Abstract
Background
Glomerular disease is a complex and evolving topic. In evaluating a specific case it is not unusual for the clinician to ask: Am I missing something? Should I biopsy? When? Should I treat first, then biopsy? This work, which is both evidence based and experience based, is intended to address each of these concerns, and many other issues relevant to the differential diagnosis of glomerular disease.
Summary
The central approach is the use of diagnostic algorithms that are based on quantitative measures routinely obtained early in the course of the diagnostic evaluation. The algorithms are designed to be easy to navigate, systematic, and inclusive. Also provided is a detailed and prioritized list of recommended diagnostic testing, and the rationale for each test.
Key message
This work is intended to facilitate accurate diagnosis in the individual patient presenting with evidence of glomerular disease.
Keywords: glomerular disease, proteinuria, glomerulonephritis
INTRODUCTION
A glomerular disease can be assumed to be present if the patient manifests glomerular hematuria, glomerular proteinuria, or both.
Glomerular hematuria is the result of disruption of the glomerular filtration barrier (GFB) to the extent that red cells are able to pass through the GFB. The hallmarks of glomerular hematuria are that the urine sediment shows:
Increased numbers of red cells that are acanthocytes. These are red cells that have been distorted by passage through the GFB [1]. If ≥ 5% of urine red cells are acanthocytes, this has about a 50% sensitivity and 95% specificity for glomerular hematuria. Increased numbers of small red cells is also characteristic of glomerular hematuria [2].
The presence of casts that contain red cells or a mixture of red cells and white cells. These “cellular casts” are formed when red cells and white cells are forced through the GFB and then become encased in a protein matrix (Tamm-Horsfall protein). Eventually these casts are extruded into the urine. In urine sediment these casts are diagnostic of glomerular hematuria. When seen in tubular lumens on kidney biopsy, they are diagnostic of glomerular hematuria.
In patients with glomerular hematuria, acanthocytes are far more common than cellular casts. Cellular casts usually indicate a more severe form of glomerular injury [3].
Glomerular hematuria is usually accompanied by increased albuminuria. This is consistent with the notion that the disruptions of the GFB that are sufficiently severe to cause hematuria also increases albuminuria. In this regard, it has been reported that, in those with glomerular hematuria, the proportion of urine protein that is albumin usually exceeds 40% [4].
Glomerular proteinuria is the result of disruption of the GFB to the extent that plasma proteins, which normally are largely excluded from the glomerular filtrate, are able to readily pass through the disrupted GFB. The most abundant of the plasma proteins is albumin. So, the hallmark of glomerular proteinuria is albuminuria. The threshold for abnormal albuminuria is 30 mg albumin/g urine creatinine [5]. However, albuminuria is not diagnostic of glomerular proteinuria. Albuminuria can also occur in those with tubular proteinuria. The albuminuria in tubular proteinuria reflects tubular injury that results in decreased tubular reabsorption of the albumin that normally is filtered (about 1 g/d) and normally is nearly completely reabsorbed by the renal tubules [6]. Also, marked albuminuria can occur in overflow proteinuria (increased urinary excretion of immunoglobulin light chains or heavy chains because of their overproduction). The albuminuria occurs because the free monoclonal light chains or free monoclonal heavy chains induce a glomerulopathy (light chain deposition disease, heavy chain deposition disease, AL amyloidosis or AH amyloidosis), which then cause glomerular proteinuria. Also, the filtered paraproteins can cause tubular injury, which causes albuminuria. So, the presence of substantial albuminuria does not exclude tubular proteinuria or overflow proteinuria.
Abnormally increased albuminuria can be assumed if the urinary dipstick shows a value of 2+ or greater (> 100 mg/dl). However, a false positive test for albumin by dipstick can occur in very concentrated urine (specific gravity > 1.030) or in very alkaline urine (pH > 7.0 in which the high pH is the result of bicarbonaturia) [7]. Highly alkaline urine is seen in those on a high alkaline-ash diet (strict vegetarians) or in those receiving high-dose sodium bicarbonate therapy. A false positive test for albumin due to concentrated urine or alkaline urine can be confirmed by testing the urine with 20% sulfosalicylic acid (SSA) and showing that no turbidity develops with addition of the SSA. In alkaline urine bubbles may develop as the bicarbonate in the urine is changed to CO2 by the SSA [7].
Albuminuria is confirmed by immunoassay. Tubular proteinuria, and overflow proteinuria are confirmed by urine immunofixation assay, which characterizes the low molecular weight proteins in urine. The routine clinical laboratory tests for urine protein are chemical assays (e.g., pyrogallol red or Coomassie blue), which detect both albumin and non-albumin proteins (total proteinuria) [6]. In general, if glomerular disease is the cause of the proteinuria, albumin represents > 40 % of total proteinuria (i.e., the ratio: urine albumin (A)/protein (P)(APR) > 0.4) If the urine total protein/creatinine ratio (PCR) is > 0.4 usually the APR is 60–80% of total proteinuria [4,6,8,9]. So, in most of these patients, measuring total proteinuria provides about the same information as measuring albuminuria, and is much less expensive. However, in those with total proteinuria < 500 mg/d, the preferred method for monitoring glomerular proteinuria is by measuring albuminuria. The rationale is that even though these patients have glomerular proteinuria, most of the proteinuria can be non-glomerular in origin. So, total proteinuria can obscure clinically important changes in albuminuria [6].
If tubulo-interstitial disease is the cause of the proteinuria, albumin usually is less than 40% of the total urine protein (APR < 0.4) [4,8,9].
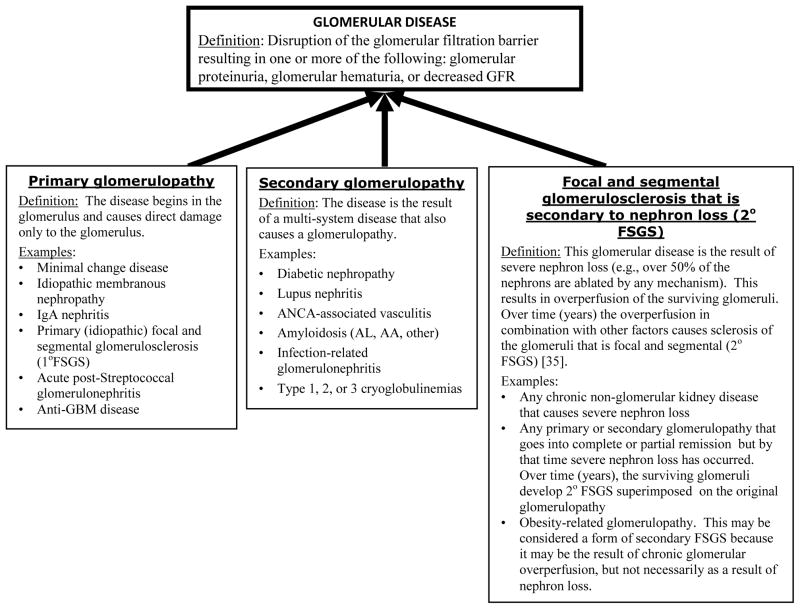
In light of the above, it is clear that determining whether a glomerular disease is present is relatively straightforward. However, determining which glomerular disease is present can be challenging because there are multiple pathways to glomerular disease, including non-glomerular kidney diseases that transforms into a glomerular disease through the process of secondary focal and segmental glomerular sclerosis (secondary FSGS) as shown in Figure 1. Adding to the challenge of differential diagnosis of glomerular disease are the following paradoxes:
Figure 1.
Pathways to glomerular disease.
-
Glomerular hematuria can be present even though glomerulonephritis (inflammatory glomerular disease) is not present.
