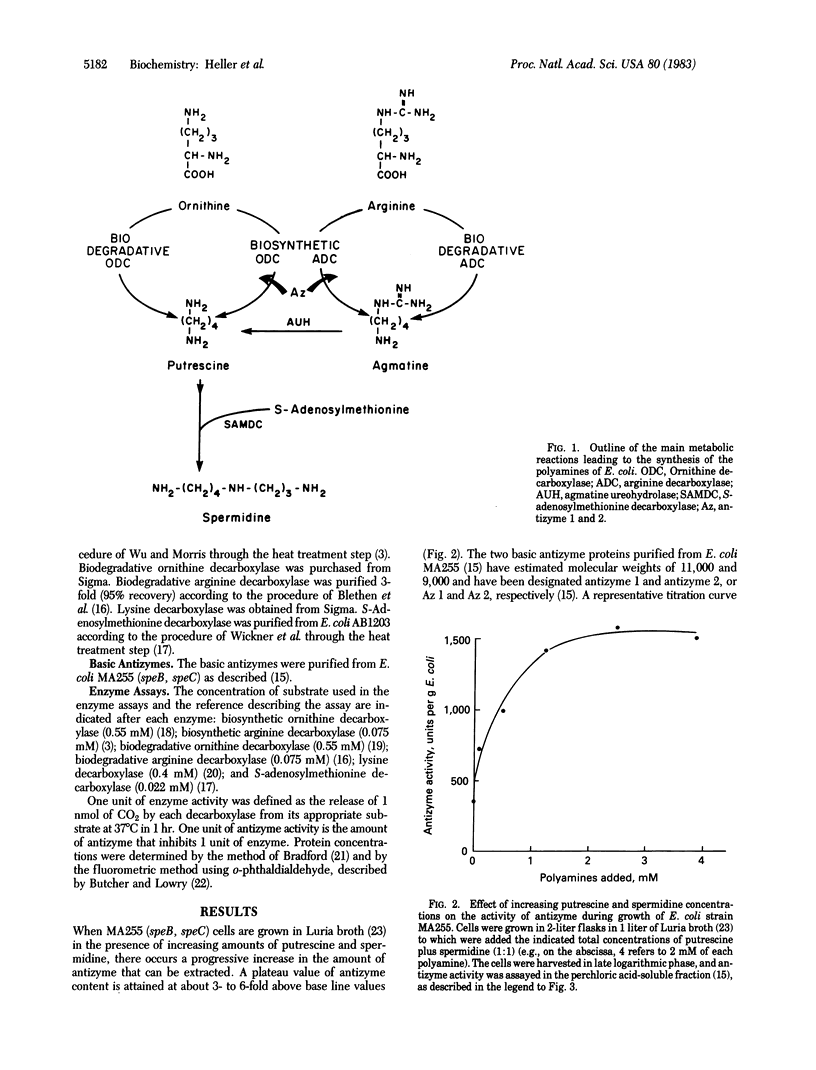
Abstract
In Escherichia coli, the biosynthetic ornithine and arginine decarboxylases (EC 4.1.1.17 and 4.1.1.19, respectively) are responsible for the biosynthesis of polyamines from ornithine and arginine, respectively. When E. coli cells are grown in the presence of increasing amounts of polyamines, a progressive increase in the amount of antizyme 1 and antizyme 2 occurs. The amino acid compositions of antizymes 1 and 2 show them to be basic proteins; antizyme 1 has an amino acid composition similar to that of the E. coli histone-like protein HU and of the eukaryotic histone H2B; antizyme 2 is characterized by an unusually high arginine content. We find these proteins to be specific inhibitors of both the biosynthetic ornithine decarboxylase and the biosynthetic arginine decarboxylase. They do not inhibit the corresponding biodegradative ornithine and arginine decarboxylases, nor do they inhibit lysine decarboxylase or S-adenosylmethionine decarboxylase. These properties of the antizymes favor their function in the regulation of polyamine biosynthesis in E. coli. The ability of the purified antizymes to inhibit the ornithine and arginine decarboxylases is stabilized in acidic buffers and is lost upon prolonged exposure to solutions at neutral or basic pH.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum D. M., Dunlap J. C., Morris D. R. Comparison of the biosynthetic and biodegradative ornithine decarboxylases of Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1580–1584. doi: 10.1021/bi00627a008. [DOI] [PubMed] [Google Scholar]
- Applebaum D. M., Dunlap J. C., Morris D. R. Comparison of the biosynthetic and biodegradative ornithine decarboxylases of Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1580–1584. doi: 10.1021/bi00627a008. [DOI] [PubMed] [Google Scholar]
- Berthold V., Geider K. Interaction of DNA with DNA-binding proteins. The characterization of protein HD from Escherichia coli and its nucleic acid complexes. Eur J Biochem. 1976 Dec 11;71(2):443–449. doi: 10.1111/j.1432-1033.1976.tb11132.x. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., McCann P. P., Sjoerdsma A. Restriction of bacterial growth by inhibition of polyamine biosynthesis by using monofluoromethylornithine, difluoromethylarginine and dicyclohexylammonium sulphate. Biochem J. 1982 Nov 15;208(2):435–441. doi: 10.1042/bj2080435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blethen S. L., Boeker E. A., Snell E. E. Argenine decarboxylase from Escherichia coli. I. Purification and specificity for substrates and coenzyme. J Biol Chem. 1968 Apr 25;243(8):1671–1677. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Lowry O. H. Measurement of nanogram quantities of protein by hydrolysis followed by reaction with orthophthalaldehyde or determination of glutamate. Anal Biochem. 1976 Dec;76(2):502–523. doi: 10.1016/0003-2697(76)90343-2. [DOI] [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Gale E. F. The production of amines by bacteria: The decarboxylation of amino-acids by strains of Bacterium coli. Biochem J. 1940 Mar;34(3):392–413. doi: 10.1042/bj0340392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. S., Canellakis E. S. Cellular control of ornithine decarboxylase activity by its antizyme. J Cell Physiol. 