Abstract
The enantioselective oxidative C-H functionalization of tetrahydroisoquinoline derivatives is achieved through the merger of photoredox and asymmetric anion-binding catalysis. This combination of two distinct catalysis concepts introduces a potentially general approach to asymmetric transformations in oxidative photocatalysis.
Introduction
Photoredox catalysis has emerged recently as a powerful tool for organic synthesis.1 Irradiation of photoactive catalysts with visible light can be parlayed to the practical generation of synthetically useful high-energy organic intermediates such as free radicals2 and radical anions and cations.3,4 Successful efforts to induce enantioselective catalytic control in such photocatalyzed processes have relied thus far on covalent organocatalysis: photoredox aminocatalysis as introduced by MacMillan,2a,5 and the combination of photoredox catalysis with N-heterocyclic carbene catalysis as developed by Rovis.6,7The main text of the article should appear here.
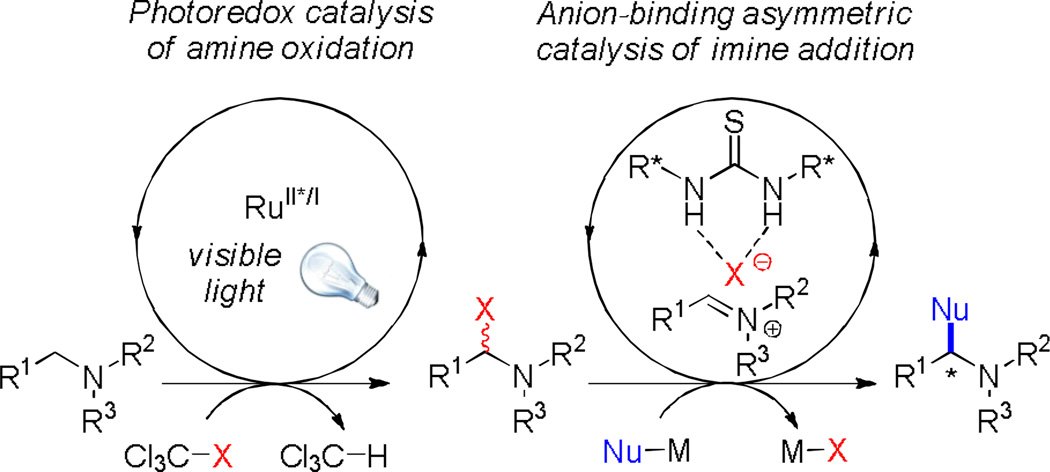
Since many reactions initiated by visible light photocatalysis involve the reductive generation of halide anions, 8 we considered whether the use of chiral anion-binding catalysts9 in combination with photocatalysis would provide opportunities for new types of enantioselective transformations10,11 In one specific embodiment of this idea, we envisioned that iminium ion equivalents generated under mild conditions from tertiary amines by photocatalyzed oxidation12 might be trapped in stereoselective nucleophilic addition reactions under the influence of a chiral H-bond donor catalyst (Figure 1). We describe here the successful development of a dual catalyst strategy for the enantioselective oxidative alkylation of tetrahydroisoquinolines with silyl ketene acetals.
Fig. 1.
Merger of photoredox and anion-binding catalysis in oxidative enatioselective C-H functionalization of amines
We chose to evaluate the proposed dual catalytic reaction design in enantioselective oxidative Mannich reactions to access tetrahydroisoquinoline derived β-amino esters such as 3a (Table 1).13,14 In this context, we reported recently a photocatalytic method for the oxidative generation of iminium ion precursors from simple N-aryltetrahydroisoquinolines such as 1a using bromotrichloromethane as the stoichiometric oxidant.15,16,17 Coupling this protocol to an enantioselective thiourea-catalyzed addition of enolate equivalents such as silyl ketene acetal 2 would require identification of the appropriate photoredox and chiral thiourea catalysts, as well as reaction conditions suitable for both transformations.
Table 1.
