Abstract
The FDA-approved drug suberoylanilide hydroxamic acid (SAHA, Vorinostat) was modified to improve its selectivity for a single histone deaetylase (HDAC) isoform. We show that attaching an ethyl group at the C3 position transforms SAHA from non-selective to an HDAC6-selective inhibitor. Theses results indicate that small structural changes in SAHA can significantly influence selectivity, which will lead future anti-cancer design efforts targeting HDAC proteins.
Keywords: histone deaceylases, HDAC inhibitors, Vorinostat, isoform selectivity
Histone deacetylase (HDAC) proteins are key enzymes that regulate the acetylation status of histone lysine residues to regulate transcription. The overexpression of HDAC proteins occurs in some cancer cells and results in aberrant transcription of certain genes.1 The HDAC protein family consists of 18 members and is divided into four classes.2 Class I includes HDAC1, HDAC2, HDAC3, and HDAC8. Class II includes HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, and HDAC10. Class IV includes HDAC11 as the sole member because it displays similarities to both class I and class II. Class III are NAD+-dependent proteins, referred to as sirtuins (SIRT1–7).3 Class I, II, and IV are metal ion-dependent proteins and are sensitive to the inhibitors in this work. Class I HDAC proteins are found in cancers, including ovarian (HDAC1, HDAC2, and HDAC3),4 gastric (HDAC2),5 and lung cancers (HDAC1 and 3).6 Overexpression of class II HDAC6 was observed in breast and ovarian cancer tissues.7, 8 As a result, several HDAC inhibitor (HDACi) drugs are in clinical trials for treatment of cancer.9, 10
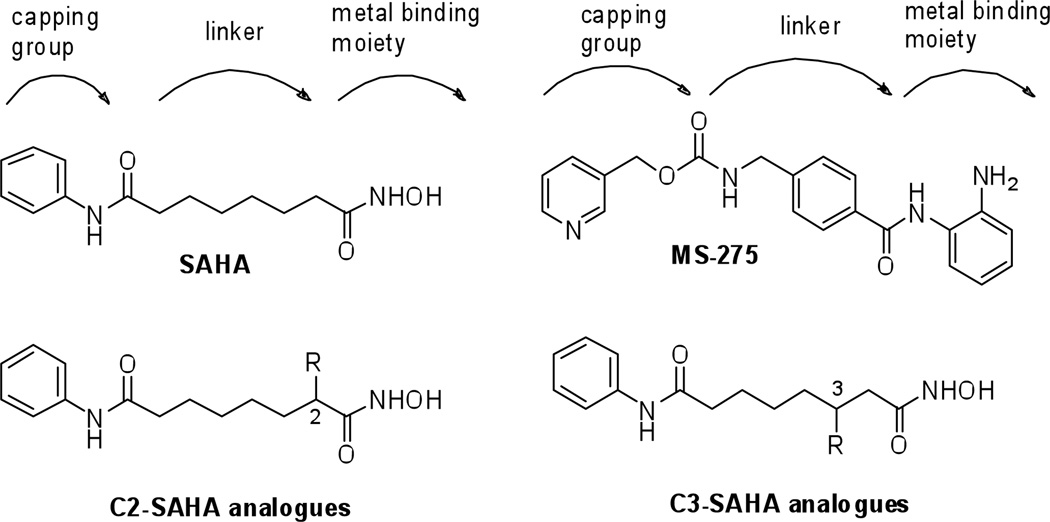
Most HDAC inhibitors consist of a capping group that is solvent-exposed, a carbon linker that is surrounded by a hydrophobic tunnel, and a metal binding moiety that is buried in the protein active site (Figure 1). SAHA (Figure 1, suberoylanilide hydroxamic acid, Vorinostat) was the first HDACi approved by the FDA for the treatment of cutaneous T-cell lymphoma.11 Unfortunately, SAHA nonspecifically inhibits the eleven metal-dependent HDAC proteins.12 In contrast, MS-275 displays selectivity for class I HDAC proteins.13, 14 MS-275 contains a different metal binding moiety than SAHA (benzamide vs. hydroxamic acid) and an aryl ring in the linker region (Figure 1). The intra-chain aryl group of MS-275 suggests that selectivity may be influenced by the structure of the linker region. Despite the possible influence of the linker on selectivity, few studies rigorously probe the potency and selectivity of linker region analogues.15
Figure 1.
