SUMMARY
Seedlings of Arabidopsis have been exposed to norflurazon. Depending on the developmental stage at which seedlings were first exposed to the inhibitor, enhanced production of reactive oxygen species occurred and, among others, 1O2-mediated and EXECUTER-dependent retrograde signaling was induced.
Key words: retrograde signaling, singlet oxygen, Executer, norflurazon, programmed cell death, photo-oxidative stress.
Abstract
Chloroplast development depends on the synthesis and import of a large number of nuclear-encoded proteins. The synthesis of some of these proteins is affected by the functional state of the plastid via a process known as retrograde signaling. Retrograde plastid-to-nucleus signaling has been often characterized in seedlings of Arabidopsis thaliana exposed to norflurazon (NF), an inhibitor of carotenoid biosynthesis. Results of this work suggested that, throughout seedling development, a factor is released from the plastid to the cytoplasm that indicates a perturbation of plastid homeostasis and represses nuclear genes required for normal chloroplast development. The identity of this factor is still under debate. Reactive oxygen species (ROS) were among the candidates discussed as possible retrograde signals in NF-treated plants. In the present work, this proposed role of ROS has been analyzed. In seedlings grown from the very beginning in the presence of NF, ROS-dependent signaling was not detectable, whereas, in seedlings first exposed to NF after light-dependent chloroplast formation had been completed, enhanced ROS production occurred and, among others, 1O2-mediated and EXECUTER-dependent retrograde signaling was induced. Hence, depending on the developmental stage at which plants are exposed to NF, different retrograde signaling pathways may be activated, some of which are also active in non-treated plants under light stress.
INTRODUCTION
The majority of chloroplast proteins are encoded by nuclear genes, synthesized in the extra-plastidic cytoplasm and imported into the organelle. The expression of at least some of these genes is affected by the functional state of the plastid via a process known as retrograde signaling (Surpin et al., 2002). Retrograde plastid-to-nucleus signaling is usually inferred from correlations between a particular perturbance of chloroplast homeostasis and a corresponding change in nuclear gene activities. As different disturbances of plastid homeostasis affect different sets of target genes, several plastid-derived signals have been implicated with inducing nuclear gene expression changes (Nott et al., 2006; Leister, 2012). Two of the plastid-to-nucleus signaling pathways in the flu mutant and in norflurazon (NF)-treated wild type have in common that their activation is closely linked to perturbations of plastid homeostasis that may enhance the photosensitizing activity of tetrapyrroles (Feierabend and Winkelhüsener, 1982; Meskauskiene et al., 2001). Tetrapyrroles may transfer excitation energy onto ground-state triplet oxygen, a biradical that is chemically inert, and transform it to the highly reactive singlet oxygen (1O2) (Halliwell and Gutteridge, 1999; Apel and Hirt, 2004).
By far the most abundant tetrapyrrole in plants that may act as photosensitizer is chlorophyll. Most chlorophyll is bound to light-harvesting chl a/b proteins and is in direct contact with xanthophylls, a group of carotenoids that efficiently quench excess light energy absorbed by chlorophyll and suppress 1O2 formation (Li et al., 2009). In contrast to chlorophyll, its biosynthetic precursors occur mostly in a free form and are potentially much more destructive when illuminated. Accumulation of these intermediates is tightly controlled via negative feedback control of Glu t-RNA reductase, the first enzyme committed to tetrapyrrole biosynthesis, by heme and FLU (Goslings et al., 2004). Inactivation of FLU leads to the continuous accumulation of the precursor of chlorophyll, protochlorophyllide (Pchlide), in dark-grown plants. In the light, Pchlide acts as a photosensitizer and generates 1O2 (Gollnick, 1968; op den Camp et al., 2003; Hideg et al., 2006). Immediately after the release of 1O2, mature flu plants stop growing, whereas seedlings bleach and die. An extensive second-site mutant screen has led to the isolation of several suppressors of flu. One group has lost the ability of the parental flu line to over-accumulate Pchlide in the dark (ulf, reversal of flu) (Goslings et al., 2004). Another suppressor mutant dubbed executer1 (ex1) continues to over-accumulate Pchlide in the dark and, during re-illumination, generates similar amounts of 1O2 as the parental flu line, but does not show the same stress responses as flu (Wagner et al., 2004). A second related nuclear-encoded protein, dubbed EXECUTER2 (EX2), has been identified that is also implicated with 1O2-mediated plastid-to-nucleus retrograde signaling. Like EXECUTER1 (EX1), EX2 is confined to the plastid. Inactivation of both EX proteins in an ex1/ex2/flu triple mutant is sufficient to abrogate 1O2-mediated responses of the flu mutant (Lee et al., 2007).
Plastid-to-nucleus signaling in NF-treated plants is perhaps the most intensively studied retrograde signaling pathway in higher plants. Retrograde signaling in these plants has often been associated with photo-oxidative stress that impedes chloroplast development (e.g. Gray, 2003; Voigt et al., 2010). In NF-treated seedlings with non-functional chloroplasts, the transcription of nuclear genes encoding plastid proteins such as the light-harvesting chlorophyll a/b protein (LHCB) is down-regulated relative to non-treated controls, whereas the expression of most other nuclear genes is not affected (Mayfield and Taylor, 1984; Batschauer et al., 1986). This down-regulation has been attributed to the release of a plastid factor that indicates a perturbation of the functional state of the plastid and represses the expression of nuclear genes required for normal chloroplast development. Attempts to identify this factor gave contradictory results and different views as to how retrograde signaling operates in NF-treated plants are still under debate (Strand et al., 2003; Mochizuki et al., 2008; Moulin et al., 2008; Zhang et al., 2011; Leister, 2012).
Initially, repression of nuclear gene expression was reported to correlate with enhanced levels of Mg2+Protoporphyrin IX (Mg2+ProtoIX) in NF-treated plants (Strand et al., 2003). Mg2+ProtoIX was also reported to be translocated from the plastid to the surrounding cytoplasm, being in line with its proposed role as a plastid-derived repressor of photosynthesis-related nuclear genes (Ankele et al., 2007). However, subsequent studies were unable to confirm the reported accumulation of Mg2+ProtoIX in NF-treated seedlings (Mochizuki et al., 2008; Moulin et al., 2008). Almost all genes of the tetrapyrrole biosynthesis pathway were repressed and levels of Mg2+ProtoIX and other tetrapyrrole intermediates were much lower than in non-treated control seedlings. Based on these results, reactive oxygen species (ROS) such as 1O2 or hydrogen peroxide (H2O2) rather than Mg2+ProtoIX were discussed as possible signaling molecules (Moulin et al., 2008). This proposal is in agreement with the known photosensitizing activity of tetrapyrroles (Hu et al., 1998; Mock et al., 1999; Mach et al., 2001; Przybyla et al., 2008).
Carotenoids quench excess light energy absorbed by chlorophyll and, in this way, protect photosynthetic membranes against photo-oxidative damage (Li et al., 2009). In the absence of carotenoids, chlorophyll and its intermediates would be expected to show an enhanced photosensitizing activity and generate 1O2 within chloroplasts, once NF-treated plants are exposed to light (Knox and Dodge, 1985). Generation of 1O2 in chloroplasts may initiate retrograde signaling either via an EX-dependent signaling pathway first characterized in the flu mutant or due to its cytotoxicity that leads to photo-oxidative damage (Triantaphylidès et al., 2008) and generates non-enzymatic peroxidation products of lipids and carotenoids that may act as signals and modify nuclear gene expression (Mueller et al., 2008; Ramel et al., 2012). In the present work, a signaling role of 1O2 was tested in NF-treated seedlings exposed to the inhibitor either before or after chloroplast had been formed and seedlings had visibly greened. This study shows that, depending on the developmental stage at which plants were first exposed to NF, different retrograde signaling pathways are activated, one of them being the 1O2- and EX-dependent signaling pathway first described in the flu mutant.
