Abstract
Vascular compromise and the accompanying perfusion deficits cause or complicate a large array of disease conditions and treatment failures. This has prompted the exploration of therapeutic strategies to repair or regenerate vasculatures thereby establishing more competent microcirculatory beds. Growing evidence indicates that an increase in vessel numbers within a tissue does not necessarily promote an increase in tissue perfusion. Effective regeneration of a microcirculation entails the integration of new stable microvessel segments into the network via neovascularization. Beginning with angiogenesis, neovascularization entails an integrated series of vascular activities leading to the formation of a new mature microcirculation and includes vascular guidance and inosculation, vessel maturation, pruning, arterio-venous specification, network patterning, structural adaptation, intussusception, and microvascular stabilization. While the generation of new vessel segments is necessary to expand a network, without the concomitant neovessel remodeling and adaptation processes intrinsic to microvascular network formation, these additional vessel segments give rise to a dysfunctional microcirculation. While many of the mechanisms regulating angiogenesis have been detailed, a thorough understanding of the mechanisms driving post-angiogenesis activities specific to neovascularization has yet to be fully realized, but is necessary in order to develop effective therapeutic strategies for repairing compromised microcirculations as a means to treat disease.
INTRODUCTION
Effective adult tissue neovascularization, whether by native or therapeutic means, results in an expanded vascular network and increased blood perfusion pathway length resulting in the appropriate delivery of more blood to tissues. While often considered synonymous with angiogenesis (formation of new vessels from existing vessels), neovascularization involves a much broader series of temporally controlled vascular processes beginning with angiogenesis and progressing through multiple phases resulting in the formation of a new functional circulatory network. At the onset of neovascularization, relevant microvessel segments relax their stable vessel structure and initiate vessel sprouting leading to the formation of new vessel segments. Subsequently, the newly formed neovessels remodel via vascular cell differentiation and incorporation of perivascular cells into the newly formed vessel walls resulting in the appropriate density and distribution of arterioles, venules, and capillaries. Finally, the newly formed vascular network matures and remodels into a more efficient perfusion circuit that meets tissue perfusion needs and function.
While there is no one stereotypical vascular architecture, microvascular networks generally involve a branched network of progressively smaller caliber small arteries/arterioles at the inflow side delivering blood to the distal capillaries which subsequently drain into a branched network of increasingly larger caliber outflow venules/small veins. However, there are variations of this basic network organization, often reflecting tissue and/or organ specific function. As examples, the hepatic distal microcirculation is somewhat less ordered, reflecting more a plexus of homogeneous diameter blood perfusion pathways [12] and the nephron microcirculation in the kidney consists of arterioles organized into unique, convoluted bundles forming the glomeruli prior to emptying into the capillaries of the vasa recta [200]. Despite these tissue-specific aspects, each of the three general vascular compartments (arterioles, capillaries, and venules) performs different functions in the microcirculation due to their unique structural and functional characteristics and their locations within the vasculature. Arterioles provide the greatest resistance to blood flow in the vascular circuit with most of this resistance attributed to 1st and 2nd branch order arterioles [155] (Box 1). This is primarily due to the relative larger diameter differences between the feeding arteries and the smaller arterioles and the relative fewer numbers of these proximal arterioles. The more prevalent downstream and terminal arterioles act to broadly distribute blood throughout the tissue and control, via vessel tone dynamics, blood flow into the most distal capillaries (Figure 1). The very small diameters and large numbers of capillaries make them ideal for supporting effective blood-tissue exchange. Finally, venules, due in part to a relatively more compliant wall, serve as a high capacitance drainage system. Importantly, in a competent microcirculatory bed, as vessel diameters reduce within a vascular compartment the number of vessels in that compartment increase due to branching. This results in a sufficiently large enough cross-sectional area to keep resistance to blood flow across the compartment relatively low even though resistance within a single vessel segment might be high (due to the inverse relationship between resistance and the 4th power of the radius). Thus, to maintain proper resistances across the microvasculature, and therefore effective perfusion, proper branch ordering is critical. In addition, blood flow distribution in a tissue depends on the extent of branching in a logarithmic fashion [156]. This normalized relationship between vessel caliber and vessel numbers (i.e. branching) is a critical feature of functional microvascular network architectures. Mismatches in this relationship lead to poor hemodynamic function typically observed as hypo-perfusion and/or hypoxia within the tissue.
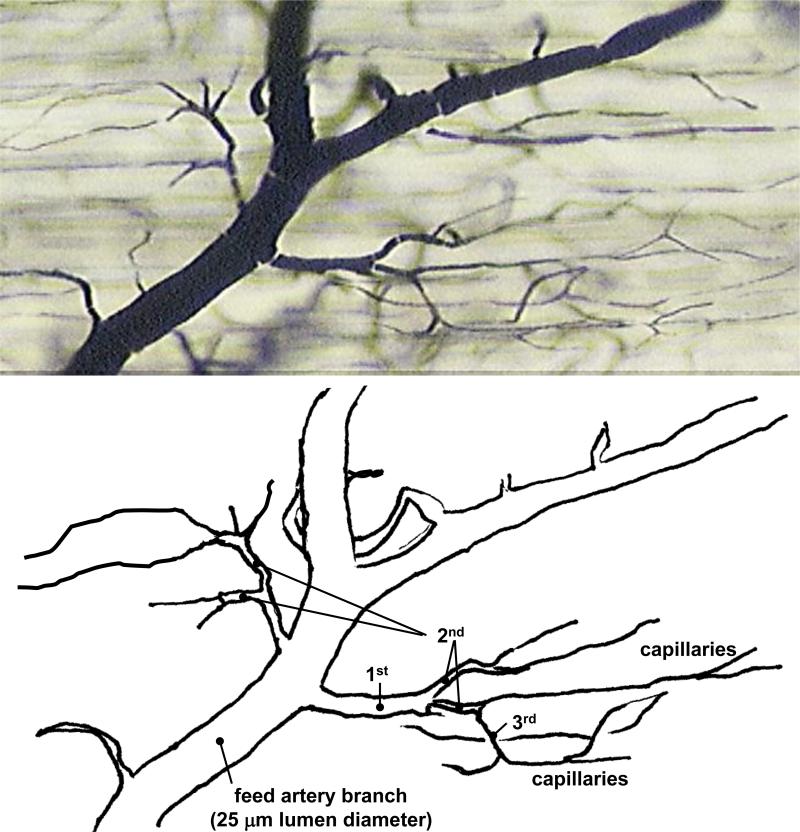
Figure 1.
Vascular ink cast of a normal, hierarchical microvasculature in the mouse gracilis skeletal muscle showing a distal branch of the feed artery and as much as 3 orders of arteriole branches leading to the capillaries. In larger species, there are often more than 3 orders of branching from the feed artery to the distal capillaries.
VASCULAR DEFICIT AND REPAIR
Deficits in blood perfusion (e.g. ischemia, hypoxia) are a cause of and/or complication associated with a number of disease states including tissue infarction, necrosis, wound healing, tissue grafting, and organ dysfunction. In addition to the more familiar examples of perfusion deficit such as stroke [175], myocardial infarction [119], peripheral vascular disease [137], and diabetes [57, 77], vascular insufficiencies are a significant component of renal disease [72], retinopathies [19, 42, 47], fibro-proliferative disorders [46, 86], chronic ischemia [14, 63], hypertension [112, 164], and others. Furthermore, vascular rarefaction of mature microcirculations, a consequence of microvascular instability, is thought to contribute to tissue hypo-perfusion in diabetes, hypertension fibrotic disorders, pulmonary diseases and others [63, 81, 112, 202]. The mechanisms underlying microvascular rarefaction are not fully understood, but perturbations in vascular endothelial growth factor (VEGF) homeostasis appear important [141]. In the postnatal retina, diminished levels of VEGF secondary to hyperoxic conditions lead to vascular regression [10]. Also, iatrogenic rarefaction of microvascular beds is possible following anti-VEGF therapy [122, 123]. However, mural cell dysfunction can also contribute to instability in the mature microcirculation and contribute to rarefaction [236]. Thus, it appears that a tissue must maintain an appropriate VEGF “tone” in balance with other tissue parameters, such as perivascular cell maturation and tissue PO2 (which impacts VEGF dynamics), in order to maintain proper vascular density and microcirculatory stability [141].
Clinically, a limited number of strategies are employed to address perfusion deficits and involve revascularization interventions to open conduit pathways, pharmacological vasodilation of the existing vasculature, or pro-angiogenesis strategies. While revascularization procedures and vasodilation drugs are routinely used clinically, implementation of pro-angiogenesis therapies has yet to be fully realized as this relatively new approach continues to be researched, developed and tested. Unlike current standard therapies, which target one aspect of the vascular dysfunction and initially rely on the existing vascular infrastructure, pro-angiogenesis strategies represent a more comprehensive approach at re-establishing or regenerating the vascular tree. Regardless of the therapeutic strategy employed to treat vascular deficits, the goal is to re-establish and/or expand the perfusion capacity of the target vasculature, thereby promoting the health of the compromised tissues.
Pro-angiogenesis therapies
Therapeutic Angiogenesis
In therapeutic angiogenesis, the intent is to induce formation of new vessel pathways for increased perfusion to a tissue bed. These pathways can serve as collateral feed and drainage vessels supporting an existing distal microvascular bed (i.e. collateralization) and/or expand the entire vascular unit via the addition of new exchange microvessels [11, 111, 185]. The primary strategy in therapeutic angiogenesis has been the delivery of angiogenic factors, such as VEGF and fibroblast growth factor (FGF), to the affected tissues as a means to stimulate new vessel production [162]. An alternate but complementary approach has been to deliver/recruit cells with pro-angiogenic potential, such as macrophages using granulocyte colony-stimulating factor (G-CSF) delivered to the tissue site [62]. Many of these therapies have been explored in Phase I clinical trials addressing myocardial ischemia and chronic limb ischemia with some moving on to Phase II status [11, 162, 165]. For example, findings that increased collateral indices, perfusion, and coronary reserve occurred in VEGF-treated MI hearts in animal models [151, 180], lead to Phase I and II studies testing the safety and efficacy of VEGF-based therapeutic angiogenesis [98, 152] such as the VIVA (Vascular Endothelial Growth Factor in Ischemia for Vascular Angiogenesis) trial, one of the first large Phase II therapeutic angiogenesis trials completed. Unexpectedly patients in the VIVA trial with myocardial ischemia received placebo or doses of recombinant VEGF via intracoronary infusion, but found no significant perfusion improvements up to 4 months follow-up [98]. Similarly, no significant improvements in perfusion were observed in other large clinical trials utilizing other growth factor therapies to treat myocardial ischemia/infarction, including the Kuopio Angiogenesis Trial [92] and Euroinject One Trial [126] among others [62, 101]. A consensus opinion continues to be that while preclinical studies were very promising, clinical efficacy has been ambiguous. In many of these clinical trials, treated patients exhibited signs of improved vascular status early following treatment (given either as a bolus administration or over time) [25, 214]. However, subsequent later assessments showed that indices associated with improved perfusion were comparable to placebo treatments [15, 115, 152]. While there are potentially many reasons for the unexpected outcomes (e.g. discrepancies in preclinical disease models, patient selection, mode of delivery, timing of delivery, etc.) the trial findings highlight the therapeutic challenges related to forming a new, functional and stable circulation.
Exercise has proven to be an effective means by which to therapeutically promote neovascularization in a compromised tissue. For example, a prescribed daily exercise program of walking and increased activity alleviates pain and improves limb function in patients with peripheral artery disease and the concomitant muscle ischemia [100]. Based primarily on pre-clinical studies, the clinical improvement is thought to be due, in part, to improved perfusion secondary to increases in both artery numbers and capillarity via flow adaptation mechanisms and elevated angiogenic factors, respectively [193, 240]. An important aspect of the exercise-induced adaptation in these patients is the increase in collateral flow secondary to arteriogenesis [22]. Presumably, comparable increases in venous outflow vessels occur as well. An important aspect of the neovascularization associated with therapeutic exercise, and exercise in general, is that all aspects of the vascular tree, not just the smaller distal microvessels, expand proportionally.