Structures of metal-dependent HDAC inhibitors: SAHA, MS-275, and the C2- and C3-SAHA analogues
To explore the impact of substituents in the linker region, we previously studied SAHA analogues with hydrophobic substituents attached on the C2 position (Figure 1).16 In this case, inhibitor potency was significantly reduced regardless of substituent size. The lack of potency of the C2-SAHA analogues indicates that limited flexibility exists in the HDAC active site near the hydroxamic acid group. In contrast, high potency (nM range) HDAC inhibitors have been created with bulky substituent near the solvent-exposed region.17–19 Therefore, we propose that HDAC proteins would be more tolerant of SAHA analogues containing substituents positioned closer to the solvent exposed surface.
To systematically probe the impact of substituents present in the linker, SAHA analogues with substituents on the C3 position (Figure 1) were synthesized. Like the C2-SAHA analogues, we selected hydrophobic substituents due to the hydrophobic nature of the HDAC active site near the C3 position.20–22 We theorized that analogues with substituents attached at the C3 position would display more potent inhibition compared to analogues with C2 substituents due to their location closer to the solvent-exposed region. In addition, we sought to test the influence of C3 substituents on the isoform selectivity of SAHA, given the possible influence of the intra-aryl group on the Class I selectivity of MS-275 (Figure 1). The ability to modulate the isoform selectivity of the FDA-approved SAHA by simply attaching a linker substituent would be useful and enable the study of isoform selectivity on inhibitor efficacy and toxicity in a clinical setting.23
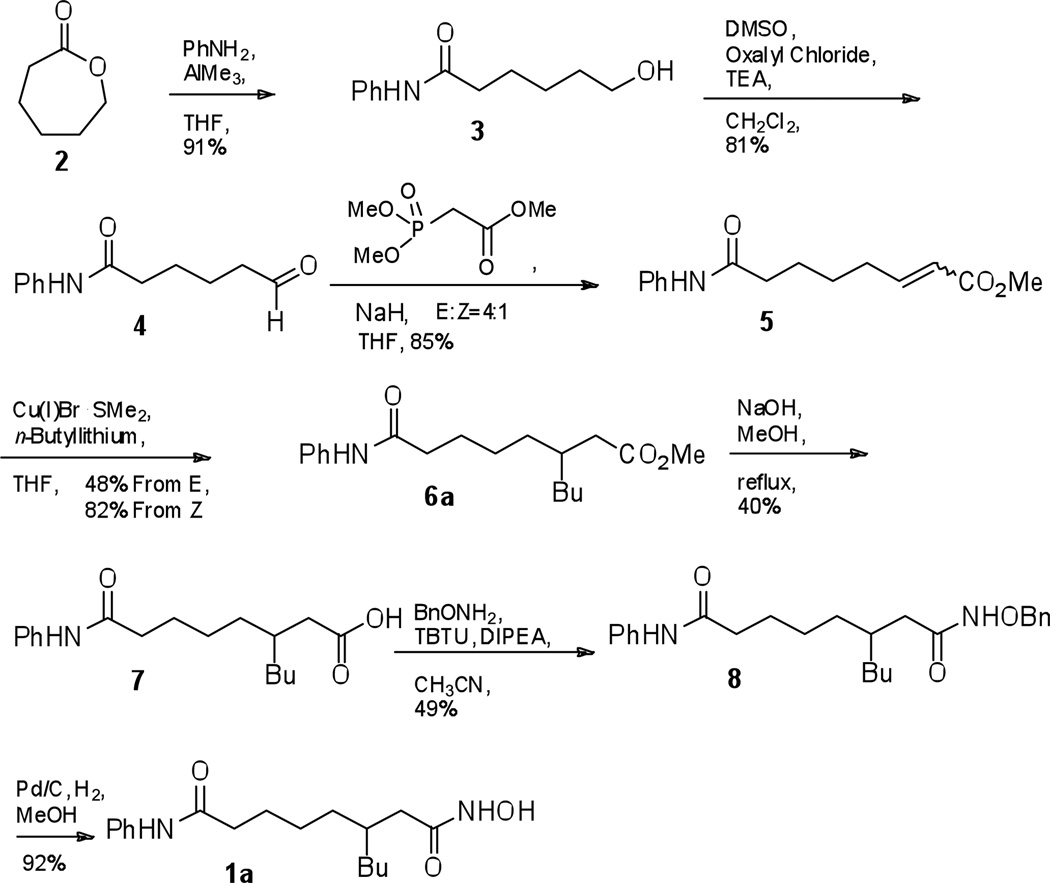