RESULTS
Retrograde Signaling in NF-Treated Bleached Arabidopsis Seedlings
In previous work, retrograde signaling had been analyzed in seedlings grown from the very beginning in the presence of 5 μM NF under continuous light (Susek et al., 1993; Strand et al., 2003; Ruckle et al., 2007). These seedlings bleach and accumulate anthocyanins and chloroplast formation is arrested at an early stage of seedling development, giving rise to undifferentiated proplastid-like organelles (Figure 1A) (Susek et al., 1993). These effects of NF treatment have been often attributed to photo-oxidative damage confined to the plastid compartment (Reiss et al., 1983; Sagar and Briggs, 1990; Voigt et al., 2010). To study the possible role of 1O2-mediated signaling in NF-treated seedlings, plants were kept under two different growth conditions. The first group of NF-treated seedlings was kept under growth conditions similar to the one used previously (Susek et al., 1993) (High NF growth condition: 100 µmol photons m–2 s–1/5 µM NF). For the second group, a lower stress level was used: the NF concentration was reduced 100-fold and the light intensity 10-fold (Low NF growth condition: 10 µmol photons m–2 s–1/50 nM NF) (Saini et al., 2011). Responses of NF-treated seedlings were compared to those of flu seedlings initiated by 1O2-mediated and EX-dependent retrograde signaling. Like flu, NF-treated seedlings kept under high or low NF growth conditions bleached (Figure 1A) (Koussevitzky et al., 2007; Saini et al., 2011). To prove or disprove the involvement of 1O2 in triggering this bleaching, consequences of retrograde signaling in NF-treated and flu seedlings were analyzed and compared.
Figure 1.
A Comparison of NF-Treated Seedlings and the flu Mutant Exposed to a Dark/Light Shift.
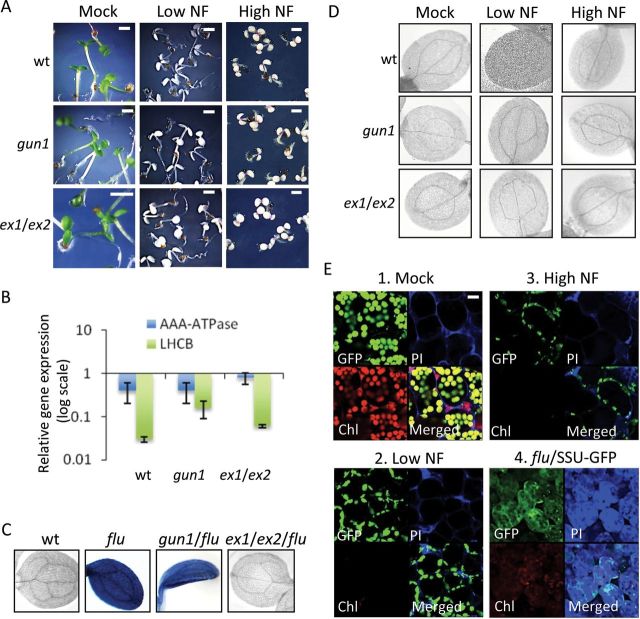
(A) A comparison of 5-day-old wild-type (wt), gun1, and ex1/ex2 seedlings kept under low NF (50 nM NF/10 μmol photons m–2 s–1) or high NF (5 μM NF/100 μmol photons m–2 s–1) growth conditions. Seedlings remain bleached under both NF growth conditions, whereas non-treated control seedlings (Mock) are green. Scale bar = 0.5 cm.
(B) Expression level of AAA-ATPase and LHCB marker genes in 5-day-old seedlings kept under high NF growth condition (High NF) relative to gene expression of control seedlings grown on NF-free medium. Three biological replicas were analyzed by qPCR and the mean values are shown with standard deviations. Actin2 was used to normalize the values.
(C) 1O2-mediated cell death in flu and gun1/flu as revealed by trypan blue staining of 3-day-old seedlings grown under long-day conditions. The cell death response is suppressed in flu/ex1/ex2 and does not occur in wild-type (wt).
(D) Cotyledons of 3-day-old wild-type (wt), gun1 and ex1/ex2 seedlings grown on NF-free medium (Mock) or kept under low (Low NF) or high (High NF) NF growth conditions were stained with trypan blue. None of these seedlings showed a cell death response.
(E) Chloroplast integrity in wild-type and flu seedlings expressing the SSU–GFP fusion protein was assessed under the confocal laser scanning microscope (CLSM). Three-day-old wild-type seedlings grown on NF-free medium (Mock) (E1) or kept under low NF (Low NF) (E2) or high NF (High NF) (E3) growth conditions in the presence of continuous light and 3-day-old flu seedlings grown under long-day conditions (flu/SSU–GFP) (E4) were monitored. The cell death was visualized by staining with propidium iodine (PI). The green fluorescence of GFP, the red fluorescence of chlorophyll, and the blue fluorescence of PI were monitored separately by CLSM, and the three fluorescence images were merged. Bars = 20 μm.
The gun1 mutation de-represses LHCB expression in seedlings kept under low or high NF growth conditions (Figure 1B) (Koussevitzky et al., 2007; Saini et al., 2011), but, unlike the ex1/ex2 mutations, does not prevent the 1O2-mediated bleaching and cell death response of the flu mutant (Figure 1C). Inactivation of EX1 and EX2 abrogates these responses of the flu mutant, indicating that bleaching of flu seedlings is due to EX-dependent signaling rather than 1O2 directly (Figure 1C) (Kim et al., 2012). Bleaching of NF-treated seedlings was not suppressed when 1O2-mediated signaling was blocked in a genetic ex1/ex2 background (Figure 1A). These results may be expected if 1O2 acts as a cytotoxin, causing photo-oxidative damage irrespectively of the presence or absence of EX1 and EX2 (Przybyla et al., 2008; Kim et al., 2012).
