Abstract
The shoulder girdle of turtles has a triradiate morphology. Although its dorsal process represents the scapular blade, the skeletal identities of the two ventral processes remain uncertain. To elucidate the question, developmental patterns of the girdles were compared between Chinese soft-shelled turtles, chickens, and mice. Despite the morphological diversity of adults, the initial primordia of the shoulder girdles showed similar morphological patterns. The ventral two processes developed from the anlagen comparable to those of the acromion and the coracoid in other amniotes. The developmental pattern of the acromion is very similar among embryos, whereas that of the coracoid in mammals differs from that in non-mammals, implying that coracoids are not homologous between non-mammals and mammals. Therefore, amniotes have retained the ancestral pattern of the girdle anlage, and the shoulder girdle of turtles has been achieved through a transformation of the pattern in the late ontogenic period.
Keywords: coracoid, homology, scapula, shoulder girdle, turtles
Introduction
The vertebrate shoulder girdle contains both endochondral and dermal elements. Although the major part of the girdle is primarily formed by dermal elements, such as the cleithlum, clavicle, and interclavicle, the endochondral elements tend to become more prominent along the lineage towards tetrapods (Fig. 1A; reviewed by McGonnell, 2001). The endochondral shoulder girdle in basal amniotes consists of a dorsal element, the scapula, and a ventral, the coracoid. The latter can be further divided into the procoracoid and metacoracoid, rostrocaudally (Fig. 1C; Vickaryous & Hall, 2006). The majority of extant amniotes, however, retain only a single coracoid; the metacoracoid has been preserved in therian mammals, as has the procoracoid in saurians, which includes avians (Romer, 1922a,b; Versluys, 1927; Starck, 1979; and references therein). As an exception, monotremes retain both the coracoids. In yet another exception, turtles also possess both coracoids. Turtles are, therefore, expected to be the only living saurian taxon that has preserved the basal state of the shoulder girdle.
Fig. 1.
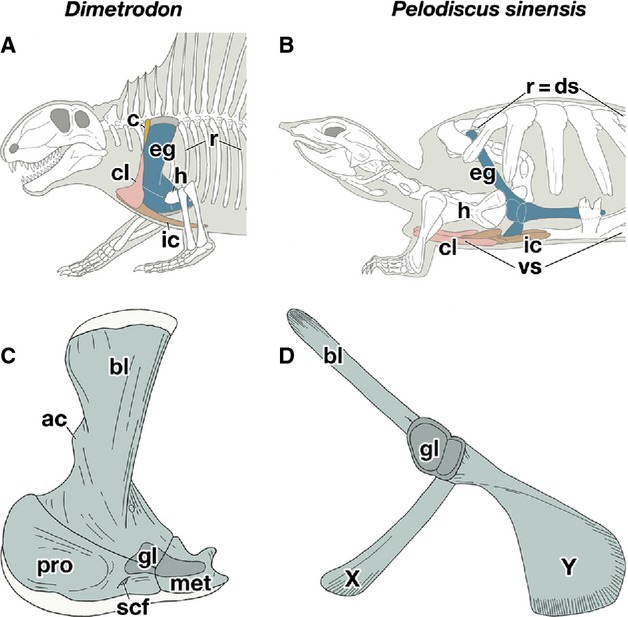
Comparison of the shoulder girdle skeleton. (A,B) Lateral views of the left shoulder region of Dimetrodon (A) and Pelodiscus sinensis (B). (C,D) Lateral views of the left endochondral girdle of Dimetrodon (C) and P. sinensis (D). ac, acromion; bl, scapular blade; c, cleithlum; cl, clavicle; ds, dorsal shell; eg, endochondral girdle; gl, glenoid cavity; h, humerus; ic, interclavicle; met, metracoracoid; pro, procoracoid; r, ribs; scf, supracoracoid foramen; vs, ventral shell.
The shoulder girdle of turtles has a triradiate morphology with a glenoid cavity, a socket for the humerus, at the crux (Fig. 1B,D), which is so unique among tetrapods that the homology of the three projections of the skeleton remains uncertain. The dorsal process has been unanimously homologized with the scapular blade, but controversies persist as to the nature of the two ventral processes. In the dawn of the comparative morphology, the ventrorostrally oriented process, referred to as ‘X’ in this study (Fig. 1D), was homologized to the clavicle based on its morphology (Bojanus, 1819-1821; Meckel, 1824). This theory, however, has become less popular since the discovery of the clavicle in the rostral part of the ventral shell (Fig. 1B; Oken, 1823), although it persisted through the early 20th century (Ogushi, 1911).
Other hypotheses have compared element X either with an outgrowth of the scapula called the acromion (Cuvier, 1836; Rathke, 1848; Osborn, 1903; Versluys, 1927; Walker, 1947; Romer, 1956; Starck, 1979; Lee, 1996, 1998) or with a procoracoid (Gegenbaur, 1865; Parker, 1868; Fürbringer, 1874; Gaffney, 1990; Rieppel, 1996; deBraga & Rieppel, 1997; Rieppel & Reisz, 1999; Vickaryous & Hall, 2006). The former idea was based mainly on the absence of a suture separating element X from the scapular blade, whereas the latter maintains that this character is the result of a fusion of the two skeletal elements. Curiously, both hypotheses have been supported in osteological comparisons between the shoulder girdles of turtles and other amniotes (Gaffney, 1990; Lee, 1996, 1998; deBraga & Rieppel, 1997; Rieppel & Reisz, 1999), indicating that the adult morphology does not provide evidence that reliably settles this controversy.
Moreover, the ventrocaudal process of the shoulder girdle in turtles, referred to as ‘Y’ in this study (Fig. 1D), showed a coracoid, but it was unclear whether the coracoid represented a procoracoid (Versluys, 1927; Walker, 1947; Romer, 1956; Starck, 1979) or a metacoracoid (Gegenbaur, 1865; Parker, 1868; Fürbringer, 1874; Gaffney, 1990; Lee, 1996, 1998; Rieppel, 1996; deBraga & Rieppel, 1997; Rieppel & Reisz, 1999; Vickaryous & Hall, 2006).