Vascular Regeneration
An alternate strategy to using angiogenic factors for establishing a new microvasculature is the use of engineered vascular cell systems [150]. Most commonly involving a “vasculogenesis” approach in which vascular cells are assembled within a scaffold, microvascular precursors serve to launch the neovascularization process; the assembled simple vascular tubes progress to form true microvessels and microvascular networks following implantation. Angiogenic factors, while not always required in these systems, are often included to promote additional angiogenesis and vascular cell survival [83, 102]. Clearly, the incorporation of endothelial cells alone (particularly human endothelial cells) into a tissue scaffold does not effectively accelerate the formation of a stable microvasculature once implanted. However, the presence of additional perivascular cells or precursors, such as smooth muscle cells, mesenchymal smooth muscle precursors (e.g. 10T1/2 cells), and/or tissue stromal cells, in the engineered system promotes neovascularization and leads to long-term microvascular stability [47, 146, 167]. This is perhaps best highlighted in the use of isolated, intact microvessel segments, which retain the native microvessel structure [105], to rapidly form a mature, functional microcirculation in vivo (Figure 2) [172, 212]. While the perivascular cells in these composite vascular tissue constructs are playing multiple roles related to neovascularization, an important function of these cells is to maintain neovessel stability [47, 102, 225, 226].
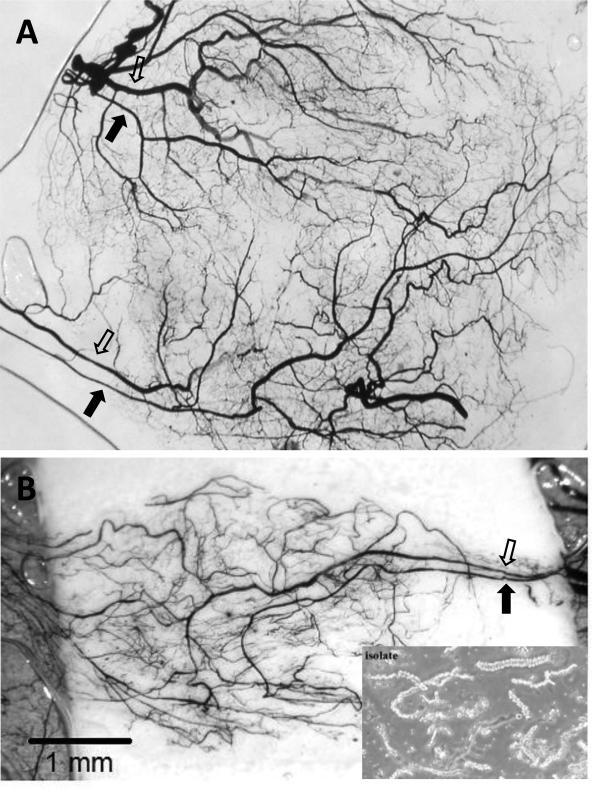
Figure 2.
Two separate examples (A and B) of regenerated microvascular beds derived from implanted isolated microvessels visualized by india ink casting and tissue clearing. In both examples, paired inflow (closed arrow) and outflow (open arrow) pathways developed a branched microvascular tree. Notice that in (A), two “microvascular units” evolved from the initial implanted parent microvessel source (lower right inset). Because the microvasculatures derived from these implants progress in a regimented fashion in the absence of additional stromal cells, they are useful for investigating angiogenesis and post-angiogenesis processes and mechanisms. The implants consisted of isolated, intact microvessel segments suspended in polymerized, 3-dimensional, type I collagen gels and implanted subcutaneously for 4 weeks in mice.
Alternatively, the direct manipulation of endothelial cells promoting cell survival is also effective. For example, microvessel formation and survival were considerably enhanced when the ECs used to assemble the implant were genetically manipulated to over-express the anti-apoptotic protein Bcl-2 [171, 206]. The ability of the newly formed vasculature to carry blood is often assessed in these implanted engineered systems commonly via the use of blood tracers or intravascular lectin tags [38, 172]. However, the extent of perfusion, in terms of blood volume flows or flow rates, has yet to be systematically evaluated.
An additional approach at bolstering compromised microvasculatures has involved the in situ regeneration of vessels using vascular cell precursors and regenerative cells. Bone marrow-derived cells, including monocytes, and circulating endothelial cell progenitors were some of the first therapeutic cells to be explored for this application [199]. However, there has been an explosion in the breadth of cell types and cell sources now being employed including pluripotent stem cells [40]. Recently, adult adipose-derived cells have emerged as potential vascular regenerative cells. Either as fresh isolates or following selective culturing, adipose-derived cells significantly improve ischemic repair in animal models and are currently in clinical trials [104, 145, 161, 246]. While capable of contributing to microvascular repair through a number of mechanisms [17, 195], these cells can regenerate new vessel segments in vivo directly via vascular assembly [131].
Vessel Density-Perfusion Relationship
Many pro-angiogenesis strategies are based on the idea that generation of more vessels segments will lead to an improved vascular status thereby mitigating the perfusion deficit. However, while a repairing tissue may have a high microvascular density, there may not necessarily be more tissue perfusion. Solid tumors contain regions of higher-than-normal microvessel densities yet perfusion through these chaotic networks is limited [48, 190]. Granulation tissue formed during chronic inflammation represents pathologic microvascular enrichment (the high vessel density gives the tissue its “granular” appearance in histological sections [84]), yet perfusion is heterogeneous, complicated by areas of flow stasis and blood pooling [48, 56]. Similarly, many tissues undergoing post-ischemic repair exhibit elevated vessel densities (often assessed by histological methods), but perfusion through the tissue is less than expected. This is commonly observed in mouse ischemic hindlimb experiments in which the vessel densities in the affected hindlimb may be as high as twice that in the non-repairing contralateral hindlimb, yet perfusion is approximately half of that in the contralateral hindlimb [43, 221]. Similar observations have been made in post-ischemic myocardial tissues in which elevated numbers of vessels are observed in the peri-infarct myocardium, yet resting and maximal perfusion in these regions is well below normal [21, 69, 144, 168, 196]. Endothelial cell dysfunction in the repairing/regenerating vasculature is likely a primary underlying cause [8, 128, 148, 198, 218, 234]. For example, acetylcholine-dependent relaxation of arterioles, a common indicator of vascular function [71], is compromised in ischemic myocardium [210] and post-ischemic repairing mouse hindlimbs [30]. This dysfunction likely translates into faulty flow control as reactive or functional hyperemic blood flow in rodent ischemic hindlimbs is diminished or altered [29, 109, 221] (Figure 3). Similarly, coronary functional perfusion reserve (associated with an increase in heart workload and used clinically to evaluate both the structural and functional integrity of the microcirculation) can be compromised in the peri-infarct regions of infarcted rat hearts, despite an elevated vessel density [69, 144, 168]. Altered network architectures could also potentially contribute to lower perfusion competencies in tissues as highlighted by the tumor vasculature. Often comprised of tortuous microvessels with disproportional diameters connected to each other in a non-hierarchical manner, the network architecture in a tumor is clearly atypical and is associated with poor perfusion [190, 204].
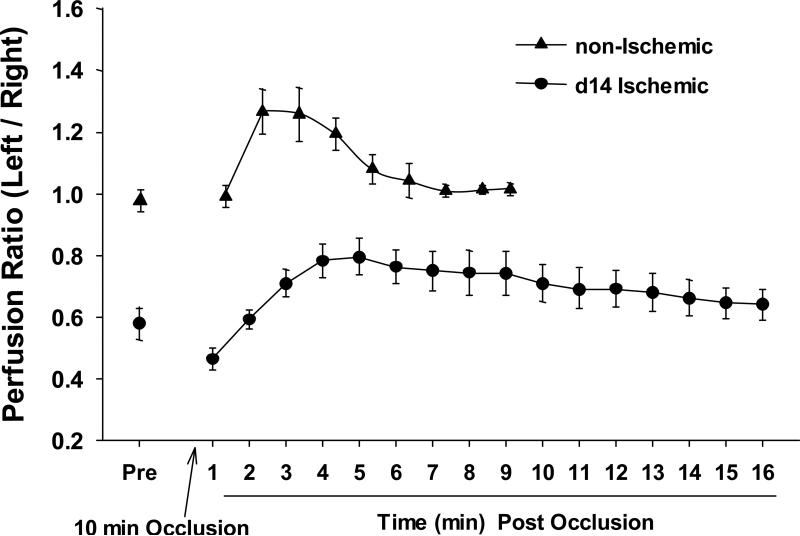
Figure 3.
Altered flow control in mouse ischemic hindlimb neovasculatures. Laser Doppler perfusion imaging was used to assess the relative perfusion of normal and 14 day post-ischemic mouse hindlimbs following a reactive hyperemia challenge (n = 6). For the challenge, the proximal iliac artery to the affected left hindlimb was briefly occluded with a ligature. Immediately following the occlusion, the lower limb was imaged by laser Doppler and the relative perfusion of the challenged left limb to the contralateral, unaffected right limb was determined.
Interestingly, this apparent uncoupling between perfusion and vessel density does not occur in vascular beds expanded via physiological (i.e. non-pathological) angiogenesis. For example, exercise- or direct muscle stimulation leads to an increase in skeletal muscle vascularity with a proportional increase in muscle bed perfusion [58, 59]. Exercise-induced muscle hypertrophy is accompanied by a relative increase in capillarity without a significant change in topology features, such as mean segment length [217]. The concomitant increase in arteriole numbers with increases in capillary densities [89, 90], which would act to maintain a normalized network architecture [60], as well as preserve vessel tone [116] may explain why perfusion and vessel density are balanced during exercise-induced neovascularization. In the growing intestinal wall of the weaned juvenile rat, the number of arterioles and the number of arteriole branches remains constant over the course of 4 weeks of organ growth [227]. Similarly, while not measured directly, capillarity likely does not increase. Interestingly, vessel density decreases indicating that inter-vessel distances expand as the intestinal wall increases in dimensions [227]. Thus, in the growing intestine, perfusion capacity changes to meet the needs of the larger tissue mass by increasing microvascular lengths and diameters but not by increasing vessel numbers [227, 228]. In the growing juvenile (post-weaning) heart, microcirculatory beds expanded in a manner preserving architecture and intervessel spacing on the venous-side and less so on the arteriole-side of the networks [13]. While there is also some capillary growth in the growing, post-weaned heart, it is not commensurate with myocyte hypertrophy resulting in an overall reduction in the microvascular density [13]. However, the capillary-to-myocyte fiber ratio remains relatively constant. Based on these types of observations in native neovascularization, an important objective in increasing perfusion capacity therapeutically in a tissue is the expansion of the entire network that preserves native vascular network architectures, whether vessel numbers are increased or not. As pointed out earlier, this architecture includes appropriate branch ordering which serves to distribute flows accordingly and moderate resistances across the perfusion circuit as vessel caliber decreases.
NEOVASCULARIZATION
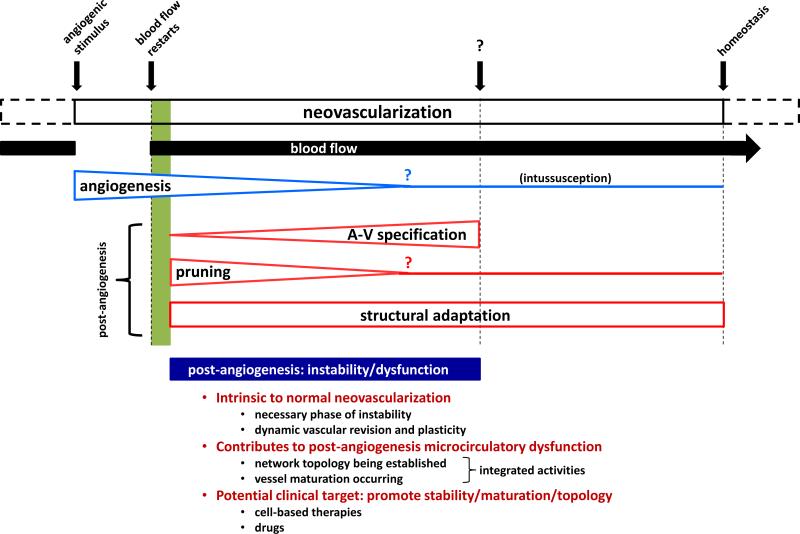
As highlighted above, effective expansion of a microvascular bed requires the newly formed vessels to progress from a disordered collection of neovessels into an organized network of perfusable mature vessels (Figure 4). In the absence of normal progression, perfusion-vessel density mismatch can occur. The transition from sprouting angiogenesis to a functional circulation involves the post-angiogenesis differentiation of neovessels into specific vessel types and organization into an appropriate vascular tree. Further refinement of vessel elements into larger and smaller caliber vessels, longer and smaller vessel segment lengths and vessel removal or “pruning” leads to final network maturation. Ultimately, these structural changes in vessels lead to long-term adjustments in blood flow pathways [138, 187, 203]. While much is known concerning the underlying molecular and cellular mechanisms of angiogenesis [3, 185], considerably less is known about the numerous post-angiogenesis mechanisms giving rise to a subsequent microcirculation.
