Abstract
Extant tree sloths are uniquely slow mammals with a very specialized suspensory behavior. To improve our understanding of their peculiar evolution, we investigated the inner ear morphology of one of the largest and most popular fossil ground sloths, Megatherium americanum. We first address the predicted agility of this animal from the scaling of its semicircular canals (SC) relative to body mass, based on recent work that provided evidence that the size of the SC in mammals correlates with body mass and levels of agility. Our analyses predict intermediate levels of agility for Megatherium, contrasting with the extreme slowness of extant sloths. Secondly, we focus on the morphology of the SC at the inner ear scale and investigate the shape and proportions of these structures in Megatherium and in a large diversity of extant xenarthrans represented in our database. Our morphometric analyses demonstrate that the giant ground sloth clearly departs from the SC morphology of both extant sloth genera (Choloepus, Bradypus) and is in some aspects closer to that of armadillos and anteaters. Given the close phylogenetic relationships of Megatherium with the extant genus Choloepus, these results are evidence of substantial homoplasy of the SC anatomy in sloths. This homoplasy most likely corresponds to an outstanding convergent evolution between extant suspensory sloth genera.
Keywords: allometry, bony labyrinth, Folivora, fossil, semicircular canals, shape, Xenarthra
Introduction
The giant ground sloth Megatherium, the ‘big beast’ in Greek, is one of the most iconic extinct mammals. Its locomotor behavior has been a point of debate since the description of the first complete skeleton by Cuvier (e.g. Cuvier, 1804; Casinos, 1996; Fariña & Blanco, 1996; Bargo et al. 2000; Blanco & Czerwonogora, 2003; McDonald, 2007; Argot, 2008; Pujos et al. 2012). Although scientific discussion has focused mainly on its mode of locomotion (e.g. bipedal or quadrupedal), Megatherium is widely considered slow and sluggish in the scholarly and popular science literature (e.g. Bell, 2002; Zabludoff & Bollinger, 2009). This interpretation is encouraged by the close relationship Megatherium has with extant two-toed sloths of the genus Choloepus (Gaudin, 2004), and the fact that giant animals tend to show restricted locomotor abilities due to mechanical constraints (Biewener, 1990). The influence of size on agility levels might find further support in the fact that large animals, often less vulnerable as a prey (Hone & Benton, 2005), might have less of a need to run fast to escape predators. This idea has previously been suggested by Cuvier, who considered that Megatherium ‘n’était pas prompt à la course, mais cela ne lui était pas nécessaire, n'ayant besoin ni de poursuivre ni de fuir’ (‘was not of swift course, but this was not necessary, the animal needing neither to pursue, nor to escape’, Cuvier, 1804:386).
Interestingly, an extreme slow locomotion and suspensory behavior are hypothesized to have evolved convergently in the two extant sloth genera Bradypus and Choloepus within the Folivora (Gaudin, 2004; Delsuc & Douzery, 2009; Nyakatura, 2012; Pujos et al. 2012), a group that comprises the extant tree sloths and a large diversity of fossil representatives, including Megatherium. In addition to a number of morphological convergences possibly triggered by their sluggish behavior (e.g. Aiello, 1985; Suutari et al. 2010; Nyakatura, 2012), both extant sloth genera exhibit small and variable semicircular canals (SC) of the inner ear (Gray, 1907; Spoor et al. 2007; Billet et al. 2012). Thanks to the development of high resolution computed tomography (μCT), recent research has shown that the size and shape of the SC, responsible for the detection of angular acceleration and deceleration of the head, relate to various mechanical parameters of sensitivity to movement and to general patterns of agility during locomotion (e.g. Spoor et al. 2002, 2007; David et al. 2010). The putative association of small and variable SC with extreme slowness in sloths might constitute one of the most striking illustrations of this phenomenon. The history of this unique case of deeply convergent and parallel evolution to a slower way of life can be further clarified in the light of the inner ear anatomy of fossil sloths.
Recent work has provided evidence that the size of the SC, approximated by their radius of curvature, correlates with body mass and levels of agility in mammals (e.g. the ratio SC size to body mass tends to be higher in the agile taxa such as bats than in those that move more cautiously such as sloths) and that these relations could be used to estimate the locomotor behavior in extinct species (Spoor et al. 2007; Walker et al. 2008; Silcox et al. 2009; Ryan et al. 2012). Therefore, the investigation of the SC anatomy in Megatherium is interesting on two counts. First, and as outlined above, the iconic giant ground sloth constitutes an ideal candidate on which to conduct further research into the evolution of the vestibular system and locomotion within the group. A glimpse at Megatherium indeed constitutes a pertinent first step for testing whether some SC specializations (e.g. small SC) and a low agility are exclusive to tree sloths or are features also present in ground sloths and widespread in Folivora. Secondly, the study of the inner ear in Megatherium offers a unique opportunity to assess the locomotion and agility of an emblematic taxon from evidence other than postcranial anatomy, as well as to document further the locomotor behavior of a gigantic terrestrial mammal, a category underrepresented in extant mammalian faunas.
Based on current knowledge on SC functional morphology, we provide a new contribution to the understanding of the past level of agility of the giant ground sloth Megatherium, which can be compared with the one of extant tree sloths and extant giant mammals. We also make a comparison between the proportions and shape of the SC observed in Megatherium and that of extant sloths and other xenarthrans, which allows us to propose models of vestibular evolution within the group.
Material and methods
Specimens
In addition to the xenarthran specimens already included in the database of Billet et al. (2012) (11 genera, 14 species, 33 specimens), two specimens referred to the genus Megatherium were studied. The first specimen investigated, MNHN-F-TAR 1291, corresponds to an isolated fragment of the right temporal region, undoubtedly belonging to a member of the Megatherium genus from its large size and morphology. A label accompanying it identifies it as Megatherium tarijense. Nevertheless, the Pleistocene locality of Tarija (Bolivia), where it comes from, houses two species of Megatherium: M. tarijense and Megatherium americanum (De Iuliis et al. 2009). Megatherium tarijense is distinctly smaller than M. americanum (De Iuliis et al. 2009) but from the size of the MNHN-F-TAR 1291 fragment alone, it is difficult to ascertain a referral to M. tarijense and to reject that to M. americanum. Accordingly, we refer the specimen to Megatherium indet. However, this uncertainty did not limit our analyses. For the analyses that necessitate data on the body mass of the animal, we used the weight estimated for M. americanum for MNHN-F-TAR 1291, which sets an upper body mass limit for this specimen, clearly overestimated if the specimen belongs to M. tarijense (see also section “Limitations of predictions for Megatherium” below).
The second specimen corresponds to the skull MNHN-F-PAM 276, from the Pleistocene of Río Salado (Prov. Buenos Aires, Argentina), a specimen attributed to M. americanum (Edmund & Hoffstetter, 1970). This specimen exhibits a dental anomaly (fusion of some teeth), which affects (reduces) the size and shape of the muzzle, but does not show additional morphological deviation. Its overall size is just slightly below that of most other specimens belonging to M. americanum (Edmund & Hoffstetter, 1970). As for MNHN-F-TAR 1291, the body mass used for MNHN-F-PAM 276 is that estimated for M. americanum, probably also constituting an overestimation for this rather small individual.