To understand the origin of the unique morphology of the shoulder girdle in turtles, we compared the developmental patterns in the embryos of the Chinese soft-shelled turtle (Pelodiscus sinensis) and two other amniotes: chickens (Gallus gallus) and mice (Mus musculus). Because the shoulder girdle of avians has both an acromion and a procoracoid, whereas that of mice displays a metacoracoid in addition to an acromion, comparisons of the developmental processes among these skeletal elements unveiled the homology of elements X and Y.
We found that the shoulder girdles of turtles and chickens have a common primordium pattern in early development. A species-specific morphology for the shoulder girdle was acquired through the remodeling of this common pattern in the late developmental period. Thus, element X is homologous to the acromion and element Y is comparable to the chicken coracoid. These saurian coracoids are the most likely a procoracoid, because their developmental processes differ from those of the mouse coracoid. The shared pattern of the shoulder girdle anlage would have been established by common ancestors of the amniotes and inherited as a constrained developmental pattern, which is the source of the homology of the shoulder girdle in amniotes. The morphological divergence of the shoulder girdle in amniotes can be attributed to a species-specific transformation of the common pattern in the late ontogeny.
Materials and methods
Sample collection
Fertilized eggs of P. sinensis were purchased from a local farm in Japan. The eggs were incubated at 30 °C, and the embryos were staged according to a table established by Tokita & Kuratani (2001). Fertilized chicken eggs were also obtained from a local supplier and incubated at 38 °C. The embryos were staged according to Hamburger & Hamilton (1951). Mice embryos were collected at various times of gestation and staged according to Theiler (1989). Animal care was entirely in accordance with the guidelines provided by the RIKEN Center for Developmental Biology and Niigata University, and approval for the experiments was obtained from the institutions.
cDNA cloning and in situ hybridization
Pelodiscus sinensis Tbx15 and Pax1 homolog genes were obtained by degenerate RT-PCR. The identified sequences have been deposited in GenBank under accession numbers AB776698 and AB776699, respectively. The in situ hybridization was performed as described previously (Nagashima et al., 2007). Riboprobes for chicken Sox9, Tbx15, P. sinensis Sox9, and mouse Sox9, Pax1, Tbx15 were generated based on the nucleotide sequences U12533, XM_416537, AB472747, NM011448, NM_008780, and NM_009323 deposited in GenBank, respectively. Japanese quail Coturnix coturnix Pax1 probe applied for chicken embryos was provided by Dr. Aoyama et al. (2005).
3D reconstruction
Adjacent sections were either hybridized with RNA probes or stained with hematoxylin and eosin, followed by 0.1% alcian blue. All images were recorded with a DP70 digital camera (Olympus Corporation, Shinjuku, Tokyo, Japan) attached to a light microscope, and reconstructed with avizo® (Visualization Sciences Group, Burlington, MA, USA).
Whole-mount skeletal staining
Chicken hatchling was cleared and double-stained for bone and cartilage following standard procedures (Taylor and Van Dyke, 1985).
Data from fossils
Anatomical data for Odontochelys semitestacea (IVPP V 13240 and V 15653) was newly collected for this study. These specimens are housed at the IVPP (Institute of Vertebrate Paleontology and Paleoanthropology), Beijing, China.
Results
Comparing shoulder girdle development
To compare the three animal species at a corresponding developmental stage, we attempted to establish common stages. Observations were made concerning histological sections, whole-mount alcian blue staining, and whole-mount in situ hybridization for a precartilaginous cell marker gene, Sox9. The common stages were defined mainly based on the developmental signatures of the shoulder-forelimb region (Fig. 2; Supporting Information Fig. S1 and Table S1). These stages ranged from the appearance of shoulder girdle anlage observed at stage I for stage 13 P. sinensis (Tokita & Kuratani, 2001), stage 24 chickens (Hamburger & Hamilton, 1951), and 10.5-day post coitus (dpc) mice (Theiler, 1989), to a common stage V when the anatomical features of the muscles and skeletal elements become apparent in each species for stage 18 P. sinensis, stage 34 chickens, and 14.5-dpc mice. The common stages I and II were initiation periods of the shoulder girdle, when the contour of the shoulder girdle primordium was not yet distinct, and species-specific differences were not obvious (Fig. S1, Table S1). The outline of the girdle anlage became clear at the common stage III, and thus the following describes the morphological characteristics of the anlage and its change during the development of each species.
Fig. 2.
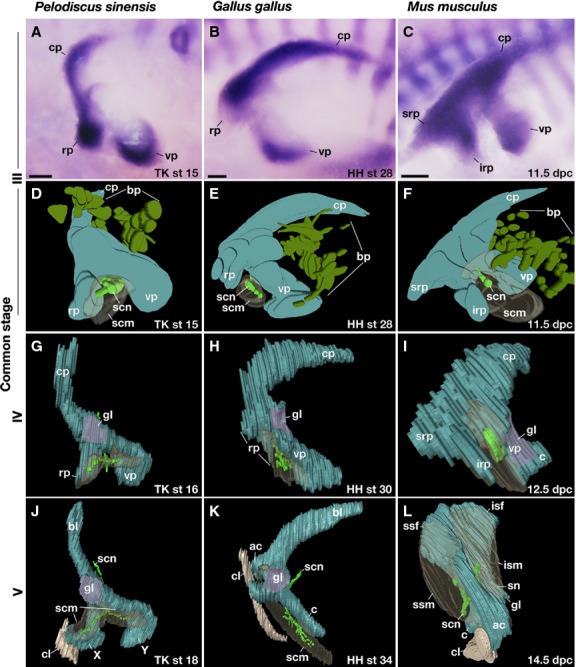
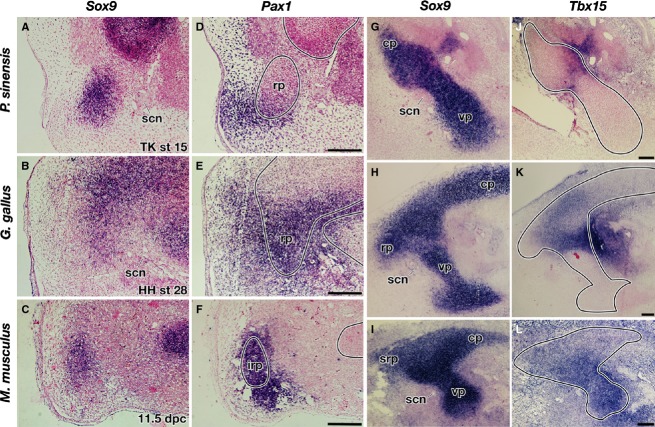
Comparison of the shoulder girdle development. (A,D,G,J) Pelodiscus sinensis, (B,E,H,K) chickens, and (C,F,I,L) mice. (A–C) Expression of Sox9 in the left girdle. (D–L) Three-dimensional reconstructions of the cartilage, nerves, and muscles. All panels are the lateral view, and rostral is to the left. bp, brachial plexus; c, coracoid; cl; clavicle; cp, caudal process; irp, infrarostral process; isf, infraspinatus fossa; ism, infraspinatus muscle; rp, rostral process; scm, supracoracoid muscle; scn, supracoracoid nerve; sn, scapular neck; srp, suprarostral process; ssf, supraspinatus fossa; ssm, supraspinatus muscle; vp, ventral process. Scale bar: 200 μm.