Examples include:
Diabetic nephropathy. Glomerular hematuria, including red blood cell casts, is a not uncommon manifestation in patients with biopsy proven diabetic glomerulosclerosis [10,11].
Warfarin-related nephropathy (WRN). This is a newly recognized but not uncommon syndrome in which acute kidney injury (AKI) follows shortly after an increase in INR to > 3.0. On kidney biopsy there are multiple tubules occluded by red blood cell casts but the glomeruli usually are unremarkable. So, the patient manifests severe glomerular hematuria in the absence of glomerulonephritis. WRN can occur in those with or without chronic kidney disease, although the presence of chronic kidney disease increases the risk of WRN by about twofold [12–15]. Glomerulonephritis may especially increase the risk of WRN [16].
Constitutive abnormally thin or thick glomerular basement membrane (GBM) disease. These conditions can also cause prolonged gross hematuria thereby mimicking severe glomerulonephritis [17–19].
Atheroembolism in which the cholesterol crystals damage the glomeruli causing glomerular hematuria [20].
Allergic interstitial nephritis. This can mimic glomerulonephritis by causing gross hematuria, including acanthocytes, and overt proteinuria [21–23]
Idiopathic FSGS. Glomerular hematuria is common in this disorder [24].
Hematuria can be absent or minimal even though the patient has severe glomerulonephritis. This is not rare phenomena. The published work states that the urine sediment can appear “normal or bland” [25–28]. In our experience a better description is that the urine sediment is “unremarkable” in that it contains few or no red cells. If acanthocytes are present, they are rare. Also, there are no cellular casts. This paradox can be explained if the severely affected glomeruli do not contribute importantly to urine formation, and the other glomeruli have relatively little inflammatory change. The combination of severely involved glomeruli side by side with minimally involved glomeruli is especially common in ANCA-related vasculitis [29].
In diabetic glomerulosclerosis proteinuria and microalbuminuria can be absent even in Stage 3 CKD. This is common and is seen in both Type 1 and Type 2 diabetics with nephropathy [30–33]. However, progression to end-stage kidney disease is strongly dependent upon the development of macroalbuminuria [34].
Proteinuria can be nephrotic range and accompanied by microscopic and even gross hematuria suggesting a form of severe primary or secondary glomerulopathy, but the problem is malignant hypertension. As the malignant hypertension is controlled, the kidney manifestations rapidly resolve (see UpToDate: Malignant hypertension and hypertensive encephalopathy). So, generally, malignant hypertension can be easily distinguished from severe glomerulonephritis which, generally, does not present with malignant hypertension and does not resolve rapidly.
Non-glomerular kidney disease that results in substantial nephron loss can, over time, develop secondary FSGS with nephrotic-range proteinuria (see Figure 1) [35–37].
-
Glomerulopathies that began as a primary or secondary glomerulopathy can transform into secondary FSGS. This occurs when the initial glomerulopathy results in substantial nephron loss (e.g., > 50% loss) [35,38]. If the surviving glomeruli heal, the patient will show an elevated serum creatinine but proteinuria will be low level because the surviving glomeruli are in good condition. However, over time the surviving glomeruli may be transformed into a new glomerulopathy, secondary FSGS (Figure 1). In such patients nephrotic-range proteinuria can develop. Hematuria usually is not conspicuous. The clinician must then decide whether the patient is experiencing recurrence of their primary or secondary glomerulopathy, or another glomerulopathy is developing, especially secondary FSGS [39].
Note that the term “secondary FSGS” can also refer to glomerulopathies in which the characteristic change is FSGS but, unlike primary FSGS, the cause of the FSGS is known (e.g., FSGS secondary to HIV infection). In these forms of secondary FSGS, the glomerular changes may be initiated by the disease process itself, not hyperperfusion injury [40].
The biomarkers that are used to detect the presence of a secondary glomerulopathy may be unreliable. As examples, the ANA, which is the hallmark of SLE and certain other autoimmune-related glomerulopathies, is very sensitive but very non-specific. Indeed, the vast majority of patients with a positive ANA do not have lupus or any other autoimmune disease [41]. Antineutrophil cytoplasmic antibodies (ANCA), the hallmark of ANCA-related vasculitis, are positive in conditions that can mimic ANCA-related vasculitis such as infection (bacterial endocarditis, atypical pneumonia, invasive amoebiasis, and HIV), cancer and other conditions such as inflammatory bowel disease and cystic fibrosis, and during therapy with propylthiouracil or hydralazine [42–48]. Low levels of complement C3 and C4 are the hallmarks of classical pathway activation by diseases caused by deposition of circulating immune complexes. However, low C3 and C4 can be present in conditions as disparate as primary antiphospholipid syndrome [49], sepsis, severe liver disease, and severe atheroembolism [50].
Kidney biopsy, which is the ultimate step in the differential diagnosis of glomerular disease, often does not provide a disease-specific result. There is only a limited number of ways that the glomerulus can respond to injury. So it is not surprising that the same glomerular pattern of injury can be the result of a wide range of pathogenetic mechanisms. For example, membranous nephropathy can be the result of a variety of disease mechanisms including idiopathic, SLE, infections with Hepatitis B, Hepatitis C, HIV, syphilis, or parasites, or can be drug induced (NSAIDs, gold injections, penicillamine) [51] Similarly, glomerulonephritis with a membranoproliferative pattern can be the result of a systemic immune complex disease (for example, bacterial endocarditis, systemic lupus erythematosus, or hepatitis C virus), or genetic or acquired complement dysregulation, chronic thrombotic microangiopathy, monoclonal immunoglobulin deposition disease, or cryoglobulinemia [52,53]. Pauci-immune crescentic glomerulonephritis (immune complexes are absent or minimal) is characteristic of ANCA-related glomerulonephritis. However, little or no immune complex deposition can also be seen in some infection-related glomerulonephritides, including legionella, visceral abscess, and other infections [54–58] Nodular glomerulosclerosis, the hallmark of diabetic glomerulopathy, can result from conditions as disparate as chronic thrombotic microangiopathy and cigarette smoking [59].
To summarize, in most patients with evidence of glomerular disease there is no single measure that provides a specific diagnosis, not even kidney biopsy. To achieve a specific diagnosis, and all that this implies for appropriate management, it is often necessary to test broadly (as described in the tables shown below) and use a systematic approach (as described in the algorithms shown below). However, to use these tables and algorithms optimally they need to be interpreted in light of the paradoxes described above, and the cardinal manifestations of glomerular disease, which are discussed next.
Nephrotic syndrome
In adults, nephrotic-range proteinuria is defined as > 3.5 g/d. Severe nephrotic syndrome is variously defined but usually includes the following: proteinuria exceeding 10 g/d, serum albumin < 2.5 g/dl, and severe edema. Deciding whether the patient has severe nephrotic syndrome is of diagnostic value because severe nephrotic syndrome is characteristic of minimal change disease, idiopathic membranous nephropathy, primary FSGS, and AL amyloid of the kidney. By contrast, it is unusual for severe nephrotic syndrome to be a manifestation of IgA nephritis, secondary FSGS, or Alport’s syndrome.
The extent to which a glomerular disease can manifest the signs and symptoms of severe nephrotic syndrome is influenced by a number of nonrenal factors including whether the patient has high-salt intake or is receiving calcium channel blocker therapy, both of which exacerbate proteinuria [37]. The edema of nephrotic syndrome can be increased by calcium channel blockers, hydralazine, or the oral hypoglycemic drugs pioglitazone or rosiglitazone. The low serum albumin of nephrotic syndrome can be worsened by low protein intake, liver disease, or nonrenal albumin losses such as protein-losing enteropathy. Other factors influencing edema formation in nephrotic syndrome are the patient’s dominant posture (e.g., standing or sitting for prolonged periods) or whether lymphatic or venous insufficiency or congestive heart failure is present [60].