High-resolution computed tomography-scanning and digital endocast extraction
For the specimens studied, we investigated the shape of the bony labyrinth of the inner ear by high-resolution computed tomography (μCT). The Megatherium specimens have been scanned at the AST-RX platform MNHN, Paris, France (http://www.ums2700.mnhn.fr/ast-rx/ressources). The first scans were focused on the basicranium of the skull MNHN-F-PAM 296 and resulted in 2009 slices with a resolution of 0.09345889 mm (voxel size) for the virtual reconstruction of the bony labyrinth. The skull (as presented in Fig. 1) has secondly been scanned entirely through a series of four scans assembled in an image stack of 5457 slices, with a resolution of 0.138 mm (voxel size). The scans of the specimen MNHN-F-TAR 1291 resulted in 1865 slices, with a resolution of 0.07964477 mm (voxel size). Three-dimensional reconstruction and visualization of the endocasts were performed using the stacks of digital CT images with the software vg studio max (Volume Graphics; Fig. 1) and mimics (Materialize NV). Measurements on the reconstructed bony labyrinth have been made with AVIZO 6.0 ® (Visualization Science Group), mimics and 3-matic (Materialize NV).
Fig. 1.
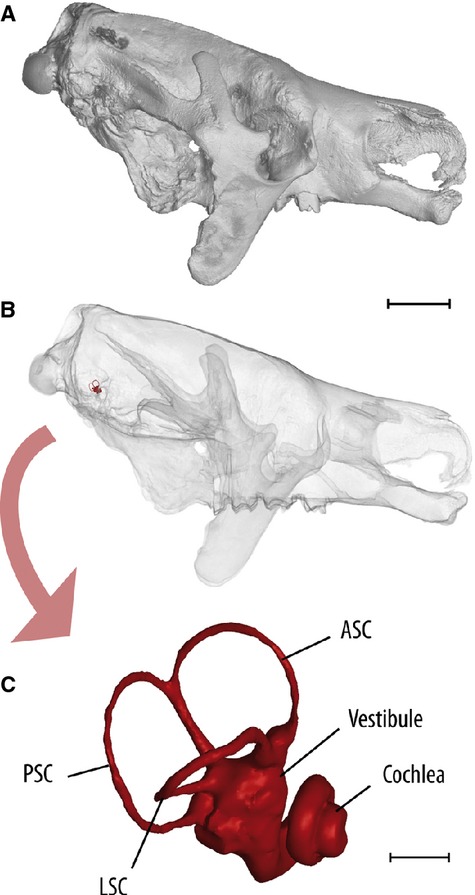
Views of the 3D reconstructed skull and inner ear of Megatherium americanum MNHN-F-PAM 276. (A) Right lateral view of skull. (B) Same than (A) but reconstructed with transparency of bone leaving apparent the right bony labyrinth of the inner ear in opaque red at the rear of the skull. (C) Close-up on the right bony labyrinth of the inner ear, lateral view. ASC, anterior semicircular canal; LSC, lateral semicircular canal; PSC, posterior semicircular canal. Scale bar: (A,B) 100 mm, (C) 5 mm.
Measurements
A large array of mammalian SC morphometric studies using diverse methodologies has been published in recent years (e.g. Schmelzle et al. 2007; Spoor et al. 2007; Welker et al. 2009; Cox & Jeffery, 2010; Lebrun et al. 2010; Ekdale & Rowe, 2011; Billet et al. 2012; Gunz et al. 2012; Malinzak et al. 2012), which reinforces the need to detail which and how measurements are taken. For both Megatherium specimens, we measured the length and width diameters of the arc of each of the semicircular canals as defined by Spoor & Zonneveld (1995) but made our measurements directly on the bony labyrinths 3D models from the medial (inner) border of the canals (Schmelzle et al. 2007), thus not at the midpoint of the lumen. This might represent a slight underestimation of the SC size but represents no problem for our purpose (estimation of a minimal agility score; see below). From these measurements, we have calculated the radii of curvature R = 0.5 × (length + width)/2 for the anterior, posterior and lateral SC (respectively ASCR, PSCR and LSCR; e.g. Spoor et al. 2007) (Supporting Information Table S1).
In addition to scaling against body mass for agility predictions (see below), we also wanted to compare the general size of the SC (mean of SC radii, noted SCR) to that of the inner ear. For this, we chose to scale the SC size against a linear measurement such as the inner ear height, rather than against the cube root of the volume of the bony labyrinth, because the latter would likely be more sensitive to slight differences in threshold parameters (see some examples of threshold effects in Gunz et al. 2012). The inner ear height was measured through the linear distance between the apex (dorsal) of the common crus and the apex (ventral) of the cochlea (i.e. the most apical and ventral point on the apex of the cochlea). The apex of the common crus was chosen because it allows, with the apex of the cochlea, measurement of a linear dimension close to the maximal dorsoventral height of the bony labyrinth, which, for the purpose addressed here, is a good approximation of the size of the inner ear. The apex of the common crus also corresponds to a precise and well-defined point, which is easily comparable across species. Moreover, the orientation of the cochlea relative to the SC system, which varies a little within the group, does not bias substantially the values of the ‘inner ear height’ measured and of the subsequent calculated ratio in the present case. As an illustration, the extant sloths in our sample cover a large part of the range of variation of the cochlear orientation recovered in extant Xenarthra (see notably the angle lateral semicircular canal (LSC)/cochlea measured in Billet et al. 2012: ESM2); despite this, the ratio ‘SC size/inner ear height’ of extant sloths departs from all but one of the other xenarthrans (see Results). The same holds true for the influence of the number of coils and the profile shape of the cochlea on the values of this ratio.
Further measurements were taken on the bony labyrinth for inclusion into a principal component analysis (PCA) on the shape of the SC (see below). These measurements resulted in 10 variables: ASC/PSC angle, ASC/LSC angle, PSC/LSC angle, R1a, R1b, R1c, R2a, R2b, R2c, and R3. These respectively correspond to angles, profile aspect of SCs, size ratios between SCs, and relative thickness of ducts (more precise definitions of these variables and measurements can be found in Billet et al. 2012). To measure the angles between the SCs, the SC planes have been defined with avizo 6.0® (Visualization Sciences Group) following the protocol of Ekdale (2010). Points for the ‘ObliqueSlice’ tool that determines the canal planes were modified in being set on the outer curvature of the semicircular canals.
Predicting locomotor agility
The body mass taken for M. americanum is 3950 kg based on the work of Fariña et al. (1998), which has been used in many studies (e.g. Bargo, 2001; Saint-André & De Iuliis, 2001; Vizcaíno et al. 2006; Vizcaíno et al. 2008). The maximal score of 6000 kg (Fariña et al. 1998) has also been used to provide a minimal estimation of the agility score.
We first plotted the SCR values and the body mass estimations of the two specimens of Megatherium using the mammal graphics established by Spoor et al. (2007: fig. 1b). This allows them to be visualized if they fall in the upper left (agile) part of the curve rather than in the lower right (slow) part.