Thiourea catalyst optimization
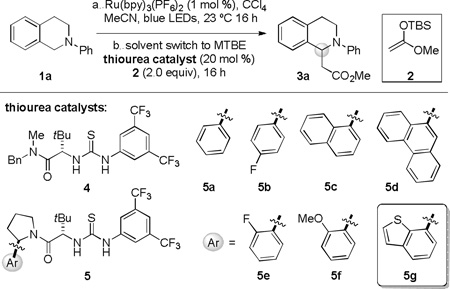 | |||||
|---|---|---|---|---|---|
| entry | thiourea catalyst |
T (°C)a | [1a]o (M) | yield[%]b | ee[%]c |
| 1 | - | −78 | 0.1 | 0 | N/A |
| 2 | 4 | −78 | 0.1 | 75 | 10 |
| 3 | 5a | −78 | 0.1 | 68 | 50 |
| 4 | 5b | −78 | 0.1 | 63 | 20 |
| 5 | 5c | −78 | 0.1 | 57 | 80 |
| 6 | 5d | −78 | 0.1 | 63 | 29 |
| 7 | 5c | −60 | 0.05 | 37 | 93 |
| 8 | epi-5c d | −60 | 0.05 | 68 | 44 |
| 9 | 5e | −60 | 0.05 | 63 | 87 |
| 10 | 5f | −60 | 0.05 | 63 | 85 |
| 11 | ent-5g | −60 | 0.05 | 72 | −97 |
Reaction temperature for the alkylation step in MTBE.
Yields determined by 1H NMR spectroscopic analysis of the crude reaction mixture relative to 2,5-dimethylfuran as the internal standard.
Determined by HPLC on commercial chiral columns.
Pyrrolodine configuration is (R), tert-leucine configuration is (S).
Results and Discussion
In initial experiments, the proper choice of solvent systems was revealed as a key challenge. In relatively polar media (CH3CN, CH2Cl2, DMF), with CCl4 as the stoichiometric oxidant, the oxidative photocatalytic Mannich reaction of N-phenyltetrahydroisoquinoline (1a) and silyl ketene acetal 2 afforded 3a cleanly in the presence of chiral thiourea catalysts, but always in racemic form. In contrast, unreacted tetrahydroisoquinoline 1a could be recovered from attempted oxidations in non-polar solvents such as MTBE, which are optimal for attaining high enantioselectivity in anion-binding thiourea catalysis. The failure of the photoredox reaction under these conditions could be ascribed simply to the lack of solubility of the photocatalyst, even when relatively non-polar complexes such as Ru(bpy)3(PF6)2 were employed. Ultimately, the enantioselective oxidative Mannich reaction could be achieved by performing the photocatalytic oxidation in CH3CN and switching solvents to MTBE for the thiourea-catalyzed alkylation reaction. In this manner, full conversion of 1a to the corresponding iminium ion in CH3CN was observed after irradiation with blue LEDs for 16 hours using 2 equiv of CCl4, and tetrahydroisoquinoline 3a was obtained in 50% ee upon alkylation with 2 in the presence of chiral thiourea 5a at −78 °C.
Enantioselective oxidative Mannich reactions of 1a and silyl ketene acetal 2 were evaluated with a variety of thiourea catalysts (Table 1). Only catalysts bearing both 3,5-bis(trifluoromethyl)aniline and tertiary amide components were found to promote useful reaction rates at −78 °C.18 Constrained arylpyrrolidine-derived thiourea catalysts of the general structure 5, which have proven effective in a wide range of enantioselective transformations,19,20 induced significantly higher ee’s than catalysts bearing a less constrained tertiary amide fragment (e.g. 4). In that context, the relative configuration of the arylpyrrolidine unit relative to the tert-leucine component had a significant impact on reaction enantioselectivity (entries 7–8), pointing to the importance of the precise disposition of the aryl group relative to the thiourea anion-binding site. No straightforward correlation between the expanse of the α-aryl substituent on the pyrrolidine and reaction enantioselectivity was observed (Table 1, entries 3–6). In contrast, substitution on the ortho-position of the arylpyrrolidine resulted in significant improvements in enantioinduction, although the electronic properties of the substituent had little impact (entries 9–11). Further reaction optimization using catalyst 5c revealed a significant improvement in enantioselectivity when the reaction was conducted at −60 °C with an initial concentration of 0.05M (entries 5 and 7). After extensive evaluation of different arylpyrrolidine-derived analogs of 5, thiourea 5g was identified as an optimal catalyst for the model transformation of 1a to 3a.
