Abstract
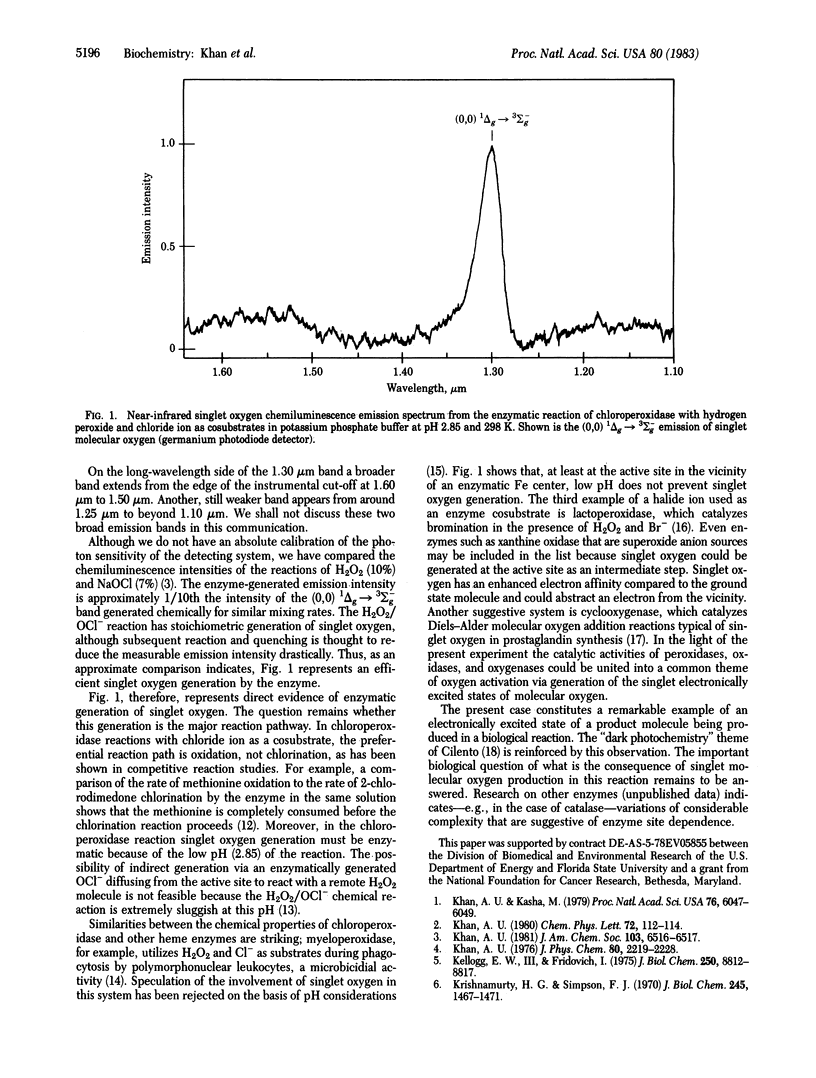
The (0,0) 1Δg → 3Σ-g singlet molecular oxygen chemiluminescence emission from a biological reaction system, chloroperoxidase (EC 1.11.1.10) acting on hydrogen peroxide at low pH (phosphate buffer, pH 2.85) with Cl- as a cosubstrate, was recorded with an ultrasensitive IR spectrometer. The strong chemiluminescence emission peak observed at 1.30 μm provides clear evidence of the enzymatic generation of (excited) singlet oxygen from peroxide in this system.
Keywords: (0,0) transition; chloride peroxidase; hydrogen peroxide; chloride ion
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Chan H. W. Singlet oxygen analogs in biological systems. Peroxidase-catalyzed oxygenation of 1,3-dienes. J Am Chem Soc. 1971 Sep 8;93(18):4632–4633. doi: 10.1021/ja00747a071. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Watson B. D., Schultz J. Myeloperoxidase and singlet oxygen: a reappraisal. FEBS Lett. 1978 Aug 15;92(2):327–332. doi: 10.1016/0014-5793(78)80780-7. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- Khan A. U., Kasha M. Direct spectroscopic observation of singlet oxygen emission at 1268 nm excited by sensitizing dyes of biological interest in liquid solution. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6047–6049. doi: 10.1073/pnas.76.12.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurty H. G., Simpson F. J. Degradation of rutin by Aspergillus flavus. Studies with oxygen 18 on the action of a dioxygenase on quercetin. J Biol Chem. 1970 Mar 25;245(6):1467–1471. [PubMed] [Google Scholar]
- Libby R. D., Thomas J. A., Kaiser L. W., Hager L. P. Chloroperoxidase halogenation reactions. Chemical versus enzymic halogenating intermediates. J Biol Chem. 1982 May 10;257(9):5030–5037. [PubMed] [Google Scholar]
- Morris D. R., Hager L. P. Chloroperoxidase. I. Isolation and properties of the crystalline glycoprotein. J Biol Chem. 1966 Apr 25;241(8):1763–1768. [PubMed] [Google Scholar]
- Piatt J. F., Cheema J. S., O'Brien P. J. Peroxidase catalyzed singlet oxygen formation from hydrogen peroxide. FEBS Lett. 1977 Mar 1;74(2):251–254. doi: 10.1016/0014-5793(77)80857-0. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON B. ON THE INCORPORATION OF OXYGEN IN THE CONVERSION OF 8, 11, 14-EICOSATRIENOIC ACID TO PROSTAGLANDIN E1. J Am Chem Soc. 1965 Jul 5;87:3011–3013. doi: 10.1021/ja01091a043. [DOI] [PubMed] [Google Scholar]