To obtain a more precise estimate of an agility score for Megatherium based on its SC size and body mass, we used another different and independent method, linear discriminant analysis (LDA), which resulted in improved predictions relative to the equations provided by Silcox et al. (2009). The data used in previous studies (Spoor et al. 2007; Silcox et al. 2009) (i.e. several continuous variables and a classification) are suitable for such an analysis when considering the radii of curvature for each SC separately (e.g. Germain & Laurin, 2005). We ran an LDA using the following variables: ASCR, PSCR, LSCR, and log body mass, with the classification attributes being the agility score (discrete, from 1 to 6) given a priori, from Spoor et al. (2007: SI Dataset). The LDA was performed with the library MASS (Venables & Ripley, 2002) in the software r (R Core Team, 2012). This LDA provided us with a probability of belonging to any of these six discrete categories of agility score (integers). To get a continuous variable corresponding to real numbers between 1 and 6, and comparable to Silcox et al. (2009), we calculated for each taxon the expected value (or mathematical expectation): E(X) = ∑xipi, where xi is the discrete agility score category and Pi the probability of belonging to the xi agility score. With this methodology, we obtained on average more precise predictions and, in particular, less underestimated agility scores for large body-sized mammals compared with Silcox et al. (2009) (Supporting Information Data S1 and S2). The overall average absolute error being ∼1, our LDA agility scores should be considered to have a reliability of ±1 unit.
Limitations of the predictions for Megatherium
The possible overestimation of the body mass corresponding to the studied specimens (see above) provides us with a possibly underestimated agility: at same SC size, the higher the body mass, the lower the predicted agility score (e.g. Silcox et al. 2009). Likewise, the slightly underestimated SCR (see above) may also slightly lower the predicted score: at same body mass, the lower the SC size (SCR), the lower the estimated agility (e.g. Silcox et al. 2009). The results we obtained are therefore considered here as minimal agility scores for Megatherium. However, we tried to quantify these possible underestimations of the agility scores regarding the possible overestimation of the weight of the specimen that possibly belongs to M. tarijense and the possible underestimation of the SC size.
To provide a rough weight estimate for M. tarijense, we assumed geometric similitude with M. americanum, as previously done by Saint-André & De Iuliis (2001) for M. altiplanicum. Based on the figures and measurements of M. tarijense (De Iuliis et al. 2009: Fig. 2, Table 1) which were comparable to those used by Fariña et al. (1998: Table 3) for M. americanum, we estimated the size of the former species as around 78% the size of the latter and on this basis predicted a weight value of around 3.1 tons (Supporting Information Table S2). This latter value has been used to estimate the underestimation of the agility score that could depend on this.
Fig. 2.
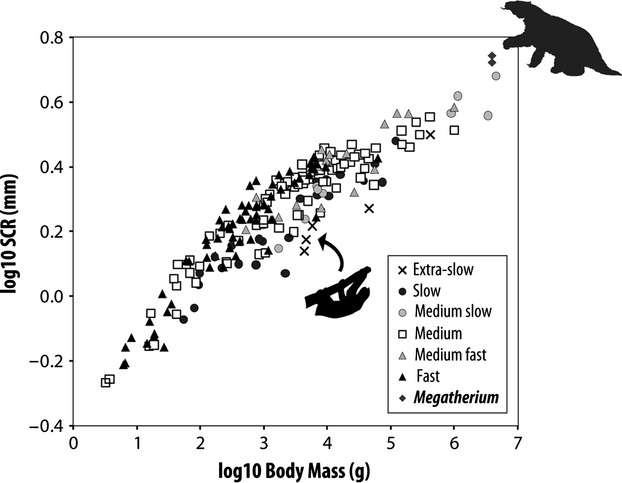
Graphical relationship between semicircular canal sizes, body mass and agility. Double logarithmic plots of average semicircular canal radius (SCR) against body mass for 210 mammals (modified from fig. 1b in Spoor et al. 2007) and two specimens of Megatherium. Note that Megatherium (considered at 3950 kg; Fariña et al. 1998) plots well above similar-sized and medium slow taxa (i.e. elephants, represented by the two circles below Megatherium), and that the three extant sloth specimens (the three black crosses indicated with an arrow) plot below all similar-sized mammals.
The underestimation of the size of the SC, if the midpoint of the lumen is considered (e.g. Silcox et al. 2009: Fig. 2), has been estimated by adding 1.5 × SC D (= mean duct thickness of the SC; Billet et al. 2012) to the sum of the arc length and width diameters for the calculation of the SC radii; e.g. ASCR = 0.5 × [(length + width + 1.5 × ASC D)/2]. The new SC radii values obtained have been used to estimate in turn the underestimation of the agility score depending on this measurement method (Data S2).
Analyses on proportions and shape of the SC
The proportions of the SC (SCR) was compared with the entire inner ear height (IEH) using the ratio SCR/IEH for Megatherium and all extant Xenarthra in our database. To take into account the potentially confounding effects of size allometry on these inner ear proportions, we performed a regression of SCR/IEH on the logarithm of the body mass (Supporting Information Table S3). To account for the influence of phylogeny on our result, we regressed phylogenetically independent contrasts (PICs; Felsenstein, 1985) to test whether the body mass and inner ear proportions show evidence of correlated evolution. PIC analysis was implemented using the PDAP:PDTREE module, version 1.14 (Midford et al. 2008) of mesquite version 2.71 (Maddison & Maddison, 2009). The phylogenetic tree follows a consensus of recent morphological and molecular phylogenies of Xenarthra (Gaudin, 2004; Billet et al. 2011; Delsuc et al. 2012). For this, body masses in extant xenarthrans were taken from Spoor et al. (2007), Wetzel (1985a,b) and Vizcaíno & Milne (2002), in that order of priority.
Based on recent results suggesting an allometric effect on the relative size of the ASC, PSC and LSC in some mammals (Lebrun et al. 2010; Alloing-Séguier et al. 2013), we tested for a correlated evolution in our xenarthran sample of the relative sizes of the ASC, PSC and LSC with the body mass. For this, the relative size of the SC has been investigated using the ratios ASCR/PSCR, ASCR/LSCR and PSCR/LSCR, which were regressed on the logarithm of the body mass (Supporting Information Table S4). We also conducted regression analyses that took into account the influence of the phylogeny (using PICs; as explained above).
Furthermore, the shape of the inner ear was further analyzed by a PCA on the SC morphology following Billet et al. (2012) to visualize overall resemblance of the SC of Megatherium within extant xenarthrans. A multivariate analysis of variance (manova) was performed on the same variables to assess the effect of phylogeny. The PCA and manova were performed in past 2.06 (Hammer et al. 2001) on a set of 35 xenarthran specimens (including the two specimens of Megatherium) and 10 variables (see above).
Results
General morphology of the bony labyrinth
The bony labyrinths of the inner ear of two Megatherium specimens were reconstructed and revealed the shape and size of the bony semicircular canals (SC), vestibule and cochlea (Fig. 1). Both specimens show the same pattern, which is different from the living sloths Choloepus and Bradypus (studied by Billet et al. 2012) in the proportion and shape of the SC. The thin SCs of Megatherium have pronounced curvatures and appear proportionally larger than in the living sloths Choloepus and Bradypus. The lateral and posterior SCs do not unite to form a secondary crus commune. The cochlea in Megatherium shows 2.5 turns, which is a rather high value within Xenarthra (Billet et al. 2012). As in many extant Xenarthra, the coiling of the cochlear canal in Megatherium is more pronounced distally than proximally, resulting in a distinct gap between the basal and the following turns.