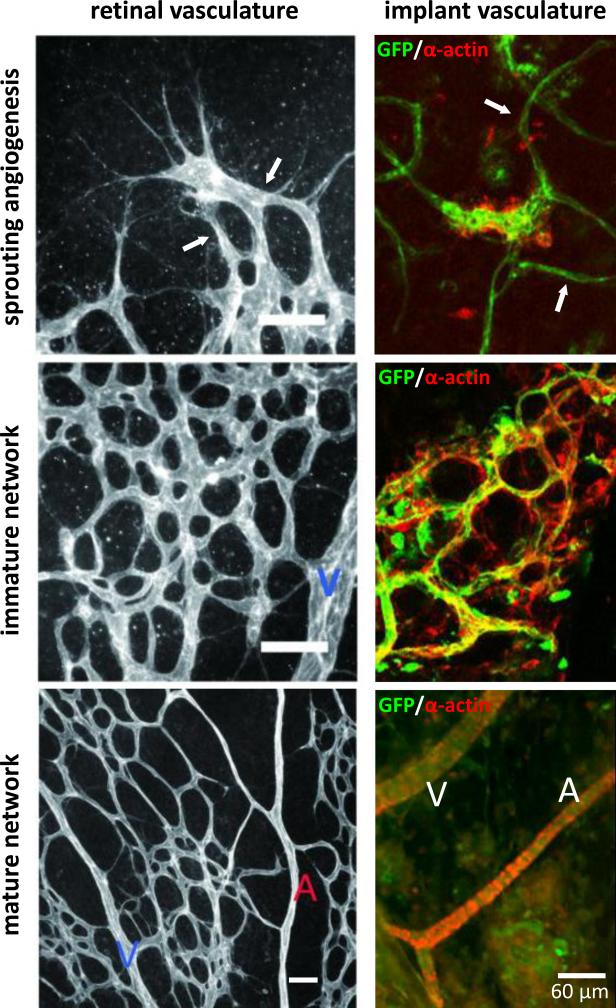
Figure 4.
Vascular and network morphologies present during progression through neovascularization in two different settings: post-natal development of the retinal vasculature and regenerative implant neovascularization. Initial angiogenic sprouts (white arrows) inosculate to form an immature network which matures into a hierarchical tree of mature arterioles (A), venules (V) and capillaries. Scale bars in the left panels equal 50 μm. Parts of this figure were derived from [70] and [172] with permission.
While there is a common progression through neovascularization (described below), there are organ- and tissue-specific aspects that contribute to imparting unique features to the new vascular beds. This likely reflects the differences in tissue microenvironments, including parenchymal cell-vascular interactions and tissue biomechanics [38, 173], that influence the formation and remodeling of new vascular beds. It is clear, though, that the subtle genetic variations can have a significant impact on neovascularization as the VEGF-A expression, ischemic revascularization and vascular remodeling vary dramatically in both extent and character between different inbred strains of mice [34, 35, 75, 91, 95]. Whether these phenotypic vascular differences between strains are due to subtle allelic variation and/or epigenetic control unique to each strain remains to be determined. Similarly, there are differences in how neovascularization is realized between physiological and pathological settings. Often this reflects differences in the angiogenic factors that are either uniquely expressed or function differently in these situations [55, 76, 140, 166]. For example, placental growth factor, while expressed in multiple tissues, regulates angiogenesis in pathological settings but not embryo development or exercise-induced angiogenesis [32, 80]. Similarly, the VEGF164 isoform is critical for pathological but not physiological retinal neovascularization [114]. Together, all of these observations indicate that there is considerable plasticity in the neovascularization process.
Angiogenesis
Angiogenesis generally refers to the process of new vessel formation from pre-existing vessels and is often described as involving not only the formation and growth of new, immature vessels but also the maturation of these neovessels into functional vessels. However, as discussed below, neovessel maturation is intimately coupled with post-angiogenesis processes. Either occurring through a sprouting or splitting (intussusception – addressed later) process, angiogenesis is the primary means by which new vessel segments are added to a microvasculature in the adult. Vasculogenesis, the de novo assembly of vessels from cellular precursors, does occur in post-natal life, but the prevalence is low [45] and therefore likely less impactful on native vascular regeneration and repair.
New vessel growth is a central activity in a vast array of normal and pathological processes. Historically, the vascularizing tumor; with its profoundly disturbed microenvironment, high angiogenic factor levels and often high inflammation, has served as the focus of angiogenesis research and the basis for the general conceptual models of angiogenesis regulation. However, investigations of other conditions involving angiogenesis such as embryonic development, exercised-induced angiogenesis, uterine tissue expansion, ovarian cycling, retinal vascular development, ischemic repair, and others are showing that multiple pathways inducing and influencing angiogenesis exist [106]. Consequently, numerous and varied molecular programs are known to regulate the initiation, promotion, and inhibition of angiogenesis [3, 33]. The complexity of the molecular aspects of angiogenesis is perhaps best reflected by, but certainly not limited to, VEGF. Originally identified as a hyper-permeability factor [211], this versatile peptide factor is critical for vascular development in the embryo [31, 65], a regulator of nitric oxide activity [229], and a vascular trophic factor [122, 241]. Furthermore, VEGF is synthesized in multiple functional isoforms that signal through a panel of different receptors to establish and maintain vascular homeostasis [141]. In addition to the complexity of the individual molecule, VEGF is part of a broader dynamic involving a complex interplay between other molecules regulating angiogenesis. For example, depending on the proportional amounts of Angiopoietin 1 (Ang 1) and 2 (Ang 2) relative to VEGF levels, a nascent neovasculature will either continue to grow, stabilize and mature, or collapse and regress [88].
Neovessel guidance/Network assembly/Inosculation
Intrinsic to the angiogenesis phase of neovascularization is the directed growth of neovessels away from a parent microvessel, and presumably, towards other vessels in an effort to make connections with each other and the existing network. Recently, studies have described a sophisticated molecular system involving VEGF and the Notch family of receptors controlling, via lateral inhibition, the behavior of the individual tip and stalk endothelial cells of the neovessel and neovessel guidance [18, 39, 97, 118]. Stalk cells sense spatial arrangements within the neovessel via epidermal growth factor like domain 7 (EGFL7) signaling, while tip cells of the sprouting neovessel uses filopodia to scan the environment for guidance signals such as a gradient of VEGF [78]. The Ephrin-Eph receptor and co-receptor system have also emerged as key regulators of vascular cell dynamics during angiogenesis [135]. Not surprising, the Notch and ephrin-Eph programs appear to have broader importance during vascular development, including the establishment of arterial-venous identity [1, 2, 132, 233, 235], due to the highly integrated nature of the many vascular cell activities intrinsic to neovascularization. All these molecular systems function primarily through cis- and trans- cell-cell interactions. Soluble ligand-receptor signaling partners, such as of Slit:Robo, Netrin:DCC/Unc5b, and Semaphorin:neuropilin/VEGF-R, are also emerging as critical guidance regulators, particularly during vascular patterning during development. While originally identified as critical regulators of axonal guidance [4], these molecular systems also influence neovessel guidance by acting as neovessel attraction or repulsion systems and are important for proper vascular development and neovascular stability [16, 67, 120, 127, 163]. Interestingly, the Robo signaling axis appears to be one of many targets for regulation by small regulatory microRNAs in angiogenesis [215, 216, 245]. While gradients of soluble factors are thought to provide the predominate directional cues for growing neovessels, matrix dynamics including collagen fibril alignment and tissue deformation may also be important determinants of neovessel growth direction during angiogenesis, particularly in directing one growing neovessel towards another [129, 133, 134].
Little is known concerning the underlying mechanisms specific to neovessel inosculation, the formation of microvascular anastomoses forming contiguous lumens. In the embryo, the interaction of cellular processes at the growing tips of opposing neovessels leads to the fusion of lumens and vessel connections [124]. However, in a vasculogenic setting wherein individual endothelial cells assemble to form neovessel elements, inosculation appears to involve a different mechanism in which free endothelial cells wrap around and integrate with an intact neovessel [41]. Because inosculation between two neovessels requires them to be in close proximity to each other, presumably those molecular systems mediating neovessel guidance may be involved in inosculation as well. Interestingly, it appears that macrophages can act as cellular chaperones for neovessel anastomoses in the developing zebrafish downstream of VEGF [64]. Whether macrophages are also involved in guiding neovessel growth, analogous to their role in non-vascular tissue branching morphogenesis [184], is not known. In many respects, inosculation of neovessels and the associated initiation of perfusion mark the end of angiogenesis and the beginning of the complicated post-angiogenesis process leading to a new microcirculatory circuit.
Post-Angiogenesis
While angiogenesis generates new vessel segments, it's those processes that occur post-angiogenesis that are critical to the formation of a new microcirculation. During post-angiogenesis, individual neovessels must remodel to form stable vessel elements, integrate into the new perfusion network, and adapt (as mature vessels) to meet the new hemodynamic conditions associated with the metabolic demands of the tissue. The progression through these vascular activities is likely tightly regulated and may involve distinct phases. In a model of implant neovascularization, three morphologically, transcriptionally, and functionally defined vascular phenotypes were identified, two of which were associated with post-angiogenesis [172] (Table 1). As expected, the 1st phenotype reflected an angiogenesis phenotype resulting in a formation of a simple network of perivascular cell-free neovessel segments. The 2nd phenotype reflected a neovascular remodeling phenotype characterized by the appearance of intravascular perfusion, significant cell turnover characteristic of vessel pruning, and the re-acquisition of perivascular cells. In related studies, it was suggested that vessel specification into artery- and vein-side flow pathways and network topology was also a feature of this phenotype [38, 174]. The 3rd phenotype, vascular maturation, was characterized by the formation of a hierarchical (based on diameters) network of vessels, presence of mature perivascular cell coverage, and relatively low vessel turnover (low proliferation and low apoptosis). Two key transitions in vascular behavior during neovascularization were identified and associated with changes in global gene expression [172]: one between angiogenesis and network remodeling (marked by the initiation of blood flow) and one between network formation and vascular maturation. As compared to the neovessel sprouting, growth and guidance activities of angiogenesis, notably less is known concerning the regulators and mechanisms underlying the subsequent vascular processes and transitions specific to post-angiogenesis. However, many of the relevant vascular activities are likely common to situations of vascular adaptation and remodeling different from neovascularization, for which there is a good understanding (discussed below). In addition, much is known concerning the array of vascular responses to the hemodynamic forces associated with blood flow that are certainly relevant during post-angiogenesis remodeling and maturation.
Table 1.
Angiogenesis and post-angiogenesis vascular activities related to neovascularization
| Vascular Phenotype | Vascular Activity | Mediators*† | Assessment |
|---|---|---|---|
| Angiogenesis | neovessel growth | EC proliferation; matrix remodeling; perivascular cell regression; angiogenic factors | vessel density‡; BrdU incorporation (proliferation) |
| neovessel guidance | Notch; VEGF; Slit-Robo; Netrin-DCC/Unc5b; semaphorin-neuropilin/VEGFR; matrix organization | Serial or time-lapse microscopy (intravital or in vitro); lineage tracking | |
| neovessel inosculation | macrophage; adhesion molecules | lineage tracking; time-lapse microscopy; blood tracers | |
| Post-Angiogenesis | vessel maturation | perivascular cell association; PDGFβ; ephrin-Eph; Ang-1; hemodynamics | morphology (e.g. diameter, perivascular coverage); molecular markers (e.g. α-actin, vWf) |
| vessel pruning | EC apoptosis; macrophage; Tsp-1; tissue PO2; reduced shear stress | TUNEL assay (apoptosis); vessel density‡ | |
| AV specification | perivascular cell differentiation; eprin-Eph; flow pulsatility | morphology; molecular markers (e.g. EphrinB2, EphB4); intravital flow dynamics | |
| network patterning | tissue mechanics/deformation; molecular spatial fields, hemodynamics | vascular casting (e.g. ink, polymer); morphology (e.g. path lengths, node count) | |
| structural adaptation | hemodynamic forces; vascular cell proliferation; macrophage; MCP-1; connexins | morphology; theoretical modeling | |
| intussusception | interstitial pillar cell proliferation; shear stress; Tie2 | morphology (e.g. via vascular casting); time-lapse/serial intravital microscopy | |
| Angiogenesis/Post-Angiogenesis | vascular stability | Ang-VEGF; integrins; perivascular cells; hemodynamics | morphology; EC dysfunction |
vascular cell gene expression is involved in all activities
italics indicate a putative role
while vessel density is often used to assess angiogenesis or pruning, it is not a direct measure of either.