We initially synthesized the C3-n-butyl SAHA analogue 1a, as shown in Scheme 1. The ring of ε-caprolactone 2 was opened with aniline and trimethyl aluminum to give alcohol 3, which was subjected to Swern oxidation to give aldehyde 4. The Horner-Wadsworth-Emmons reaction with trimethyl phosphonoate gave the corresponding α,β-unsaturated ester 5. The (E)- and (Z)-isomers of ester 5 were separated by column chromatography and then individually treated with a copper (I) bromide dimethylsulfide complex to give the n-butyl ester 6a. Saponification of 6a gave carboxylic acid 7, which was coupled with O-benzyl-protected hydroxamine. O-benzyl-protected hydroxamic acid 8 was deprotected by hydrogenolysis to give the C3-n-butyl SAHA 1a final product.
Scheme 1.
Initial synthesis of C3-n-butyl SAHA (1a)
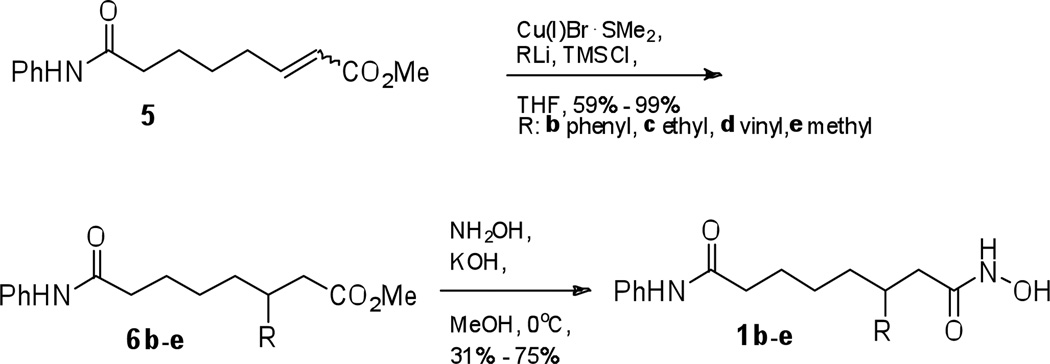
To create the remaining C3-SAHA analogues, several aspects of the synthetic scheme were improved. First, the 1,4-conjugate addition reaction (5 to 6) was performed using a mixture of (E) and (Z) isomers without the separation (Scheme 2). Second, we found that when preparing compound 6e from methyl lithium, no addition product was observed. However, addition of trimethylsilane chloride (TMSCl) to the reaction gave excellent yield (Scheme 2).24 With this success, TMSCl was included in the addition reactions with all remaining analogues. Finally, we used a direct, one-step conversion of ester 6 to the final product 1 (Scheme 2).25 In the synthesis of C3-n-butyl SAHA 1a, a benzyl-protected hydroxamic acid intermediate 8 was used en route to the hydroxamic acid final product, as previously reported.16 However, 40% yield after three steps (saponification, coupling O-benzyl hydroxylamine, and benzyl deprotection) was unsatisfying. The direct conversion using neutralized hydroxylamine in methanol was more efficient compared to the three-step conversion and was employed for all remaining analogues. Using these modified conditions, the methyl, vinyl, ethyl, and phenyl analogues 1b–e were synthesized (Scheme 2).