Agility estimations
Agility, size of SC relative to body mass and comparison with other mammals
Both Megatherium specimens show close values of their radii of curvature (R) of the SCs (Table S1). The plot of these specimens in the graphs established by Spoor et al. (2007: fig. 1), which compares the radii of curvature to the body masses of 210 mammalian taxa (excluding cetaceans), gives similar results for both specimens. When considered at a body mass of 6000 kg, both Megatherium specimens occupy positions tending to the upper left (agile) part of the curve rather than the lower right (slow) part. However, the absence of any similarly sized extant land-mammal in the database prevents further conclusions to be made on this basis (but see agility score predictions below). When considering the preferred body mass of 3 950 kg (see Material and methods), both Megatherium specimens plot clearly above the similar-sized elephants in the graph (Fig. 2; Spoor et al. 2007); that is, with a similar body mass, they present relatively larger SCs than these elephants. Conversely, extant sloths plot below all mammals of comparable body mass (Fig. 2); that is, they have relatively smaller SCs than these latter. This observation suggests that, contrary to extant sloths, Megatherium had relatively larger SCs compared with similar-sized mammals. Considering the relationship of SC relative size to levels of agility (Spoor et al. 2007), this suggests intermediate levels of agility in Megatherium, or at least not extra-slow, as found in extant tree sloths.
Agility predictions from linear discriminant analysis
Based on a theoretical scale of agility scores from 1 (extra-slow) to 6 (fast), our linear discriminant analysis predicts agility scores of 3.36 and 3.45, respectively, for the two Megatherium specimens MNHN-F-TAR 1291 and MNHN-F-PAM 276, when the considered body mass is 3950 kg, and scores of 3.17 and 3.24 with a body mass of 6000 kg (Data S2). Therefore, if considered at a more cautious and reliable ±1 unit approximation (see Material and methods and Data S1 and S2), the agility score of Megatherium should fall within the range 2.17–4.45. This theoretically corresponds to moderately slow to intermediate levels of agility (Spoor et al. 2007) and is clearly higher than the extra-slow category of extant sloth, whose agility score is 1.
The measuring methods of the SC size (see Material and methods) are estimated to have little effect on the predicted agility scores [mean increase of ∼0.26 of the agility score values for Megatherium when larger SC size (from midpoint of the lumen) is estimated; see Data S2]. On the other hand, the weight used for MNHN-F-TAR 1291 has a rather strong effect. When using the estimated lighter weight of M. tarijense (see Material and methods), the corresponding agility score estimated for this specimen increases by ∼1 (see Data S2). Therefore, both a larger SC size and a lighter weight have the effect, though at different scales, of increasing the agility scores relative to our main predictions above. These alternative estimates confirm the large difference in agility levels between Megatherium and extant sloths.
Proportions and shape of the SC in Megatherium and extant Xenarthra
Scaling of SC with the inner ear height
The size of the SC, approximated by the average radius of curvature (SCR) and scaled relative to the IEH, clearly distinguishes two groups within the xenarthrans. The ratio calculated proved to be (much) higher than 0.20 in Megatherium and almost all xenarthran specimens included in the database, except for extant sloths and the pink-fairy armadillo Chlamyphorus (Fig. 3). As the average radius of curvature of the SC conveniently expresses the overall size of these structures (Spoor & Zonneveld, 1998), this shows that recent sloths and Chlamyphorus present small SC relative to the entire inner ear height in comparison with other xenarthrans. This confirms a clear visual impression left from a simple observation of the bony labyrinth diversity within Xenarthra. A regression of this ratio (SCR/IEH)) on the logarithm of the body mass showed that there is no evidence that body mass has a confounding influence on the inner ear proportions (r² = 0.098; P = 0.067). A similar regression of the ratio performed only on extant xenarthrans (i.e. without considering Megatherium) leads to a much stronger disproof of the effects of body mass on inner ear proportions (r² = 0.028; P = 0.356). Phylogenetic independent contrasts (PIC) analysis is not congruent with these results, and indicates that after phylogenetic correction, allometry plays a role in determining the pattern of morphological proportions of the inner ear in our xenarthran sample, with (r² = 0.684; P = 1.40 × 10−4) or without (r² = 0.373; P = 0.020) considering Megatherium.
Fig. 3.
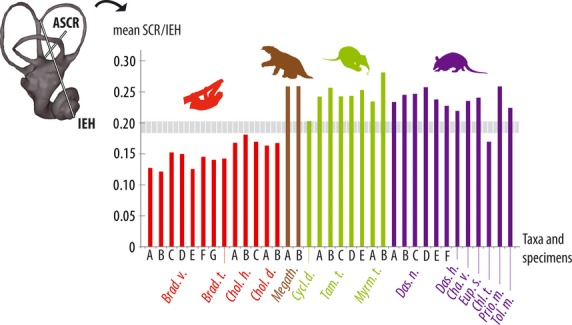
Relative size of the semicircular canals (SC) scaled with the inner ear in extant xenarthrans and Megatherium. Bar graph showing the values of the ratio ‘SC size/inner ear height’ (=SCR/IEH) in various specimens. The thick dotted line in the background emphasizes a distinction between most xenarthrans and extant tree sloths in the relative size of the semicircular canals. Taxonomic abbreviations are for Bradypus variegatus (Brad. v.), Bradypus tridactylus (Brad. t.), Chaetophractus vellerosus (Cha. v.), Chlamyphorus truncatus (Chl. t.), Choloepus hoffmani (Chol. h.), Choloepus didactylus (Chol. d.), Cyclopes didactylus (Cycl. d.), Dasypus novemcinctus (Das. n.), Dasypus hybridus (Das. h.), Euphractus sexcinctus (Eup. s.), Megatherium (Megath.), Myrmecophaga tridactyla (Myrm. t.), Priodontes maximus (Prio. m.), Tamandua tetradactyla (Tam. t.), Tolypeutes matacus (Tol. m.); see Supporting Information Table S5 for more details on specimens (e.g. designed with letters). ASCR, radius of curvature of anterior semicircular canal; IEH, inner ear height = distance between the apex of common crus and the apex of cochlea; SCR, average radius of curvature of the three semicircular canals.
Apart from possible effects of allometry (see Discussion), these results support a relatively large size of the SCs in Megatherium, which is more similar to what is observed in most xenarthrans (anteaters and armadillos) than to the configuration in extant tree sloths, whose SCs appear very small whether they are scaled against body mass (Fig. 2) or simply against inner ear height (Fig. 3).
Relative size of each SC and relation to body mass
No significant allometric effects have been found in our xenarthran sample in the relative sizes of each SC based on regressions of the ratios ASCR/PSCR, ASCR/LSCR and PSCR/LSCR on the logarithm of body mass (for ASCR/PSCR: r² = 1.2 × 10−4, P = 0.95; for ASCR/LSCR: r² = 0.004, P = 0.69; for PSCR/LSCR: r² = 0.014, P = 0.49). The same holds true with PIC analyses correcting for the influence of phylogeny (for ASCR/PSCR: r² = 0.027, P = 0.56; for ASCR/LSCR: r² = 0.034, P = 0.51; for PSCR/LSCR: r² = 0.003, P = 0.85). The same analyses performed without considering Megatherium also demonstrate the absence of correlation (P values ≫ 0.05).