Maturation
For the new vessels to persist and become functional, they must establish a mature vessel wall as well as integrate into a network. The initiation of blood flow, and subsequent formation of the basement membrane and recruitment of mural cells all contribute to stabilizing a neovessel [185]. During this time, signals from platelet derived growth factor β (PDGF-B), Ang-1, transforming growth factor beta1 (TGFβ1), ephrin-B2, Notch, endothelial differentiation gene 1 (EDG1), thrombospondin and many others will arrest angiogenesis and promote structural maturation of the neovessels [33, 36, 73, 102, 117, 181, 194, 220].
Central to neovessel differentiation and maturation is the recruitment and/or expansion of perivascular cells (i.e. pericytes, smooth muscle cells) to and along the immature neovessel. Mice lacking PDGF-B develop fragile capillaries leading to embryonic lethality due to failure to recruit perivascular cells to vessels during development [96, 149]. Other experiments are revealing that the release of pericytes from a capillary in response to an angiogenic factor is a necessary event for angiogenic sprouting, the functional opposite of vessel maturation [68]. Finally, it appears that pericytes act to mature a neovessel and attenuate vascular proliferation. For example, the proliferation of retinal capillaries leading to blindness is associated with the depletion of capillary pericytes in the retina [177]. Because perivascular cell re-association with the neovessel coincides with perfusion of immature vessels [172], shear-induced responses by endothelial cells likely leads to perivascular cell recruitment. Indeed, endothelial cells release a number of factors, including PDGF-B, which recruit and differentiate perivascular cells and perivascular cell precursors following exposure to increased or changing shear stress [107]. Whether other aspects of blood perfusion, such as plasma components leaking from the remodeling neovessels [85], mediates perivascular cell recruitment remains to be determined. The subsequent maturation of the perivascular cells into smooth muscle cells is also influenced by endothelial cell-released factors [102]. Additionally, transmural pressure-induced stretch secondary to intravascular blood pressure contributes to vessel wall muscularization [108, 110]. Recently, ephrin-Eph mediated heterotypic cell interactions have also been shown to be important in the maturation of the mural cell layers around the neovessel [66, 205]. The lineages of recruited perivascular cells during neovascularization is likely varied as fibroblasts, pericytes, and mesenchymal stem cells all have the potential to incorporate into the mural layers of an immature vessel [26, 147, 159, 206, 225, 245].
Pruning
Normally, vessel densities during neovascularization rise to a maximum before eventually reaching a lower steady state level upon completion of neovascularization [243]. Similarly, in a model of implant neovascularization, neovessel density is initially high but drops by 60% to a new, lower constant level [172]. These two examples indicate that select vessels in a neovascular bed undergo removal of superfluous vessels (called pruning), with the remaining vessels maturing into a hierarchical network. The potential mechanisms initiating neovascular pruning are varied, but generally involves vascular cell apoptosis. Low shear stress is thought to be an initial trigger behind vessel regression [197]. Alternatively, thrombospondin-1 (Tsp-1), acting via the CD36 receptor, can induce apoptosis in endothelial cells [23]. Exposure to shear stress leads to reduced expression of Tsp-1 and CD36 in endothelial cells, suggesting that these molecules may be mediators of shear-dependent pruning [23]. Interestingly, endothelial cell-derived Tsp-1 can recruit macrophages, the primary cell mediating phagocytosis of apoptotic and dying cells [130]. Tissue PO2 is also a likely regulator of vascular pruning as vascular densities adjust to reflect tissue oxygenation through a VEGF-dependent signaling axis [42]. As tissue PO2 rises, VEGF levels drop leading to vascular instability and apoptosis [19]. However, this is most pertinent during remodeling of the immature neovascular network [54] which occurs early post-angiogenesis [172]. Finally, while macrophages remove apoptotic vascular cells associated with pruning [130], they also function to induce the apoptosis of vascular cells contributing to pruning [49, 50, 236]. Interestingly, macrophages perform a similar function in in the mammary gland as a mechanism to regulate duct branching morphogenesis [82, 113].
Arterio-Venous Specification and Plasticity
The neovessels formed during the angiogenesis phase of neovascularization must assemble into an effective perfusion circuit complete with in-flow, exchange, and out-flow pathways. Critical to the establishment of this flow axis is the specification of neovessel segments as arterial or venous. Without arterio-venous (AV) specification, vascular networks are improperly structured and dysfunctional [239]. Seminal studies using Ephrin knockout mice indicate that AV specification during development is genetically pre-determined, occurring before the heart starts pumping blood [117]. The hemodynamic forces associated with blood flow also influence AV identity in the developing embryo, but likely influence more once the basic vascular architecture has formed [121, 143]. Interestingly, it appears that pulsatile flow as opposed to maximal flow velocity determines arteriovenous identity in the embryo [28]. Subsequent work has identified a number of molecular programs involved in AV specification including the Ephrin/Eph, Notch, and VEGF signaling axes [74, 176, 213]. As in the formed embryonic vasculature [222], discrete compartmentalized expression of EphrinB2 and EphB4 also marks arterial- and venous-side vessels in adulthood, respectively [74, 201, 213, 222]. However, the impact of pre-specification of AV identity following angiogenesis in the adult appears to be less than that in the embryo as neovessels generated from only arterioles are able to generate the entire microvascular tree (i.e. arterioles, capillaries, and venules) upon maturation [174]. In fact, vascular cells of angiogenic immature neovessels express both EphrinB2 (arterial marker) and EphB4 (venous marker) suggesting that perhaps specific AV identity is lost following development and then re-acquired during post-angiogenesis [74, 174], presumably in response to the evolving hemodynamic regimen associated with the developing network.
Microvessels display considerable phenotypic heterogeneity. Depending on the tissue environment (i.e. organ) and the position in the vascular tree, microvessels (and capillaries in particular) vary in structure, permeability, hemostasis, inflammatory control, and others [5, 6]. While the endothelial cell plays a central role in determining vessel phenotype [7, 219], vascular smooth muscle cells are also capable of remarkable plasticity and, as with endothelial cells, adapt to environmental and functional cues [9, 157, 232]. Similar to AV specification, immature neovessels must re-specify structural and functional characteristics unique to the tissue environment. As with AV specification, presumably this occurs during early post-angiogenesis as the neovessel re-assembles the entire cellular and matrix components. It was during this window in neovascularization in which fat-derived neovessels acquired a more blood-brain-barrier like vascular phenotype in the presence of astrocyte precursors [173]. Clearly, the microvasculature displays a considerable level of phenotypic adaptation and plasticity which appears important in maturing the post-angiogenesis microcirculation.
Network Patterning and Topology
An effective microcirculation consists of a properly arranged hierarchical tree of heterogeneous vessel types producing a network topology that reflects the relevant 3-D tissue architecture while still maintaining perfusion efficacy (Figure 4) [183, 188, 189]. Faulty network architectures, such as those that retain short pathway shunts in the tumor microcirculation, lead to heterogeneous perfusion and dysfunctional microcirculations [28, 191]. In the early embryo, spatially controlled molecular programs act to pattern the developing vasculature [44, 139, 222]. However, in the adult with more complex tissue geometries and compositions, such global spatial control is not obvious. Instead, local cues derived from stroma structure, preexisting vascular network architectures and hemodynamic forces are prevalent. This latter cue seems critical as the incorporation of hemodynamic force parameters (i.e. shear stress and pressure) into computational models of branching morphogenesis generated more realistic arterial trees [125, 142, 169]. Interestingly, neovessel guidance is coupled with branching morphogenesis in the embryo [153]. However, a pre-existing pattern of neovessels does not necessarily define the final network topology. Pre-patterned neovessel organization, either by direct bioprinting or mechanical alignment [37, 134], was not maintained in the post-angiogenic, mature network [38]. The loss of any existing pre-pattern soon after perfusion through the new neovascular network begins suggests a strong influence of hemodynamic-related cues on the final angioarchitecture [38]. However, a role for tissue structure is also likely as local stress-strain relationships and tissue deformation can influence final network topology [38].
Structural adaptation
The vasculature has an intrinsic ability to adapt to meet the changing functional needs of a tissue [242]. One aspect of this adaptation entails changes in vessel segment diameters with concomitant changes to vessel wall structure. This structural adaptation occurs in response to long-term adjustments in shear stress and transmural pressure (resulting in circumferential stretch) [209]. In general, increases or decreases in shear stress associated with changes in blood flow lead to outward and inward remodeling of the vessel, respectively. These shear-dependent changes in diameter changes are thought to occur as a means by which vessels maintain shear levels, but in a pressure-dependent fashion [186]. In the context of the entire vascular network, responses to hemodynamic forces alone are insufficient to explain the heterogeneous diameter distributions and vessel ordering observed in functional vasculatures [187]. Theoretical considerations indicate that the influence of at least the surrounding tissue metabolic demands and retrograde intravascular communication, such as that mediated by connexin-based gap junctions [28, 192], are required to produce realistic network architectures [187, 209]. Also, in the developing embryonic vasculature, when immature vessels have just formed, shifts in VEGF and Ang1 signaling lead to the preferential enlargement of venous-side segments without further angiogenic sprouting [223]. Clearly, structural adaptation is an integral aspect of neovascularization, particularly as the newly formed vessels and immature network remodel and mature, if not in the final, mature network revisions. Presumably, the same considerations related to vessel adaptation in an existing, mature network described above also apply to the post-angiogenesis network.
Expansion of a microcirculatory network is often accompanied by a concomitant increase in blood delivery by enlargement of arterioles and small arteries into larger feed arteries via arteriogenesis [99, 179]. Essentially a manifestation of structural adaptation, arteriogenesis describes the significant outward remodeling of pre-existing interconnecting collateral vessels through prolonged proliferation of vascular smooth muscle and endothelial cells. As with microvascular adaptation, hemodynamic forces associated with increased blood flow is a primary stimulus [182, 207]. Increases in hemodynamic stresses lead to arterialization of arterioles including expansion of smooth muscle coverage even without significant alteration in perfusion rates [89, 230]. However, the substantial enlargement of an arteriole to the caliber of an artery (e.g. large enough to be identified by angiography) appears to require monocyte recruitment and possibly other bone-marrow-derived cells. In mouse models, arteriogenesis was significantly attenuated in the absence of monocytes/macrophages [20]. Furthermore, arteriogenesis during vascular repair and neovascularization is compromised in mice lacking either monocyte chemotactic protein-1 (MCP-1), an inflammatory cytokine important in macrophage activity [24], or its receptor CCR2 [93, 170, 231]. Interestingly, the recruitment of inflammatory cells to the arteriogenic vessel may reflect a non-inflammatory process by which shear and pressure-induced stresses on the vascular cells initiate a program leading to recruitment of macrophages and other blood cells to enhance outward remodeling beyond the more typical adaptive responses [94, 158].
Intussusception
In sprouting angiogenesis, additional vessel segments are formed by the generation of new vessel elements characterized by growing neovessel sprouts. In contrast, with splitting angiogenesis or intussusception, additional vessel segments are formed by longitudinally splitting a single vessel into two, usually smaller caliber, vessels. Relative to sprouting angiogenesis, intussusception can rapidly expand vascular volume and branching geometry without the need for significant endothelial cell proliferation [51, 53]. During intussusception, an interstitial tissue pillar forms by invading mural cells through the center of a vessel. This transluminal pillar then expands longitudinally eventually resulting in two distinct vessel segments [136]. It is this tissue pillar which is the common diagnostic of intussusception. In the embryo, the angiopoietin receptor Tie2 appears to promote the tissue invagination, analogous to that in intussusception, which is necessary to progress the early embryonic vascular plexus to form a vascular tree [178]. While there may be additional paracrine factors directly initiating intussusception, a prevalent trigger in the adult is thought to be a sustained increase in capillary wall shear stress associated with increased blood flow through a network [237, 244]. Whether a capillary enlarges in diameter or splits in response to this change in shear stress is not clear. However, the levels of VEGF in the tissue may be an important accessory determinant [79, 160, 238]. While intussusception can be a means of microvascular growth [154], it appears that in most neovascularizing tissues, it is detected during post-angiogenesis when the early network is remodeling [52, 208]. As such, and given the close association to changes in blood flow, intussusception is most likely contributing to network revision and branch remodeling, including pruning, in adult microvascular repair [53, 103]. In this regard, intussusception appears to an adaptive mechanism to normalize the flow velocity across the daughter vessels, presumably to normalize hemodynamic performance [27, 53].