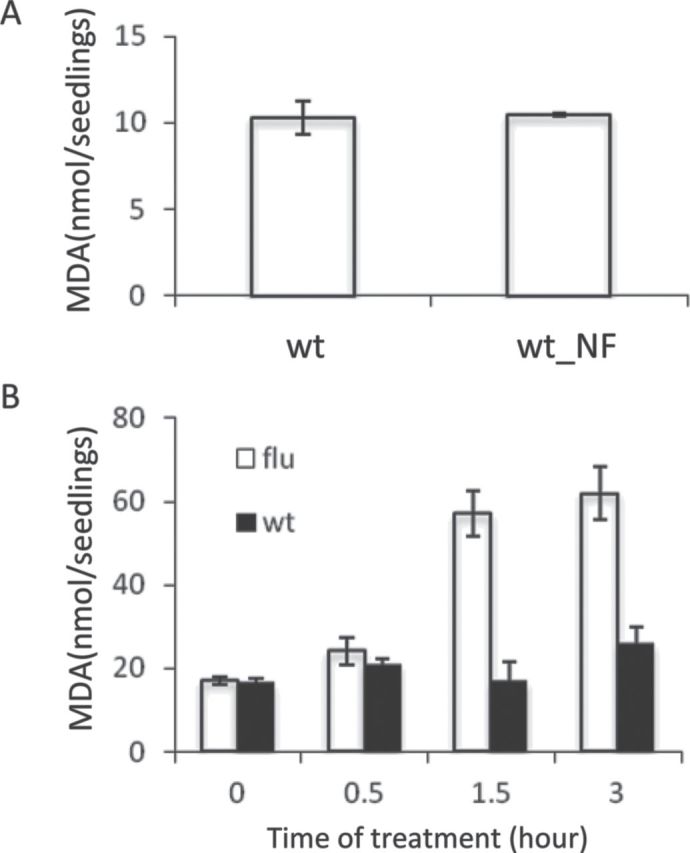
Photo-oxidative damage in NF-treated seedlings was monitored by measuring cell death, chloroplast leakage, and lipid peroxidation. NF-treated wild-type, gun1, and ex1/ex2 seedlings bleached, but, unlike flu and flu/gun1 seedlings exposed to a dark/light shift, did not reveal any cell death response (Figure 1D). In the flu mutant, the first consequence of 1O2 generation visible to the eye is a rapid loss of chloroplast integrity (Kim et al., 2012). Chloroplast integrity in NF-treated seedlings was assessed under the confocal laser scanning microscope by monitoring the fluorescence distribution of the green fluorescent protein (GFP) in transgenic plants expressing a chimeric reporter protein consisting of the chloroplast-localized small subunit of the ribulose-1,5-bisphosphate carboxylase (SSU) and GFP. In non-treated wild-type control seedlings, the fusion protein was confined to the plastid compartment, as indicated by merging separate fluorescence images of chlorophyll and GFP (Figure 1E1). When wild-type seedlings were kept under low or high NF growth conditions, plastid development was blocked, as indicated by the lack of chlorophyll fluorescence (Figure 1E2 and 1E3). The plastid size was slightly reduced in seedlings grown in the presence of 50 nM NF (Figure 1E2), whereas, in seedlings grown on 5 µM NF, the plastid size was much smaller than in non-treated control seedlings (Figure 1E3). In both cases, the fusion protein was imported and retained within plastids, indicating that, even under high NF growth conditions, plastids did not lose their protein import capacity and remained intact (Figure 1E3). By contrast, in flu seedlings after a dark/light shift, generation of 1O2 is rapidly followed by a loss of chloroplast integrity as indicated by the release of the fusion protein from the chloroplast to the surrounding cytoplasm that precedes the rupture of the central vacuole and the final collapse of the cell (Figure 1E4) (Kim et al., 2012). The onset of cell death in these seedlings was determined by propidium iodine (PI) staining. This reagent is excluded from intact cells and may accumulate in the extracellular space of the tissue, whereas dying or dead cells will be stained (Kim et al., 2012). Unlike cells of the flu mutant (Figure 1E4), none of the cells of NF-treated wild-type seedlings was stained by PI (Figure 1E2 and 1E3). Finally, lipid peroxidation was used as an indicator of photo-oxidative membrane damage. Malondialdehyde (MDA) levels in seedlings kept under high NF growth conditions were low and equal to those of non-treated control seedlings (Figure 2A), whereas, in flu seedlings following a dark/light shift, MDA levels rapidly increased (Figure 2B). MDA is formed during oxidation of polyunsaturated fatty acids and has been widely used to estimate the extent of photo-oxidative damage (Heath and Packer, 1968; Taulavuori et al., 2001).
Figure 2.
Lipid Peroxidation in Wild-Type and flu Seedlings.
Lipid peroxidation in 5-day-old wild-type seedlings grown under continuous light on NF-free medium (wt) or under high NF growth conditions (wt_NF) (A) and 5-day-old light-grown wild-type and flu seedlings transferred to the dark for 8 h and re-exposed to light for up to 3 h (B) was used as an indicator of oxidative stress and was determined by measuring formation of malondialdehyde (MDA). Results present the means and standard deviations of three biological samples. For each sample, 20 seedlings were used.
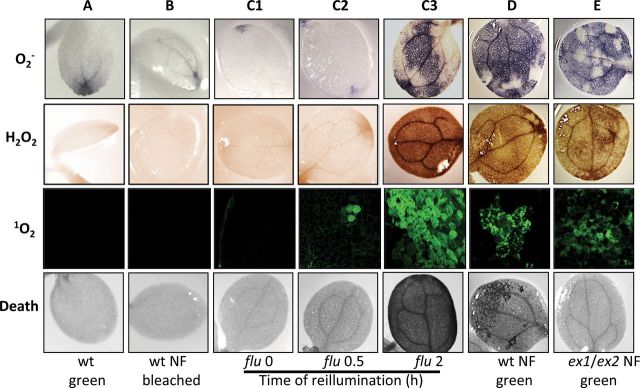
These results suggest that, in bleached seedlings grown from the very beginning in the presence of NF, photo-oxidative damage does not occur and, unlike the bleaching of flu seedlings, bleaching of these seedlings does not require 1O2-mediated and EX-dependent retrograde signaling. Transcript levels of the 1O2-responsive marker gene AAA-ATPase and ROS levels in NF-treated seedlings corroborated this conclusion. Transcript levels of AAA-ATPase in NF-treated wild-type, gun1, and ex1/ex2 seedlings were not up-regulated as in flu seedlings after a dark/light shift (Baruah et al., 2009), but were even slightly lower than in non-treated controls (Figure 1B). Accumulation of superoxide anion radical, H2O2, and 1O2 was visualized after staining NF-treated seedlings and non-treated control seedlings with nitro blue tetrazolium (NBT), 3,3’-diaminobenzidine (DAB), and singlet oxygen sensor green (SOSG), respectively (Flors et al., 2006; Ramel et al., 2009). In non-treated controls as well as in NF-treated bleached seedlings, these three ROS were not detectable (Figure 3A and 3B). In pre-darkened flu seedlings, however, 1O2 accumulation as seen by SOSG staining was detected already 30 min after the beginning of re-illumination prior to the onset of cell death, whereas accumulation of superoxide radical and H2O2 were first seen after 2 h of re-illumination in seedlings that had already initiated the 1O2-mediated and EX-dependent cell death response (Figure 3C1–3C3) (Kim et al., 2012).
Figure 3.
Generation of Reactive Oxygen Species (ROS) and Induction of Cell Death in Wild-Type Seedlings Kept on NF and flu Seedlings Exposed to a Dark/Light Shift.
(A, B) The accumulation of ROS and the induction of cell death in wild-type seedlings grown on NF-free (wt green) or 5 μM NF-containing medium (wt NF bleached) for 5 d. DAB, NBT, and SOSG were used to visualize the presence of superoxide anion radical (O2–), H2O2, 1O2, respectively, and trypan blue staining revealed cell death responses.
(C1–C3) As a control, 5-day-old light-grown flu seedlings were transferred to the dark for 8 h and re-exposed to light (0, 0.5, and 2 h). Notice that flu seedlings accumulate 1O2 within 30 min of re-illumination, whereas the accumulation of H2O2 and O2– is first seen after 2 h of light, when cell death has already been initiated.