Resemblances of Megatherium overall SC shape within Xenarthra
The PCA allowed us to examine the morphological space defined by the overall SC morphological variation in Xenarthra. The first two axes represent 53% of the among-group variance (Data S3). The first principal component is positively correlated with angle ASC/PSC, R2a, R2b, R2c, and R3; and negatively with angle ASC/LSC, angle LSC/PSC, R1a, R1b, and R1c. The second axis is positively correlated with angle ASC/PSC, R1a, R1b, R1c, R2c, and R3; and negatively with angle ASC/LSC, angle LSC/PSC, R2a, R2b. manovas indicate a significant morphological differentiation between the different suborders (Wilk's Lambda test: value = 0.03047, F = 10.88, P < 0.001). On one hand, the different xenarthran species occupy distinct positions in the morphological space of the SC shape, and the extant subsets Cingulata, Vermilingua and Folivora are also well-identified. On the other hand, Megatherium plots closer to armadillos (extant Cingulata) than to extant sloths (extant Folivora; Fig. 4). Furthermore, whereas Megatherium and Choloepus are phylogenetically closer to each other than to Bradypus (see Discussion), the two genera of extant sloths plot closer to one another than to Megatherium. This analysis thus demonstrates an overall morphological dissemblance of the SC of Megatherium with that of both extant sloth genera.
Fig. 4.
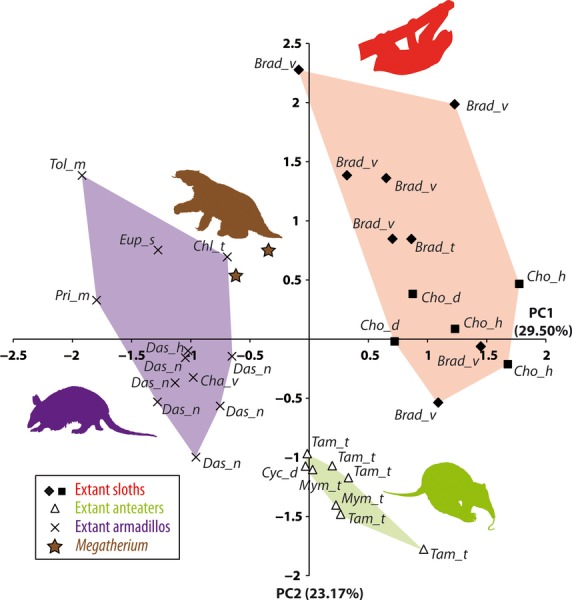
Shape differentiation of the bony labyrinth of xenarthrans on the first two axes (52.67% of the among-group variance) of the principal component analysis (PCA) performed on a set of 10 variables on the semicircular canals (SCs) and 35 specimens. Taxonomic abbreviations are for Bradypus variegatus (Brad_v); B. Bradypus tridactylus (Brad_t), Chaetophractus vellerosus (Cha_v), Chlamyphorus truncatus (Chl_t), Choloepus didactylus (Cho_d), Choloepus hoffmani (Cho_h), Cyclopes didactylus (Cyc_d), Dasypus novemcinctus (Das_n); Dasypus hybridus (Das_h), Euphractus sexcinctus (Eup_s), Myrmecophaga tridactyla (Mym_t), Priodontes maximus (Pri_m), Tamandua tetradactyla (Tam_t), Tolypeutes matacus (Tol_m).
Discussion
The reconstruction and study of the inner ear of a fossil giant ground sloth such as Megatherium is of fundamental interest as, for the first time, it is possible to test whether the small size of the SC, slow locomotion and agility could be exclusive to extant tree sloths within the Folivora. All our results congruently indicate that the proportions and shape of the semicircular canals of the inner ear in Megatherium depart from those of extant sloths. More specifically, Megatherium exhibits the same SC proportions relative to the inner ear height as in most other extant xenarthrans (i.e. armadillos and anteaters), and an overall shape of SC (angles, profile aspect, size ratios between SCs, and relative thickness of ducts) more similar to those of armadillos than to those of extant tree sloths. It could be intuitive to link these overall SC shape differences to habitat preferences, given that ground sloths are terrestrial while extant sloths are fiercely arboricolous. However, a similar case occurred during the evolutionary history of Xenarthra. Our PCA shows that the giant anteater, the only terrestrial vermilinguan, displays similar SC shape to both the arboricolous silky anteater (Cyclopes didactylus) and Southern Tamandua (Tamandua tetradacyla; Fig. 4). Therefore, both the proximity of the inner ear proportions of Megatherium to that of armadillos in the morphospace, and the cluster of extant convergent tree sloths, might have been influenced by factors other than habitat alone. These factors might be phylogenetic, with a possible retention of a plesiomorphic configuration of the SCs in Megatherium and armadillos (see below the discussion on SC proportions), and/or functional, with a similar influence of patterns of head movements in these two clusters of taxa. However, for some of the characters included in this analysis (e.g.profile aspect of SC) a clear link to functional parameters of the SC has not yet been established.
Considering the size of the SCs scaled relative to body mass, our method predicts intermediate levels of agility for Megatherium, in contrast to the extremely slow and cautious movements of extant sloths (Spoor et al. 2007; Silcox et al. 2009). So far, only trackways have been used to give a precise estimation of the locomotor speed in Megatherium. From this data, studies predicted a 3–8 km h−1 velocity (Aramayo & Manera de Bianco, 1994; Casinos, 1996; Blanco & Czerwonogora, 2003), which is clearly faster than the estimated maximal average speed of 0.5–0.6 km h−1 for extant tree sloths (Britton & Kline, 1939; Brattstrom, 1966; Adam, 1999; Billet et al. 2012). Thus, two independent sources of data (SC and fossilized trackways) now make a strong argument for the case that Megatherium was not the slow and indolent animal often depicted in literature. Nevertheless, McDonald (2012) pointed out that the limb proportions and morphology of the appendicular skeleton in extinct sloths like Megatherium would prevent them from reaching substantial speeds. It is thus questionable if this limitation in locomotor speed could discredit the idea that giant ground sloths would have been at least as agile as extant elephants, as it might be interpreted from the SC data. In fact, the SC data should more directly reflect the pattern of head motion rather than the general locomotion, as the SCs are directly sensitive to the angular acceleration/deceleration of the head (e.g. David et al. 2010; Malinzak et al. 2012). This means that the SC data may primarily support that Megatherium performed more agile head movements but not necessarily an overall more agile or faster locomotion than elephants. Moreover, the probable absence of predators for such a large animal as Megatherium (at least for healthy adults) – in a similar fashion to adult elephants that are rarely the target of predators – suggests no need of substantial locomotor speed. Considering these arguments, a possible explanation for larger SCs in Megatherium relative to elephants may relate to more rapid head movements executed during feeding. Indeed, both the elephants and Megatherium are herbivorous taxa (see Bargo, 2001) but elephants may use their agile trunk to grab plant material and thus have less need for head movements. On the other hand, when compared with extant sloths, it is clear that Megatherium also presented an overall faster locomotion, as is indicated by fossilized trackways.