Microvascular Stability/Normalization
Neovascularization is inherently a de-stabilizing process as pre-existing, stable vessel and network structures must be regionally unmade or altered before the expanded microvasculature can be reformed. Complete vascular collapse during neovascularization is prevented during this instability by a complex interplay of molecular signals that maintains vascular cell integrity in the presence of the destabilizing signals (i.e. angiogenic factors) driving neovascularization. For example, the integration of angiopoietin (Ang1 and Ang2) signaling with VEGF activity during angiogenesis determines whether a growing neovessel persists and matures or regresses [88]. Also, without ligation of select vascular integrins, angiogenic endothelial cells apoptose and sprouting is attenuated in the presence of angiogenic factors [61]. As with angiogenic sprouting, immature vessels of the early post-angiogenesis phase persist amidst the de-stabilizing conditions intrinsic to neovascular adaptation and remodeling.
Interestingly, this is a time during which perivascular cells are reacquired and blood is beginning to flow through the immature network, suggesting that mural cells as well as the hemodynamic forces associated with perfusion through the forming network are likely key factors in maintaining vascular integrity during this period of plasticity. Also, as mentioned previously, shear stress and mural cells promote endothelial cell quiescence and maturity. In the absence of perfusion, the newly forming network associated with implant neovascularization collapses and fails to fully develop [172]. The interval of perivascular cell re-acquisition may in fact define a window of post-angiogenesis plasticity and vessel pruning [19]. Certainly, the many molecular, cellular and physiological factors that regulate vascular activities described in previous sections are likely contributing to this important interplay between stability and instability. However, any roles specific to post-angiogenesis processes have not been fully and explicitly addressed. Interestingly, it may be this period of instability and limited function in which vascular normalization strategies developed to treat tumors act [87, 224]. The tumor microcirculation is often characterized as being a disorganized network of immature, dysfunctional vessels [190], a description very similar to that of the early post-angiogenesis, remodeling network (Figure 4) [172]. In this regard, the tumor microcirculation reflects a microvasculature that has arrested in the unstable, network remodeling/maturation phase. Normalization therapies then could be described as permitting or enabling neovascularization to progress normally towards microvascular stability. Regardless, it appears that instability is an intrinsic and necessary feature of neovascularization and that integrated mechanisms regulate the degree and nature of destabilization such that the potential for normal vascular form and function is preserved as the vasculature locally disassembles and reassembles. In the absence of this regulation, new vessels persist in a dysfunctional state and/or regress.
SUMMARY and CHALLENGES
Effective neovascularization requires not just the addition of new vessel segments, but also a number of other vascular activities occurring after angiogenesis necessary to establish and maintain a new, functional microcirculation (Figure 5). While much is known and is being learned concerning the mechanisms of angiogenesis and the activities of angiogenic neovessels related to guidance and directed growth, considerably less is known about those mechanisms directing the processes underlying the post-angiogenesis formation of a new vessel network in the context of neovascularization and microvascular repair, particularly in the adult. Questions related to how growing neovessels locate each other, what are the factors promoting and regulating post-angiogenesis progression, and what are the mechanisms of microvascular stability during periods of intense neovessel adaptation and remodeling are of particular interest. In addition to more completely defining the vascular biological landscape, new investigations of post-angiogenesis processes will lead to advanced therapeutic solutions, including the development of strategies to assess neovascular status, the identification of new therapeutic targets, and the discovery of new avenues for repairing the microvasculature.
Figure 5.
Schematic of neovascularization including the relative timing (moving left to right) of the different activities intrinsic to forming a new microcirculation. Question marks indicate estimates of relative times at which the indicated events occur.
Therapeutically, this post-angiogenesis centric concept of neovascularization emphasizes the importance of expanding a vascular network and not necessarily expanding only vascular numbers. Indeed, during organ growth in the juvenile, perfusion capacity is expanded without necessarily an increase in vessel numbers. This may explain why many of the clinical trials focusing on the delivery of angiogenic factors to a diseased/ischemic area with the goal of increasing vascularity have had disappointing results. While it's reasonable to assume that promoting the formation of new vessels would necessarily lead to them being functionally incorporated into the existing vascular network, particularly given the relatively high remodeling capacity and plasticity of the microvasculature, experience is showing that this does not happen spontaneously, at least in disease settings. This argues that a pro-angiogenic approach, while effective at generating new vessels, is not sufficiently comprehensive to also promote network adaptation and organization. Perhaps, then, the central vascular “compromise” in many diseases is not a deficit in vessel growth but one of network dysfunction and instability. In this regard, the therapeutic repair of a vascular circuit is not a vessel growth problem so much as it is a problem of network integration, adaptation, persistence, and function: fundamental aspects intrinsic to the microcirculatory system.
Box 1 Vascular Scaling and Function.
Vessels of approximately 100 μm in diameter in larger species are routinely used in studies examining arteriole physiology. However, major arteries in the mouse, clearly conduit vessels in function, are also ~100 μm or smaller in diameter. Therefore, the assignment of functional roles to specific vascular compartments, in particular arterioles, is a generalization and depends on the scale of the vascular tree and the relative caliber differences as opposed to absolute caliber values.
LIST OF ABBREVIATIONS
- Ang
angiopoietin
- AV
arterio-venous
- EC
endothelial cell
- EDG1
endothelial differentiation gene 1
- EGFL7
epidermal growth factor domain-like 7
- FGF
fibroblast growth factor
- G-CSF
granulocyte colony stimulating factor
- MCP1
monoctyte chemotactic protein-1
- MI
myocardial infarction
- PDGFB
platelet-derived growth factor subunit B
- PO2
oxygen partial pressure
- Tsp-1
thrombospondin-1
- TGF-β
transforming growth factor beta
- VEGF
vascular endothelial growth factor
- VIVA
Vascular Endothelial Growth Factor in Ischemia for Vascular Angiogenesis
REFERENCES
- 1.Adams RH, Wilkinson GA, Weiss C, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams RH, Klein R. Eph receptors and ephrin ligands. essential mediators of vascular development. Trends CardiovascMed. 2000;10:183–188. doi: 10.1016/s1050-1738(00)00046-3. [DOI] [PubMed] [Google Scholar]
- 3.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. NatRevMolCell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 4.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–73. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 6.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–90. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 7.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akbari CM, Saouaf R, Barnhill DF, et al. Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. JVascSurg. 1998;28:687–694. doi: 10.1016/s0741-5214(98)70095-3. [DOI] [PubMed] [Google Scholar]
- 9.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 10.Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–8. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 11.Attanasio S, Schaer G. Therapeutic Angiogenesis for the Management of Refractory Angina: Current Concepts. Cardiovascular therapeutics. 2010 doi: 10.1111/j.1755-5922.2010.00153.x. [DOI] [PubMed] [Google Scholar]
- 12.Barbera-Guillem E, Vidal-Vanaclocha F. Sinusoidal structure of the liver. Revis Biol Celular. 1988;16:1–34. 54–68. [PubMed] [Google Scholar]
- 13.Batra S, Rakusan K. Capillary network geometry during postnatal growth in rat hearts. Am J Physiol. 1992;262:H635–40. doi: 10.1152/ajpheart.1992.262.3.H635. [DOI] [PubMed] [Google Scholar]
- 14.Bauer SM, Bauer RJ, Velazquez OC. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovascular Surg. 2005;39:293–306. doi: 10.1177/153857440503900401. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner I, Isner JM. Stimulation of peripheral angiogenesis by vascular endothelial growth factor (VEGF). VASA Zeitschrift fur Gefasskrankheiten Journal for vascular diseases. 1998;27:201–6. [PubMed] [Google Scholar]
- 16.Bedell VM, Yeo SY, Park KW, et al. roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:6373–8. doi: 10.1073/pnas.0408318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell LN, Cai L, Johnstone BH, et al. A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am J Physiol Endocrinol Metab. 2008;294:E336–44. doi: 10.1152/ajpendo.00272.2007. [DOI] [PubMed] [Google Scholar]
- 18.Benedito R, Roca C, Sorensen I, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann CE, Hoefer IE, Meder B, et al. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol. 2006;80:59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- 21.Blankesteijn WM, Creemers E, Lutgens E, et al. Dynamics of cardiac wound healing following myocardial infarction: observations in genetically altered mice. Acta physiologica Scandinavica. 2001;173:75–82. doi: 10.1046/j.1365-201X.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 22.Bondke Persson A, Buschmann EE, Lindhorst R, et al. Therapeutic arteriogenesis in peripheral arterial disease: combining intervention and passive training. Vasa. 2011;40:177–87. doi: 10.1024/0301-1526/a000092. [DOI] [PubMed] [Google Scholar]
- 23.Bongrazio M, Da Silva-Azevedo L, Bergmann EC, et al. Shear stress modulates the expression of thrombospondin-1 and CD36 in endothelial cells in vitro and during shear stress-induced angiogenesis in vivo. International journal of immunopathology and pharmacology. 2006;19:35–48. [PubMed] [Google Scholar]
- 24.Boring L, Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–61. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouis D, Kusumanto Y, Meijer C, et al. A review on pro- and anti-angiogenic factors as targets of clinical intervention. PharmacolRes. 2006;53:89–103. doi: 10.1016/j.phrs.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Boyd NL, Nunes SS, Jokinen JD, et al. Microvascular Mural Cell Functionality of Human Embryonic Stem Cell-Derived Mesenchymal Cells. Tissue Eng Part A. 2011 doi: 10.1089/ten.tea.2010.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burri PH, Djonov V. Intussusceptive angiogenesis--the alternative to capillary sprouting. Mol Aspects Med. 2002;23:S1–27. doi: 10.1016/s0098-2997(02)00096-1. [DOI] [PubMed] [Google Scholar]
- 28.Buschmann I, Pries A, Styp-Rekowska B, et al. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development. 2010;137:2187–96. doi: 10.1242/dev.045351. [DOI] [PubMed] [Google Scholar]
- 29.Cardinal TR, Hoying JB. A modified fluorescent microsphere-based approach for determining resting and hyperemic blood flows in individual murine skeletal muscles. Vascular pharmacology. 2007;47:48–56. doi: 10.1016/j.vph.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardinal TR, Struthers KR, Kesler TJ, et al. Chronic hindlimb ischemia impairs functional vasodilation and vascular reactivity in mouse feed arteries. Frontiers in Physiology. 2011;2 doi: 10.3389/fphys.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. NatMed. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 33.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalothorn D, Clayton JA, Zhang H, et al. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–91. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 35.Chalothorn D, Faber JE. Strain-dependent variation in collateral circulatory function in mouse hindlimb. Physiol Genomics. 2010;42:469–79. doi: 10.1152/physiolgenomics.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chambers RC, Leoni P, Kaminski N, et al. Global expression profiling of fibroblast responses to transforming growth factor-beta1 reveals the induction of inhibitor of differentiation-1 and provides evidence of smooth muscle cell phenotypic switching. The American journal of pathology. 2003;162:533–46. doi: 10.1016/s0002-9440(10)63847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang CC, Boland ED, Williams SK, et al. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater. 2011;98:160–70. doi: 10.1002/jbm.b.31831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CC, Krishnan L, Nunes SS, et al. Determinants of microvascular network topologies in implanted neovasculatures. Arterioscler Thromb Vasc Biol. 2012;32:5–14. doi: 10.1161/ATVBAHA.111.238725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chappell JC, Wiley DM, Bautch VL. Regulation of blood vessel sprouting. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhury H, Raborn E, Goldie LC, et al. Stem cell-derived vascular endothelial cells and their potential application in regenerative medicine. Cells Tissues Organs. 2012;195:41–7. doi: 10.1159/000331423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng G, Liao S, Kit Wong H, et al. Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood. 2011;118:4740–9. doi: 10.1182/blood-2011-02-338426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claxton S, Fruttiger M. Oxygen modifies artery differentiation and network morphogenesis in the retinal vasculature. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;233:822–8. doi: 10.1002/dvdy.20407. [DOI] [PubMed] [Google Scholar]