(D, E) The impact of NF on ROS accumulation and cell death in light-grown wild-type (D) and ex1/ex2 (E) seedlings initially kept on NF-free medium for up to 5 d before treating them with 5 μM NF (wt NF green, ex1/ex2 NF green). Seedlings were kept in the dark for 12 h and re-exposed to light for up to 2 d. Each experiment was carried out with a total of 10 seedlings and for each seedling gave similar results.
Altogether, the results presented thus far do not support a signaling role of 1O2 in seedlings grown from the very beginning in the presence of NF and seem to be at variance with the view that bleaching of NF-treated seedlings is associated with photo-oxidative stress or damage. As carotenoids together with chlorophyll are required for the assembly of chlorophyll–protein complexes (Apel and Kloppstech, 1980; Bellemare et al., 1982; Plumley and Schmidt, 1987; Reinsberg et al., 2001) and carotenoid and tetrapyrrole synthesis is strongly suppressed in NF-treated seedlings (Chamovitz et al., 1991; Moulin et al., 2008), bleaching could simply result from an arrest of plastid development due to the failure of NF-treated seedlings to assemble thylakoid membranes.
1O2-Mediated Signaling in NF-Treated Green Seedlings
Consequences of NF treatment are very different, when seedlings were exposed to the inhibitor not before but after light-dependent chloroplast formation had been completed and seedlings were visibly green. Wild-type seedlings were initially grown for 5 d on half-strength MS agar medium under continuous light, before they were treated with 5 µM NF or a mock solution. They were then transferred to the dark for 12 h to allow the uptake of the inhibitor and its distribution within the plants to be completed, before seedlings were returned to continuous light for up to 120 h. By the end of this light period, growth and development of NF-treated seedlings had been severely retarded relative to control seedlings (Figure 4A). Non-treated wild-type seedlings had green cotyledons, fully developed first and emerging second true leaves. NF-treated seedlings, however, stopped growing shortly after the first true leaves had emerged and, by that time, had lost part of their chlorophyll. These seedlings suffered from severe photo-oxidative stress. They accumulated larger amounts of superoxide anion radical, H2O2 and 1O2 (Figure 3D) and at the same time showed extensive lesion formation on their leaves (Figures 3D, 4A, and 4B). The impact of photo-oxidative stress became evident already 1 d after starting the NF treatment and progressively increased over the next few days as indicated by the rapid decline of the maximum quantum efficiency of photosystem II expressed as the ratio of variable to maximum fluorescence of chlorophyll (Fv/Fm) (Figure 5A) and an increase in lipid peroxidation (Figure 5B). The cell death responses in these NF-treated seedlings could be a consequence of photo-oxidative damage due to a general increase in ROS levels or could be triggered more specifically by 1O2-mediated and EX-dependent programmed cell death signaling (Kim et al., 2012). To distinguish between these two modes of cell death, ex1/ex2 seedlings with fully developed chloroplasts were exposed to NF as described for wild-type. Non-treated ex1/ex2 seedlings were phenotypically indistinguishable from wild-type, whereas NF-treated ex1/ex2 and wild-type seedlings showed some differences (Figure 4A and 4B). NF-treated ex1/ex2 seedlings were developmentally arrested and chlorotic, they generated similar amounts of ROS, and showed a reduced maximum quantum efficiency of photosystem II and a similar increase in lipid peroxdation to NF-treated wild-type seedlings (Figures 4A, 5A, and 5B), but the necrotic lesions in cotyledons and true leaves of NF-treated wild-type seedlings were absent in NF-treated ex1/ex2 seedlings (Figure 4A). Staining with trypan blue revealed that NF-treated wild-type seedlings underwent extensive cell death, whereas, in NF-treated ex1/ex2 seedlings, this death response had been largely suppressed (Figures 3D, 3E, and 4B). This result was in line with the intactness of chloroplasts and the viability of cells of NF-treated ex1/ex2 seedlings as revealed by the distribution of GFP–SSU and by PI staining (Figure 4C). The GFP fluorescence was retained within chloroplasts and PI was present only in the extracellular space but not within the cell. In NF-treated wild-type seedlings, GFP fluorescence had leaked out of the chloroplasts and PI had penetrated most of the cells (Figure 4C).
Figure 4.
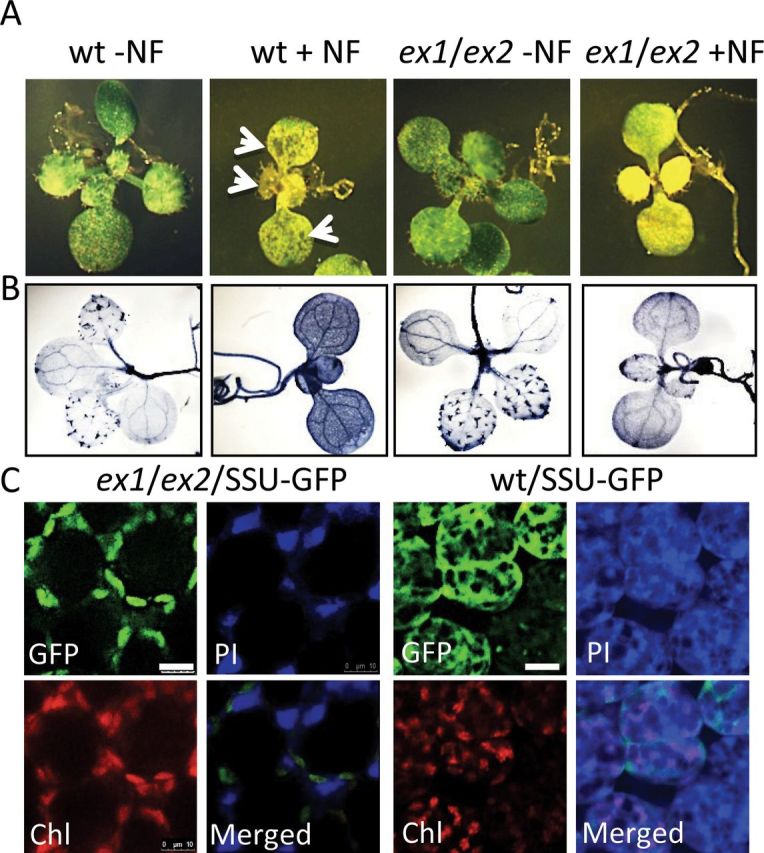
1O2-Mediated and EX-Dependent Cell Death Signaling in NF-Treated Seedlings.
(A) Wild-type (wt) and ex1/ex2 seedlings expressing SSU–GFP were initially grown for 5 d on NF-free MS agar medium at a photon flux density of 100 μmol photons m–2 s–1. They were then treated with a 5-μM NF (+NF) or NF-free mock solution (–NF), transferred to the dark for 12 h and then re-exposed to the light. Formation of necrotic lesions in NF-treated wild-type seedlings (arrowheads) is suppressed in NF-treated ex1/ex2 seedlings.
(B) The EX-dependent cell death response of seedlings shown in (A) as revealed by trypan blue staining. Blue spots in ‘wt –NF’ and ‘ex1/ex2 –NF’ control seedlings indicate trichomes of true leaves.
(C) Chloroplast integrity was monitored under the confocal laser scanning microscope in NF-treated ex1/ex2 and wild-type seedlings expressing the SSU–GFP reporter protein as described in Figure 1E. The fluorescence images of GFP, chlorophyll (Chl), and propidium iodine (PI) were taken 120 h after NF-treated seedlings had been re-exposed to light. Scale bar = 20 μm.
