprehensive Cancer Center’s Population Sciences Research Award Grant #5-32613.
Declaration of Interests
The authors report no competing interests.
Acknowledgments
The authors acknowledge the following contributions: H. Shelton Earp III, M.D., Director of the University of North Carolina Lineberger Cancer Center and Michael S. O’Malley, Ph.D., Associate Director, gave valuable feedback on the survey. Jessica Platz, Executive Assistant, ensured that each Cancer Center director received a personal invitation from Dr. Earp to participate in the study. Otherwise, the funders had no role in data collection, management, analysis, or interpretation nor in the preparation, review, or approval of this manuscript. We also acknowledge the contributions of the National Cancer Institute (NCI) working group on tobacco use in cancer centers. Preliminary results from this study were presented at the NCI tobacco treatment meeting in Bethesda, MD, December 2009.
References
- Bradley EH, Holmboe ES, Mattera JA, Roumanis SA, Radford MJ, Krumholz HM. A qualitative study of increasing beta-blocker use after myocardial infarction: Why do some hospitals succeed? Journal of the American Medical Association. 2001;285:2604–2611. doi: 10.1001/jama.285.20.2604. doi: 10.1001/jama.285.20.2604. [DOI] [PubMed] [Google Scholar]
- Cooley ME, Emmons KM, Haddad R, Wang Q, Posner M, Bueno R, et al. Patient-reported receipt of and interest in smoking-cessation interventions after a diagnosis of cancer. Cancer. 2011;117:2961–2969. doi: 10.1002/cncr.25828. doi:10.1002/cncr.25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KA, Johnson MM, Lee JJ, Wu XF, Dong Q, Hong WK, et al. Longitudinal study of smoking patterns in relation to the development of smoking-related secondary primary tumors in patients with upper aerodigestive tract malignancies. Cancer. 2004;101:2837–2842. doi: 10.1002/cncr.20714. doi:10.1002/cncr.20714. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence. 2008 update. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. May 2008. [Google Scholar]
- Fleshner N, Garland J, Moadel A, Herr H, Ostroff J, Trambert R, et al. Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer. 1999;86:2337–2345. doi:10.1002/(SICI)1097-0142(19991201)86:11<2337::AID-CNCR23>3.0.CO; 2–6. [PubMed] [Google Scholar]
- Garces YI, Yang P, Parkinson J, Zhao X, Wampfler JA, Ebbert JO, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126:1733–1741. doi: 10.1378/chest.126.6.1733. doi:10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Ebbert JO, Hays JT, McFadden DD. Treating tobacco dependence in a medical setting. CA: A Cancer Journal for Clinicians. 2009;59:314–326. doi: 10.3322/caac.20031. doi:10.3322/caac.20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint Commission. Specifications Manual for National Hospital Inpatient Quality Measures. 2011 Retrieved from http://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures/. [Google Scholar]
- Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. Journal of the National Cancer Institute. 1998;90:1335–1345. doi: 10.1093/jnci/90.18.1335. doi:10.1093/jnci/90.18.1335. [DOI] [PubMed] [Google Scholar]
- McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: The context of cancer care and survivorship. Cancer Control. 2003;10:325–333. doi: 10.1177/107327480301000407. [DOI] [PubMed] [Google Scholar]
- McCullough A, Fisher M, Goldstein AO, Kramer KD, Ripley-Moffitt C. Smoking as a vital sign: Prompts to ask and assess increase cessation counseling. Journal of the American Board of Family Medicine. 2009;22:625–632. doi: 10.3122/jabfm.2009.06.080211. doi:10.3122/jabfm.2009.06.080211. [DOI] [PubMed] [Google Scholar]
- Morgan G, Schnoll RA, Alfano CM, Evans SE, Goldstein A, Ostroff J, et al. National Cancer Institute Conference on Treating Tobacco Dependence at Cancer Centers. Journal of Oncology Practice. 2011;7:178–182. doi: 10.1200/JOP.2010.000175. doi:10.1200/JOP.2010.000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. Cancer Centers list. 2009. Retrieved from http://cancercenters.cancer.gov/cancer_centers/cancer-centers-names.html.
- National Cancer Institute. 2010. Policies and guidelines relating to the cancer center support grant. Retrieved from http://cancercenters.cancer.gov/documents/CCSG_Guidelines.pdf.
- Partnership for Prevention. 2008. Healthcare provider reminder systems, provider education, and patient education: Working with healthcare delivery systems to improve the delivery of tobacco-use treatment to patients—An Action Guide. The Community Health Promotion Handbook: Action Guides to Improve Community Health. Washington, DC: Partnership for Prevention.
- Reid RD, Mullen KA, Slovinec D’Angelo ME, Aitken DA, Papadakis S, Haley PM, et al. Smoking cessation for hospitalized smokers: An evaluation of the “Ottawa Model”. Nicotine & Tobacco Research. 2010;12:11–18. doi: 10.1093/ntr/ntp165. doi:10.1093/ntr/ntp165. [DOI] [PubMed] [Google Scholar]
- Sanderson Cox L, Patten CA, Ebbert JO, Drews AA, Croghan GA, Clark MM, et al. Tobacco use outcomes among patients with lung cancer treated for nicotine dependence. Journal of Clinical Oncology. 2002;20:3461–3469. doi: 10.1200/JCO.2002.10.085. doi:10.1200/JCO.2002.10.085. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, James C, Malstrom M, Rothman RL, Wang H, Babb J, et al. Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Annals of Behavioral Medicine. 2003;25:214–222. doi: 10.1207/S15324796ABM2503_07. doi:10.1207/S15324796ABM2503_07. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Rothman RL, Newman H, Lerman C, Miller SM, Movsas B, et al. Characteristics of cancer patients entering a smoking cessation program and correlates of quit motivation: Implications for the development of tobacco control programs for cancer patients. Psycho-Oncology. 2004;13:346–358. doi: 10.1002/pon.756. doi:10.1002/pon.756. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Murray N, Shaw EG, Ettinger DS, Mabry M, Huber MH, et al. Second primary cancers related to smoking and treatment of small-cell lung cancer. Lung Cancer Working Cadre. Journal of the National Cancer Institute. 1997;89:1782–1788. doi: 10.1093/jnci/89.23.1782. doi:10.1093/jnci/89.23.1782. [DOI] [PubMed] [Google Scholar]
- U.S. Government. 2009 The American Recovery and Reinvestment Act of 2009. Retrieved from http://www.recovery.gov/About/Pages/The_Act.aspx. [Google Scholar]