During the course of the optimization studies outlined above, we observed that the identities of both the photocatalyst counterion and the halogen atom source had significant effects on enantioselectivity in the thiourea-catalyzed silyl ketene acetal addition reaction (Table 2). Consistently lower enantioselectivity was observed when using BrCCl3 as the stoichiometric oxidant compared with CCl4, a result that is attributable to more favorable interactions of the chloride-bound thiourea catalyst in the enantioselectivity-determining transition structure.21 When Ru(bpy)3(PF6)2 was used as the photocatalyst in place of Ru(bpy)3Cl2 together with CCl4 as the stoichiometric oxidant, highly variable enantioselectivities were obtained (e.g. 61–97% ee with catalyst 5g). This unpredictable behavior associated with the use of the Ru(bpy)3(PF6)2 photocatalyst appears to be tied to the heterogeneous nature of the alkylation reaction mixture. Although only 2 mol % of PF6− is present in the reaction medium and the iminium ion intermediate is predominantly associated with chloride (Cl−/PF6− = ca. 51:1), the PF6− may impart enhanced solubility properties to the iminium ion, and thereby enable the racemic, background reaction to a greater extent.
Table 2.
Counterion effects
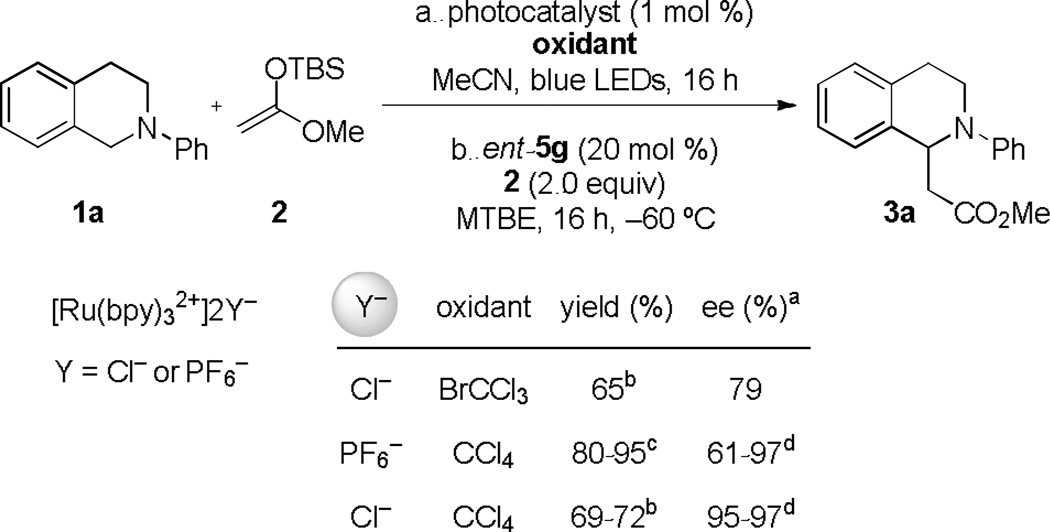 |
Determined by HPLC on commercial chiral columns.
Isolated yield after purification by chromatography on silica gel.
Determined by 1H NMR spectroscopic analysis of the crude reaction mixture relative to 2,5-dimethylfuran as the internal standard.
Range of enantiomeric excess achieved over >10 runs.
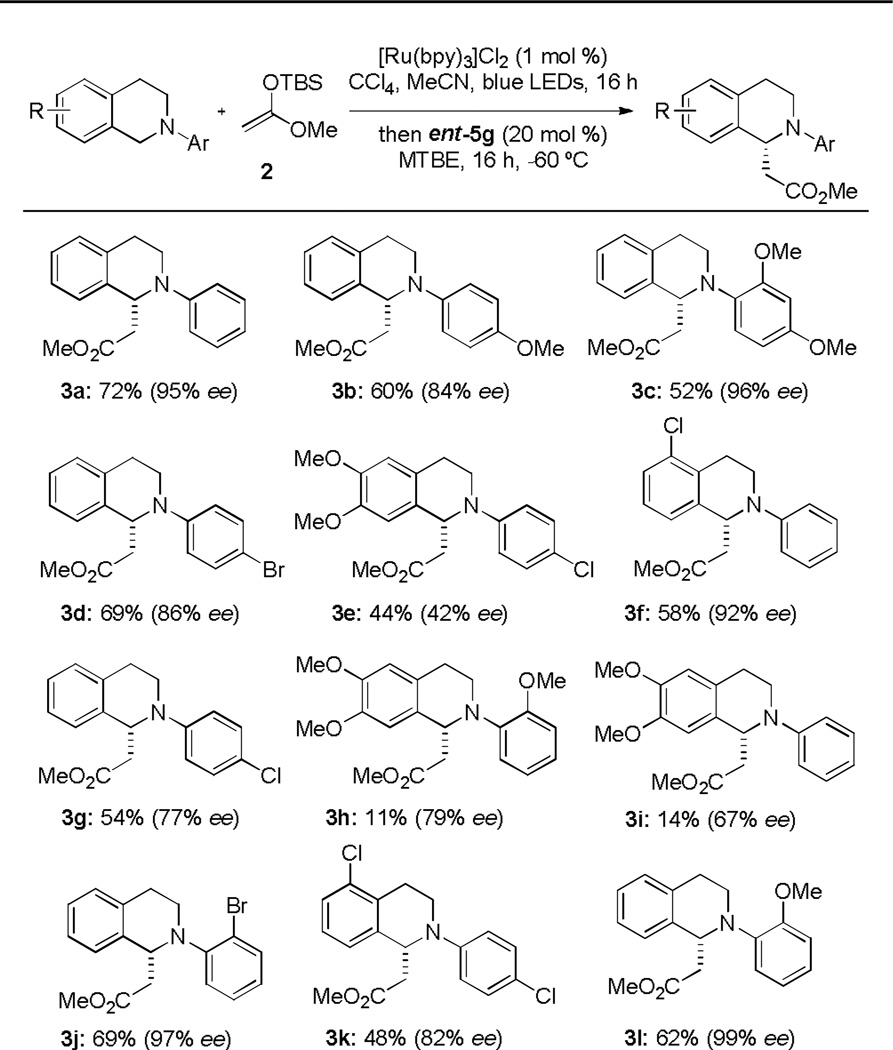
With optimized conditions in hand we next explored the substrate scope of the reaction (Figure 2). The time required for full conversion of the N-aryltetrahydroisoquinoline derivatives 1 in the oxidation reaction was highly dependent on the electronic nature of the substrate, with electron-rich derivatives being more readily oxidized. To ensure complete conversion to the iminium ion, the amine was treated under the photochemical oxidation conditions for 16 h in all cases. The position of substituents on the N-aryl group influenced the enantioselectivity of the oxidative alkylation addition more profoundly than did their electronic properties. Thus, ortho-substituted substrates generally provided higher ee compared to para-substituted analogs (3l vs 3b; 3j vs 3d). In contrast, reaction enantioselectivities were very sensitive to the electronic nature substituents on the tetrahydroisoquinoline ring, with electron-rich systems generally affording lower ee’s and/or yields (e.g. 3e, 3h, 3i).