Figure 5.
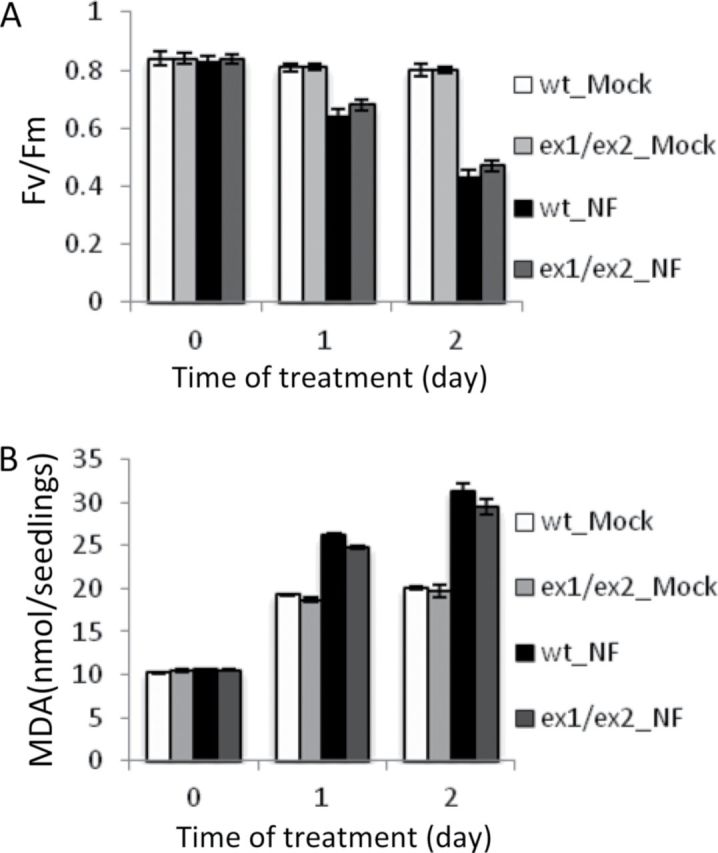
Impact of NF on Light-Grown Wild-Type and ex1/ex2 Seedlings Initially Kept on NF-Free Medium.
Photoinhibition of photosystem II (A) and lipid peroxidation (B) were analyzed by measuring maximum quantum efficiency (Fv/Fm) and MDA levels before (0) or 1 and 2 d after NF treatment. Note that the addition of the detergent Silwet L-77 to the NF and mock solutions led to an enhanced background level of MDA in mock-treated seedlings that remained constant during a 2-d incubation, but did not affect photosystem II activity. Results represent the means and standard deviations of three biological samples. For each sample, 20 seedlings were used.
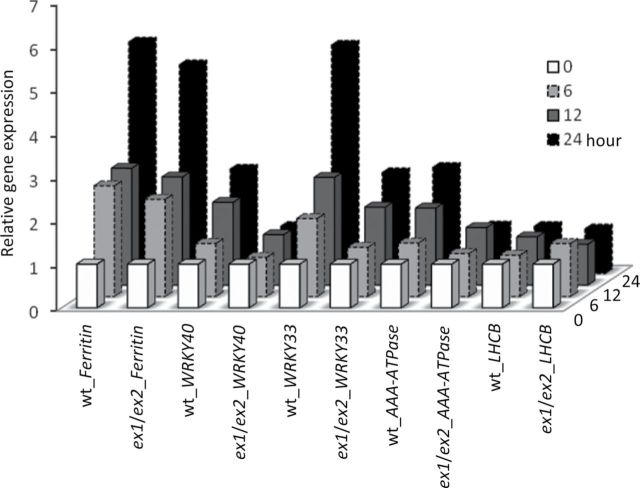
1O2-mediated and EX-dependent retrograde signaling in light-grown and green seedlings exposed to NF was confirmed by its impact on the expression of several nuclear marker genes. RNA was extracted from wild-type and ex1/ex2 seedlings either before or 6, 12, and 24 h after NF treatment. Changes in transcript levels of the H2O2-responsive FERRITIN1 (FER1), the 1O2-responsive WRKY33, WRKY40, and AAA-ATPase, and the light-responsive LHCB genes were determined by qPCR (Supplemental Table 1 and Figure 6). The results represent mean values of three independent biological replicas. Standard deviations of these values are given in Supplemental Table 2. The H2O2- and 1O2-responsive marker genes were rapidly up-regulated following the NF treatment. The up-regulation of AAA-ATPase, WRKY33, and WRKY40 in wild-type was suppressed in NF-treated ex1/ex2 seedlings, whereas the enhanced expression of FER1 in wild-type was not affected in ex1/ex2, in line with our earlier report that 1O2- and H2O2-dependent signaling operates via two distinct pathways (op den Camp et al., 2003). Unlike LHCB transcript levels in seedlings grown from the very beginning in the presence of NF, LHCB transcript levels in green seedlings exposed to NF after chloroplasts had been formed were not down-regulated, but remained constant throughout the first 24 h of NF treatment and were similar in wild-type and ex1/ex2 seedlings (Figure 6).
Figure 6.
NF-Induced Gene Expression Changes in Light-Grown Seedlings.
Expression level of H2O2– (FER1) and 1O2– (AAA-ATPase, WRKY33, and WRKY40) responsive marker genes in 5-day-old wild-type (wt) and ex1/ex2 seedlings treated with 5 μM NF before (0) or 6, 12, or 24 h after they had been re-exposed to light. LHCB gene expression changes have been included as a control. Three biological replicas were analyzed by qPCR and the mean values are shown. Data are normalized to the values measured in the mock-treated wild type at time 0. Standard deviations of these values are given in Supplemental Table 2.