In common stage III P. sinensis, the girdle anlage showed a tripartite mass of prechondrogenic cells, located rostral to the brachial plexus and dorsal to the anlage of the supracoracoid muscle as well as the supracoracoid nerve that innervates the muscle (Fig. 2A,D; Supporting Information Movie S1). The protrusions in the mesenchymal anlagen dorsal and ventral to the brachial plexus were referred to as the caudal (CP) and ventral (VP) processes, respectively. The ramus rostral to the supracoracoid nerve was referred to as the rostral process (RP). In common stage IV P. sinensis, the glenoid cavity was formed at the dorsal extremity of the VP but the entire morphology did not change from the previous stage (Fig. 2G; Supporting Information Movie S2). In the common stage-V for P. sinensis, the CP differentiated into a scapular blade (Fig. 2J; Supporting Information Movie S3). The RP developed into element X articulated to the lateral end of the clavicle. The VP grew into element Y.
For common stage III chicken embryos, the girdle anlage shared an almost identical morphology and position with that of P. sinensis at the corresponding stage (Fig. 2B,E; Supporting Information Movie S4), and thus the CP, VP, and RP could be assigned to the three precartilaginous projections using the nerves and the muscle anlage as landmarks. For common stage IV chickens, as in the common stage IV P. sinensis, the glenoid cavity developed at the dorsal tip of the VP (Fig. 2H; Supporting Information Movie S5). For common stage V chickens, the CP, RP, and VP became the scapular blade, acromion and coracoid, respectively (Fig. 2K; Supporting Information Movie S6). The clavicle was articulated to the rostral tip of the RP derivative (acromion) with a lateral end similar to that in turtles, and subsequently it also articulated with the coracoid in later development (Supporting Information Fig. S2).
Common stage III mice also had a CP and VP dorsal and ventral to the brachial plexus, as with other amniotes, but rostral to the supracoracoid nerve were the suprarostral (SRP) and infrarostral (IRP) processes (Fig. 2C,F; Supporting Information Movie S7). From the common stage IV (Fig. 2I; Supporting Information Movie S8) to stage V (Fig. 2L; Supporting Information Movie S9), the mouse CP gave rise only to the caudal half of the scapular blade (infraspinatus fossa), whereas the rostral half (supraspinatus fossa) differentiated from SRP, implying that the supraspinatus fossa is derived from a cartilaginous anlage that is unique to mammals. The remaining process in the rostral region of the mammalian girdle anlage, the IRP, developed into the acromion, which was rostrally articulated with the lateral end of the clavicle (Fig. 2L). Although the coracoid developed in the ventral extremity of the VP (Fig. 2I), the glenoid cavity arose in the middle rather than the dorsal part of the VP, and the root of the VP formed the scapular neck (Fig. 2I,L).
These observations suggest that the development of element X in P. sinensis was very similar to that of the acromion in other animals, whereas the VP development in P. sinensis is comparable to that in chickens and different from that in mice.
To further investigate the developmental characteristics of each protrusion in the girdle anlage, the expressions of some of the genes involved in the girdle development were observed (Kuijper et al., 2005). Pax1, for example, was expressed in the RP of P. sinensis and chickens as well as in the IRP of mice (Fig. 3A–F), reinforcing the hypothesis that these processes are homologous. Consistent with the developmental fate, the gene expression was not observed in the SRP in mice. Although Pax1 has been used as a marker gene for the scapular blade in avian studies (Huang et al., 2000; Moeller et al., 2003; Ehehalt et al., 2004; Wang et al., 2005), the gene was expressed not in the anlage of the scapular blade (CP) but in the mesenchyme along it (Supporting Information Fig. S3, top), and such an expression domain is unique to chickens (Fig. S3, bottom). Tbx15 was expressed in the dorsolateral part of the VP in all the animals studied, but the size of the expression domain differed among the species. The expression reached the ventral end of the VP in mice, but it was restricted to the dorsal tip of the VP in the non-mammals (Fig. 3G–L), indicating that the developmental background of the coracoid differs between the saurians and mammals.
Fig. 3.
Expressions of Pax1 and Tbx15 in shoulder girdle primordia in common stage III. (A–C,G–I) Expression of Sox9. (D–F) Expression of Pax1. (J–L) Expression of Tbx15. (A–C,G–I) Adjacent sections to (D–F,J–L), respectively. Lines in (D–F,J–L) indicate the contours of the girdle anlage. All images are sagittal sections of the left girdle anlagen, and the rostral is left. Scale bar: 100 μm.
Based on an out-group comparison of the above-presented developmental data, it would be overly conservative to infer that in the development of a common ancestor of the saurians, the shoulder girdle developed from the tripartite mesenchymal condensation with CP, RP, and VP. In birds, the CP, RP, and VP later developed into the scapular blade, acromion, and coracoid, respectively. In turtles, on the other hand, the CP, RP, and VP later developed into the scapular blade, element X, and element Y, respectively, suggesting that elements X and Y are comparable to the avian acromion and coracoid. Although the developmental process of the acromion was also conserved in mammals, differences in coracoid development between the saurians and therian mammals indicate that they are not homologous to each other as has been suggested by paleontological analyses (Broom, 1912; Romer, 1922a,b), and thus element Y would represent the procoracoid (see below for further discussion).