Scheme 2.
Optimized synthesis of C3-SAHA analogues
The inhibitory activities of the C3-SAHA analogues were measured using the Fluor de LysTM in vitro fluorescence activity assay kit (Biomol) using HeLa cell lysates as the source of HDAC activity (Table 1, Figure S1).16 The methyl variant 1e was the most potent analogue, displaying an IC50 of 350 nM, which is only 4-fold less potent than SAHA (90 nM). These results indicate that the active site of HDAC proteins can accommodate a small methyl substituent at the C3 position. The potency of the remaining analogues decreased with increasing size of the C3 substituent. The n-butyl and phenyl analogues (1a and 1b) displayed the weakest inhibitory activity (184 µM and 73µM, respectively). Interestingly, the ethyl-substituted analogueue 1c displayed 91-fold decreased activity compared to the methyl analogue 1e, despite containing only one additional methylene. Likewise, the vinyl analogue 1d showed significantly reduce activity compared to the methyl analogue 1e. In total, the data indicate that a C3-methyl substituted SAHA analogue maintains submicromolar potency, but substituents larger than methyl result in a reduction in potency.
Table 1.
HDAC inhibiton by SAHA, MS-275, and the C3-SAHA analogues using HeLa cell lysates
| Compounds | R | IC50, µM [a] |
|---|---|---|
| SAHA | 0.09 ± 0.004 | |
| MS-275 | 3.2 ± 0.1 | |
| 1a | n-butyl | 184 ± 14 |
| 1b | phenyl | 73 ± 14 |
| 1c | ethyl | 32 ± 4 |
| 1d | vinyl | 15 ± 1 |
| 1e | methyl | 0.35 ± 0.05 |
Values are the mean of three experiments with standard error.
The inhibition results are consistent with the hypothesis that linker substituents are accommodated in the HDAC active site when positioned closer to the solvent exposed capping group of SAHA. While the C3-methyl analogue displayed potency comparable to SAHA (4-fold reduced), the previously reported C2-methyl analogue displayed 1488-fold reduced activity versus SAHA (IC50 of 134 µM).16 Interestingly, the C3-n-butyl variant 1a is less potent (184 µM IC50) than the previously reported C2-n-butyl analogue (72 µM IC50),16 suggesting that the area of the HDAC active site near the C2 and C3 linker positions displays structural differences.
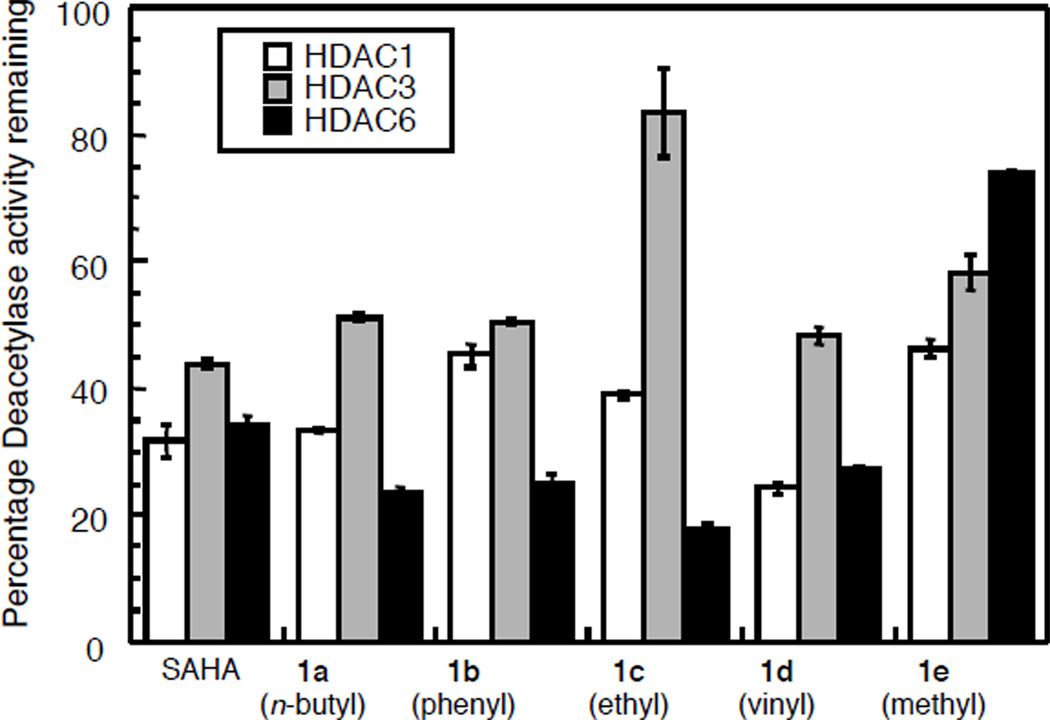