Fig. 3.
Substrate scope
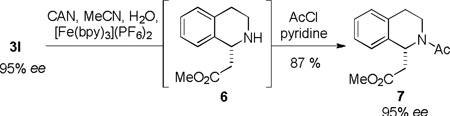
In order to better establish the synthetic utility of this chiral tetrahydroisoquinoline synthetic methodology, we sought to develop a protocol for N-dearylation of the products to provide access to the more useful secondary amine derivatives.22 Electron-rich anilines have favorable redox properties that render them prone to N-aryl bond cleavage under oxidative conditions.23 Accordingly, compound 3l, which was generated with nearly perfect enantioselection in the oxidative Mannich reaction, was chosen as a model substrate for dearylation studies. Application of standard oxidative protocols for PMP or PMB removal (CAN, PhI(TFA)2, or DDQ) led to rapid consumption of 3l but low yields of the desired secondary amine due to product decomposition via over-oxidation. We anticipated that the use of an oxidant more closely matched to the oxidation potential of the substrate would have a better chance of avoiding decomposition of the relatively sensitive secondary amine product. Evaluation of Fe(III)-based oxidants led to the identification of [Fe(bpy)3]3+, formed in situ upon treatment of [Fe(bpy)3](PF6)2 with CAN,24 as a highly effective oxidant for this transformation, leading to clean conversion to the desired amine 6. Comparison of the optical rotation of 6 with the reported value enabled the assignment of its absolute configuration. Acylation of 6 with acetyl chloride provided 7 (95% ee, 87% yield starting from 3l) with no significant compromise of enantiomeric purity.
 |
(1) |
Conclusions
In summary, we have developed an approach to the oxidative, enantioselective C-H functionalization of tetrahydroisoquinoline derivatives. A mild method for the selective dearylation of products bearing N-o-anisyl groups was developed, thereby enabling further derivatization of the tetrahydroisoquinoline scaffold. Further applications of photoredox /chiral anion binding dual catalysis are anticipated and are the subject of current study.