Besides the homology of skeletal elements, our present observation found some differences between mammals and non-mammals. In all the animals examined, the glenoid cavity was directed laterally at their first appearance (Fig. 2G–I). While the orientation of the cavity in non-mammals was preserved throughout development, in mice it changed from lateral to ventral (Fig. 2I,L; Supporting Information Movies S8 and S9). This change was accompanied by a rostral shift in the entire VP (Figs 2I,L and 4); the VP was originally situated caudal to the IRP but secondarily moved rostrally and occupied the medial position to the IRP derivative (acromion).
Another difference is that the supracoracoid nerve and muscle in the non-mammalian embryos were spread over the RP- and VP-derivatives, prefiguring adult patterns (Figs 2G, H, J, K; Supporting Information Fig. S4). In mice, the equivalent nerve and a part of the homologous muscle anlage shifted rostrally along the VP to become the supracoracoid nerve (also called the suprascapular nerve in human anatomy) and the supraspinatus muscle, respectively, and the rest of the muscle anlage grew dorsally over the CP to form the infraspinatus muscle (Figs 2I,L, 4; Fig. S4).
Fig. 4.
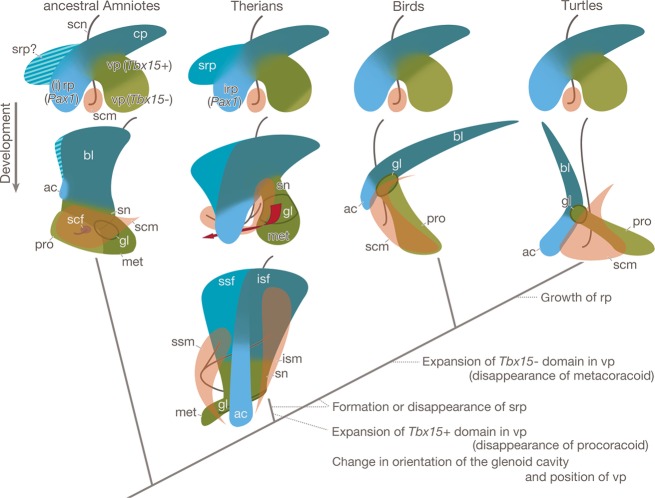
Evolutionary scenario of the shoulder girdle. The girdle primordium in the ancestral amniotes would have been situated dorsal to the anlagen of the supracoracoid muscle and nerve, and would have had at least three projections. The caudal process would have formed the scapular blade. The rostral process would have expressed Pax1 and would have formed the acromion. The ventral process would have been divided into a dorsolaterally situated Tbx 15-positive domain and a ventromedially Tbx15-negative domain, of which the former would have grown into the metacoracoid, scapular neck, and glenoid cavity, and the latter into the procoracoid. This pattern of the girdle anlage is conserved in extant therian mammals and saurians, although the relative size of the Tbx 15 domain in the ventral process differs between the animals, and the therian mammals have a suprarostral process. In the therian mammals, the suprarostral process forms the rostral half of the scapular blade, the supraspinatus fossa, and the caudal process forms the caudal half of the blade, the infraspinatus fossa. The presence or absence of the suprarostral process in the ancestral amniotes is uncertain (striped area), thus the process and its derivative may have been invented in the lineage leading to the therian mammals or may have been lost during the evolution of saurians. In therians, the Tbx 15-negative domain becomes a lesser part of the ventral process and the process forms the glenoid cavity, scapular neck, and the metacoracoid. These ventral process derivatives changed their orientation and position during later development (red arrow), which was accompanied by a division and rostral shift of the anlagen of the supracoracoid muscle and nerve to form the supraspinatus muscle and suprascapular nerve, respectively. In the saurians, the Tbx 15-positive domain became a lesser part of the ventral process and formed only the glenoid cavity, while the Tbx 15-negative domain formed the procoracoid. In the lineage leading to turtles, the growth of the rostral process would have been promoted to form the large acromion.
These developmental events confirm that even if the shoulder girdle as well as its associated nerves and muscles exhibit a wide range of morphological variation in adults (Fig. S4), these derive from the embryonic pattern conserved among the amniotes (Fig. 2D–F), and species-specific configuration is achieved in later development (Fig. 4).
Morphology of the shoulder girdle in basal turtles
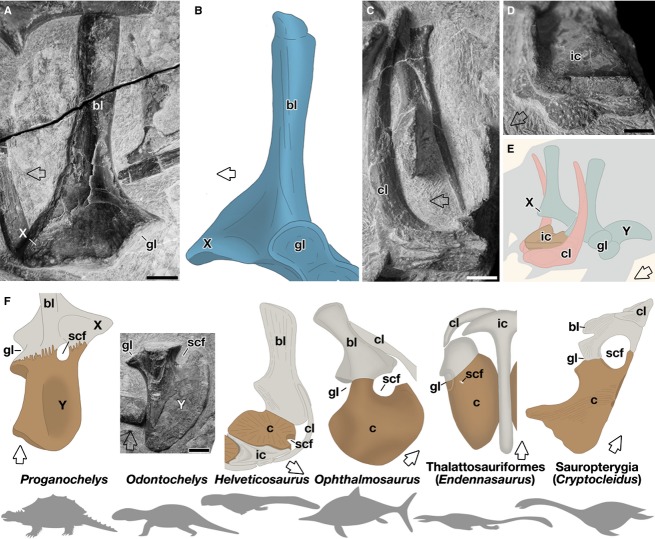
Odontochelys is suggested to be the uncontested basalmost stem turtle (Li et al., 2008). Li et al. (2008) reported that the shoulder girdle of this animal lacked element X, indicating that this element may have been newly acquired in the turtle lineage. However, we found the element in the shoulder girdle of the animal, and confirmed that it had not yet taken a rod-like shape but remained as a short triangular eminence with a broad base on the rostral margin of the scapular blade (Fig. 5A). Furthermore, not only element X but also other skeletal elements, such as the scapular blade, the element Y, and the clavicle, had a morphology that was almost identical to that of the stem turtle, Proganochelys (Fig. 5; Gaffney, 1990). These observations indicated that element X had already developed in the basal turtle, and that the entire architecture of the shoulder girdles in the two basal turtles would be very similar. Importantly, in both species, there was no trace of a suture between the element X and the scapular blade (Fig. 5A,B; Gaffney, 1990).