The conditions that cause severe nephrotic syndrome typically are noninflammatory glomerulopathies. So, typically the urine sediment is “bland”, indicating that it is not “nephritic” (see below). The typical nephrotic urine sediment contains few red cells, no red cell/white cell casts, but oval fat bodies and fatty casts are present. The latter are the result of the filtration of plasma lipoproteins because of the hyperlipidemia that is characteristic of severe nephrotic syndrome.
Nephritic syndrome
A nephritic urine can be defined by a urine sediment showing red cells > 5/high power field (hpf) and the presence of one or more of the following: acanthocytes, red cell casts or mixed red cell/white cell casts [61] (discussed above). A normal urine sediment usually contains fewer than 2 red cells/hpf and acanthocytes and small red cells are generally absent (see UptoDate: Hematuria: glomerular versus extraglomerular). The urine dipstick test for “blood” should also be negative. However, occasionally dipstick testing for blood will show “blood-trace” in normal persons (unpublished personal observations). If glomerular hematuria is present, usually at least 1 per 20 red cells is an acanthocyte. Cellular casts are far less common than acanthocytes [3].
Pyuria can also be conspicuous in nephritic patients, particularly in inflammatory forms of glomerulonephritis such as post-streptococcal glomerulonephritis. However, pyuria is never the sole manifestation of a nephritic urine sediment. Acanthocytes are best detected by phase contrast microscopy [1]. However, acanthocytes can also be readily detected by standard transmission microscopy (unpublished personal observations).
LABORATORY TESTING IN PATIENTS WITH SUSPECTED GLOMERULAR DISEASE
In general, the recommended laboratory diagnostic approach should include routine testing (e.g., a “metabolic panel”, CBC, and platelet count) and should be the same for all patients presenting with evidence of a major glomerular disease (see Table 1), with a few notable exceptions (see Table 2). The rationale for a single uniform diagnostic approach is that, as discussed in Introduction, there is no pattern of urine sediment, quantitative proteinuria, serum creatinine level, or biomarker testing that definitively rules in or rules out any nephritic or nephrotic glomerular disorder. So, in many instances the final diagnosis (and management plan) is deduced from a mosaic of information that includes both positive and negative outcomes of specific tests. It could be argued that, if it has already been determined that a kidney biopsy is to be done, a more economical approach would be to perform the kidney biopsy, and when the result returns, tailor the laboratory diagnostic approach to the kidney biopsy findings. Although this is reasonable, we suggest that generally it is not the most efficient or cost-effective approach for the following reasons:
Table 1.
Recommended initial testing in the patient being evaluated for glomerular disease1
| Test | Specific Purpose | Comments |
|---|---|---|
| 1. 24-h urine collection | Quantify proteinuria. Spot PC (protein/creatinine ratio) is not recommended for this purpose because it is unreliable in individual patients [66,85,87–89]. Measuring albuminuria is useful in monitoring low-level glomerular proteinuria. However, once the total proteinuria exceeds 500 mg/d, albuminuria is about 60–80% of total proteinuria. So, proteinuria provides the same information as albuminuria, and is less expensive [6]. | Also recommended is the measurement of sodium, potassium, and urea content of the collection. If the collection is a complete or nearly 24-h collection, these are reliable estimates of 24-h intake of salt, potassium, and protein, which are important for nutrient management the CKD patient [38]. |
| 2. Serum albumin | Assess severity of the disruption of the GFB, and whether protein nutrition, and hepatic albumin synthesis is adequate. | At any given level of proteinuria, the lower the serum albumin, the greater is the glomerular permeability to albumin. The serum albumin also assesses nutritional/liver status. For example, if proteinuria is modest (e.g., 3.0 g/d) and serum albumin is low (e.g., 1.5 g/dl), this suggests that a low rate of albumin synthesis is partly to blame for the low albumin level. This may be nutritional or related to liver disease. |
| 3. LDH | Assess for hemolysis, or damage to muscles or viscera. | In the absence of muscle injury or visceral organ necrosis, elevated LDH is evidence for hemolysis (e.g., a thrombotic microangiopathy). |
| 4. Reticulocyte count Platelet count | Assess for increased or decreased red cell production. Assess for thrombotic microangiopathy. | If elevated reticulocyte count or decreased platelet count, do blood smear to search for schistocytes and haptoglobin to assess for intravascular hemolysis. These will assess for thrombotic microangiopathy, which can cause glomerular disease (e.g., vasculitis, antiphospholipid syndrome, TTP, HUS, aHUS, scleroderma, malignant hypertension, blood stream infection, and the cryoglobulinemias). |
| 5. C3, C4 | Test for disorders that activate the classical or alternative complement pathways. | Low C3 and C4 are characteristic of the glomerulonephritis caused by deposition of circulating immune complexes (e.g., SLE, infective endocarditis). Normal or nearly normal C3 with very low C4 is characteristic of Type 2 and Type 3 cryoglobulinemia. Very low C3 and normal C4 is characteristic of post-streptococcal glomerulonephritis. Low C3 and normal C4 is seen in HUS, aHUS, C3 glomerulopathy, and idiopathic MPGN Type 1 [50]. |
| 6. Serum protein electrophoresis (SPEP) + free light chains | Screen for monoclonal gammopathy (SPEP + free light chains), or hypogammaglobulinemia, hypergammaglobulinemia (SPEP). Serum or urine for immunofixation is not recommended for routine screening because it is much more expensive than SPEP + free light chains, which are sensitive and specific for detection of monoclonal gammopathy [90]. | If monoclonal protein is present on SPEP, and/or there is an abnormal ratio of kappa/lambda light chains, serum immunofixation is indicated to characterize the monoclonal proteins [90] (see Algorithm 1). If hypogammaglobulinemia is present, measure serum IgG, IgA, IgM levels to assess for immunodeficiency. If hyperglobulinemia is present, measure serum IgG, IgA, IgM levels. If IgG is is increased, measure IgG isotypes (IgG1,2,3,4) to test for IgG4 disease. |
| 7. Hepatitis B surface antigen, Hepatitis C antibody, and HIV (if risk factors for HIV are present) | These infections are common causes of glomerular disease. | Even if the patient’s glomerular diseases is not the result of one of these infections, it is important to know whether these infections are present, especially if immunosuppressive therapy is planned. |
| 8. ANA | Screen for autoimmune disorders | See text for Limitations of ANA testing to identify SLE and (other autoimmune disorders) |
| 9. ANCA | Screen for ANCA-related vasculitis | See text for Limitations of ANCA testing. If ANCA is present, test for anti-myeloperoxidase (if pANCA positive) or proteinase 3 (if c-ANCA positive). |
| 10. Rheumatoid factor | Screen for cryoglobulinemias (Types 2 and 3), and certain autoimmune disorders. | In cryoglobulinemic glomerulonephritis, plasma cryoglobulins are often undetectable. So, the rheumatoid factor serves as a surrogate marker for Type 2 and Type 3 cryoglobulinemia. This is especially helpful in cryoglobulinemic glomerulonephritis because often the deposits are cleared rapidly and are not present in the kidney biopsy. |
Not included in this list is testing that is routinely indicated in the CKD patient, such as intact PTH, serum creatinine, BUN, electrolytes, blood glucose, lipid panel, calcium, phosphorus, bilirubin, ALT, AST, CBC and urinalysis (as discussed above).
Table 2.