We next tested the isoform selectivity of the C3-SAHA analogues. Creating isoform selective HDAC inhibitors has been challenging.15 However, the availability of selective inhibitors would provide powerful chemical tools to dissect the individual functions of the HDAC isoforms, in addition to providing lead antitumor drug candidates. To assess the isoform selectivity of the C3-SAHA analogues, HDAC1 and HDAC3 representing class I and HDAC6 representing class II were tested at a single concentration near to their IC50 values using the Fluor de LysTM kit (Figure 2, Table S1). As expected, SAHA almost equally inhibited HDAC1, HDAC3, and HDAC6.12 The methyl variant 1e showed a slight preference for HDAC1 over HDAC3 and HDAC6 at 375 nM. In contrast, the ethyl variant 1c showed greater potency for HDAC6 over HDAC1 and HDAC3 at 32 µM. The butyl, phenyl, and vinyl variants (1a, 1b, and 1d) also showed similar, although more modest, preference for HDAC6 over HDAC3.
Figure 2.
Screen of C3-SAHA analogues against HDAC1, HDAC3, and HDAC6 with 125 nM SAHA, 32 µM 1a–1d, and 375 nM 1e.
To more rigorously assess the selectivity observed in the initial screen, we determined the IC50 values of the C3-ethyl variant 1c because it displayed the most promising results in the initial screen (Table 2, Figures S3). The C3-ethyl analogue 1c displayed 12-fold selectivity for HDAC6 over HDAC3 and 3-fold selectivity for HDAC6 over HDAC1. In addition, it displayed selectivity within class I, with 4-fold preference for HDAC1 over HDAC3. As a control, SAHA displayed similar inhibitor activity against the isoforms, as expected (Table 2, Figures S2).12 The isoform selectivity analysis shows that a substituent on the C3 position can transform SAHA from non-selective inhibitor to an HDAC6-selective one. As a comparison, the HDAC6-selective inhibitor tubacin displays 7-fold selectivity for HDAC6 over HDAC126 and has been used widely in cell biology studies.27, 28 Therefore, the data indicate that isoform selective SAHA analogues can be generated by attaching a substituent to the linker chain.
Table 2.
IC50 values of SAHA and the C3-ethyl SAHA variant 1c for HDAC1, HDAC3, and HDAC6
| Compounds | IC50, µM [a] | ||
|---|---|---|---|
| HDAC1 | HDAC3 | HDAC6 | |
| SAHA | 0.096 ± 0.016 | 0.146 ± 0.012 | 0.074 ± 0.009 |
| 1c | 22 ± 2 | 97 ± 6 | 8 ± 1 |
Values are the mean of three experiments with standard error.