Fig. 5.
Comparison of shoulder girdle skeletons. (A–E) Skeletons in Odontochelys (A,C–E) and Proganochelys (B). (A,B) Lateral view of the scapular blade and element X. The original photo of Odontochelys (A) was flipped horizontally for comparison. (C) The clavicle. Medial view. As in Proganochelys (Gaffney, 1990), a dorsal process was not sutured at the base, suggesting that the stem was an outgrowth of the clavicle. (D) The interclavicle. Dorsolateral view. (E) Cartoon showing the shoulder girdle skeletons in Odontochelys. (F) Comparison of Y elements in the basal turtles and the coracoids in other amniotes. The original photo of Odontochelys was flipped horizontally for comparison. Endennasaurus shows the ventral view and other animals the dorsal view. Rostral is indicated by arrows. Scale bar: 5 mm.
Discussion
In his monumental analysis of Proganochelys, Gaffney (1990) argued that the element X = procoracoid theory can be based on five lines of evidence. First, topological relationships among the skeletal elements in the shoulder girdle were conserved in Proganochelys and in a basal reptile that possessed the two coracoids. Using similar analyses, however, this result could be either supported (deBraga & Rieppel, 1997; Rieppel & Reisz, 1999) or denied (Lee, 1996, 1998). However, it would be indispensable to compare the shoulder girdle of turtles with a girdle possessing a single coracoid at the same time, because the entire architecture of the shoulder girdle would be affected by the presence of the two coracoids. Baur (1891), Seeley (1893), Andrews (1895), and Osborn (1903) have already established the close morphological resemblance of element X and the acromion of plesiosaurs and Metriorhynchus.
Secondly, the supracoracoid foramen, a passage on the coracoid for the supracoracoid nerve and a hallmark of the procoracoid, lies in the suture between elements X and Y in Proganochelys (Fig. 5F), whereas in Labidosaurs and Dimetrodon it can be seen in the procoracoid but near the suture with the metacoracoid (Fig. 1C). However, Romer (1956) proposed that this foramen belonged not to element X but to element Y, and regarded element X as the acromion. There is some data supporting that assessment (see below).
Thirdly, during the growth of Captorhinus aguti, the procoracoid secondarily fused with the scapular blade (Holmes, 1977), which would explain the loss of the suture between the scapular blade and element X in turtles. Nevertheless, during turtle development, the suture never appeared (Walker, 1947; Rieppel, 1993).
Fourthly, there is the articulation of element X to the clavicle–interclavicle (Vickaryous & Hall, 2006). This feature was also used for the evidence supporting the element X = acromion theory, because in basal amniotes, not only the procoracoid but also the acromion articulated with the clavicle (Romer, 1922b, 1956). Thus, this character is neutral to the diagnosis.
Fifthly, the supracoracoid muscle is attached to element X in turtles and to the procoracoid in basal amniotes (Vickaryous & Hall, 2006). As Gaffney admitted, although the muscle attachment does not always indicate homology of the skeletal element, the muscle was also attached to the ventral part of the scapula of Dicynodon and Cynognathus but never to the metacoracoid (Romer, 1922b), whereas in turtles the muscle is attached not only to element X but also to element Y (Figs 2J and S4), which implies that element X = acromion, and element Y = procoracoid.
As further evidence for the element X = procoracoid theory, the intrinsic ossification center in the X element has been emphasized (Hulke, 1893; deBraga & Rieppel, 1997). Rieppel (1996), however, previously established that: ‘The use of separate ossification centers in the assessment of homologies of adult endochondral ossifications may be misleading (deBeer, 1937). For example, the transverse processes of the dorsal vertebrae ossify from separate ossification centers in Alligator, yet there is no indication that they represented separate, i.e. individualized structures, in the phylogenetic past (Rieppel, 1993).
To support the element X = procoracoid theory, Vickaryous & Hall (2006) contended that the element X and scapular blade develop from different mesodermal cell populations (Burke, 1991). However, this result, does not corroborate the hypothesis, since the scapula in other tetrapods also has a dual mesodermal cell origin, as does that in turtles (Huang et al., 2000; Piekarski & Olsson, 2011; Shearman et al., 2011; and references therein).
In turtles and chickens, the early shoulder girdle anlage commonly has a triradiate girdle morphology and occupies a comparable position to the muscle and nerve tissues in the embryonic body. Also, the gene expression patterns in RP and VP are similar between the two animals. The CP shows a progenitor of the scapular blade in both animals. The RP gives rise to element X in turtles and to the acromion in chickens, and the VP to element Y in turtles and to the coracoid in chickens. Thus, element X is homologous to the acromion and element Y is comparable to the chicken coracoid (Fig. 4). Because those animals belong to Archosauromorpha, the taxon including birds and crocodiles (Shaffer et al., 2013; Wang et al., 2013; and references therein), the shared developmental programs of the shoulder girdle appear to have already been established at least in the last common ancestor of arcosauromorphans.
In particular, the developmental process of the acromion also seems to be shared with mice. Pax1 is a key regulatory factor involved in the mesenchymal condensation of the acromion anlage (Timmons et al., 1994). In Pax1 mutant mice (undulated) and a Pax1 null mouse, only the acromion was lost and turned into a ligament (Grüneberg, 1950; Timmons et al., 1994; Wilm et al., 1998). The derivative of SRP, the supraspinous fossa, is not affected in these mice, confirming again that only the IRP is comparable to the RP in the saurian amniotes. Although fossil evidence suggests that mammalian acromion and saurian acromion evolved independently (Osborn, 1903; Romer, 1956; Gaffney, 1990), our results imply that in the ancestral amniotes a part of the scapula was already more or less specialized not only as an attachment site to the clavicle but also as a derivative of a distinct anlage (RP) with a unique gene expression. In this regard, it would be worth indicating that the acromion is a unique scapular region in that it has a contribution from both the cephalic neural crest and the trunk mesoderm in mice (Matsuoka et al., 2005). These results suggest that element X shows the acromion, and that turtles have only a single coracoid.