Supplementary Material
Acknowledgements
Financial support for this research from the NIH-NIGMS (PO1 GM69721 and R01-GM43214) to ENJ and R01-GM096129 to CRJS), the Alfred P. Sloan Foundation, Amgen, Boehringer Ingelheim, Eli Lilly, and Novartis is gratefully acknowledged. GB is grateful to the Spanish Ministry of Education and Science (MEC) for a FPU predoctoral fellowship (AP2009-0950). Postdoctoral support from the Alexander von Humboldt Foundation (Feodor Lynen fellowship to CSS) and the Swedish Research Council (to CJW) is gratefully acknowledged.
Footnotes
Electronic Supplementary Information (ESI) available: Detailed experimental procedures and spectroscopic data of all new compounds. See DOI: 10.1039/b000000x/
Footnotes should appear here. These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data.
Contributor Information
Eric N. Jacobsen, Email: Jacobsen@chemistry.harvard.edu.
Corey R. J. Stephenson, Email: crjsteph@umich.edu.
Notes and references
- 1.For recent reviews, see Narayanam JMR, Stephenson CRJ. Chem. Soc. Rev. 2011;40:102. doi: 10.1039/b913880n. Teplý F. Collect. Czech. Chem. Commun. 2011;76:859. Tucker JW, Stephenson CRJ. J. Org. Chem. 2012;77:1617. doi: 10.1021/jo202538x. Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322. doi: 10.1021/cr300503r.
- 2.For selected examples, see: Nicewicz D, MacMillan DWC. Science. 2008;322:77. doi: 10.1126/science.1161976. Narayanam JMR, Tucker JW, Stephenson CRJ. J. Am. Chem. Soc. 2009;131:8756. doi: 10.1021/ja9033582. Andrews RS, Becker JJ, Gagné MR. Angew. Chem. Int. Ed. 2010;49:7274. doi: 10.1002/anie.201004311. Tucker JW, Stephenson CRJ. Org. Lett. 2011;13:5468. doi: 10.1021/ol202178t. Neumann M, Füldner S, König B, Zeitler K. Angew. Chem. Int. Ed. 2011;50:951. doi: 10.1002/anie.201002992. Nguyen JD, D’Amato EM, Narayanam JMR, Stephenson CRJ. Nature Chem. 2012;4:854. doi: 10.1038/nchem.1452.
- 3.For a recent review on accessing the chemistry of radical ions, see: Ischay MA, Yoon TP. Eur. J. Org. Chem. 2012:3359.
- 4.For selected examples, see: Ischay MA, Anzovino ME, Du J, Yoon TP. J. Am. Chem. Soc. 2008;130:12886. doi: 10.1021/ja805387f. Ischay MA, Lu Z, Yoon TP. J. Am. Chem. Soc. 2010;132:8572. doi: 10.1021/ja103934y. Lin S, Ischay MA, Fry CG, Yoon TP. J. Am. Chem. Soc. 2011;133:19350. doi: 10.1021/ja2093579. Maity S, Zhu M, Shinabery RS, Zheng N. Angew. Chem. Int. Ed. 2012;51:222. doi: 10.1002/anie.201106162.
- 5.(a) Nagib DA, Scott ME, MacMillan DWC. J. Am. Chem. Soc. 2009;131:10875. doi: 10.1021/ja9053338. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shih H-W, Vander Wal MN, Grange RL, MacMillan DWC. J. Am. Chem. Soc. 2010;132:13600. doi: 10.1021/ja106593m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiRocco DA, Rovis T. J. Am. Chem. Soc. 2012;134:8094. doi: 10.1021/ja3030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.These are distinct conceptually from enantioselective catalysis of photochemical transformations, as described by Bach et al. For examples, see: Bauer A, Westkämper F, Grimme S, Bach T. Nature. 2005;436:1139. doi: 10.1038/nature03955. Müller C, Bauer A, Bach T. Angew. Chem. Int. Ed. 2009;48:6640. doi: 10.1002/anie.200901603. Müller C, Bauer A, Maturi M, Cuquerella MC, Miranda MA, Bach T. J. Am. Chem. Soc. 2011;133:16689. doi: 10.1021/ja207480q.