The angle values between the three SCs influence the distribution of the specimens in the morphospace of our PCA analysis. Malinzak et al. (2012) recently demonstrated that the closest the SC configuration is to orthogonality in primates, the faster the rotational head speed. They proposed on this basis that SC orthogonality can be used to estimate the rotational head speed attributes and locomotor behavior in extinct species. It is interesting to note that Megatherium exhibit SC angle values rather close to orthogonality (67–93°) when compared with some outlying specimens of extant sloths (Supporting Information Data S3). We would argue, however, that the conclusions of Malinzak et al. (2012) are not directly transposable to our case. In their study, the authors notably include a sloth as an outgroup to explore the possibility of extrapolating the results obtained in primates to other mammals. However, they did not consider the characteristic large intraspecific angular variation between the SCs observed in sloths, which covers values both far from and close to orthogonality (Billet et al. 2012: ESM-2). As a result, from one specimen they concluded that extant sloths show values far from orthogonality (Malinzak et al. 2012). Given the inconstancy of this deviation from orthogonality, future studies might also focus on the question of whether slower head movements could rather correlate with a large intraspecific variation in SC angles as observed in extant sloths. This idea, which could be tested on extant species but is difficult to analyze in fossils, suggests that preservation of orthogonality could be constrained in agile taxa, whereas large variation in SC angles would be authorized in slow-moving ones. This echoes previous hypotheses of an overall vestibular variation in extant sloths in relation with a relaxation of functional constraints (Billet et al. 2012) and would contradict a selected deviation from orthogonality in the SCs of slow taxa.
As detailed above, the size of SCs (SCR) relative to the inner ear height is clearly small in extant sloths but not in Megatherium and many other extant xenarthrans. The results of our analyses regarding the potential allometric influence on the ratio SC size/inner ear height are confounding. Our regression analysis suggests that allometry has no significant effect. In contrast, our analysis correcting for phylogeny indicates that allometry does have an effect, especially when Megatherium is included in the data, namely, that the larger the body mass, the higher this ratio. The data in the latter analysis may suffer from an absence of fossil sloths other than Megatherium, which certainly introduces a bias due to the omission of most of the extinct morphological and weight diversity of the lineage. A predominant effect of allometry is also challenged by the value of the ratios being similar in the 4-ton Megatherium as in many smaller xenarthrans, and especially in closely related anteaters (Tamandua and Myrmecophaga, respectively 4 and 30 kg). Based on these considerations, we conclude that allometric processes may partly influence the SC proportions at the inner ear scale, but at an intensity that remains to be determined. However, the allometric effects detected by Alloing-Séguier et al. (2013) and Lebrun et al. (2010) in diprotodontian marsupials and primates on the relative sizes of the ASC, PSC, and LSC are not present in our xenarthran sample.
The detected pattern of SC proportions relative to the inner ear height can be arbitrarily categorized into two distinct subsets of xenarthran species: those characterized by relatively small SC, showing a SCR/IEH value of under 0.18–0.20 (Fig. 3), and those characterized by larger SCs, showing a value greater than 0.20. From this perspective, extant sloths prove not to be the only xenarthrans that display small SC relative to the entire inner ear. A relative reduction of the SC also occurs in the extant pink-fairy armadillo Chlamyphorus truncatus, a poorly known fossorial species (Superina, 2011) but whose size is small and actions are described as ‘not frantic or hasty’ (Merrit, 1985: 394). Further investigation into this taxon is needed to determine whether its SC proportions at inner ear scale could relate to a peculiar locomotor behavior and/or to allometry.
When categories of SC size relative to the inner ear height are mapped on a cladogram corresponding to a consensus of recent morphological and molecular phylogenies of Xenarthra (Gaudin, 2004; Billet et al. 2011; Delsuc et al. 2012), parsimony principles argue for a homoplastic evolution of the SC proportions within Xenarthra (Fig. 5). The interpreted plesiomorphic condition for Xenarthra is to present large SCs relative to the entire inner ear height. Within Folivora, either a convergence or a reversion can explain the configuration observed from our dataset: a convergent reduction of the SC in extant tree sloths or a reduction of the SCs in the clade Folivora with a subsequent reversion (secondary increase of SC size) in Megatherium. Considering that reversals might be rare and constitute controversial evolutionary processes (Wake et al. 2011), we favor a priori the hypothesis of a convergent reduction of the SC size relative to the inner ear dimensions in both extant tree sloth genera, and preserved plesiomorphic proportions in Megatherium (see below). As discussed above, this phenomenon may be partly related to allometric processes. We further note that the small size of the SCs relative to body mass coincides with the small size of the SCs relative to the inner ear height in our sloth sample (Figs 2 and 3). As small SCs relative to body mass are suggestive of low agility (Spoor et al. 2007), this indicates that slow locomotion and reduction of SC size at inner ear scale might have evolved concomitantly in the extant tree sloths, but in an opposite way to the unique case of cetaceans (Spoor et al. 2002). The above indicates that, in addition to the possible allometric influence, the SC proportions relative to the inner ear size could be linked to locomotor agility patterns in Xenarthra.
Fig. 5.
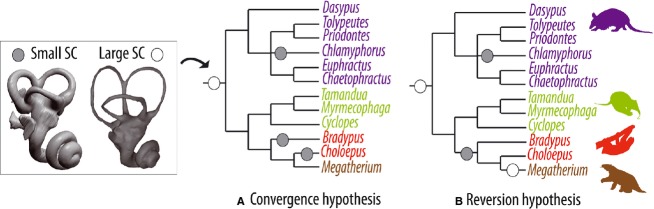
Evolution of semicircular canal (SC) proportions in Xenarthra. Illustration of the two most parsimonious hypotheses (A,B) of phylogenetic evolution of small SC relative to the inner ear height (as shown in Fig. 3), mapped on a cladogram of extant Xenarthra and Megatherium (consensus of Gaudin, 2004; Billet et al. 2011; Delsuc et al. 2012). Note that an alternative phylogenetic pattern of relationships with Megatherium closer to Bradypus than to Choloepus – as proposed by Poinar et al. (2003) for the ground sloth Nothrotheriops, a possible relative of Megatherium (Gaudin, 2004) – would also support homoplastic scenarios within Folivora.
It is noteworthy that the suspensory and sluggish behavior of extant sloths, whose convergent status is in agreement with the higher agility of Megatherium and supported by both the fossil record and molecular estimates (e.g. Gaudin, 2004; Delsuc & Douzery, 2009), has triggered parallel evolution of a high number of postcranial changes. These notably include hands and feet modified in rigid hook-like appendages, and relatively long arms with short scapula (Nyakatura, 2012). In relation to their frequent immobility, both sloth genera also convergently developed very specialized fur which provided a host environment for symbiotic algae (Aiello, 1985; Suutari et al. 2010). Considering these multiple convergent events between the two genera, we explicitly hypothesize that a relative reduction in size and resemblance in shape of the SCs is likely to have occurred convergently in both extant sloths genera in concert with their parallel acquisition of a slow and suspensory locomotion. The validity of this hypothesis would confirm that the two extant sloth genera represent one of the most extreme cases of convergent evolution in the evolutionary history of placental mammals.
Acknowledgments
We warmly thank Christine Argot, Vincent Pernègre, Florent Goussard, Platform AST-RX MNHN and Miguel García-Sanz (MNHN, Paris, France) for allowing and helping us in the manipulation, CT scanning and imaging of the specimens of Megatherium. Acknowledgements are also due to Romain David (Leipzig, Max-Planck Institut, Germany) and Christine Argot (MNHN, Paris, France) for fruitful discussion on the inner ear system and on Megatherium functional morphology. We thank Sarah Jones, Julien Bigot (MNHN, Paris) and Rob Asher (University of Cambridge, UK) who helped to improve the quality of this manuscript. Lionel Hautier acknowledges Sidney Sussex College for financial support. Many thanks to two anonymous reviewers whose comments helped us to substantially improve the manuscript.