Recommended optional initial testing for the patient being evaluated for glomerular diseases.
| Test | Specific Purpose | Comment |
|---|---|---|
| 1. Blood cultures | Test for the blood stream infection. particularly bacterial, fungal, or viral. A low threshold for doing blood culture is recommended. Usually infection-related GN, particularly bacterial infection, manifests with fever and leukocytosis. Remarkably, some times manifestations are absent in infection-related GN. | Most infection-related glomerulonephritides are due to bacterial infections. However, CMV, EBV, and parvovirus may also cause glomerulonephritis. Legionella infection can cause glomerulonephritis but it is more quickly identified by the presence of Legionella antigen in the urine. Bacteria and fungi are detected by the same culture technique. Virus culturing and detection requires separate material. Testing for parasite infections require separate testing methods. |
| 2. Anti-GBM assay | Test for Goodpasture’s syndrome. | The renal biopsy findings for Goodpasture’s syndrome are diagnostic and generally are available more quickly than anti-GBM assay. So, in general, there is little point in ordering the anti-GBM assay if the kidney biopsy is imminent, unless the patient presents with a pulmonary-renal syndrome. |
| 3. Streptozyme assay | Screen for recent streptococcal group A infection. | Usually the diagnosis of acute post-streptococcal glomerulonephritis can be made by its clinical presentation (recent infection, nephritic urine, low C3 and normal C4, and a positive streptozyme test). |
| 4. D-dimer | To search for evidence of the hypercoagulable state. To search for evidence that a clinically important clotting event has occurred. | Elevated D-dimer (e.g., > 2.0 ng/ml) identifies those who have formed a clot or are at increased risk of clotting [91,92]. In the patient with severe nephrotic syndrome, an elevated D-dimer warrants a search for venous clot (extremities, renal vein) and a search for a thrombotic microangiopathy (see Table 1). Even if clot is not found, the elevated D-dimer may warrant preemptive systemic anticoagulation in the severely nephrotic patient (see UpToDate: Severe nephrotic syndrome). Also, in those with antiphospholipid antibodies, a D-dimer > 2.0 μg/l may be a better predictor of a future major clotting event than the presence of antiphospholipid antibodies [91]. |
| 5. Quantitative immunoglobulins (IgG, IgA, IgM) | Characterize the polyclonal abnormalities identified by SPEP (also see Table 1, SPEP) | Low IgG levels are occasionally seen in untreated SLE with or without GN, and in severe nephrotic syndrome [93]. This increases the risk of infection. So careful monitoring for signs of infection in these patients is warranted. Also, if immunosuppressive therapy is needed, IgG levels should be monitored to avoid over-immunosuppression. |
| 6. ADAMTS-13 activity | Assess for TTP. | This test is indicated in any patient with glomerular disease who presents with evidence of a thrombotic microangiopathy [73] |
| 7. Chest X-ray | Assess for a pulmonary-renal syndrome or a pulmonary complication of the glomerular disease (e.g., pleural effusion, cardiomegaly), that would influence management. | A routine chest X-ray is warranted in any patient presenting with glomerular disease to search for a pulmonary-renal syndrome, and especially if the patient has pulmonary symptoms, abnormal lung findings on physical exam, or has a low oxygen saturation by pulse oximeter. |
In most instances, the glomerular pattern itself does not identify a specific glomerular disease, as discussed above. So, not having in hand the results of the recommended broad testing can cause important delays in arriving at an accurate diagnosis, and deciding on appropriate management plan.
The recommended broad testing provides information that is of general management value, regardless of the final diagnosis, as discussed in Tables 1 and 2.
The recommended broad testing is usually a one-time expense, which often can be justified by the critical importance of quickly and accurately identifying the cause of the patient’s kidney condition.
We recognize, however, that broad pre-kidney biopsy testing (Tables 1 and 2) is not needed for every patient presenting with evidence of glomerular disease and, in whom, kidney biopsy is planned. For example, broad testing is not needed in the patient with indolent, non-severe glomerular disease. In such patients it is appropriate to do limited testing prior to the kidney biopsy. When the biopsy results return, obtain the testing needed to elucidate the cause of the kidney disease and decide on its management.
Assessing GFR from measurement of serum creatinine in the patient with glomerular disease is important for both diagnosis (does the patient have acute or chronic kidney disease) and management (e.g., does medication dose need to be adjusted because of impaired kidney function). In this regard, it is important to recognize the limitations of creatinine-based eGFR equations. These include marked overestimation of true GFR in nephrotic syndrome, especially in those with hypoalbuminemia [62] and uncertainty regarding whether CKD is present because of confounding by age, gender, race [63] and creatinine production if it deviates substantially from normal [64].
APPROACH TO DIFFERENTIAL DIAGNOSIS OF GLOMERULAR DISEASE IN THE INDIVIDUAL PATIENT USING ALGORITHMS 1, 1A, AND 2
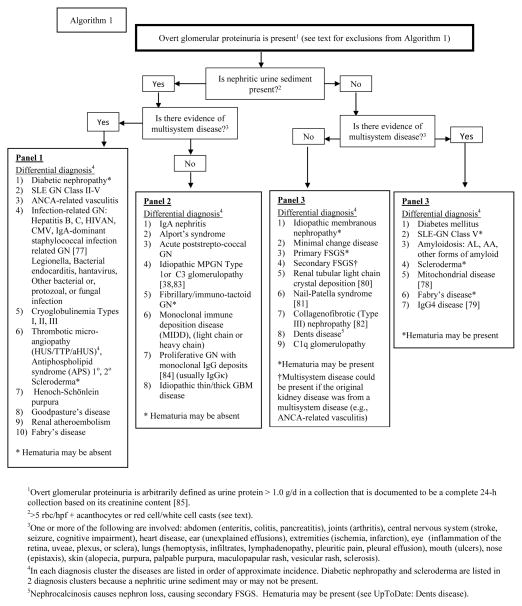
Algorithm 1.
Approach to the differential diagnosis of the patient presenting with overt glomerular proteinuria.
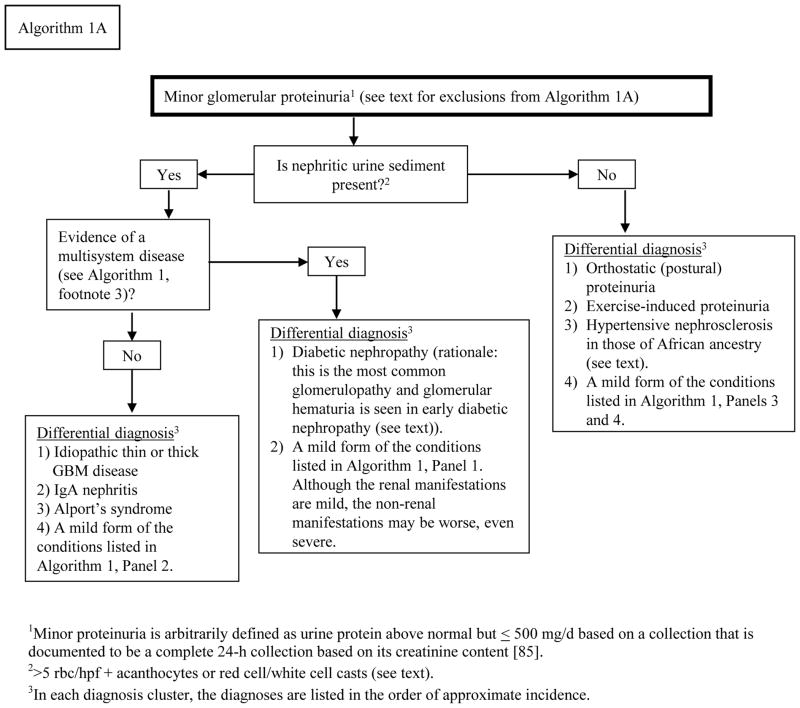
Algorithm 1A.
Approach to the differential diagnosis of the patient presenting with minor proteinuria.