- 8.For selected examples, see refs 2, 5 and Nguyen JD, Tucker JW, Konieczynska MD, Stephenson CRJ. J. Am. Chem. Soc. 2011;133:4160. doi: 10.1021/ja108560e. Wallentin C-J, Nguyen JD, Finkbeiner P, Stephenson CRJ. J. Am. Chem. Soc. 2012;134:8875. doi: 10.1021/ja300798k. Pirtsch M, Paria S, Matsuno T, Isobe H, Reiser O. Chem. Eur. J. 2012;18:7336. doi: 10.1002/chem.201200967.
- 9.For recent reviews, see: Brak K, Jacobsen EN. Angew. Chem. In. Ed. 2013;52:534. doi: 10.1002/anie.201205449. Phipps RJ, Hamilton GL, Toste FD. Nature Chem. 2012;4:603. doi: 10.1038/nchem.1405.
- 10.For a recent review, see: Allen AE, MacMillan DWC. Chem. Sci. 2012;3:633. doi: 10.1039/C2SC00907B.
- 11.Visible light photoredox catalysis has been successfully combined with other means of catalysis. For examples with transition metals, see: Kalyani D, McMurtrey KB, Neufeldt SR, Sanford MS. J. Am. Chem. Soc. 2011;133:18566. doi: 10.1021/ja208068w. Ye Y, Sanford MS. J. Am. Chem. Soc. 2012;134:9034. doi: 10.1021/ja301553c. Rueping M, Koenigs RM, Poscharny K, Fabry DC, Leonori D, Vila C. Chem. Eur. J. 2012;18:5170. doi: 10.1002/chem.201200050. Sahoo B, Hopkinson MN, Glorius F. J. Am. Chem. Soc. 2013;135:5505. doi: 10.1021/ja400311h. For an example with enamine catalysis, see: Rueping M, Vila C, Koenigs RM, Poscharny K, Fabry DC. Chem. Commun. 2011;47:2360. doi: 10.1039/c0cc04539j. For examples with Brønsted acid catalysis, see: Ruiz Espelt L, Wiensch EM, Yoon TP. J. Org. Chem. 2013;78:4107. doi: 10.1021/jo400428m. Neumann M, Zeitler K. Chem. Eur. J. 2013;19:6950. doi: 10.1002/chem.201204573.
- 12.For a recent review on tertiary amine oxidation using photoredox catalysis, see: Shi L, Xia W. Chem. Soc. Rev. 2012;41:7687. doi: 10.1039/c2cs35203f.
- 13.For reviews on β-amino acids, see: Seebach D, Hook DF, Glättli A. Biopolymers (Peptide Science) 2006;84:23. doi: 10.1002/bip.20391. Cheng RP, Gellman SH, de Grado WF. Chem. Rev. 2001;101:3219. doi: 10.1021/cr000045i. Seebach D, Kimmerlin T, Sebesta R, Campo MA, Beck AK. Tetrahedron. 2004;60:7455. Seebach D, Gardiner J. Acc. Chem. Res. 2008;41:1366. doi: 10.1021/ar700263g. Weiner B, Szymanski W, Janssen DB, Minnaarda AJ, Feringa BL. Chem. Soc. Rev. 2010;39:1656. doi: 10.1039/b919599h. (f) Enantioselective Synthesis of β-Amino Acids Juaristi E, Soloshonok VA, editors. Hoboken, NJ: Wiley; 2005.
- 14.For selected examples of biologically active tetrahydroisoquinolines, see: Allen JRF, Holmstedt BR. Phytochemistry. 1980;19:1573. Scott JD, Williams RM. Chem. Rev. 2002;102:1669. doi: 10.1021/cr010212u. Kartsev VG. Med. Chem. Res. 2004;13:325. Renaud J, Bischoff SF, Buhl T, Floersheim P, Fournier B, Geiser M, Halleux C, Kallen J, Keller H, Ramage P. J. Med. Chem. 2005;48:364. doi: 10.1021/jm040858p. Bentley KW. Nat. Prod. Rep. 2006;23:444. doi: 10.1039/b509523a. Ludwig M, Hoesl CE, Höfner G, Wanner KT. Eur. J. Med. Chem. 2006;41:1003. doi: 10.1016/j.ejmech.2006.03.005. Grycová L, Dostál J, Marek R. Phytochemistry. 2007;68:150. doi: 10.1016/j.phytochem.2006.10.004.