Author contributions
G.B. and L.H. initiated the project; G.B., L.H. and D.G. designed the research plan; G.B. collected the CT data, reconstructed and measured the bony labyrinths of Megatherium; G.B., L.H. and D.G. analyzed the data; G.B., L.H., D.G., I.R. and C. M. discussed the results and wrote the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Values of the radii of curvature in Megatherium.
Table S2. Data used for the estimation of weight for Megatherium tarijense.
Table S3. Values used for the regression analyses of the ratio ‘SC size/inner ear height’ vs. the logarithm of the body mass.
Table S4. Values used for the regression analyses of the ratios ‘ASCR/PSCR’, ‘ASCR/LSCR’, ‘PSCR/LSCR’ vs. the logarithm of the body mass.
Table S5. Details on specimens reported in Fig. 3.
Data S1. Comments on accuracy of linear discriminant analysis for agility predictions.
Data S2. Dataset linear discriminant analysis.
Data S3. Dataset principal component analysis (with original ratios and angles values).
References
- Adam PJ. Choloepus didactylus. Mamm Species. 1999;621:1–8. [Google Scholar]
- Aiello A. Sloth hair: unanswered questions. In: Montgomery GG, editor. The Evolution and Ecology of Armadillos, Sloths and Vermilinguas. Washington, DC: Smithsonian Institution Press; 1985. pp. 213–218. [Google Scholar]
- Alloing-Séguier L, Sánchez-Villagra MR, Lee MS, et al. The bony labyrinth in diprotodontian marsupial mammals: diversity in extant and extinct forms and relationships with size and phylogeny. J Mamm Evol. 2013;20:191–198. [Google Scholar]
- Aramayo SA, Manera de Bianco T. 1994. p. 17. Aspectos de la locomoción de mamíferos extinguidos en base a icnitas del yacimiento paleoicnologico de Pehuen-Co (Pleistoceno Tardio) Provincia de Buenos AiresVI congreso Argentino de Paleontología y Bioestratigrafía. Trelew, Chubut, Resúmenes. [Google Scholar]
- Argot C. Changing views in paleontology: the story of a giant (Megatherium, Xenarthra) In: Sargis EJ, Dagosto M, editors. Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. Dordrecht: Springer Science; 2008. pp. 37–50. [Google Scholar]
- Bargo MS. The ground sloth Megatherium americanum: skull shape, bite forces, and diet. Acta Palaeontol Pol. 2001;46:173–192. [Google Scholar]
- Bargo MS, Vizcaíno SF, Archuby FM, et al. Limb bone proportions, strength and digging in some Lujanian (Late Pleistocene-Early Holocene) mylodontid ground sloths (Mammalia, Xenarthra) J Vertebr Paleontol. 2000;20:601–610. [Google Scholar]
- Bell CM. Did elephants hang from trees? – The giant sloths of South America. Geol Today. 2002;18:63–66. [Google Scholar]
- Biewener AA. Biomechanics of mammalian terrestrial locomotion. Science. 1990;250:1097–1103. doi: 10.1126/science.2251499. [DOI] [PubMed] [Google Scholar]
- Billet G, Hautier L, de Muizon C, et al. Oldest cingulate skulls provide congruence between morphological and molecular scenarios of armadillo evolution. Proc R Soc B. 2011;278:2791–2797. doi: 10.1098/rspb.2010.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billet G, Hautier L, Asher R, et al. High morphological variation of vestibular system accompanies slow and infrequent locomotion in three-toed sloths. Proc R Soc B. 2012;279:3932–3939. doi: 10.1098/rspb.2012.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco RE, Czerwonogora A. The gait of Megatherium Cuvier 1796 (Mammalia, Xenarthra, Megatheriidae) Senckenb Biol. 2003;83:61–68. [Google Scholar]
- Brattstrom BH. Sloth behavior. J Mammal. 1966;47:348. [Google Scholar]
- Britton SW, Kline RF. Augmentation of activity in the sloth by adrenal extract, emotion and other conditions. Am J Physiol. 1939;127:127–130. [Google Scholar]
- Casinos A. Bipedalism and quadrupedalism in Megatherium: an attempt at biomechanical reconstruction. Lethaia. 1996;29:87–96. [Google Scholar]
- Cox PG, Jeffery N. Semicircular canals and agility: the influence of size and shape measures. J Anat. 2010;216:37–47. doi: 10.1111/j.1469-7580.2009.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvier G. Sur le Megatherium, autre animal de la famille des paresseux, mais de la taille du rhinocéros, dont un squelette fossile presque complet est conservé au cabinet royal d'Histoire naturelle à Madrid. Annales du Muséum national d'Histoire naturelle. 1804;5:376–387. [Google Scholar]
- David R, Droulez J, Allain R, et al. Motion from the past: a new method to infer vestibular capacities of extinct species. CR Palevol. 2010;9:397–410. [Google Scholar]
- De Iuliis G, Pujos F, Tito G. Systematic and taxonomic revision of the Pleistocene ground sloth MegatheriumPseudomegatheriumtarijense (Xenarthra: Megatheriidae) J Vertebr Paleontol. 2009;29:1244–1251. [Google Scholar]
- Delsuc F, Douzery EJP. Armadillos, anteaters and sloths (Xenarthra) In: Hedges SB, Kumar S, editors. The Time Tree of Life. Oxford: Oxford University Press; 2009. pp. 475–478. [Google Scholar]
- Delsuc F, Superina M, Tilak M-K, et al. Molecular phylogenetics unveils the ancient evolutionary origins of the enigmatic fairy armadillos. Mol Phyl Evol. 2012;62:673–680. doi: 10.1016/j.ympev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Edmund GA, Hoffstetter R. Essonodontherium gervaisi es un sinonimo de Megatherium americanum Cuvier (Xenarthra, Mammalia) Ameghiniana. 1970;7:317–328. [Google Scholar]
- Ekdale EG. Ontogenetic variation in the bony labyrinth of Monodelphis domestica (Mammalia: Marsupialia) following ossification of the inner ear cavities. Anato Rec. 2010;293:1896–1912. doi: 10.1002/ar.21234. [DOI] [PubMed] [Google Scholar]
- Ekdale EG, Rowe T. Morphology and variation within the bony labyrinth of zhelestids (Mammalia, Eutheria) and other therian mammals. J Vertebr Paleontol. 2011;31:658–675. [Google Scholar]
- Fariña RA, Blanco RE. Megatherium, the stabber. Proc R Soc B. 1996;263:1725–1729. doi: 10.1098/rspb.1996.0252. [DOI] [PubMed] [Google Scholar]
- Fariña RA, Vizcaíno SF, Bargo MS. Body mass estimations in Lujanian (late Pleistocene – early Holocene of South America) mammal megafauna. Mastozool Neotrop. 1998;5:87–108. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- Gaudin TJ. Phylogenetic relationships among sloths (Mammalia, Xenarthra, Tardigrada): the craniodental evidence. Zool J Linn Soc. 2004;140:255–305. [Google Scholar]
- Germain D, Laurin M. Microanatomy of the radius and lifestyle in amniotes (Vertebrata, Tetrapoda) Zool Scr. 2005;34:335–350. [Google Scholar]
- Gray AA. The labyrinth of animals. Vol. 1. London: Churchill; 1907. [Google Scholar]
- Gunz P, Ramsier M, Kuhrig M, et al. The mammalian bony labyrinth reconsidered, introducing a comprehensive geometric morphometric approach. J Anat. 2012;220:529–543. doi: 10.1111/j.1469-7580.2012.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:9. [Google Scholar]
- Hone DWE, Benton MJ. The evolution of large size: how does Cope's Rule work? Trends Ecol Evol. 2005;20:4–6. doi: 10.1016/j.tree.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Lebrun R, Ponce de León MS, Tafforeau P, et al. Deep evolutionary roots of strepsirrhine primate labyrinthine morphology. J Anat. 2010;216:368–380. doi: 10.1111/j.1469-7580.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. 2009. Mesquite: a modular system for evolutionary analysis. Version 2.71, http://mesquiteproject.org.