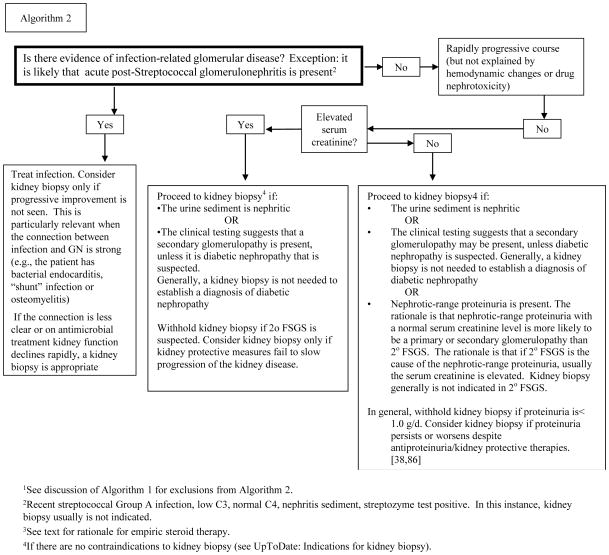
Algorithm 2.
Approach to deciding whether the kidney biopsy is needed to manage the patient presenting with overt glomerular proteinuria1.
Algorithm 1 is a systematic and inclusive approach intended to narrow the differential diagnosis of glomerular disease to a single cluster of the most likely diagnoses, ranked in approximate incidence. Further diagnostic separation within the diagnosis cluster is achieved by using the references provided herein, and those available from standard sources. For rare or very recently described conditions, references are provided in the algorithm. Algorithm 1 is intended for those with overt proteinuria. Algorithm 1A is intended for those with minor proteinuria.
Algorithm 2 proceeds naturally from Algorithm 1 because it bridges the gap between arriving at the most likely diagnoses, and whether a kidney biopsy is needed to achieve a specific diagnosis. Kidney biopsy usually is not needed for the types of patients described in Algorithm 1A.
Algorithms 1 and 2 are intended for evaluation of the patient with overt glomerular proteinuria arbitrarily defined as ≥ 1.0 g/24 h, based on a complete 24-h collection documented by the creatinine content of the collection [65,66]. This level of proteinuria was chosen because it will generally exclude those with mild glomerular disease. In addition, proteinuria ≥ 1 g is associated with progression of kidney disease [67] and is generally regarded as a threshold for considering kidney biopsy to elucidate the cause of glomerular proteinuria.
Although Algorithms 1 and 2 are intended for those with proteinuria ≥ 1.0 g/d, likely they will also be informative in those with proteinuria 500–1000 mg/d. Algorithm 1A is intended for those with abnormal proteinuria but < 500 mg/d.
The following steps are recommended when applying Algorithms 1 and 1A to the individual patient:
-
Step 1
Exclude those for whom the algorithm is not intended, as follows:
The cause of the proteinuria is tubular proteinuria, documented by urine immunofixation showing that the dominant protein is non-albumin small molecular weight proteins.
The cause of the proteinuria is overflow proteinuria, documented by urine immunofixation showing increased amounts of free monoclonal light chains or heavy chains or an intact monoclonal immunoglobulin. This is an exclusion from Algorithms 1 and 2 only if a diagnosis of multiple myeloma or B-cell lymphoma can be established by the usual methods. In these instances, Algorithms 1 and 2, and kidney biopsy generally are not needed for diagnosis and management.
Renal imaging shows evidence of a primary cystic disease of the kidney (autosomal dominant or recessive polycystic kidney disease: ADPKD, ARPKD, tuberous sclerosis, or von Hippel-Lindau disease), obstructive uropathy, or the characteristic radiographic findings of reflux nephropathy.
The proteinuria is likely related to drug toxicity or hypersensitivity. These cause proteinuria that generally is reversible when the drug is discontinued. As examples, minimal change or membranous nephropathy associated with NSAIDs, minimal change disease/FSGS associated with lithium, the proteinuric renal disease associated with heavy metals (gold salts, mercury), rapamune, drug-induced allergic interstitial nephritis or chemotherapy [68] including anti-VEGF therapy. The latter may be overlooked as a cause of glomerular disease when it is used as an intraocular injection to treat macular degeneration [69].
Relapse of minimal change disease likely is present. In these cases, a trial of steroid therapy is more appropriate than application of Algorithm 1.
Systemic cancer is present. Here the management strategy is to treat the cancer. If the glomerular disease is the result of cancer, resolution of the glomerular disease may occur [70].
The patient is pregnant. The manifestations of glomerular disease as induced by or modified by pregnancy is beyond the scope of this work.
-
Step 2
Undertake the recommended initial testing/optional testing (see Tables 1 and 2)
-
Step 3
Proceed through the algorithm to determine which diagnostic cluster best describes the patient’s condition.
-
Step 4
Narrow the differential diagnosis by using standard sources, and the information provided herein, including the “paradoxes” and the discussion of nephrotic and nephritic syndrome.
-
Step 5
If a specific diagnosis is not achieved, proceed to Algorithm 2. Also proceed to Algorithm 2 if a specific diagnosis is achieved, but the management plan is dependent on whether a specific histologic pattern is present (for example, the WHO/RPS Class II-V of SLE GN.
Algorithm 2 first turns on whether there is evidence of an infection-related glomerulonephritis. Next it turns on whether rapid progression is developing, and it is not explained by hemodynamic factors or drug nephrotoxicity. In such patients the recommendation is to begin high-dose steroid therapy, and then proceed to the kidney biopsy as soon as feasible. The rationale is that, if the patient has an acute immune-mediated glomerulonephritis, TTP, or an anti-phospholipid syndrome, the steroid therapy will move things in the right direction. On the other hand, if the patient has a covert infection, short-term high-dose steroid therapy (e.g., 3 days) is unlikely to cause important difficulties [54,55,71,72]. Similarly, if the patient has HUS, or aHUS a few days of high-dose steroid until that diagnosis can be established, should not cause harm.
If antiphospholipid syndrome is the prime suspect, systemic anticoagulation with heparin should be given until that diagnosis is excluded.
If TTP or aHUS are the prime suspects, plasma exchange with fresh frozen plasma should be given until these diagnoses are excluded. Generally TTP and aHUS do not require a kidney biopsy for diagnosis [73].
Algorithm 2 also turns on whether the serum creatinine is abnormally elevated. In general, a kidney biopsy is not recommended if secondary FSGS or obesity-related glomerulopathy is the likely cause of the proteinuria and elevated serum creatinine. In these circumstances, a more prudent approach is conservative management with kidney protective/antiproteinuric therapies. In the obese patient, weight loss [38] and ACE inhibitor therapy[74] have been shown to favorably influence their nephropathy. If the patient progresses despite these measures, a kidney biopsy might then be indicated.
Another key example of conditions in which kidney biopsy generally is not part of the initial evaluation is the hypertensive nephrosclerosis that is seen in those of African ancestry [75]. This disorder begins as a low-proteinuria disease, even though the serum creatinine can be substantially elevated (Stage 3 CKD) [76]. Over time, these patients may develop progressive proteinuria, usually related to secondary FSGS. In such patients, aggressive kidney protective/antiproteinuria therapy is recommended, including weight loss, if obese. If these measures fail to control progression, kidney biopsy might then be indicated.
Acknowledgments
This work is supported in part by NIH grant DK55546, NIH grant R01 DK074661, NIH grant U01 DK085673, NIH CTSA grant UL1RR025755-01, and the James E. Casto Fund
Footnotes
Conflict of Interest Statement: All authors have no conflict of interest of any type.