In summary, SAHA analogues with substituents on the C3 position displayed HDAC6-selective inhibition, in contrast to the broad-spectrum inhibitor SAHA. These results reveal that small structural changes in the linker region of SAHA can significantly influence selectivity. Selective HDAC inhibitors are powerful tools to elucidate the individual functions of the HDAC isoforms.27, 28 In addition, isoform selective inhibitors have the potential to improve therapeutic outcomes in the clinic compared to broad spectrum inhibitors. HDAC6 has known involvement in cancer progression,29 making HDAC6-selective inhibitors of great interest. Despite the involvement of HDAC6 in disease states, there are relatively few examples of HDAC6-selective inhibitors.15, 30–32 SAHA analogues with HDAC6 selectivity will be useful to develop new and more effective anti-cancer drugs.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM067657) and Wayne State University for funding and Dr. A. Bieliauskas, Dr. I. Lysenka, J. H. Lee, and E. Aubie for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary material, including experimental procedures, compound characterization, and biological evaluation, are available online.
References and notes
- 1.Yoo CB, Jones PA. Nat Rev Drug Discov. 2006;5:37. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 2.Gregoretti I, Lee Y-M, Goodson HV. J. Mol. Biol. 2004;338:17. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Grozinger CM, Schreiber SL. Chem. Biol. 2002;9:3. doi: 10.1016/s1074-5521(02)00092-3. [DOI] [PubMed] [Google Scholar]
- 4.Khabele D, Son DS, Parl AK, Goldberg GL, Augenlicht LH, Mariadason JM, Rice VM. Cancer Biology & Therapy. 2007;6:795. doi: 10.4161/cbt.6.5.4007. [DOI] [PubMed] [Google Scholar]
- 5.Song J, Noh JH, Lee JH, Eun JW, Ahn YM, Kim SY, Lee SH, Park WS, Yoo NJ, Lee JY, Nam SW. APMIS. 2005;113:264. doi: 10.1111/j.1600-0463.2005.apm_04.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartling B, Hofmann HS, Boettger T, Hansen G, Burdach S, Silber RE, Simm A. Lung Cancer. 2005;49:145. doi: 10.1016/j.lungcan.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi SI, Itoh A, Funata N, Schreiber SL, Yoshida M, Toi M. Oncogene. 2005;24:4531. doi: 10.1038/sj.onc.1208646. [DOI] [PubMed] [Google Scholar]
- 8.Bazzaro M, Lin Z, Santillan A, Lee MK, Wang M-C, Chan KC, Bristow RE, Mazitschek R, Bradner J, Roden RBS. Clinical Cancer Research. 2008;14:7340. doi: 10.1158/1078-0432.CCR-08-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer OH, Gottlicher M, Heinzel T. Trends Endocrinol. Metab. 2001;12:294. doi: 10.1016/s1043-2760(01)00438-6. [DOI] [PubMed] [Google Scholar]
- 10.Garber K. Nat Biotech. 2007;25:17. doi: 10.1038/nbt0107-17. [DOI] [PubMed] [Google Scholar]
- 11.Grant S, Easley C, Kirkpatrick P. Nat Rev Drug Discov. 2007;6:21. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- 12.Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian X, Mills E, Berghs SC, Carey N, Finn PW, Collins LS, Tumber A, Ritchie JW, Jensen PB, Lichenstein HS, Sehested M. Biochem J. 2008;409:581. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- 13.Hu E, Dul E, Sung C-M, Chen Z, Kirkpatrick R, Zhang G-F, Johanson K, Liu R, Lago A, Hofmann G, Macarron R, De Los Frailes M, Perez P, Krawiec J, Winkler J, Jaye M. J Pharmacol Exp Ther. 2003;307:720. doi: 10.1124/jpet.103.055541. [DOI] [PubMed] [Google Scholar]