Both in chickens and mice, the VP in the girdle anlage is a source of the coracoid (Fig. 2) and is characterized by Tbx15 expression in its dorsolateral region (Fig. 3), but the size of the expression domain and the position of the glenoid cavity are different in these animals (Figs 2H, 2I, 3H, 3I, 3K, 3L and 4). Moreover, the gene expression pattern is reminiscent of the developmental pattern of the two coracoids in the VP of monotremes: the dorsolaterally developing metacoracoid and the ventromedially procoracoid (Klima, 1973, 1985). Thus, if the gene expression reflects the embryonic environment where the metacoracoid develops, these results support the classical morphological identification of the coracoid – the metacoracoid in therian mammals and the procoracoid in non-mammals. Because the developmental patterns of the VP in turtle embryos are similar to those in chicken embryos, the element Y is at least comparable to the chicken coracoid and presumably would represent the procoracoid. As noted above, the attachment of the supracoracoid muscle also favors our hypothesis.
Whereas molecular phylogenetic studies have almost unanimously supported the archosauromorph affinity of turtles (Shaffer et al., 2013; Wang et al., 2013; and references therein), paleontological analyses have not agreed on the evolutionary origin of turtles (reviewed by Tsuji & Müller, 2009). Amid the controversies, some studies suggest a sister taxon relationship between turtles and Euryapsida (Helveticosaurus, Sauropterygia, and Ichthyosauria)-Thalattosauria clade (Rieppel & deBraga, 1996; deBraga & Rieppel, 1997; Rieppel & Reisz, 1999; Li et al., 2011) belonging to the Archosauromorpha (Merck JW unpublished data). Thus, a comparison of coracoids among these animals would bring some insight into the homology of the coracoid in turtles.
The morphological patterns of the coracoid are very similar among the species (Fig. 5F; Seeley, 1893; Andrews, 1910; Rieppel, 1989; Gaffney, 1990; Müller et al., 2005). Many of these coracoids appear as a plate-like structure with a concave outer edge, a convex medial edge, and a notch for the supracoracoid nerve in the medio-rostral margin (Fig. 5F). It is noteworthy that with the exception of the stem turtles, the members obviously had only a single coracoid, and some in Thalattosauriformes had the supracoracoid foramen in the coracoid (Nicholls, 1999; Müller et al., 2005), suggesting that these coracoids are the procoracoid. Thus, it is most parsimonious to infer that the stem turtles also possessed only a single coracoid, and the foramen or notch for the supracoracoid nerve in the stem turtles belonged not to element X but to element Y. Supporting our observations, a supracoracoid foramen has been reported in element Y both in the stem turtle Eurysternum (Versluys, 1927) and in the modern turtle Chrysemys embryos (Walker, 1947).
Collectively, these developmental and paleontological data suggest a procoracoid identity for element Y.
Despite morphological divergence in adults, the initial developmental pattern of the shoulder girdle is well conserved between turtles and chickens. Thus, the basic developmental program to form the shared embryonic pattern would have been established in the common ancestor of the animals. Modifications in the developmental process to achieve a species-specific morphology appear to have been accumulated in the late phases of development. Hence, the ventral projections of the shoulder girdle in turtles are homologous to the acromoin and procoracoid, and the origin of the unique morphology can be ascribed to the promoted growth of the acromoin in late development (Fig. 4). It is noteworthy that a common embryonic pattern can be found in the girdle primordium of mammals (Fig. 4). This suggests that change in the early pattern was strictly restricted during the evolution of amniotes, which is also known as developmental constraint (Wagner, 1994) and is a developmental basis to generate the morphological homology. In this respect, it is curious that the rostral half of the scapular blade in mammals derives from their unique anlage. Further study is required to determine whether this anlage represents derived or ancestral traits (Fig. 4; also see Romer, 1922b, 1956; Sánchez-Villagra & Maier, 2002, 2006). The constraint appears to be deeply rooted in the cell-to-cell interactions between different cell populations, which characterize the body plan of amniotes, but this remains to be clarified.
Concluding remarks
Although the morphology of shoulder girdle in turtles has been assumed to possess a basal pattern, developmental and paleontological analyses indicated that it rather shows derived morphology via the transformation of patterns in other extant saurians.
Acknowledgments
We deeply thank Zhonghe Zhou and Chun Li for fossil specimen access. We also acknowledge Hirohiko Aoyama and Haruhiko Koseki for providing us with riboprobe for Coturnix Pax1, Yoshie Kawashima-Ohya and Shigehiro Kuraku for technical support, and Masahiro Shibata for critical reading of the manuscript. A part of this work was supported by grants-in-aid to H.N. from the Ministry of Education, Science and Culture of Japan.
Author contributions
H.N. and S.K. designed the research. H.N., F.S., M.T., and R.U. performed the developmental research. H.N., T.H., and S.K. examined the fossil specimens. H.N., N.S., and S.K. wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Movie S1. Three-dimensional reconstruction of the stage 15 Pelodiscus sinensis embryos.
Movie S2. Three-dimensional reconstruction of the stage 16 Pelodiscus sinensis embryos.
Movie S3. Three-dimensional reconstruction of the stage 18 Pelodiscus sinensis embryos.
Movie S4. Three-dimensional reconstruction of the stage 28 chicken embryos.
Movie S5. Three-dimensional reconstruction of the stage 30 chicken embryos.
Movie S6. Three-dimensional reconstruction of the stage 34 chicken embryos.
Movie S7. Three-dimensional reconstruction of 11.5 dpc mouse embryos.
Movie S8. Three-dimensional reconstruction of 12.5 dpc mouse embryos.
Movie S9. Three-dimensional reconstruction of 14.5 dpc mouse embryos.
Fig. S1. Comparison of skeletal development in the shoulder–forelimb region.