- 43.Couffinhal T, Silver M, Zheng LP, et al. Mouse model of angiogenesis. The American journal of pathology. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 44.Coultas L, Nieuwenhuis E, Anderson GA, et al. Hedgehog regulates distinct vascular patterning events through VEGF dependent and independent mechanisms. Blood. 2010 doi: 10.1182/blood-2009-12-256644. [DOI] [PubMed] [Google Scholar]
- 45.Crosby JR, Kaminski WE, Schatteman G, et al. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circulation research. 2000;87:728–30. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 46.Cutolo M, Ferrone C, Pizzorni C, et al. Peripheral blood perfusion correlates with microvascular abnormalities in systemic sclerosis: a laser-Doppler and nailfold videocapillaroscopy study. The Journal of rheumatology. 2010;37:1174–80. doi: 10.3899/jrheum.091356. [DOI] [PubMed] [Google Scholar]
- 47.Darland DC, Massingham LJ, Smith SR, et al. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. DevBiol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 48.Dewhirst MW, Tso CY, Oliver R, et al. Morphologic and hemodynamic comparison of tumor and healing normal tissue microvasculature. International journal of radiation oncology, biology, physics. 1989;17:91–9. doi: 10.1016/0360-3016(89)90375-1. [DOI] [PubMed] [Google Scholar]
- 49.Diez-Roux G, Lang RA. Macrophages induce apoptosis in normal cells in vivo. Development. 1997;124:3633–8. doi: 10.1242/dev.124.18.3633. [DOI] [PubMed] [Google Scholar]
- 50.Diez-Roux G, Argilla M, Makarenkova H, et al. Macrophages kill capillary cells in G1 phase of the cell cycle during programmed vascular regression. Development. 1999;126:2141–7. doi: 10.1242/dev.126.10.2141. [DOI] [PubMed] [Google Scholar]
- 51.Djonov V, Schmid M, Tschanz SA, et al. Intussusceptive angiogenesis: its role in embryonic vascular network formation. CircRes. 2000;86:286–292. doi: 10.1161/01.res.86.3.286. [DOI] [PubMed] [Google Scholar]
- 52.Djonov V, Andres AC, Ziemiecki A. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc Res Tech. 2001;52:182–9. doi: 10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 53.Djonov VG, Kurz H, Burri PH. Optimality in the developing vascular system: branching remodeling by means of intussusception as an efficient adaptation mechanism. Developmental dynamics : an official publication of the American Association of Anatomists. 2002;224:391–402. doi: 10.1002/dvdy.10119. [DOI] [PubMed] [Google Scholar]
- 54.Dor Y, Porat R, Keshet E. Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol Cell Physiol. 2001;280:C1367–74. doi: 10.1152/ajpcell.2001.280.6.C1367. [DOI] [PubMed] [Google Scholar]
- 55.Drixler TA, Borel Rinkes IH, Ritchie ED, et al. Angiostatin inhibits pathological but not physiological retinal angiogenesis. Invest Ophthalmol Vis Sci. 2001;42:3325–30. [PubMed] [Google Scholar]
- 56.Dudar TE, Jain RK. Microcirculatory flow changes during tissue growth. Microvascular research. 1983;25:1–21. doi: 10.1016/0026-2862(83)90040-7. [DOI] [PubMed] [Google Scholar]
- 57.Duncan HJ, Faris IB. Skin vascular resistance and skin perfusion pressure as predictors of healing of ischemic lesion of the lower limb: influences of diabetes mellitus, hypertension, and age. Surgery. 1986;99:432–8. [PubMed] [Google Scholar]
- 58.Egginton S, Hudlicka O, Brown MD, et al. Capillary growth in relation to blood flow and performance in overloaded rat skeletal muscle. Journal of applied physiology. 1998;85:2025–2032. doi: 10.1152/jappl.1998.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 59.Egginton S, Hudlicka O. Early changes in performance, blood flow and capillary fine structure in rat fast muscles induced by electrical stimulation. J Physiol. 1999;515(Pt 1):265–75. doi: 10.1111/j.1469-7793.1999.265ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Egginton S, Gaffney E. Tissue capillary supply--it's quality not quantity that counts! ExpPhysiol. 2010;95:971–979. doi: 10.1113/expphysiol.2010.053421. [DOI] [PubMed] [Google Scholar]
- 61.Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. Journal of Clinical Investigation. 1999;103:1227–1230. doi: 10.1172/JCI6869. [Review] [18 refs].
- 62.Engelmann MG, Theiss HD, Hennig-Theiss C, et al. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. Journal of the American College of Cardiology. 2006;48:1712–21. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 63.Faber JE, Zhang H, Lassance-Soares RM, et al. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1748–56. doi: 10.1161/ATVBAHA.111.227314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fantin A, Vieira JM, Gestri G, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–40. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 66.Foo SS, Turner CJ, Adams S, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–73. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 67.Freitas C, Larrivee B, Eichmann A. Netrins and UNC5 receptors in angiogenesis. Angiogenesis. 2008;11:23–9. doi: 10.1007/s10456-008-9096-2. [DOI] [PubMed] [Google Scholar]
- 68.Frerich B, Lindemann N, Kurtz-Hoffmann J, et al. In vitro model of a vascular stroma for the engineering of vascularized tissues. IntJOral MaxillofacSurg. 2001;30:414–420. doi: 10.1054/ijom.2001.0130. [DOI] [PubMed] [Google Scholar]
- 69.Fukuda S, Kaga S, Sasaki H, et al. Angiogenic signal triggered by ischemic stress induces myocardial repair in rat during chronic infarction. Journal of molecular and cellular cardiology. 2004;36:547–59. doi: 10.1016/j.yjmcc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Fukushima Y, Okada M, Kataoka H, et al. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011;121:1974–85. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 72.Futrakul P, Futrakul N, Sitprija V, et al. Improved renal perfusion prevents disease progression in focal segmental glomerulosclerosis. Nephron. 1995;69:351. doi: 10.1159/000188492. [DOI] [PubMed] [Google Scholar]
- 73.Gaengel K, Genove G, Armulik A, et al. Endothelial-mural cell signaling in vascular development and angiogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:630–8. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 74.Gale NW, Baluk P, Pan L, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Developmental biology. 2001;230:151–60. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 75.Gao G, Li Y, Fant J, et al. Difference in ischemic regulation of vascular endothelial growth factor and pigment epithelium--derived factor in brown norway and sprague dawley rats contributing to different susceptibilities to retinal neovascularization. Diabetes. 2002;51:1218–25. doi: 10.2337/diabetes.51.4.1218. [DOI] [PubMed] [Google Scholar]
- 76.Gardiner TA, Gibson DS, de Gooyer TE, et al. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am J Pathol. 2005;166:637–44. doi: 10.1016/s0002-9440(10)62284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Georgi MK, Vigilance J, Dewar AM, et al. Terminal arteriolar network structure/function and plasma cytokine levels in db/db and ob/ob mouse skeletal muscle. Microcirculation. 2011;18:238–51. doi: 10.1111/j.1549-8719.2011.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. The Journal of cell biology. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gianni-Barrera R, Trani M, Reginato S, et al. To sprout or to split? VEGF, Notch and vascular morphogenesis. Biochem Soc Trans. 2011;39:1644–8. doi: 10.1042/BST20110650. [DOI] [PubMed] [Google Scholar]
- 80.Gigante B, Tarsitano M, Cimini V, et al. Placenta growth factor is not required for exercise-induced angiogenesis. Angiogenesis. 2004;7:277–84. doi: 10.1007/s10456-004-4179-1. [DOI] [PubMed] [Google Scholar]
- 81.Goligorsky MS. Microvascular rarefaction: The decline and fall of blood vessels. Organogenesis. 2010;6:1–10. doi: 10.4161/org.6.1.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–82. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 83.Grosskreutz CL, Anand-Apte B, Duplaa C, et al. Vascular endothelial growth factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res. 1999;58:128–36. doi: 10.1006/mvre.1999.2171. [DOI] [PubMed] [Google Scholar]
- 84.Gschwandtner ME, Ambrozy E, Schneider B, et al. Laser Doppler imaging and capillary microscopy in ischemic ulcers. Atherosclerosis. 1999;142:225–32. doi: 10.1016/s0021-9150(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 85.Guerreiro-Lucas LA, Pop SR, Machado MJ, et al. Experimental and theoretical modelling of blind-ended vessels within a developing angiogenic plexus. MicrovascRes. 2008;76:161–168. doi: 10.1016/j.mvr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 86.Hallerstam S, Larsson PT, Zuber E, et al. Carotid atherosclerosis is correlated with extent and severity of coronary artery disease evaluated by myocardial perfusion scintigraphy. Angiology. 2004;55:281–8. doi: 10.1177/000331970405500307. [DOI] [PubMed] [Google Scholar]
- 87.Hamzah J, Jugold M, Kiessling F, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 88.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 89.Hansen-Smith F, Egginton S, Hudlicka O. Growth of arterioles in chronically stimulated adult rat skeletal muscle. Microcirculation. 1998;5:49–59. [PubMed] [Google Scholar]
- 90.Hansen-Smith F, Egginton S, Zhou AL, et al. Growth of arterioles precedes that of capillaries in stretch-induced angiogenesis in skeletal muscle. MicrovascRes. 2001;62:1–14. doi: 10.1006/mvre.2001.2308. [DOI] [PubMed] [Google Scholar]
- 91.Harmon KJ, Couper LL, Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. AmJPathol. 2000;156:1741–1748. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hedman M, Hartikainen J, Syvanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation. 2003;107:2677–83. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 93.Heil M, Ziegelhoeffer T, Wagner S, et al. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res. 2004;94:671–7. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 94.Heil M, Eitenmuller I, Schmitz-Rixen T, et al. Arteriogenesis versus angiogenesis: similarities and differences. Journal of cellular and molecular medicine. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Helisch A, Wagner S, Khan N, et al. Impact of mouse strain differences in innate hindlimb collateral vasculature. ArteriosclerThrombVascBiol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 96.Hellstrom M, Kal n M, Lindahl P, et al. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 97.Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 98.Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–65. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 99.Hershey JC, Baskin EP, Glass JD, et al. Revascularization in the rabbit hindlimb: dissociation between capillary sprouting and arteriogenesis. Cardiovascular research. 2001;49:618–25. doi: 10.1016/s0008-6363(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 100.Hiatt WR, Regensteiner JG, Hargarten ME, et al. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81:602–9. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 101.Hill JM, Syed MA, Arai AE, et al. Outcomes and risks of granulocyte colony-stimulating factor in patients with coronary artery disease. Journal of the American College of Cardiology. 2005;46:1643–8. doi: 10.1016/j.jacc.2005.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hirschi KK, Rohovsky SA, Beck LH, et al. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. CircRes. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- 103.Hlushchuk R, Ehrbar M, Reichmuth P, et al. Decrease in VEGF expression induces intussusceptive vascular pruning. Arterioscler Thromb Vasc Biol. 2011;31:2836–44. doi: 10.1161/ATVBAHA.111.231811. [DOI] [PubMed] [Google Scholar]
- 104.Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. CurrOpinOrgan Transplant. 2010;15:86–91. doi: 10.1097/MOT.0b013e328334f074. [DOI] [PubMed] [Google Scholar]
- 105.Hoying JB, Boswell CA, Williams SK. Angiogenic potential of microvessel fragments established in three-dimensional collagen gels. In Vitro Cell DevBiolAnim. 1996;32:409–419. doi: 10.1007/BF02723003. [DOI] [PubMed] [Google Scholar]
- 107.Hsieh HJ, Li NQ, Frangos JA. Shear stress increases endothelial platelet-derived growth factor mRNA levels. The American journal of physiology. 1991;260:H642–6. doi: 10.1152/ajpheart.1991.260.2.H642. [DOI] [PubMed] [Google Scholar]