DISCUSSION
For a number of years, NF-treated light-grown seedlings with non-functional chloroplasts have been established as an important model to study the role of a plastid factor that represses the expression of some of the nuclear genes encoding plastid proteins (Reiss et al., 1983; Mayfield and Taylor, 1984; Batschauer et al., 1986). Progress of this research was rapidly accelerated once genetic screens had been developed to define a genetic basis of this plastid-to-nucleus signaling in Arabidopsis (Susek et al., 1993). In particular, the isolation of genomes-uncoupled (gun) mutations that de-repress the expression of nuclear genes in NF-treated seedlings was an important step towards identifying putative constituents of a plastid-to-nucleus retrograde signaling pathway (Mochizuki et al., 2001; Larkin et al., 2003; Koussevitzky et al., 2007; Ruckle et al., 2007; Woodson et al., 2011). Most of the GUN genes encode proteins involved in various steps of tetrapyrrole synthesis, suggesting a close link between tetrapyrroles and retrograde signaling in NF-treated seedlings. This work culminated in the identification of Mg2+ProtoIX as a plastid signal and its translocation from the plastid to the surrounding cytoplasm, in line with its proposed function as a repressor of specific nuclear target genes (Strand et al., 2003; Ankele et al., 2007). However, subsequent studies failed to confirm these results and questioned the proposed signaling role of tetrapyrrole intermediates in NF-treated Arabidopsis seedlings (Mochizuki et al., 2008; Moulin et al., 2008). Instead of Mg2+ProtoIX, 1O2 was discussed as a possible elicitor of retrograde signaling in NF-treated seedlings (Moulin et al., 2008). In the present work, this proposed role of 1O2 has been analyzed and confirmed for green seedlings grown in the light for 5 d before they were treated with NF. A block of carotenoid synthesis in these seedlings is likely to increase the photosensitizing activity of tetrapyrroles and enhance 1O2 production and photo-oxidative stress. Indeed, following the uptake of NF, seedlings started to generate larger amounts of 1O2, H2O2, and superoxide (Figure 3), reduced the maximum quantum efficiency of photosystem II, underwent lipid peroxidation (Figure 5), and formed extensive lesions in cotyledons and emerging true leaves (Figure 4). At first glance, intracellular changes such as chloroplast leakage and the rupture of vacuoles and the following collapse of cells seemed to reflect severe photo-oxidative damage due to the cytotoxicity of 1O2 and other ROS generated in NF-treated plants. However, these responses were suppressed in NF-treated ex1/ex2 seedlings, demonstrating that 1O2-mediated and EX-dependent signaling was responsible rather than ROS directly (Figures 3 and 4). The onset of 1O2 production in flu leads to the rapid bleaching of seedlings, but bleaching was not seen in NF-treated wild-type seedlings. Experimental evidence of the present work suggests that 1O2-mediated and EX-dependent signaling in NF-treated wild-type seedlings does not operate alone as in flu but converges within a complex regulatory network with other signaling pathways that may modify consequences of 1O2-mediated signaling. One potential modifier of 1O2-mediated and EX-dependent signaling that may suppress bleaching of NF-treated seedlings is H2O2. As shown previously, a reduction of H2O2 levels in chloroplasts of the flu mutant accelerates the intensity of 1O2-mediated stress responses, indicating that H2O2 antagonizes 1O2-mediated signaling (Laloi et al., 2007). In NF-treated wild-type seedlings, not only 1O2, but also H2O2 and superoxide levels strongly increase. It seems likely that H2O2 may not only interfere with 1O2-mediated signaling, but also influences the overall response of NF-treated seedlings. 1O2-mediated stress responses such as lesion formation and loss of chloroplast integrity were abrogated in NF-treated ex1/ex2 seedlings, but growth and development of these seedlings were still retarded. Whereas expression of 1O2-responsive marker genes was suppressed in NF-treated ex1/ex2 seedlings, the H2O2-responsive FER1 gene was still activated and reached a similar high transcript level to NF-treated wild-type seedlings (Figure 6). Hence, in chloroplasts of light-grown green seedlings exposed to NF, at least two different retrograde signaling pathways were activated by the enhanced production of 1O2 and H2O2, respectively. Each of these signaling pathways seems to be responsible for distinct and specific stress responses of NF-treated seedlings. Whereas programmed cell death can be linked to 1O2-mediated signaling, it is not known yet whether growth inhibition and chlorosis are both exclusively triggered by the release of ROS or depend also on other plastid-derived signals.
In marked contrast to this first group of NF-treated seedlings, seedlings grown from the very beginning on NF-containing media bleached, but did not reveal detectable levels of ROS or photo-oxidative damage (Figures 1–3). As carotenoids do not only act as scavengers of excess light but together with chlorophyll play also an important role during the assembly of chlorophyll–protein complexes (Plumley and Schmidt, 1987; Reinsberg et al., 2001), bleaching of NF-treated, carotenoid-deficient seedlings may simply reflect suppression of chlorophyll accumulation and a perturbation of chloroplast development due to an impaired membrane assembly and the reported down-regulation of tetrapyrrole biosynthesis (Moulin et al., 2008). This conclusion, however, does not preclude the possibility that photo-oxidative stress confined to the beginning of seed germination in the presence of NF may initiate bleaching, when the emerging NF-treated seedling is first exposed to light. At this early stage of development, NF-treated seedlings start to accumulate anthocyanin, an indicator of light stress (Chalker-Scott, 1999). Furthermore, in 3-day-old NF-treated seedlings, the 1O2-responsive marker gene AAA-ATPase is up-regulated (Saini et al., 2011), whereas, in 5-day-old NF-treated seedlings, transcript levels of this gene have dropped to non-treated control levels or even lower (Figure 1B). Thus, it is conceivable that, at the very beginning of seedling development during the transformation of proplastids, a transient production of ROS may irreversibly disturb a critical step towards chloroplast formation and prevent the following greening of NF-treated seedlings. As shown previously, 1O2-mediated signaling may predetermine the fate of chloroplast differentiation in seedlings even prior to seed germination during late embryogenesis (Kim et al., 2009). Collectively, the results of the present study show that, depending on the developmental stage at which seedlings are first exposed to NF, several signaling pathways with different specificities may be activated. At least two of them, the H2O2- and 1O2-mediated signaling pathways, are not confined to NF-treated seedlings, but may also be activated in non-treated wild-type plants under light stress (Kim et al., 2012).
METHODS
Plant Materials and Growth Conditions
All mutant lines and wild-type of Arabidopsis thaliana were in a Col-0 background. The ex1/ex2 T-DNA mutants and gun1-1 EMS mutant have been previously characterized (Kim et al., 2009; Cottage et al., 2010). Seeds were surface-sterilized with 70% (v/v) ethanol, and plated on half-strength MS agar medium with or without 50 nM or 5 μM norflurazon (Sigma-Aldrich; www.sigmaaldrich.com/). Seeds were stratified at +4°C for 2 d and seedlings were grown for 5 d under continuous light at 20–21°C. Light intensities used are described in the figure legends. In some of the experiments, seedlings were exposed to NF only after chloroplast development had been completed and cotyledons were green. These seedlings were grown initially for 5 d under continuous moderate light (100 μmol photons m–2 s–1) on NF-free MS agar medium in Petri dishes. Seedlings were then drenched with a 5-μM NF solution or a NF-free mock solution, both containing 0.02% silwet L-77 (Molecular Probes Invitrogen, Memphis, USA), and submerged for 2 min before the solutions were poured off. For the following 12 h, the seedlings were kept in the dark, before they were re-exposed to light for up to 120 h.
To compare 1O2-mediated chloroplast leakage and cell death in bleached NF-treated wild-type and flu seedlings, wild type, flu, gun1, ex1/ex2, ex1/ex2/flu, and gun1/flu were grown under continuous light or 16-h light and 8-h dark cycles for up to 3 d.
Detection of Reactive Oxygen Species
NBT and 3,3’-diaminobenzidine (DAB) were used to detect cellular accumulation of superoxide anion radical and H2O2, respectively, as described by Ramel et al. (2009). Seedlings were vacuum infiltrated with either 0.5 mg ml–1 NBT in 10 mM potassium phosphate buffer (pH 7.8) or 1 mg ml–1 DAB in distilled H2O adjusted to pH 3.8 by adding HCl. The seedlings were then incubated in the dark at room temperature for 1 h (NBT) or under light for 8 h (DAB). Chlorophyll was subsequently removed by incubating seedlings with 95% ethanol at room temperature for several hours. Afterwards, the ethanol was replaced with 10% glycerol until the pictures were taken under a microscope. To detect 1O2 in seedlings, singlet oxygen sensor green (SOSG, Molecular Probes) reagent dissolved in methanol (5 mM stock solution) was diluted to a 260 µM working solution. After vacuum infiltration, seedlings were examined under the confocal microscope and SOSG signals in the presence of 1O2 were detected using 488 nm for excitation and 530 nm for emission.
Staining of Dead Cells
Trypan blue staining of seedlings was performed as described (op den Camp et al., 2003).