Fig. S2. Articulation of the clavicle to the scapula and coracoid in chicken hatchling.
Fig. S3. Expression of Pax1 in the shoulder region.
Fig. S4. Supracoracoid muscle in an adult Pelodiscus sinensis, chickens and mice.
Table S1. Common developmental stages of Pelodiscus sinensis, Gallus gallus, and Mus musculus in the shoulder–forelimb region.
References
- Andrews CW. On the development of the shoulder-girdle of a plesiosaur (Cryptoclidus oxoniensis, Pillips sp.) from the Oxford clay. Ann Mag Nat Hist. 1895;15:333–346. [Google Scholar]
- Andrews CW. A Descriptive Catalogue of the Marine Reptiles of the Oxford Clay. Part I. London: British Museum; 1910. [Google Scholar]
- Aoyama H, Mizutani-koseki S, Koseki H. Three developmental compartments involved in rib formation. Int J Dev Biol. 2005;49:325–333. doi: 10.1387/ijdb.041932ha. [DOI] [PubMed] [Google Scholar]
- Baur G. Notes on some little known American fossil tortoises. Proc Acad Natl Sci Phil. 1891;43:411–430. [Google Scholar]
- Bojanus LH. Anatome Testudinis Europaeae. Vilnae: Impensis auctoris, typis Josephi Zawadzki; 1819. -1821. [Google Scholar]
- deBraga M, Rieppel O. Reptile phylogeny and the interrelationships of turtles. Zool J Linn Soc. 1997;120:281–354. [Google Scholar]
- Broom R. The morphology of the coracoid. Anat Anz. 1912;41:625–631. [Google Scholar]
- Burke AC. The development and evolution of the turtle body plan. Inferring intrinsic aspects of the evolutionary process from experimental embryology. Am Zool. 1991;31:616–627. [Google Scholar]
- Cuvier G. Recherches sur les Ossemens Fossils. Paris: E. d'Ocagne; 1836. [Google Scholar]
- deBeer G. Oxford: Clarendon Press; 1937. The Development of the Vertebrate Skull. [Google Scholar]
- Ehehalt F, Wang B, Christ B, et al. Intrinsic cartilage-forming potential of dermomyotomal cells requires ectodermal signals for the development of the scapula blade. Anat Embryol. 2004;208:431–437. doi: 10.1007/s00429-004-0415-0. [DOI] [PubMed] [Google Scholar]
- Fürbringer M. Zur vergleichenden Anatomie der Schultermuskeln. Jena Z Med Naturwiss. 1874;8:175–280. [Google Scholar]
- Gaffney ES. The comparative osteology of the triassic turtle Proganochelys. Bull Am Mus Nat Hist. 1990;19:1–263. [Google Scholar]
- Gegenbaur C. Untersuchungen zur vergleichenden Anatomie der Wirbelthiere. Leipzig: Verlag von Wilhelm Engelmann; 1865. [Google Scholar]
- Grüneberg H. Genetical studies on the skeleton of the mouse. J Genet. 1950;50:142–173. [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–91. [PubMed] [Google Scholar]
- Holmes R. The osteology and musculature of the pectoral limb of small captorhinids. J Morphol. 1977;152:101–140. doi: 10.1002/jmor.1051520107. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Patel K, et al. Dual origin and segmental organisation of the avian scapula. Development. 2000;127:3789–3794. doi: 10.1242/dev.127.17.3789. [DOI] [PubMed] [Google Scholar]
- Hulke JW. On the shoulder girdle in Ichthyosauria and Sauropterygia. Proc R Soc Lond. 1893;52:233–255. [Google Scholar]
- Klima M. Die Frühentwicklung des Schultergürtels und des Brustbeins bei den Monotremen (Mammalia: Prototheria) Adv Anat Embryol Cell Biol. 1973;47:1–80. [Google Scholar]
- Klima M. Development of shoulder girdle and sternum in mammals. Fortschr Zool. 1985;30:81–83. [Google Scholar]
- Kuijper S, Feitsma H, Sheth R, et al. Genetics of shoulder girdle formation: roles of Tbx15 and aristaless-like genes. Development. 2005;132:1601–1610. doi: 10.1242/dev.01735. [DOI] [PubMed] [Google Scholar]
- Lee MSY. The homologies and early evolution of the shoulder girdle in turtles. Proc R Soc Lond B. 1996;263:111–117. [Google Scholar]
- Lee MSY. Similarity, parsimony and conjectures of homology: the chelonian shoulder girdle revisited. J Evol Biol. 1998;11:379–387. [Google Scholar]
- Li C, Wu XC, Rieppel O, et al. An ancestral turtle from the Late Triassic of southwestern China. Nature. 2008;456:497–501. doi: 10.1038/nature07533. [DOI] [PubMed] [Google Scholar]
- Li C, Rieppel O, Wu XC, et al. A new Triassic marine reptile from southwestern China. J Vert Paleontol. 2011;31:303–312. [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonnell IM. The evolution of the pectoral girdle. J Anat. 2001;199:189–194. doi: 10.1046/j.1469-7580.2001.19910189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckel JF. System der Vergleichenden Anatomie. Halle: Renger; 1824. Teil. 2. Abth. 1. [Google Scholar]
- Moeller C, Swindell EC, Kispert A, et al. Carboxypeptidase Z (CPZ) modulates Wnt signaling and regulates the development of skeletal elements in the chicken. Development. 2003;130:5103–5111. doi: 10.1242/dev.00686. [DOI] [PubMed] [Google Scholar]
- Müller J, Renesto S, Evans SE. The marine diapsid reptile Endennasaurus from the upper Triassic of Italy. Palaeontology. 2005;48:15–30. [Google Scholar]
- Nagashima H, Kuraku S, Uchida K, et al. On the carapacial ridge in turtle embryos: its developmental origin, function and the chelonian body plan. Development. 2007;134:2219–2226. doi: 10.1242/dev.002618. [DOI] [PubMed] [Google Scholar]
- Nicholls ELA. A reexamination of Thalattosaurus and Nectosaurus and the relationships of the Thalattosauria (Reptilia, Diapsida) Paleobios. 1999;19:1–29. [Google Scholar]
- Ogushi K. Anatomische Studien an der japanischen dreikralligen Lippenschildkröte (Trionyx japonicus. Morph Jahrb. 1911;43:1–106. [Google Scholar]