- 108.Hudlicka O. Mechanical factors involved in the growth of the heart and its blood vessels. Cell MolBiolRes. 1994;40:143–152. [PubMed] [Google Scholar]
- 109.Hudlicka O, Brown MD, Egginton S, et al. Effect of long-term electrical stimulation on vascular supply and fatigue in chronically ischemic muscles. Journal of applied physiology. 1994;77:1317–24. doi: 10.1152/jappl.1994.77.3.1317. [DOI] [PubMed] [Google Scholar]
- 110.Hudlicka O, Brown MD. Postnatal growth of the heart and its blood vessels. JVascRes. 1996;33:266–287. doi: 10.1159/000159155. [DOI] [PubMed] [Google Scholar]
- 111.Hughes GC, Annex BH. Angiogenic therapy for coronary artery and peripheral arterial disease. Expert Rev Cardiovasc Ther. 2005;3:521–35. doi: 10.1586/14779072.3.3.521. [DOI] [PubMed] [Google Scholar]
- 112.Humar R, Zimmerli L, Battegay E. Angiogenesis and hypertension: an update. J HumHypertens. 2009;23:773–782. doi: 10.1038/jhh.2009.63. [DOI] [PubMed] [Google Scholar]
- 113.Ingman WV, Wyckoff J, Gouon-Evans V, et al. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–9. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 114.Ishida S, Usui T, Yamashiro K, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198:483–9. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Isner JM. Arterial gene transfer of naked DNA for therapeutic angiogenesis: early clinical results. Advanced drug delivery reviews. 1998;30:185–197. doi: 10.1016/s0169-409x(97)00115-4. [DOI] [PubMed] [Google Scholar]
- 116.Jacobsen JC, Mulvany MJ, Holstein-Rathlou NH. A mechanism for arteriolar remodeling based on maintenance of smooth muscle cell activation. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294:R1379–89. doi: 10.1152/ajpregu.00407.2007. [DOI] [PubMed] [Google Scholar]
- 117.Jain RK. Molecular regulation of vessel maturation. Nature medicine. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 118.Jakobsson L, Franco CA, Bentley K, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. NatCell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 119.Jeremy RW, Hackworthy RA, Bautovich G, et al. Infarct artery perfusion and changes in left ventricular volume in the month after acute myocardial infarction. Journal of the American College of Cardiology. 1987;9:989–95. doi: 10.1016/s0735-1097(87)80298-x. [DOI] [PubMed] [Google Scholar]
- 120.Jones CA, London NR, Chen H, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nature medicine. 2008;14:448–53. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones EA, le Noble F, Eichmann A. What determines blood vessel structure? Genetic prespecification vs. hemodynamics. Physiology. 2006;21:388–95. doi: 10.1152/physiol.00020.2006. [DOI] [PubMed] [Google Scholar]
- 122.Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–76. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 123.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–95. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kamei M, Saunders WB, Bayless KJ, et al. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 125.Kassab GS. Scaling laws of vascular trees: of form and function. American journal of physiology Heart and circulatory physiology. 2006;290:H894–903. doi: 10.1152/ajpheart.00579.2005. [DOI] [PubMed] [Google Scholar]
- 126.Kastrup J, Jorgensen E, Ruck A, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. Journal of the American College of Cardiology. 2005;45:982–8. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 127.Kawasaki T, Kitsukawa T, Bekku Y, et al. A requirement for neuropilin-1 in embryonic vessel formation. Development - Supplement. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 128.Kelsall CJ, Brown MD, Kent J, et al. Arteriolar endothelial dysfunction is restored in ischaemic muscles by chronic electrical stimulation. JVascRes. 2004;41:241–251. doi: 10.1159/000078301. [DOI] [PubMed] [Google Scholar]
- 129.Kirkpatrick ND, Andreou S, Hoying JB, et al. Live imaging of collagen remodeling during angiogenesis. AmJPhysiol Heart CircPhysiol. 2007;292:H3198–H3206. doi: 10.1152/ajpheart.01234.2006. [DOI] [PubMed] [Google Scholar]
- 130.Kirsch T, Woywodt A, Klose J, et al. Endothelial-derived thrombospondin-1 promotes macrophage recruitment and apoptotic cell clearance. J Cell Mol Med. 2010;14:1922–34. doi: 10.1111/j.1582-4934.2009.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koh YJ, Koh BI, Kim H, et al. Stromal vascular fraction from adipose tissue forms profound vascular network through the dynamic reassembly of blood endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:1141–50. doi: 10.1161/ATVBAHA.110.218206. [DOI] [PubMed] [Google Scholar]
- 132.Krebs LT, Xue Y, Norton CR, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 133.Krishnan L, Hoying JB, Nguyen H, et al. Interaction of angiogenic microvessels with the extracellular matrix. AmJPhysiol Heart CircPhysiol. 2007;293:H3650–H3658. doi: 10.1152/ajpheart.00772.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krishnan L, Underwood CJ, Maas S, et al. Effect of mechanical boundary conditions on orientation of angiogenic microvessels. Cardiovascular research. 2008;78:324–32. doi: 10.1093/cvr/cvn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–51. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 136.Kurz H, Burri PH, Djonov VG. Angiogenesis and vascular remodeling by intussusception: from form to function. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2003;18:65–70. doi: 10.1152/nips.01417.2002. [DOI] [PubMed] [Google Scholar]
- 137.Lamah M, Mortimer PS, Dormandy JA. In vivo microscopic study of microcirculatory perfusion of the skin of the foot in peripheral vascular disease. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 1999;18:48–51. doi: 10.1053/ejvs.1999.0831. [DOI] [PubMed] [Google Scholar]
- 138.Langille BL, Bendeck MP, Keeley FW. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. AmJPhysiol. 1989;256:H931–H939. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- 139.Lavine KJ, Long F, Choi K, et al. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development. 2008;135:3161–3171. doi: 10.1242/dev.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lavine KJ, Ornitz DM. Shared circuitry: developmental signaling cascades regulate both embryonic and adult coronary vasculature. CircRes. 2009;104:159–169. doi: 10.1161/CIRCRESAHA.108.191239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lazarus A, Keshet E. Vascular endothelial growth factor and vascular homeostasis. Proc Am Thorac Soc. 2011;8:508–11. doi: 10.1513/pats.201102-021MW. [DOI] [PubMed] [Google Scholar]
- 142.Le NF, Fleury V, Pries A, et al. Control of arterial branching morphogenesis in embryogenesis: go with the flow. CardiovascRes. 2005;65:619–628. doi: 10.1016/j.cardiores.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 143.le Noble F, Moyon D, Pardanaud L, et al. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–75. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 144.Leblanc AJ, Touroo JS, Hoying JB, et al. Adipose stromal vascular fraction cell construct sustains coronary microvascular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;302:H973–82. doi: 10.1152/ajpheart.00735.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leobon B, Roncalli J, Joffre C, et al. Adipose-derived cardiomyogenic cells: in vitro expansion and functional improvement in a mouse model of myocardial infarction. CardiovascRes. 2009;83:757–767. doi: 10.1093/cvr/cvp167. [DOI] [PubMed] [Google Scholar]
- 146.Levenberg S, Huang NF, Lavik E, et al. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. ProcNatlAcadSciUSA. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 148.Libby P, Aikawa M, Jain MK. Vascular endothelium and atherosclerosis. Handb Exp Pharmacol. 2006:285–306. doi: 10.1007/3-540-36028-x_9. [DOI] [PubMed] [Google Scholar]
- 149.Lindahl P, Johansson BR, Leveen P, et al. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 150.Lokmic Z, Mitchell GM. Engineering the microcirculation. Tissue Eng Part B Rev. 2008;14:87–103. doi: 10.1089/teb.2007.0299. [DOI] [PubMed] [Google Scholar]
- 151.Lopez JJ, Laham RJ, Stamler A, et al. VEGF administration in chronic myocardial ischemia in pigs. Cardiovascular research. 1998;40:272–81. doi: 10.1016/s0008-6363(98)00136-9. [DOI] [PubMed] [Google Scholar]
- 152.Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–4. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 153.Lu X, Le Noble F, Yuan L, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–86. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 154.Makanya AN, Stauffer D, Ribatti D, et al. Microvascular growth, development, and remodeling in the embryonic avian kidney: the interplay between sprouting and intussusceptive angiogenic mechanisms. Microsc Res Tech. 2005;66:275–88. doi: 10.1002/jemt.20169. [DOI] [PubMed] [Google Scholar]
- 155.Mayrovitz HN, Wiedeman MP, Noordergraaf A. Microvascular hemodynamic variations accompanying microvessel dimensional changes. Microvasc Res. 1975;10:322–29. doi: 10.1016/0026-2862(75)90036-9. [DOI] [PubMed] [Google Scholar]
- 156.Mayrovitz HN, Tuma RF, Wiedeman MP. Relationship between microvascular blood velocity and pressure distribution. Am J Physiol. 1977;232:H400–5. doi: 10.1152/ajpheart.1977.232.4.H400. [DOI] [PubMed] [Google Scholar]
- 157.McDonald OG, Owens GK. Programming smooth muscle plasticity with chromatin dynamics. Circ Res. 2007;100:1428–41. doi: 10.1161/01.RES.0000266448.30370.a0. [DOI] [PubMed] [Google Scholar]
- 158.Meisner JK, Price RJ. Spatial and temporal coordination of bone marrow-derived cell activity during arteriogenesis: regulation of the endogenous response and therapeutic implications. Microcirculation. 2010;17:583–99. doi: 10.1111/j.1549-8719.2010.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Melero-Martin JM, De Obaldia ME, Allen P, et al. Host myeloid cells are necessary for creating bioengineered human vascular networks in vivo. Tissue Eng Part A. 2010;16:2457–66. doi: 10.1089/ten.tea.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Milkiewicz M, Brown MD, Egginton S, et al. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation. 2001;8:229–241. doi: 10.1038/sj/mn/7800074. [DOI] [PubMed] [Google Scholar]
- 161.Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–55. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 162.Mitsos S, Katsanos K, Koletsis E, et al. Therapeutic angiogenesis for myocardial ischemia revisited: basic biological concepts and focus on latest clinical trials. Angiogenesis. 2011 doi: 10.1007/s10456-011-9240-2. [DOI] [PubMed] [Google Scholar]
- 163.Moore SW, Tessier-Lavigne M, Kennedy TE. Netrins and their receptors. Adv Exp Med Biol. 2007;621:17–31. doi: 10.1007/978-0-387-76715-4_2. [DOI] [PubMed] [Google Scholar]
- 164.Mourad JJ, Levy BI. Mechanisms of antiangiogenic-induced arterial hypertension. Curr Hypertens Rep. 2011;13:289–93. doi: 10.1007/s11906-011-0206-y. [DOI] [PubMed] [Google Scholar]
- 165.Mughal NA, Russell DA, Ponnambalam S, et al. Gene therapy in the treatment of peripheral arterial disease. Br J Surg. 2012;99:6–15. doi: 10.1002/bjs.7743. [DOI] [PubMed] [Google Scholar]
- 166.Nagai N, Noda K, Urano T, et al. Selective suppression of pathologic, but not physiologic, retinal neovascularization by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2005;46:1078–84. doi: 10.1167/iovs.04-1101. [DOI] [PubMed] [Google Scholar]
- 167.Nakatsu MN, Sainson RC, Aoto JN, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66:102–12. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 168.Nelissen-Vrancken HJ, Debets JJ, Snoeckx LH, et al. Time-related normalization of maximal coronary flow in isolated perfused hearts of rats with myocardial infarction. Circulation. 1996;93:349–55. doi: 10.1161/01.cir.93.2.349. [DOI] [PubMed] [Google Scholar]
- 169.Nguyen TH, Eichmann A, Le NF, et al. Dynamics of vascular branching morphogenesis: the effect of blood and tissue flow. PhysRevEStatNonlinSoftMatter Phys. 2006;73:061907. doi: 10.1103/PhysRevE.73.061907. [DOI] [PubMed] [Google Scholar]
- 170.Nickerson MM, Burke CW, Meisner JK, et al. Capillary arterialization requires the bone- marrow-derived cell (BMC)-specific expression of chemokine (C-C motif) receptor-2, but BMCs do not transdifferentiate into microvascular smooth muscle. Angiogenesis. 2009;12:355–63. doi: 10.1007/s10456-009-9157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nor JE, Christensen J, Mooney DJ, et al. Vascular endothelial growth factor (VEGF)- mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. AmJPathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nunes SS, Greer KA, Stiening CM, et al. Implanted microvessels progress through distinct neovascularization phenotypes. MicrovascRes. 2010;79:10–20. doi: 10.1016/j.mvr.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nunes SS, Krishnan L, Gerard CS, et al. Angiogenic Potential of Microvessel Fragments is Independent of the Tissue of Origin and can be Influenced by the Cellular Composition of the Implants. Microcirculation. 2010;17:557–567. doi: 10.1111/j.1549-8719.2010.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nunes SS, Rekapally H, Chang CC, et al. Vessel arterial-venous plasticity in adult neovascularization. PLoS One. 2011;6:e27332. doi: 10.1371/journal.pone.0027332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Olsen TS, Larsen B, Herning M, et al. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke; a journal of cerebral circulation. 1983;14:332–41. doi: 10.1161/01.str.14.3.332. [DOI] [PubMed] [Google Scholar]