Determination of Malondialdehyde Content
Total amounts of malondialdehyde (MDA) were measured to assess the extent of lipid peroxidation in NF-treated seedlings. The level of MDA was determined according to Madhava and Sresty (2000).
Determination of the Chloroplast Integrity
Seedlings expressing the SSU–GFP trans-gene under control of the Cauliflower Mosaic Virus promoter (CaMV 35S) (Kim and Apel, 2004; Kim et al., 2012) were grown from the very beginning on NF-containing medium or initially grown on NF-free medium before they were sprayed with NF as described above. The chloroplast integrity in cotyledons was monitored under a Leica TCS-SP5 confocal microscope (Leica, www.leica.com). Cell death in seedlings was detected by staining with 50 µM propidium iodine (PI, Molecular Probes, Eugene, USA) that intercalates into nucleic acid of dying or dead cells, whereas it is excluded from intact cells and remains in the extracellular space. Subsequently, seedlings were washed with water two times and the fluorescence images of GFP, PI, and chlorophyll were obtained under confocal laser scanning microscope (CLSM) as previously described (Kim et al., 2012).
RNA Extraction and cDNA Synthesis
Total RNA was extracted from seedlings using an RNeasy plant mini kit (Qiagen, Hilden, Germany). cDNA was synthesized from 0.6 μg RNA, treated with DNase (Promega Corp., Madison, WI, USA) by using random primers (Promega) and Improm II reverse transcriptase (Promega) according to the manufacturer’s instructions.
Quantitative RT–PCR
For qRT–PCR, the BioRad iQ SYBR green supermix with all the primers at final concentrations of 0.2 µM was used. Triplicated qPCR reactions of three independent biological replicates were conducted. The samples were pre-heated for 3 min at 95°C; the cycling conditions were: 30 s at 95°C, and 45 s at 60°C; 40 cycles. Actin2 (At3g18780) was used as a reference gene to normalize expression data. AAA-ATPase (At3g28580), WRKY33 (At2g46400), and WRKY40 (At1g80840) were selected as 1O2-responsive marker genes, FER1 (At5g01600) was selected as a H2O2-responsive gene, and LHCB (At1g29910) was chosen as a marker gene for NF treatment. Primer sequences for these genes are shown in Supplemental Table 1.
SUPPLEMENTARY DATA
Supplementary Data are available at Molecular Plant Online.
FUNDING
This study was supported by the Boyce Thompson Institute for Plant Research and the National Institutes for Health, grant number R01-GM085036 (K.A.).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr John C. Gray (University of Cambridge, UK) for seeds of the gun1 mutant. We also thank BTI Plant Cell Imaging Center supported by NSF (DBI-0618969) and TRIAD Foundation. No conflict of interest declared.
REFERENCES
- Ankele E., Kindgren P., Pesquet E., Strand A. (2007). In vivo visualization of Mg-protoporphyrin IX, a coordinator of photosynthetic gene expression in the nucleus and the chloroplast. Plant Cell. 19, 1964–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K., Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399 [DOI] [PubMed] [Google Scholar]
- Apel K., Kloppstech K. (1980). The effect of light on the biosynthesis of the light-harvesting chlorophyll a/b protein: evidence for the requirement of chlorophyll a for the stabilization of the apoprotein. Planta. 150, 426–430 [DOI] [PubMed] [Google Scholar]
- Baruah A., Simková K., Apel K., Laloi C. (2009). Arabidopsis mutants reveal multiple singlet oxygen signaling pathways involved in stress response and development. Plant Mol. Biol. 70, 547–563 [DOI] [PubMed] [Google Scholar]
- Batschauer A., Mösinger E., Kreuz K., Dörr I., Apel K. (1986). The implication of a plastid-derived factor in the transcriptional control of nuclear genes encoding the light-harvesting chlorophyll a/b protein. Eur. J. Biochem. 154, 625–634 [DOI] [PubMed] [Google Scholar]
- Bellemare G., Bartlett S.G., Chua N.-H. (1982). Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J. Biol. Chem. 257, 7762–7767 [PubMed] [Google Scholar]
- Chalker-Scott L. (1999). Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 70, 1–9 [Google Scholar]
- Chamovitz D., Pecker I., Hirschberg J. (1991). The molecular basis of resistance to the herbicide norflurazon. Plant Mol. Biol. 16, 967–974 [DOI] [PubMed] [Google Scholar]
- Cottage A., Mott E.K., Kempster J.A., Gray J.C. (2010). The Arabidopsis plastid-signalling mutant gun1 (genomes uncoupled1) shows altered sensitivity to sucrose and abscisic acid and alterations in early seedling development. J. Exp. Bot. 61, 3773–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Winkelhüsener T. (1982). Nature of photooxidative events in leaves treated with chlorosis-inducing herbicides. Plant Physiol. 70, 1277–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flors C., Fryer M.J., Waring J., Reeder B., Bechtold U., Mullineaux P.M., Nonell S., Wilson M.T., Baker N.R. (2006). Imaging the production of singlet oxygen in vivo using a new fluorescence sensor, Singlet Oxygen Sensor Green ®. J. Exp. Bot. 57, 1725–1734 [DOI] [PubMed] [Google Scholar]
- Gollnick K. (1968). Type II photooxygenation reactions in solution. Advan. Photochem. 6, 1–122 [Google Scholar]
- Goslings D., Meskauskiene R., Kim C., Lee K.P., Nater M., Apel K. (2004). Concurrent interactions of heme and FLU with GLU tRNA reductase (HEMA1), the target of metabolic feedback inhibition of tetrapyrole biosynthesis, in dark- and light-grown Arabidopsis plants. Plant J. 40, 957–967 [DOI] [PubMed] [Google Scholar]
- Gray J.C. (2003). Chloroplast-to-nucleus signalling: a role for Mg-protoporphyrin. Trends in Genetics. 19, 526–529 [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J.M.C. (1999). Free Radicals in Biology and Medicine. 3rd edn (Oxford, UK: Oxford University Press; ). [Google Scholar]
- Heath R.L., Packer L. (1968). Peroxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198 [DOI] [PubMed] [Google Scholar]
- Hideg E., Kalai T., Kos P.B., Asada K., Hideg K. (2006). Singlet oxygen in plants: its significance and possible detection with double (fluorescent and spin) indicator reagents. Photochem. Photobiol. 82, 1211–1218 [DOI] [PubMed] [Google Scholar]
- Hu G., Yalpani N., Briggs S.P., Johal G.S. (1998). A porphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize. Plant Cell. 10, 1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Apel K. (2004). Substrate-dependent and organ-specific chloroplast protein import in planta . Plant Cell. 16, 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Lee K.P., Baruah A., Nater M., Göbel C., Feussner I., Apel K. (2009). 1O2-mediated retrograde signaling during late embryogenesis predetermines plastid differentiation in seedlings by recruiting abscisic acid. Proc. Natl Acad. Sci. U S A. 106, 9920–9924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Meskauskiene R., Zhang S., Lee K.P., Lakshmanan A.M., Blajecka K., Herrfurth C., Feussner I., Apel K. (2012). Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell. 24, 3026–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J.P., Dodge A.D. (1985). Singlet oxygen and plants. Phytochemistry. 24, 889–896 [Google Scholar]
- Koussevitzky S., Nott A., Mockler T.C., Hong F., Sachetto-Martins G., Surpin M., Lim J., Mittler R., Chory J. (2007). Signals from chloroplasts converge to regulate nuclear gene expression. Science. 316, 715–719 [PubMed] [Google Scholar]