References
- 1.Kohler H, Wandel E, Brunck B. Acanthocyturia--a characteristic marker for glomerular bleeding. Kidney Int. 1991;40:115–120. doi: 10.1038/ki.1991.188. [DOI] [PubMed] [Google Scholar]
- 2.Shichiri M, Oowada A, Nishio Y, Tomita K, Shiigai T. Use of autoanalyser to examine urinary-red-cell morphology in the diagnosis of glomerular haematuria. Lancet. 1986;2:781–782. doi: 10.1016/s0140-6736(86)90302-8. [DOI] [PubMed] [Google Scholar]
- 3.Hebert LA, Dillon JJ, Middendorf DF, Lewis EJ, Peter JB. Relationship between appearance of urinary red blood cell/white blood cell casts and the onset of renal relapse in systemic lupus erythematosus. Am J Kidney Dis. 1995;26:432–438. doi: 10.1016/0272-6386(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 4.Ohisa N, Yoshida K, Matsuki R, Suzuki H, Miura H, Ohisa Y, Murayama N, Kaku M, Sato H. A comparison of urinary albumin-total protein ratio to phase-contrast microscopic examination of urine sediment for differentiating glomerular and nonglomerular bleeding. Am J Kidney Dis. 2008;52:235–241. doi: 10.1053/j.ajkd.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: A kdigo controversies conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 6.Birmingham DJ, Rovin BH, Shidham G, Bissell M, Nagaraja HN, Hebert LA. Relationship between albuminuria and total proteinuria in systemic lupus erythematosus nephritis: Diagnostic and therapeutic implications. Clin J Am Soc Nephrol. 2008;3:1028–1033. doi: 10.2215/CJN.04761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bay WH, Hebert LA. The living donor in kidney transplantation. Ann Intern Med. 1987;106:719–727. doi: 10.7326/0003-4819-106-5-719. [DOI] [PubMed] [Google Scholar]
- 8.Smith ER, Cai MM, McMahon LP, Wright DA, Holt SG. The value of simultaneous measurements of urinary albumin and total protein in proteinuric patients. Nephrol Dial Transplant. 2012;27:1534–1541. doi: 10.1093/ndt/gfr708. [DOI] [PubMed] [Google Scholar]
- 9.Methven S, Traynor JP, O’Reilly DS, Deighan CJ, Macgregor MS. Urine albumin: Protein ratio as a predictor of patient outcomes in ckd. Nephrol Dial Transplant. 2012;27:3372–3373. doi: 10.1093/ndt/gfs137. author reply 3373–3374. [DOI] [PubMed] [Google Scholar]
- 10.O’Neill WM, Jr, Wallin JD, Walker PD. Hematuria and red cell casts in typical diabetic nephropathy. Am J Med. 1983;74:389–395. doi: 10.1016/0002-9343(83)90956-7. [DOI] [PubMed] [Google Scholar]
- 11.Akimoto T, Ito C, Saito O, Takahashi H, Takeda S, Ando Y, Muto S, Kusano E. Microscopic hematuria and diabetic glomerulosclerosis--clinicopathological analysis of type 2 diabetic patients associated with overt proteinuria. Nephron Clin Pract. 2008;109:c119–126. doi: 10.1159/000145454. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, Hebert L, Calomeni E, Nadasdy T. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: A report of 9 cases. Am J Kidney Dis. 2009;54:1121–1126. doi: 10.1053/j.ajkd.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, Wu H, Bhatt U, Nadasdy T, Hebert LA. Warfarin therapy that results in an international normalization ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract. 2010;115:c142–146. doi: 10.1159/000312877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, Bhatt UY, Hebert LA. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80:181–189. doi: 10.1038/ki.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware K, Brodsky P, Satoskar AA, Nadasdy T, Nadasdy G, Wu H, Rovin BH, Bhatt U, Von Visger J, Hebert LA, Brodsky SV. Warfarin-related nephropathy modeled by nephron reduction and excessive anticoagulation. J Am Soc Nephrol. 2011;22:1856–1862. doi: 10.1681/ASN.2010101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodsky SV, Rovin BH, Hebert LA. Benefit of cyclophosphamide therapy in iga nephritis may have been obscured by warfarin-related nephropathy in the randomized trials in which warfarin and dipyridamole were used in combination with cyclophosphamide. Nephrol Dial Transplant. 2012;27:475–477. doi: 10.1093/ndt/gfr559. [DOI] [PubMed] [Google Scholar]
- 17.Spetie DN, Nadasdy T, Nadasdy G, Agarwal G, Mauer M, Agarwal AK, Khabiri H, Nagaraja HN, Nahman NS, Jr, Hartman JA, Hebert LA. Proposed pathogenesis of idiopathic loin pain-hematuria syndrome. Am J Kidney Dis. 2006;47:419–427. doi: 10.1053/j.ajkd.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Kabir A, Nadasdy T, Nadasdy G, Hebert LA. An unusual cause of gross hematuria and transient arf in an sle patient with warfarin coagulopathy. Am J Kidney Dis. 2004;43:757–760. doi: 10.1053/j.ajkd.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 19.Hebert LA, Betts JA, Sedmak DD, Cosio FG, Bay WH, Carlton S. Loin pain-hematuria syndrome associated with thin glomerular basement membrane disease and hemorrhage into renal tubules. Kidney Int. 1996;49:168–173. doi: 10.1038/ki.1996.23. [DOI] [PubMed] [Google Scholar]
- 20.Cosio FG, Zager RA, Sharma HM. Atheroembolic renal disease causes hypocomplementaemia. Lancet. 1985;2:118–121. doi: 10.1016/s0140-6736(85)90225-9. [DOI] [PubMed] [Google Scholar]
- 21.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60:804–817. doi: 10.1046/j.1523-1755.2001.060002804.x. [DOI] [PubMed] [Google Scholar]
- 22.Brewster UC, Perazella MA. Acute kidney injury following proton pump inhibitor therapy. Kidney Int. 2007;71:589–593. doi: 10.1038/sj.ki.5002038. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez E, Gutierrez E, Galeano C, Chevia C, de Sequera P, Bernis C, Parra EG, Delgado R, Sanz M, Ortiz M, Goicoechea M, Quereda C, Olea T, Bouarich H, Hernandez Y, Segovia B, Praga M. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. 2008;73:940–946. doi: 10.1038/sj.ki.5002776. [DOI] [PubMed] [Google Scholar]
- 24.Schnaper HW. Idiopathic focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:183–193. doi: 10.1053/snep.2003.50016. [DOI] [PubMed] [Google Scholar]
- 25.Freedman P, Meister HP, Co BS, Markowitz AS, Dubin A. Subclinical renal response to streptococcal infection. N Engl J Med. 1966;275:795–802. doi: 10.1056/NEJM196610132751501. [DOI] [PubMed] [Google Scholar]
- 26.Goorno W, Ashworth CT, Carter NW. Acute glomerulonephritis with absence of abnormal urinary findings. Diagnosis by light and electron microscopy. Ann Intern Med. 1967;66:345–353. doi: 10.7326/0003-4819-66-2-345. [DOI] [PubMed] [Google Scholar]
- 27.Bruns FJ, Adler S, Fraley DS, Segel DP. Long-term follow-up of aggressively treated idiopathic rapidly progressive glomerulonephritis. Am J Med. 1989;86:400–406. doi: 10.1016/0002-9343(89)90336-7. [DOI] [PubMed] [Google Scholar]