- Malinzak MD, Kay RF, Hullar TE. Locomotor head movements and semicircular canal morphology in primates. Proc Natl Acad Sci U S A. 2012;109:17914–17919. doi: 10.1073/pnas.1206139109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald HG. Biomechanical inferences of locomotion in ground sloths: integrating morphological and track data. New Mexico Muse Nat Hist Sci Bull. 2007;42:201–208. [Google Scholar]
- McDonald HG. Evolution of the pedolateral foot in ground sloths: patterns of change in the astragalus. J Mamm Evol. 2012;19:209–215. [Google Scholar]
- Merrit DA., Jr . The fairy armadillo, Chlamyphorus truncatus Harlan. In: Montgomery GG, editor. The evolution and Ecology of Armadillos, Sloths and Vermilinguas. Washington, DC: Smithsonian Institution Press; 1985. pp. 393–395. [Google Scholar]
- Midford PE, Garland T, Jr, Maddison WP. 2008. PDAP-PDTREE Package for Mesquite. Version 1.14. Available at http://mesquiteproject.org/pdap_mesquite/
- Nyakatura JA. The convergent evolution of suspensory posture and locomotion in tree sloths. J Mamm Evol. 2012;19:225–234. [Google Scholar]
- Poinar H, Kuch M, McDonald G, et al. Nuclear gene sequences from a Late Pleistocene sloth coprolite. Curr Biol. 2003;13:1150–1152. doi: 10.1016/s0960-9822(03)00450-0. [DOI] [PubMed] [Google Scholar]
- Pujos F, Gaudin TJ, De Iuliis G, et al. Recent advances on variability, morphofunctional adaptations, dental terminology, and evolution of sloths. J Mamm Evol. 2012;19:159–169. [Google Scholar]
- R Core Team. Vienna: R Foundation for Statistical Computing; 2012. R: A language and environment for statistical computing. ISBN 3-900051-07-0, URL http://www.R-project.org/ [Google Scholar]
- Ryan TM, Silcox MT, Walker A, et al. Evolution of locomotion in Anthropoidea: the semicircular canal evidence. Proc R Soc B. 2012;279:3467–3475. doi: 10.1098/rspb.2012.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-André P-A, De Iuliis G. The smallest and most ancient representative of the genus Megatherium Cuvier, 1796 (Xenarthra, Tardigrada, Megatheriidae), from the Pliocene of the Bolivian Altiplano. Geodiversitas. 2001;23:625–645. [Google Scholar]
- Schmelzle T, Sánchez-Villagra MR, Maier W. Vestibular labyrinth diversity in diprotodontian marsupial mammals. Mamm Stud. 2007;32:83–97. [Google Scholar]
- Silcox MT, Bloch JI, Boyer DM, et al. Semicircular canal system in early primates. J Hum Evol. 2009;56:315–327. doi: 10.1016/j.jhevol.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Spoor F, Zonneveld F. Morphometry of the primate bony labyrinth: a new method based on high-resolution computed tomography. J Anat. 1995;186:271–286. [PMC free article] [PubMed] [Google Scholar]
- Spoor F, Zonneveld F. Comparative review of the human bony labyrinth. Yearb Phys Anthropol. 1998;41:211–251. doi: 10.1002/(sici)1096-8644(1998)107:27+<211::aid-ajpa8>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- Spoor F, Bajpal S, Hussaim ST, et al. Vestibular evidence for the evolution of aquatic behavior in early cetaceans. Nature. 2002;417:163–166. doi: 10.1038/417163a. [DOI] [PubMed] [Google Scholar]
- Spoor F, Garland T, Jr, Krovitz G, et al. The primate semicircular canal system and locomotion. Proc Natl Acad Sci U S A. 2007;104:10808–10812. doi: 10.1073/pnas.0704250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superina M. Husbandry of a pink fairy armadillo (Chlamyphorus truncatus): case study of a cryptic and little known species in captivity. Zoo Biol. 2011;30:225–231. doi: 10.1002/zoo.20334. [DOI] [PubMed] [Google Scholar]
- Suutari M, Majaneva M, Fewer DP, et al. Molecular evidence for a diverse green algal community growing in the hair of sloths and a specific association with Trichophilus welckeri (Chlorophyta, Ulvophyceae) BMC Evol Biol. 2010;10:86. doi: 10.1186/1471-2148-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S. 4th edn. New York: Springer; 2002. [Google Scholar]
- Vizcaíno SF, Milne N. Structure and function in armadillo limbs (Mammalia: Xenarthra: Dasypodidae) J Zool. 2002;257:117–127. [Google Scholar]
- Vizcaíno SF, Bargo MS, Cassini GH. Dental occlusal surface area in relation to body mass, food habits and other biological features in fossil xenarthrans. Ameghiniana. 2006;43:11–26. [Google Scholar]
- Vizcaíno SF, Bargo MS, Fariña RA. Form, function and paleobiology in xenarthrans. In: Vizcaíno SF, Loughry WJ, editors. The Biology of the Xenarthra. Gainesville: University Press of Florida; 2008. pp. 86–99. [Google Scholar]
- Wake DB, Wake MH, Specht CD. Homoplasy: from detecting pattern to determining process and mechanism of evolution. Science. 2011;331:1032–1035. doi: 10.1126/science.1188545. [DOI] [PubMed] [Google Scholar]
- Walker A, Ryan TM, Silcox MT, et al. The semicircular canal system and locomotion: the case of extinct lemuroids and lorisoids. Evol Anthropol. 2008;17:135–145. [Google Scholar]
- Welker KL, Orkin JD, Ryan TM. Analysis of intraindividual and intraspecific variation in semicircular canal dimensions using high-resolution x-ray computed tomography. J Anat. 2009;215:444–451. doi: 10.1111/j.1469-7580.2009.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel RH. The identification and distribution of recent Xenarthra (= Edentata) In: Montgomery GG, editor. The evolution and ecology of armadillos, sloths and vermilinguas. Washington, DC: Smithsonian Institution Press; 1985a. pp. 5–21. [Google Scholar]
- Wetzel RH. Taxonomy and distribution of armadillos, Dasypodidae. In: Montgomery GG, editor. The evolution and ecology of armadillos, sloths and vermilinguas. Washington, DC: Smithsonian Institution Press; 1985b. pp. 23–46. [Google Scholar]
- Zabludoff M, Bollinger P. Giant Ground Sloth. Prehistoric Beasts Series. New York: Marshall Cavendish Benchmark; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