1981 May;107(2):209–217. doi: 10.1002/jcp.1041070206. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Pispa J. Ornithine decarboxylase from Escherichia coli: stimulation of the enzyme activity by nucleotides. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1165–1171. doi: 10.1016/0006-291x(72)90957-6. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Pispa J. The regulation of polyamine synthesis during the stringent control in Escherichia coli. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1104–1111. doi: 10.1016/s0006-291x(74)80092-6. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Lutz H., Kornberg A. Novel histone H2A-like protein of escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5097–5101. doi: 10.1073/pnas.77.9.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-a-Monofluoromethylputrescine is a potent irreversible inhibitor of Escherichia coli ornithine decarboxylase. Biochem J. 1982 Jun 15;204(3):771–775. doi: 10.1042/bj2040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., McCann P. P. Difluoromethylornithine irreversibly inactivates ornithine decarboxylase of Pseudomonas aeruginosa, but does not inhibit the enzymes of Escherichia coli. Biochem J. 1981 Oct 15;200(1):69–75. doi: 10.1042/bj2000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakidis D. A., Heller J. S., Canellakis E. S. Modulation of ornithine decarboxylase activity in Escherichia coli by positive and negative effectors. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4699–4703. doi: 10.1073/pnas.75.10.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L. Putrescine biosynthesis in Escherichia coli. Regulation through pathway selection. J Biol Chem. 1969 Nov 25;244(22):6094–6099. [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. Multiple pathways of putrescine biosynthesis in Escherichia coli. J Biol Chem. 1966 Jul 10;241(13):3129–3135. [PubMed] [Google Scholar]
- Pegg A. E., McGill S. Decarboxylation of ornithine and lysine in rat tissues. Biochim Biophys Acta. 1979 Jun 6;568(2):416–427. doi: 10.1016/0005-2744(79)90310-3. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Gros F. Characterization of a novel, low-molecular-weight DNA-binding protein from Escherichia coli. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière-Yaniv J. Localization of the HU protein on the Escherichia coli nucleoid. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):439–447. doi: 10.1101/sqb.1978.042.01.047. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Formation of 1,4-diaminobutane and of spermidine by an ornithine auxotroph of Escherichia coli grown on limiting ornithine or arginine. J Biol Chem. 1969 May 10;244(9):2286–2292. [PubMed] [Google Scholar]
- Wickner R. B., Tabor C. W., Tabor H. Purification of adenosylmethionine decarboxylase from Escherichia coli W: evidence for covalently bound pyruvate. J Biol Chem. 1970 Apr 25;245(8):2132–2139. [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Purification and properties. J Biol Chem. 1973 Mar 10;248(5):1687–1695. [PubMed] [Google Scholar]