- 28.Yata N, Ikeda M, Ishikura K, Hataya H, Matsuyama T, Banba M, Hasegawa O, Honda M. Typical MPGNwith few urinary abnormalities. Am J Kidney Dis. 2004;43:918–922. doi: 10.1053/j.ajkd.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 29.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noel LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM. Histopathologic classification of anca-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 30.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289:3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 31.Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 2010;77:57–64. doi: 10.1038/ki.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacIsaac RJ, Panagiotopoulos S, McNeil KJ, Smith TJ, Tsalamandris C, Hao H, Matthews PG, Thomas MC, Power DA, Jerums G. Is nonalbuminuric renal insufficiency in type 2 diabetes related to an increase in intrarenal vascular disease? Diabetes Care. 2006;29:1560–1566. doi: 10.2337/dc05-1788. [DOI] [PubMed] [Google Scholar]
- 33.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: An indicator of more advanced glomerular lesions. Diabetes. 2003;52:1036–1040. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 34.Pavkov ME, Knowler WC, Lemley KV, Mason CC, Myers BD, Nelson RG. Early renal function decline in type 2 diabetes. Clin J Am Soc Nephrol. 2012;7:78–84. doi: 10.2215/CJN.07610711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddad N, Brown C, Hebert LA. Retarding progression of kidney disease. In: Johnson RJF, editor. Comprehensive clinical nephrology. Philadelphia: Elsevier; 2007. pp. 823–830. [Google Scholar]
- 36.Novick AC, Gephardt G, Guz B, Steinmuller D, Tubbs RR. Long-term follow-up after partial removal of a solitary kidney. N Engl J Med. 1991;325:1058–1062. doi: 10.1056/NEJM199110103251502. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal A, Haddad N, Hebert LA. Progression of kidney disease: Diagnosis and management. In: Molony D, Craig J, editors. Evidence-based nephrology. Hoboken, NJ: John Wiley & Sons; 2008. pp. 311–322. [Google Scholar]
- 38.Brown C, Haddad N, Hebert LA. Retarding progression of kidney disease. In: Floege JJ, JF, editors. Comprehensive clinical nephrology. Philadelphia: Elsevier; 2010. pp. 919–926. [Google Scholar]
- 39.Barnes CE, Wilmer WA, Hernandez RA, Jr, Valentine C, Hiremath LS, Nadasdy T, Satoskar AA, Shim RL, Rovin BH, Hebert LA. Relapse or worsening of nephrotic syndrome in idiopathic membranous nephropathy can occur even though the glomerular immune deposits have been eradicated. Nephron Clin Pract. 2011;119:c145–153. doi: 10.1159/000324762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Agati VD. Pathobiology of focal segmental glomerulosclerosis: New developments. Curr Opin Nephrol Hypertens. 2012;21:243–250. doi: 10.1097/MNH.0b013e32835200df. [DOI] [PubMed] [Google Scholar]
- 41.Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, Gordon T, Hardin JA, Kalden JR, Lahita RG, Maini RN, McDougal JS, Rothfield NF, Smeenk RJ, Takasaki Y, Wiik A, Wilson MR, Koziol JA. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40:1601–1611. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum. 1998;41:1521–1537. doi: 10.1002/1529-0131(199809)41:9<1521::AID-ART2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 43.Gallicchio MC, Savige JA. Detection of anti-myeloperoxidase and anti-elastase antibodies in vasculitides and infections. Clin Exp Immunol. 1991;84:232–237. doi: 10.1111/j.1365-2249.1991.tb08154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kallenberg CG, Brouwer E, Weening JJ, Tervaert JW. Anti-neutrophil cytoplasmic antibodies: Current diagnostic and pathophysiological potential. Kidney Int. 1994;46:1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- 45.Zhao MH, Chen M, Gao Y, Wang HY. Propylthiouracil-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int. 2006;69:1477–1481. doi: 10.1038/sj.ki.5000387. [DOI] [PubMed] [Google Scholar]
- 46.Hamidou MA, El Kouri D, Audrain M, Grolleau JY. Systemic antineutrophil cytoplasmic antibody vasculitis associated with lymphoid neoplasia. Ann Rheum Dis. 2001;60:293–295. doi: 10.1136/ard.60.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solans-Laque R, Bosch-Gil JA, Perez-Bocanegra C, Selva-O’Callaghan A, Simeon-Aznar CP, Vilardell-Tarres M. Paraneoplastic vasculitis in patients with solid tumors: Report of 15 cases. J Rheumatol. 2008;35:294–304. [PubMed] [Google Scholar]
- 48.Diez-Porres L, Rios-Blanco JJ, Robles-Marhuenda A, Gutierrez-Molina M, Gil-Aguado A, Vazquez-Rodriguez JJ. Anca-associated vasculitis as paraneoplastic syndrome with colon cancer: A case report. Lupus. 2005;14:632–634. doi: 10.1191/0961203305lu2153cr. [DOI] [PubMed] [Google Scholar]
- 49.Ramos-Casals M, Campoamor MT, Chamorro A, Salvador G, Segura S, Botero JC, Yague J, Cervera R, Ingelmo M, Font J. Hypocomplementemia in systemic lupus erythematosus and primary antiphospholipid syndrome: Prevalence and clinical significance in 667 patients. Lupus. 2004;13:777–783. doi: 10.1191/0961203304lu1080oa. [DOI] [PubMed] [Google Scholar]
- 50.Hebert LA, Cosio FG, Birmingham DJ. Complement and complement regulatory proteins. In: Neilson E, Couser WG, editors. Immunologic renal diseases. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 367–393. [Google Scholar]
- 51.Brenner B, Rector FC Jr, editors. Textbook of nephrology. 8. Saunders Elsevier; 2008. pp. 1007–1012. [Google Scholar]
- 52.Brenner B, Rector FC Jr, editors. Textbook of nephrology. 8. Saunders Elsevier; 2008. p. 1014. [Google Scholar]
- 53.Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med. 2012;366:1119–1131. doi: 10.1056/NEJMra1108178. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal A, Clements J, Sedmak DD, Imler D, Nahman NS, Jr, Orsinelli DA, Hebert LA. Subacute bacterial endocarditis masquerading as type iii essential mixed cryoglobulinemia. J Am Soc Nephrol. 1997;8:1971–1976. doi: 10.1681/ASN.V8121971. [DOI] [PubMed] [Google Scholar]
- 55.Hebert LA, Sharma HM, Sedmak DD, Bay WH. Unexpected renal biopsy findings in a febrile systemic lupus erythematosus patient with worsening renal function and heavy proteinuria. Am J Kidney Dis. 1989;13:504–507. doi: 10.1016/s0272-6386(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 56.Beaufils M, Morel-Maroger L, Sraer JD, Kanfer A, Kourilsky O, Richet G. Acute renal failure of glomerular origin during visceral abscesses. N Engl J Med. 1976;295:185–189. doi: 10.1056/NEJM197607222950402. [DOI] [PubMed] [Google Scholar]
- 57.Beaufils M. Glomerular disease complicating abdominal sepsis. Kidney Int. 1981;19:609–618. doi: 10.1038/ki.1981.59. [DOI] [PubMed] [Google Scholar]
- 58.Majumdar A, Chowdhary S, Ferreira MA, Hammond LA, Howie AJ, Lipkin GW, Littler WA. Renal pathological findings in infective endocarditis. Nephrol Dial Transplant. 2000;15:1782–1787. doi: 10.1093/ndt/15.11.1782. [DOI] [PubMed] [Google Scholar]
- 59.Kwok S, D’Agati V, Anis K, Jim B. An unusual case of nephrotic syndrome and glucosuria. Am J Kidney Dis. 2012;59:734–737. doi: 10.1053/j.ajkd.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 60.Falkenhain M, Hartman JA, Hebert LA. Nutritional management of water, sodium, potassium, chloride, and magnesium in renal disease and renal failure. In: Kopple JD, Massry SG, editors. Kopple and massry’s nutritional management of renal disease. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 287–298. [Google Scholar]