- 176.Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signalling - in control of vascular function. Nature reviews Molecular cell biology. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 177.Orlidge A, D'Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. JCell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Patan S. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. MicrovascRes. 1998;56:1–21. doi: 10.1006/mvre.1998.2081. [DOI] [PubMed] [Google Scholar]
- 179.Patel TH, Kimura H, Weiss CR, et al. Constitutively active HIF-1alpha improves perfusion and arterial remodeling in an endovascular model of limb ischemia. Cardiovascular research. 2005;68:144–54. doi: 10.1016/j.cardiores.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 180.Pearlman JD, Hibberd MG, Chuang ML, et al. Magnetic resonance mapping demonstrates benefits of VEGF-induced myocardial angiogenesis. Nature medicine. 1995;1:1085–9. doi: 10.1038/nm1095-1085. [DOI] [PubMed] [Google Scholar]
- 181.Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine & growth factor reviews. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 182.Pipp F, Boehm S, Cai WJ, et al. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1664–8. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- 183.Pittman RN. Oxygen transport and exchange in the microcirculation. Microcirculation. 2005;12:59–70. doi: 10.1080/10739680590895064. [DOI] [PubMed] [Google Scholar]
- 184.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 186.Pries AR, Secomb TW, Gaehtgens P. Design principles of vascular beds. Circ Res. 1995;77:1017–23. doi: 10.1161/01.res.77.5.1017. [DOI] [PubMed] [Google Scholar]
- 187.Pries AR, Secomb TW, Gaehtgens P. Structural adaptation and stability of microvascular networks: theory and simulations. AmJPhysiol. 1998;275:H349–H360. doi: 10.1152/ajpheart.1998.275.2.H349. [DOI] [PubMed] [Google Scholar]
- 188.Pries AR, Secomb TW. Microcirculatory network structures and models. Ann Biomed Eng. 2000;28:916–21. doi: 10.1114/1.1308495. [DOI] [PubMed] [Google Scholar]
- 189.Pries AR, Secomb TW. Control of blood vessel structure: insights from theoretical models. AmJPhysiol Heart CircPhysiol. 2005;288:H1010–H1015. doi: 10.1152/ajpheart.00752.2004. [DOI] [PubMed] [Google Scholar]
- 190.Pries AR, Cornelissen AJ, Sloot AA, et al. Structural adaptation and heterogeneity of normal and tumor microvascular networks. PLoS Comput Biol. 2009;5:e1000394. doi: 10.1371/journal.pcbi.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pries AR, Secomb TW. Origins of heterogeneity in tissue perfusion and metabolism. Cardiovascular research. 2009;81:328–35. doi: 10.1093/cvr/cvn318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pries AR, Hopfner M, le Noble F, et al. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010;10:587–93. doi: 10.1038/nrc2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Prior BM, Lloyd PG, Ren J, et al. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol. 2004;287:H2434–47. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- 194.Reginato S, Gianni-Barrera R, Banfi A. Taming of the wild vessel: promoting vessel stabilization for safe therapeutic angiogenesis. Biochem Soc Trans. 2011;39:1654–8. doi: 10.1042/BST20110652. [DOI] [PubMed] [Google Scholar]
- 195.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 196.Ren G, Michael LH, Entman ML, et al. Morphological characteristics of the microvasculature in healing myocardial infarcts. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2002;50:71–9. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 197.Resnick N, Yahav H, Shay-Salit A, et al. Fluid shear stress and the vascular endothelium: for better and for worse. Progress in biophysics and molecular biology. 2003;81:177–99. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 198.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 199.Roberts N, Jahangiri M, Xu Q. Progenitor cells in vascular disease. JCell MolMed. 2005;9:583–591. doi: 10.1111/j.1582-4934.2005.tb00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Roman RJ, Carmines PK, Loutzenhiser R, et al. Direct studies on the control of the renal microcirculation. J Am Soc Nephrol. 1991;2:136–49. doi: 10.1681/ASN.V22136. [DOI] [PubMed] [Google Scholar]
- 201.Rossant J, Howard L. Signaling pathways in vascular development. Annual review of cell and developmental biology. 2002;18:541–73. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- 202.Roy JW, Mayrovitz HN. Microvascular blood flow in the normotensive and spontaneously hypertensive rat. Hypertension. 1982;4:264–71. doi: 10.1161/01.hyp.4.2.264. [DOI] [PubMed] [Google Scholar]
- 203.Rudic RD, Bucci M, Fulton D, et al. Temporal events underlying arterial remodeling after chronic flow reduction in mice: correlation of structural changes with a deficit in basal nitric oxide synthesis. CircRes. 2000;86:1160–1166. doi: 10.1161/01.res.86.11.1160. [DOI] [PubMed] [Google Scholar]
- 204.Ryschich E, Schmidt J, Hammerling GJ, et al. Transformation of the microvascular system during multistage tumorigenesis. International journal of cancer Journal international du cancer. 2002;97:719–25. doi: 10.1002/ijc.10074. [DOI] [PubMed] [Google Scholar]
- 205.Salvucci O, Maric D, Economopoulou M, et al. EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood. 2009;114:1707–16. doi: 10.1182/blood-2008-12-192294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schechner JS, Nath AK, Zheng L, et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. ProcNatlAcadSciUSA. 2000;97:9191–9196. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Scheel K, Fitzgerald E, Martin R, et al. The possible role of mechanical stresses on coronary collateral development during gradual coronary occlusion. In: Schaper W, editor. The Pathophysiology of Myocardial Perfusion. Amsterdam: 1979. pp. 489–518. [Google Scholar]
- 208.Schlatter P, Konig MF, Karlsson LM, et al. Quantitative study of intussusceptive capillary growth in the chorioallantoic membrane (CAM) of the chicken embryo. Microvasc Res. 1997;54:65–73. doi: 10.1006/mvre.1997.2022. [DOI] [PubMed] [Google Scholar]
- 209.Secomb TW, Dewhirst MW, Pries AR. Structural Adaptation of Normal and Tumour Vascular Networks. Basic Clin Pharmacol Toxicol. 2011 doi: 10.1111/j.1742-7843.2011.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sellke FW, Wang SY, Friedman M, et al. Basic FGF enhances endothelium-dependent relaxation of the collateral-perfused coronary microcirculation. Am J Physiol. 1994;267:H1303–11. doi: 10.1152/ajpheart.1994.267.4.H1303. [DOI] [PubMed] [Google Scholar]
- 211.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 212.Shepherd BR, Chen HY, Smith CM, et al. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler Thromb Vasc Biol. 2004;24:898–904. doi: 10.1161/01.ATV.0000124103.86943.1e. [DOI] [PubMed] [Google Scholar]
- 213.Shin D, Garcia-Cardena G, Hayashi S, et al. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Developmental biology. 2001;230:139–50. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- 214.Simons M, Bonow RO, Chronos NA, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: An expert panel summary. Circulation. 2000;102:E73–86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 215.Small EM, Sutherland LB, Rajagopalan KN, et al. MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circulation research. 2010;107:1336–44. doi: 10.1161/CIRCRESAHA.110.227926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–42. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Snyder GK, Coelho JR. Microvascular development in chick anterior latissimus dorsi following hypertrophy. J Anat. 1989;162:215–24. [PMC free article] [PubMed] [Google Scholar]
- 218.Sternbergh WC, III, Adelman B. The temporal relationship between endothelial cell dysfunction and skeletal muscle damage after ischemia and reperfusion. JVascSurg. 1992;16:30–39. [PubMed] [Google Scholar]
- 219.Stevens T. Functional and molecular heterogeneity of pulmonary endothelial cells. Proc Am Thorac Soc. 2011;8:453–7. doi: 10.1513/pats.201101-004MW. [DOI] [PubMed] [Google Scholar]
- 220.Streit M, Riccardi L, Velasco P, et al. Thrombospondin-2: A potent endogenous inhibitor of tumor growth and angiogenesis [In Process Citation]. ProcNatlAcadSciUSA. 1999;96:14888–14893. doi: 10.1073/pnas.96.26.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sullivan CJ, Doetschman T, Hoying JB. Targeted disruption of the Fgf2 gene does not affect vascular growth in the mouse ischemic hindlimb. Journal of applied physiology. 2002;93:2009–17. doi: 10.1152/japplphysiol.00451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Swift MR, Weinstein BM. Arterial-venous specification during development. Circulation research. 2009;104:576–88. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 223.Thurston G, Wang Q, Baffert F, et al. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development. 2005;132:3317–26. doi: 10.1242/dev.01888. [DOI] [PubMed] [Google Scholar]
- 224.Tong RT, Boucher Y, Kozin SV, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 225.Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circulation research. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 226.Traktuev DO, Prater DN, Merfeld-Clauss S, et al. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circulation research. 2009;104:1410–20. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 227.Unthank JL, Bohlen HG. Quantification of intestinal microvascular growth during maturation: techniques and observations. Circ Res. 1987;61:616–24. doi: 10.1161/01.res.61.5.616. [DOI] [PubMed] [Google Scholar]
- 228.Unthank JL, Lash JM, Bohlen HG. Maturation of the rat intestinal microvasculature from juvenile to early adult life. Am J Physiol. 1990;259:G282–9. doi: 10.1152/ajpgi.1990.259.2.G282. [DOI] [PubMed] [Google Scholar]
- 229.van der ZR, Murohara T, Luo Z, et al. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95:1030–1037. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- 230.Van Gieson EJ, Murfee WL, Skalak TC, et al. Enhanced smooth muscle cell coverage of microvessels exposed to increased hemodynamic stresses in vivo. Circulation research. 2003;92:929–36. doi: 10.1161/01.RES.0000068377.01063.79. [DOI] [PubMed] [Google Scholar]
- 231.Voskuil M, Hoefer IE, van Royen N, et al. Abnormal monocyte recruitment and collateral artery formation in monocyte chemoattractant protein-1 deficient mice. Vasc Med. 2004;9:287–92. doi: 10.1191/1358863x04vm571oa. [DOI] [PubMed] [Google Scholar]
- 232.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98:868–78. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 233.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 234.Weidinger FF, McLenachan JM, Cybulsky MI, et al. Persistent dysfunction of regenerated endothelium after balloon angioplasty of rabbit iliac artery. Circulation. 1990;81:1667–1679. doi: 10.1161/01.cir.81.5.1667. [DOI] [PubMed] [Google Scholar]
- 235.Weinstein BM, Lawson ND. Arteries, veins, Notch, and VEGF. Cold Spring Harb Symp Quant Biol. 2002;67:155–62. doi: 10.1101/sqb.2002.67.155. [DOI] [PubMed] [Google Scholar]
- 236.West J, Harral J, Lane K, et al. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell Mol Physiol. 2008;295:L744–55. doi: 10.1152/ajplung.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Williams JL, Cartland D, Hussain A, et al. A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. JPhysiol. 2006;570:445–454. doi: 10.1113/jphysiol.2005.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Williams JL, Cartland D, Rudge JS, et al. VEGF trap abolishes shear stress- and overload-dependent angiogenesis in skeletal muscle. Microcirculation. 2006;13:499–509. doi: 10.1080/10739680600785717. [DOI] [PubMed] [Google Scholar]
- 239.Yancopoulos GD, Klagsbrun M, Folkman J. Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border. Cell. 1998;93:661–4. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]
- 240.Yang HT, Prior BM, Lloyd PG, et al. Training-induced vascular adaptations to ischemic muscle. J Physiol Pharmacol. 2008;59(Suppl 7):57–70. [PMC free article] [PubMed] [Google Scholar]
- 241.Zachary I, Mathur A, Yla-Herttuala S, et al. Vascular protection: A novel nonangiogenic cardiovascular role for vascular endothelial growth factor. ArteriosclerThrombVascBiol. 2000;20:1512–1520. doi: 10.1161/01.atv.20.6.1512. [DOI] [PubMed] [Google Scholar]
- 242.Zakrzewicz A, Secomb TW, Pries AR. Angioadaptation: keeping the vascular system in shape. News Physiol Sci. 2002;17:197–201. doi: 10.1152/nips.01395.2001. [DOI] [PubMed] [Google Scholar]
- 243.Zawicki DF, Jain RK, Schmid-Schoenbein GW, et al. Dynamics of neovascularization in normal tissue. Microvascular research. 1981;21:27–47. doi: 10.1016/0026-2862(81)90003-0. [DOI] [PubMed] [Google Scholar]
- 244.Zhou A, Egginton S, Hudlicka O, et al. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with alpha1-antagonist prazosin. Cell Tissue Res. 1998;293:293–303. doi: 10.1007/s004410051121. [DOI] [PubMed] [Google Scholar]
- 245.Zhou Q, Gallagher R, Ufret-Vincenty R, et al. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc Natl Acad Sci U S A. 2011;108:8287–92. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]