- 15.Freeman DB, Furst L, Condie AG, Stephenson CRJ. Org. Lett. 2012;14:94. doi: 10.1021/ol202883v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.For selected examples of amine oxidation using photoredox catalysis, see: Condie AG, Gonzalez-Gomez JC, Stephenson CRJ. J. Am. Chem. Soc. 2010;132:1464. doi: 10.1021/ja909145y. Rueping M, Zhu S, Koenigs RM. Chem. Commun. 2011;47:8679. doi: 10.1039/c1cc12907d. Hari DP, König B. Org. Lett. 2011;13:3852. doi: 10.1021/ol201376v. Zhou Y-Q, Lu L-Q, Fu L, Chang N-J, Rong J, Chen J-R, Xiao W-J. Angew. Chem. Int. Ed. 2011;50:7171. doi: 10.1002/anie.201102306. Kohls P, Jadhav D, Pandey G, Reiser O. Org. Lett. 2012;14:672. doi: 10.1021/ol202857t. Dai C, Meschini F, Narayanam JMR, Stephenson CRJ. J. Org. Chem. 2012;77:4425. doi: 10.1021/jo300162c. Zhu S, Das A, Bui L, Zhou H, Curran DP, Rueping M. J. Am. Chem. Soc. 2013;135:1823. doi: 10.1021/ja309580a.
- 17.For other studies of tetrahydroisoquinoline derivatives in direct C-H oxidation reactions, see: Murahashi S-I, Komiya N, Terai H, Nakae T. J. Am. Chem. Soc. 2003;125:15312. doi: 10.1021/ja0390303. North M. Angew. Chem. Int. Ed. 2004;43:4126. doi: 10.1002/anie.200301750. Li Z, Li C-J. Eur. J. Org. Chem. 2005:3173. Murahashi S-I, Komiya N, Terai H. Angew. Chem. Int. Ed. 2005;117:7091. doi: 10.1002/anie.200501496. Li Z, Li C-J. J. Am. Chem. Soc. 2005;127:3672. doi: 10.1021/ja050058j. Li Z Z, Li C-J. J. Am. Chem. Soc. 2005;127:6968. doi: 10.1021/ja0516054. Catino AJ, Nichols JM, Nettles BJ, Doyle MP. J. Am. Chem. Soc. 2006;128:5648. doi: 10.1021/ja061146m. Li Z, Bohle DS, Li C-J. Proc. Nat. Acad. Sci. USA. 2006;103:8928. doi: 10.1073/pnas.0601687103. Jones KM, Klussmann M. Synlett. 2012;23:159.
- 18.See the Supporting Information for catalysts evaluated in addition to the catalysts presented in Table 1
- 19.Reisman SE, Doyle AG, Jacobsen EN. J. Am. Chem. Soc. 2008;130:7198. doi: 10.1021/ja801514m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Knowles RR, Lin S, Jacobsen EN. J. Am. Chem. Soc. 2010;132:5030. doi: 10.1021/ja101256v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Birrell JA, Desrosiers J-N, Jacobsen EN. J. Am. Chem. Soc. 2011;133:13872. doi: 10.1021/ja205602j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lin S, Jacobsen EN. Nature Chem. 2012;4:817. doi: 10.1038/nchem.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.For addition of silyl ketene acetals to N-acylquinolinium ions, see: Taylor MS, Tokunaga N, Jacobsen EN. Angew. Chem. Int. Ed. 2005;44:6700. doi: 10.1002/anie.200502277.. The class of catalysts providing the optimal enantioselection for these substrates were ineffective in the present study.
- 21.Zhang Z, Schreiner PR. Chem. Soc. Rev. 2009;38:1187. doi: 10.1039/b801793j. [DOI] [PubMed] [Google Scholar]
- 22.There is a single report describing the oxidative deprotection of the product of methyllithium addition to an N-PMP protected tetrahydroisoquinoline, see: Taniyama D, Hasegawa M, Tomioka K. Tetrahedron: Asymm. 1999;10:221.
- 23.Jurčík V, Arai K, Salter MM, Yamashita Y, Kobayashi S. Adv. Synth. Catal. 2008;350:647. [Google Scholar]
- 24.(a) Wong CL, Kochi JK. J. Am. Chem. Soc. 1979;101:5593. [Google Scholar]; (b) Rollick KL, Kochi JK. J. Am. Chem. Soc. 1982;104:1319. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.