- 61.Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol. 2005;16:467–473. doi: 10.1681/ASN.2004080658. [DOI] [PubMed] [Google Scholar]
- 62.Hofstra JM, Willems JL, Wetzels JF. Estimated glomerular filtration rate in the nephrotic syndrome. Nephrol Dial Transplant. 2011;26:550–556. doi: 10.1093/ndt/gfq443. [DOI] [PubMed] [Google Scholar]
- 63.Botev R, Mallie JP, Wetzels JF, Couchoud C, Schuck O. The clinician and estimation of glomerular filtration rate by creatinine-based formulas: Current limitations and quo vadis. Clin J Am Soc Nephrol. 2011;6:937–950. doi: 10.2215/CJN.09241010. [DOI] [PubMed] [Google Scholar]
- 64.Hebert PL, Nori US, Bhatt UY, Hebert LA. A modest proposal for improving the accuracy of creatinine-based gfr-estimating equations. Nephrol Dial Transplant. 2011;26:2426–2428. doi: 10.1093/ndt/gfr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilmer WA, Rovin BH, Hebert CJ, Rao SV, Kumor K, Hebert LA. Management of glomerular proteinuria: A commentary. J Am Soc Nephrol. 2003;14:3217–3232. doi: 10.1097/01.asn.0000100145.27188.33. [DOI] [PubMed] [Google Scholar]
- 66.Birmingham DJ, Rovin BH, Shidham G, Nagaraja HN, Zou X, Bissell M, Yu CY, Hebert LA. Spot urine protein/creatinine ratios are unreliable estimates of 24 h proteinuria in most systemic lupus erythematosus nephritis flares. Kidney Int. 2007;72:865–870. doi: 10.1038/sj.ki.5002421. [DOI] [PubMed] [Google Scholar]
- 67.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 68.Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician. 2008;78:743–750. [PubMed] [Google Scholar]
- 69.Gurevich F, Perazella MA. Renal effects of anti-angiogenesis therapy: Update for the internist. Am J Med. 2009;122:322–328. doi: 10.1016/j.amjmed.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 70.Valentine C, Hebert LA. Paraneoplastic glomerulopathies. In: Cohen EP, editor. Cancer and the kidney. London: Oxford University Press; 2010. [Google Scholar]
- 71.Rovin BH, Tang Y, Sun J, Nagaraja HN, Hackshaw KV, Gray L, Rice R, Birmingham DJ, Yu CY, Spetie DN, Aziz A, Hebert LA. Clinical significance of fever in the systemic lupus erythematosus patient receiving steroid therapy. Kidney Int. 2005;68:747–759. doi: 10.1111/j.1523-1755.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 72.Nadasdy T, Hebert LA. Infection-related glomerulonephritis: Understanding mechanisms. Semin Nephrol. 2011;31:369–375. doi: 10.1016/j.semnephrol.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 73.Parikh S, Wu H, Cataland SR, Rovin BH, Hebert LA. Encyclopedia of medical immunology. Springer; 2014. Kidney involvement in idiopthic ttp-hus. Accepted. [Google Scholar]
- 74.Mallamaci F, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Remuzzi G, Zoccali C. Ace inhibition is renoprotective among obese patients with proteinuria. J Am Soc Nephrol. 2011;22:1122–1128. doi: 10.1681/ASN.2010090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the aask trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 77.Satoskar AA, Nadasdy G, Plaza JA, Sedmak D, Shidham G, Hebert L, Nadasdy T. Staphylococcus infection-associated glomerulonephritis mimicking iga nephropathy. Clin J Am Soc Nephrol. 2006;1:1179–1186. doi: 10.2215/CJN.01030306. [DOI] [PubMed] [Google Scholar]
- 78.Doleris LM, Hill GS, Chedin P, Nochy D, Bellanne-Chantelot C, Hanslik T, Bedrossian J, Caillat-Zucman S, Cahen-Varsaux J, Bariety J. Focal segmental glomerulosclerosis associated with mitochondrial cytopathy. Kidney Int. 2000;58:1851–1858. doi: 10.1111/j.1523-1755.2000.00356.x. [DOI] [PubMed] [Google Scholar]
- 79.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 80.Herlitz LC, D’Agati VD, Markowitz GS. Crystalline nephropathies. Arch Pathol Lab Med. 2012;136:713–720. doi: 10.5858/arpa.2011-0565-RA. [DOI] [PubMed] [Google Scholar]
- 81.Sabnis SG, Antonovych TT, Argy WP, Rakowski TA, Gandy DR, Salcedo JR. Nail-patella syndrome. Clin Nephrol. 1980;14:148–153. [PubMed] [Google Scholar]
- 82.Alchi B, Nishi S, Narita I, Gejyo F. Collagenofibrotic glomerulopathy: Clinicopathologic overview of a rare glomerular disease. Am J Kidney Dis. 2007;49:499–506. doi: 10.1053/j.ajkd.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 83.Sethi S, Nester CM, Smith RJ. Membranoproliferative glomerulonephritis and c3 glomerulopathy: Resolving the confusion. Kidney Int. 2012;81:434–441. doi: 10.1038/ki.2011.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nasr SH, Satoskar A, Markowitz GS, Valeri AM, Appel GB, Stokes MB, Nadasdy T, D’Agati VD. Proliferative glomerulonephritis with monoclonal igg deposits. J Am Soc Nephrol. 2009;20:2055–2064. doi: 10.1681/ASN.2009010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hebert LA, Birmingham DJ, Shidham G, Rovin B, Nagaraja HN, Yu CY. Random spot urine protein/creatinine ratio is unreliable for estimating 24-hour proteinuria in individual systemic lupus erythematosus nephritis patients. Nephron Clin Pract. 2009;113:c177–182. doi: 10.1159/000232599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turner JM, Bauer C, Abramowitz MK, Melamed ML, Hostetter TH. Treatment of chronic kidney disease. Kidney Int. 2012;81:351–362. doi: 10.1038/ki.2011.380. [DOI] [PubMed] [Google Scholar]
- 87.Fine DM, Ziegenbein M, Petri M, Han EC, McKinley AM, Chellini JW, Nagaraja HN, Carson KA, Rovin BH. A prospective study of protein excretion using short-interval timed urine collections in patients with lupus nephritis. Kidney Int. 2009;76:1284–1288. doi: 10.1038/ki.2009.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20:436–443. doi: 10.1681/ASN.2008030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shidham G, Hebert LA. Timed urine collections are not needed to measure urine protein excretion in clinical practice. Am J Kidney Dis. 2006;47:8–14. doi: 10.1053/j.ajkd.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 90.Parry HM, Pratt G, Hutchison CA. Monoclonal gammopathy of undetermined significance: An update for nephrologists. Adv Chronic Kidney Dis. 2012;19:291–296. doi: 10.1053/j.ackd.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 91.Wu H, Birmingham DJ, Rovin B, Hackshaw KV, Haddad N, Haden D, Yu CY, Hebert LA. D-dimer level and the risk for thrombosis in systemic lupus erythematosus. Clin J Am Soc Nephrol. 2008;3:1628–1636. doi: 10.2215/CJN.01480308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eichinger S, Minar E, Bialonczyk C, Hirschl M, Quehenberger P, Schneider B, Weltermann A, Wagner O, Kyrle PA. D-dimer levels and risk of recurrent venous thromboembolism. JAMA. 2003;290:1071–1074. doi: 10.1001/jama.290.8.1071. [DOI] [PubMed] [Google Scholar]
- 93.Giangiacomo J, Cleary TG, Cole BR, Hoffsten P, Robson AM. Serum immunoglobulins in the nephrotic syndrome. A possible cause of minimal-change nephrotic syndrome. N Engl J Med. 1975;293:8–12. doi: 10.1056/NEJM197507032930103. [DOI] [PubMed] [Google Scholar]