- Laloi C., Stachowiak M., Pers-Kamczyc E., Warzych E., Murgia I., Apel K. (2007). Crosstalk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana . Proc. Natl Acad. Sci. U S A. 104, 672–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin R.M., Alonso J.M., Ecker J.R., Chory J. (2003). GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science. 299, 902–906 [DOI] [PubMed] [Google Scholar]
- Lee K.P., Kim C., Landgraf F., Apel K. (2007). EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana . Proc. Natl Acad. Sci. U S A. 104, 10270–10275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister D. (2012). Retrograde signaling in plants: from simple to complex scenarios. Front Plant Sci. 3, 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wakao S., Fischer B.B., Niyogi K.K. (2009). Sensing and responding to excess light. Annu. Rev. Plant Biol. 60, 239–260 [DOI] [PubMed] [Google Scholar]
- Mach J.M., Castillo A.R., Hoogstraten R., Greenberg J.T. (2001). The Arabidopsis accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc. Natl Acad. Sci. U S A. 98, 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhava R.K.V., Sresty T.V. (2000). Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci. 157, 113–128 [DOI] [PubMed] [Google Scholar]
- Mayfield S.P., Taylor W.C. (1984). Carotenoid-deficient maize seedlings fail to accumulate light-harvesting chlorophyll a/b binding protein (LHCP) mRNA. Eur. J. Biochem. 144, 79–84 [DOI] [PubMed] [Google Scholar]
- Meskauskiene R., Nater M., Goslings D., Kessler F., op den Camp R., Apel K. (2001). FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana . Proc. Natl Acad. Sci. U S A. 98, 12826–12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N., Brusslan J.A., Larkin R., Nagatani A., Chory J. (2001). Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl Acad. Sci. U S A. 98, 2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N., Tanaka R., Tanaka A., Masuda T., Nagatani A. (2008). The steady-state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis . Proc. Natl Acad. Sci. U S A. 105, 15184–15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock H.P., Heller W., Molina A., Neubohn B., Sandermnann H., Grimm B. (1999). Expression of uroporphyrinogen decarboxylase or coproporphyrinogen oxidase antisense RNA in tobacco induces pathogen defense responses conferring increased resistance to tobacco mosaic virus. J. Biol. Chem. 274, 4231–4238 [DOI] [PubMed] [Google Scholar]
- Moulin M., McCormac A.C., Terry M.J., Smith A.G. (2008). Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc. Natl Acad. Sci. U S A. 105, 15178–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S., Hilbert B., Dueckershoff K., Roitsch T., Krischke M., Mueller M.J., Berger S. (2008). general detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis . Plant Cell. 20, 768–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott A., Jung H.S., Koussevitzky S., Chory J. (2006). Plastid-to-nucleus retrograde signaling. Annu. Rev. Plant Biol. 57, 739–759 [DOI] [PubMed] [Google Scholar]
- op den Camp R., Przybyla D., Ochsenbein C., Laloi C., Kim C., Danon A., Wagner D., Hideg E., Göbel C., Feussner I., et al. (2003). Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis . Plant Cell. 15, 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumley F.G., Schmidt G.W. (1987). Reconstitution of chlorophyll a/b light-harvesting complexes: xanthophyll-dependent assembly and energy transfer. Proc. Natl Acad. Sci. U S A. 84, 146–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla D., Göbel C., Imboden A., Hamberg M., Feussner I., Apel K. (2008). Enzymatic, but not non-enzymatic, 1O2-mediated peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1-dependent stress response program in the flu mutant of Arabidopsis thaliana . Plant J. 54, 236–248 [DOI] [PubMed] [Google Scholar]
- Ramel F., Birtic S., Ginies C., Soubgou-Taconnat L., Triantaphylidès C., Havaux M. (2012). Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl Acad. Sci. U S A. 109, 5535–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F., Sulmon C., Bogard M., Couéel I., Gouesbet G. (2009). Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biology. 9, 28–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsberg D., Ottmann K., Booth P.J., Paulsen H. (2001). Effects of chlorophyll a, chlorophyll b, and xanthophylls on the in vitro assembly of the major light-harvesting chlorophyll a/b complex, LHCIIb. J. Mol. Biol. 308, 59–67 [DOI] [PubMed] [Google Scholar]
- Reiss T., Bergfeld R., Link G., Thien W., Mohr H. (1983). Photooxidative destruction of chloroplasts and its consequences to cytosolic enzyme levels and plant development. Planta. 159, 518–528 [DOI] [PubMed] [Google Scholar]
- Ruckle M.E., DeMarco S.M., Larkin R.M. (2007). Plastid signals remodel light signaling networks and are essential for efficient chloroplast biogenesis in Arabidopsis . Plant Cell. 19, 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar A.D., Briggs W.R. (1990). Effects of high light stress on carotenoid-deficient chloroplasts in Pisum sativum . Plant Physiol. 94, 1663–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini G., Meskauskiene R., Pijacka W., Roszak P., Sjögren L.L., Clarke A.K., Straus M., Apel K. (2011). ‘happy on norflurazon’ (hon) mutations implicate perturbance of plastid homeostasis with activating stress acclimatization and changing nuclear gene expression in norflurazon-treated seedlings. Plant J. 65, 690–702 [DOI] [PubMed] [Google Scholar]
- Strand A., Asami T., Alonso J., Ecker J.R., Chory J. (2003). Chloroplast-to- nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature. 421, 79–83 [DOI] [PubMed] [Google Scholar]
- Surpin M., Larkin R.M., Chory J. (2002). Signal transduction between the chloroplast and the nucleus. Plant Cell. 14, S327–S338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek R.E., Ausubel F.M., Chory J. (1993). Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell. 74, 787–799 [DOI] [PubMed] [Google Scholar]
- Taulavuori E., Hellström E.K., Taulavuori K., Laine K. (2001). Comparison of two methods used to analyze lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J. Exp. Bot. 52, 2375–2380 [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C., Krischke M., Hoeberichts F.A., Ksas B., Gresser G., Havaux M., Van, Breusegem F., Mueller M.J. (2008). Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 148, 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt C., Oster U., Börnke F., Jahns P., Dietz K.J., Leister D., Kleine T. (2010). In-depth analysis of the distinctive effects of norflurazon implies that tetrapyrrole biosynthesis, organellar gene expression and ABA cooperate in the GUN-type of plastid signaling. Physiol. Plant. 138, 503–519 [DOI] [PubMed] [Google Scholar]
- Wagner D., Przybyla D., op den Camp R., Kim C., Landgraf F., Lee K.P., Würsch M., Laloi C., Nater M., Hideg E., et al. (2004). The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana . Science. 306, 1183–1185 [DOI] [PubMed] [Google Scholar]
- Woodson J.D., Perez-Ruiz J.M., Chory J. (2011). Heme synthesis by plastid ferrochelatase I regulates nuclear gene expression in plants. Curr. Biol. 21, 897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-W., Yuan S., Feng H., Xu F., Cheng J., Shang J., Zhang D.-W., Lin H.-H. (2011). Transient accumulation of Mg-protoporphyrin IX regulates expression of PhANGs: new evidence for the signaling role of tetrapyrroles in mature Arabidopsis plants. J. Plant Physiol. 168, 714–721 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.