- 14.Beckers T, Burkhardt C, Wieland H, Gimmnich P, Ciossek T, Maier T, Sanders K. International Journal of Cancer. 2007;121:1138. doi: 10.1002/ijc.22751. [DOI] [PubMed] [Google Scholar]
- 15.Bieliauskas AV, Pflum MKH. Chem. Soc. Rev. 2008;37:1402. doi: 10.1039/b703830p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bieliauskas A, Weerasinghe S, Pflum MH. Bioorg Med Chem Lett. 2007;17:2216. doi: 10.1016/j.bmcl.2007.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones P, Altamura S, Chakravarty PK, Cecchetti O, Francesco RD, Gallinari P, Ingenito R, Meinke PT, Petrocchi A, Rowley M, Scarpelli R, Serafini S, Steinkuhler C. Bioorg Med Chem Lett. 2006;16:5948. doi: 10.1016/j.bmcl.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Furumai R, Komatsu Y, Nishino N, Khochbin S, Yoshida M, Horinouchi S. Proc Natl Acad Sci U S A. 2001;98:87. doi: 10.1073/pnas.011405598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Kapustin G, Etzkorn FA. J. Med. Chem. 2007;50:2003. doi: 10.1021/jm061082q. [DOI] [PubMed] [Google Scholar]
- 20.Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Pavletich NP. Nature. 1999;401:188. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 21.Somoza JR, Skene RJ, Katz BA, Mol C, Ho JD, Jennings AJ, Luong C, Arvai A, Buggy JJ, Chi E, Tang J, Sang B-C, Verner E, Wynands R, Leahy EM, Dougan DR, Snell G, Navre M, Knuth MW, Swanson RV, McRee DE, Tari LW. Structure. 2004;12:1324. doi: 10.1016/j.str.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Vannini A, Volpari C, Filocamo G, Casavola EC, Brunetti M, Renzoni D, Chakravarty P, Paolini C, Francesco RD, Gallinari P, Steinkuhler C, Marco SD. Proc Natl Acad Sci U S A. 2004;101:15064. doi: 10.1073/pnas.0404603101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karagiannis TC, El-Osta A. Leukemia. 2006;21:61. doi: 10.1038/sj.leu.2404464. [DOI] [PubMed] [Google Scholar]
- 24.Hanessian S, Chahal N, Giroux S. The Journal of Organic Chemistry. 2006;71:7403. doi: 10.1021/jo061098o. [DOI] [PubMed] [Google Scholar]
- 25.Zhu L, Lauchli R, Loo M, Shea KJ. Organic Letters. 2007;9:2269. doi: 10.1021/ol070397c. [DOI] [PubMed] [Google Scholar]
- 26.Estiu G, Greenberg E, Harrison CB, Kwiatkowski NP, Mazitschek R, Bradner JE, Wiest O. J. Med. Chem. 2008;51:2898. doi: 10.1021/jm7015254. [DOI] [PubMed] [Google Scholar]
- 27.Hideshima T, Bradner JE, Wong J, Chauhan D, Richardson P, Schreiber SL, Anderson KC. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8567. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namdar M, Perez G, Ngo L, Marks PA. Proc. Natl. Acad. Sci. U S A. 2010;107:20003. doi: 10.1073/pnas.1013754107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y, Barrientos T, Ordentlich P, Wang XF, Counter CM, Yao TP. Cancer Res. 2008;68:7561. doi: 10.1158/0008-5472.CAN-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ontoria JM, Altamura S, Di Marco A, Ferrigno F, Laufer R, Muraglia E, Palumbi MC, Rowley M, Scarpelli R, Schultz-Fademrecht C, Serafini S, SteinkuÃàhler C, Jones P. Journal of Medicinal Chemistry. 2009;52:6782. doi: 10.1021/jm900555u. [DOI] [PubMed] [Google Scholar]
- 31.Schäfer S, Saunders L, Schlimme S, Valkov V, Wagner JM, Kratz F, Sippl W, Verdin E, Jung M. ChemMedChem. 2009;4:283. doi: 10.1002/cmdc.200800196. [DOI] [PubMed] [Google Scholar]
- 32.Auzzas L, Larsson A, Matera R, Baraldi A, Deschênes-Simard B, Giannini G, Cabri W, Battistuzzi G, Gallo G, Ciacci A, Vesci L, Pisano C, Hanessian S. J. Med. Chem. 2010;53:8387. doi: 10.1021/jm101092u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.