- Oken L. Isis von Oken. Vol. 1. Jena: Expedition der Isis; 1823. [Google Scholar]
- Osborn HF. The reptilian subclasses Diapsida and Synapsida and the early history of the Diaptosauria. Mem Am Mus Nat Hist. 1903;1:451–507. [Google Scholar]
- Parker WK. A Monograph on the Structure and Development of the Shoulder-girdle and Sternum in the Vertebrates. London: Ray Society; 1868. [Google Scholar]
- Piekarski N, Olsson L. A somitic contribution to the pectoral girdle in the axolotl revealed by long-term fate mapping. Evol Dev. 2011;13:47–57. doi: 10.1111/j.1525-142X.2010.00455.x. [DOI] [PubMed] [Google Scholar]
- Rathke H. Ueber die Entwicklung der Schildkröten. Braunschweig: Druck und Verlag von Friedrich Vieweg und Sohn; 1848. [Google Scholar]
- Rieppel O. Helveticosaurus zollingeri Peyer (Reptilia, Diapsida) skeletal paedomorphosis; functional anatomy and systematic affinities. Palaeontographica. 1989;A208:123–152. [Google Scholar]
- Rieppel O. Studies on skeleton formation in reptiles: patterns of ossification in the skeleton of Chelydra serpentina (Reptilia, Testudines) J Zool. 1993;231:487–509. [Google Scholar]
- Rieppel O. Testing homology by congruence: the pectoral girdle of turtles. Proc R Soc Lond B. 1996;263:1395–1398. doi: 10.1098/rspb.1996.0204. [DOI] [PubMed] [Google Scholar]
- Rieppel O, deBraga M. Turtles as diapsid reptiles. Nature. 1996;384:453–455. [Google Scholar]
- Rieppel O, Reisz RR. The origin and early evolution of turtles. Annu Rev Ecol Syst. 1999;30:1–22. [Google Scholar]
- Romer AS. The comparison of mammalian and reptilian coracoids. Anat Rec. 1922a;24:39–47. [Google Scholar]
- Romer AS. The locomotor apparatus of certain primitive and mammal-like reptiles. Bull Am Mus Nat Hist. 1922b;46:517–606. [Google Scholar]
- Romer AS. Osteology of Reptiles. Chicago: The University of Chicago Press; 1956. [Google Scholar]
- Sánchez-Villagra MR, Maier W. Ontogenetic data and the evolutionary origin of the mammalian scapula. Naturwissenschaften. 2002;89:459–461. doi: 10.1007/s00114-002-0362-7. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Maier W. Homologies of the mammalian shoulder girdle: a response to Matsuoka et al. (2005) Evol Dev. 2006;8:113–115. doi: 10.1111/j.1525-142X.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- Seeley HG. Further observations on the shoulder girdle and clavicular arch in the Ichthyosauria and Sauropterygia. Proc R Soc Lond. 1893;54:149–168. [Google Scholar]
- Shaffer HB, Minx P, Warren DE, et al. The western painted turtle genome, a model for the evolution of extreme physiological adaptations in a slowly evolving lineage. Genome Biol. 2013;14:R28. doi: 10.1186/gb-2013-14-3-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman RM, Tulenko FJ, Burke AC. 3D reconstructions of quail–chick chimeras provide a new fate map of the avian scapula. Dev Biol. 2011;355:1–11. doi: 10.1016/j.ydbio.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Starck D. Vergleichende Anatomie der Wirbeltiere. Heidelberg: Springer-Verlag; 1979. [Google Scholar]
- Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985;9:107–119. [Google Scholar]
- Theiler K. The House Mouse. Atlas of Embryonic Development. New York: Springer-Verlag; 1989. [Google Scholar]
- Timmons PM, Wallin J, Rigby PW, et al. Expression and function of Pax 1 during development of the pectoral girdle. Development. 1994;120:2773–2785. doi: 10.1242/dev.120.10.2773. [DOI] [PubMed] [Google Scholar]
- Tokita M, Kuratani S. Normal embryonic stages of the Chinese softshelled turtle Pelodiscus sinensis. Zool Sci. 2001;18:705–715. [Google Scholar]
- Tsuji LA, Müller J. Assembling the history of the Parareptilia: phylogeny, diversification, and a new definition of the clade. Fossil Rec. 2009;12:71–81. [Google Scholar]
- Versluys J. Das Skelet. In: Ihre JEW, Kampen PN, Nierstrasz HF, Versluys J, editors. Vergleichende Anatomie der Wirbeltiere. Berlin: Verlag von Julius Springer; 1927. pp. 58–328. [Google Scholar]
- Vickaryous MK, Hall BK. Homology of the reptilian coracoid and a reappraisal of the evolution and development of the amniote pectoral apparatus. J Anat. 2006;208:263–285. doi: 10.1111/j.1469-7580.2006.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GP. Homology and the mechanisms of development. In: Hall BK, editor. Homology: the Hierarchial Basis of Comparative Biology. San Diego: Academic Press; 1994. pp. 273–299. [Google Scholar]
- Walker WF., Jr The development of the shoulder region of the turtle, Chrysemys picta marginata, with special reference to the primary musculature. J Morphol. 1947;80:195–249. doi: 10.1002/jmor.1050800204. [DOI] [PubMed] [Google Scholar]
- Wang B, He L, Ehehalt F, et al. The formation of the avian scapula blade takes place in the hypaxial domain of the somites and requires somatopleure-derived BMP signals. Dev Biol. 2005;287:11–18. doi: 10.1016/j.ydbio.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pascual-Anaya J, Zadissa A, et al. The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan. Nat Genet. 2013;45:701–706. doi: 10.1038/ng.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm B, Dahl E, Peters H, et al. Targeted disruption of Pax1 defines its null phenotype and proves haploinsufficiency. Proc Natl Acad Sci U S A. 1998;95:8692–8697. doi: 10.1073/pnas.95.15.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.